Telodendrimers: Promising Architectural Polymers for Drug Delivery
Abstract
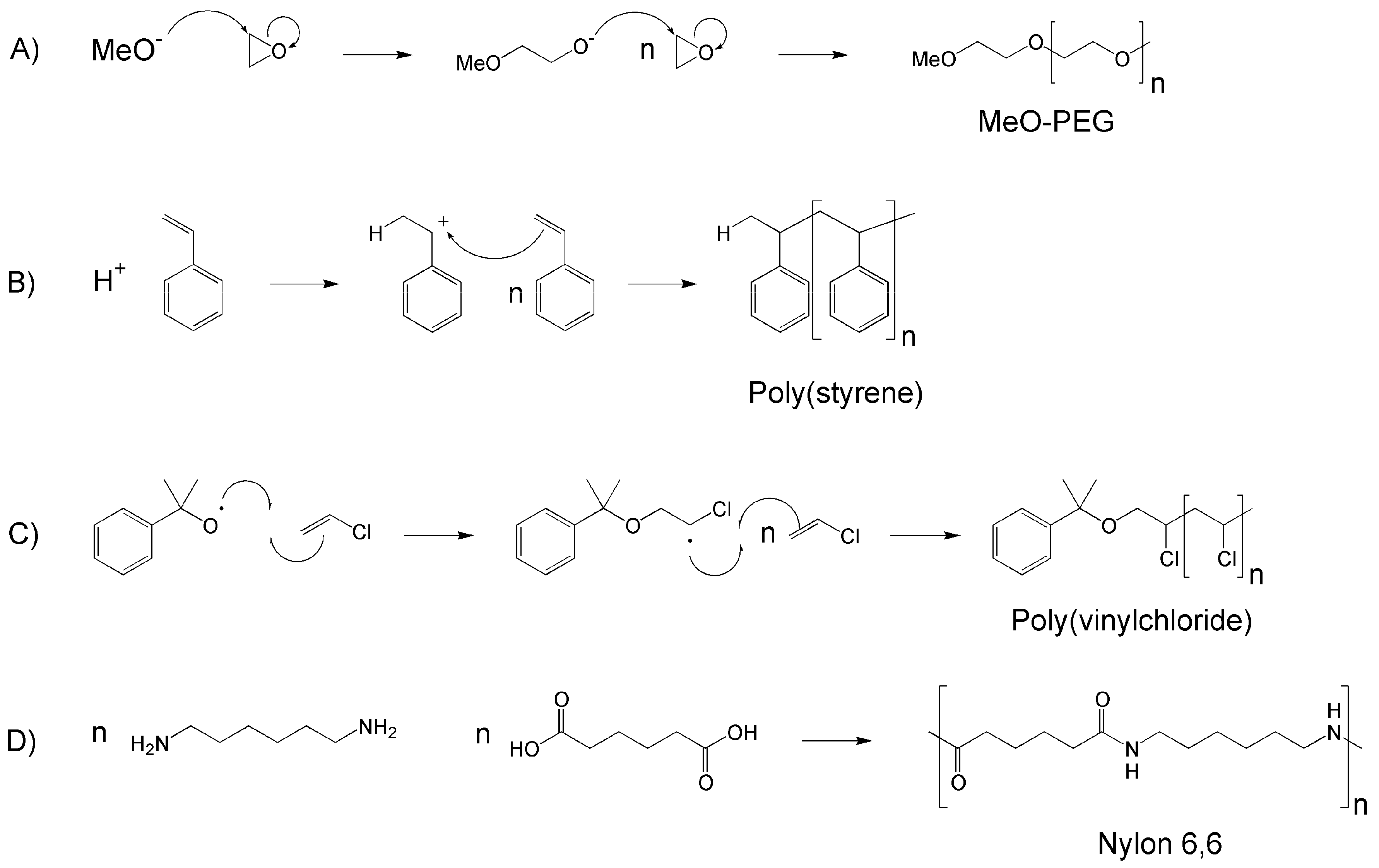
1. Introduction
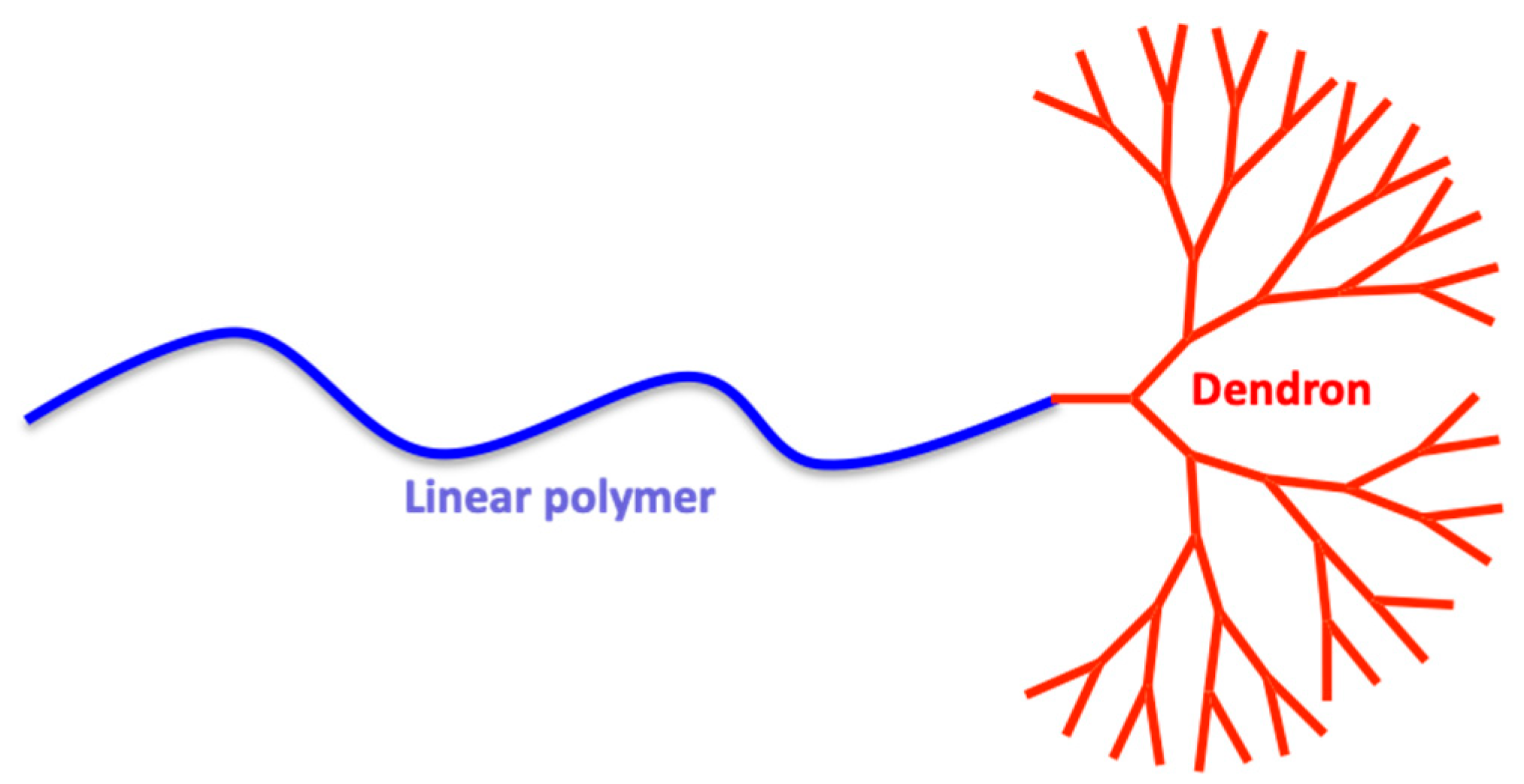
2. Dendrimers/Dendrons
3. Linear Polymers
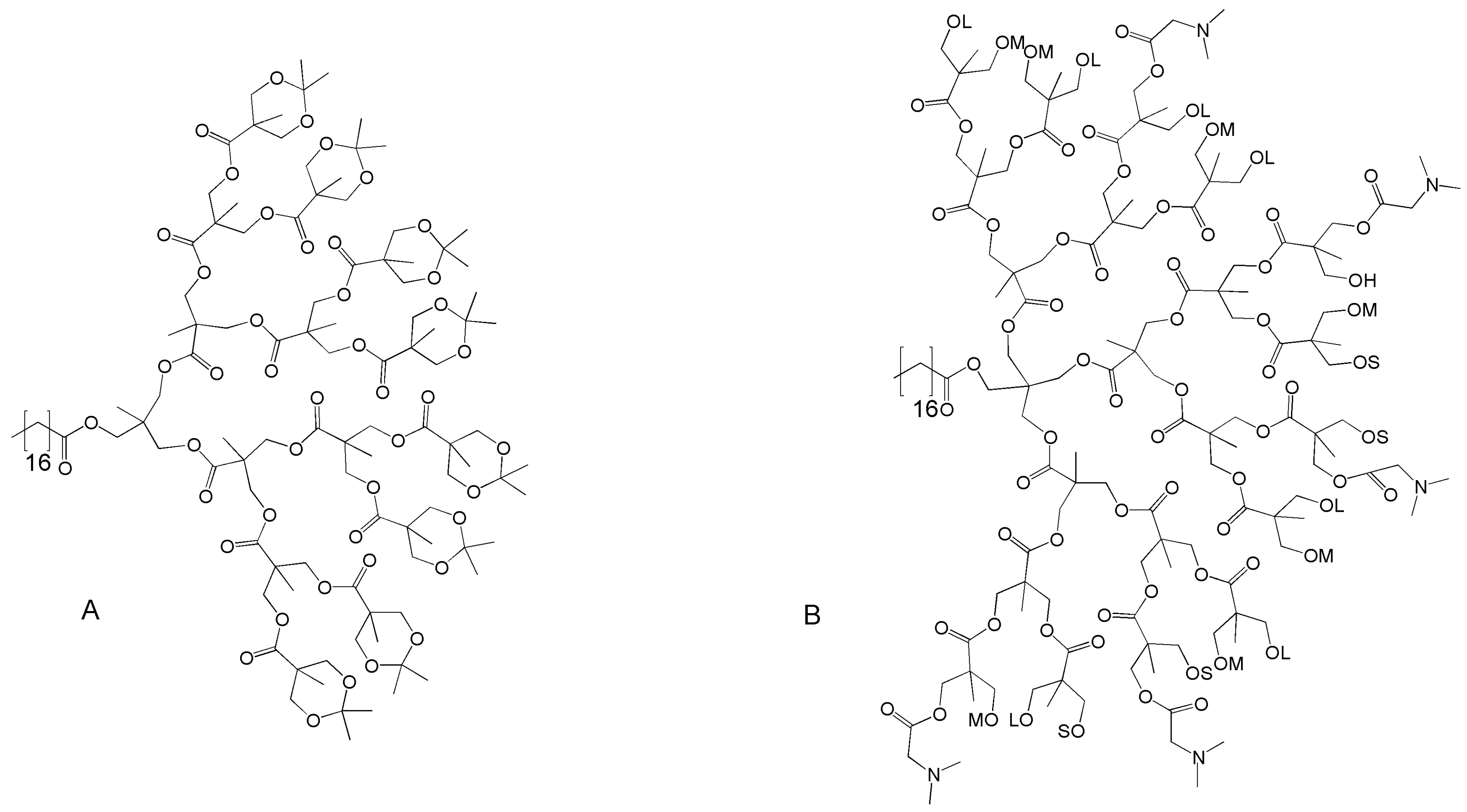
4. Telodendrimers
4.1. Telodendrimers with Reverse Hydrophobic/Hydrophilic Blocks
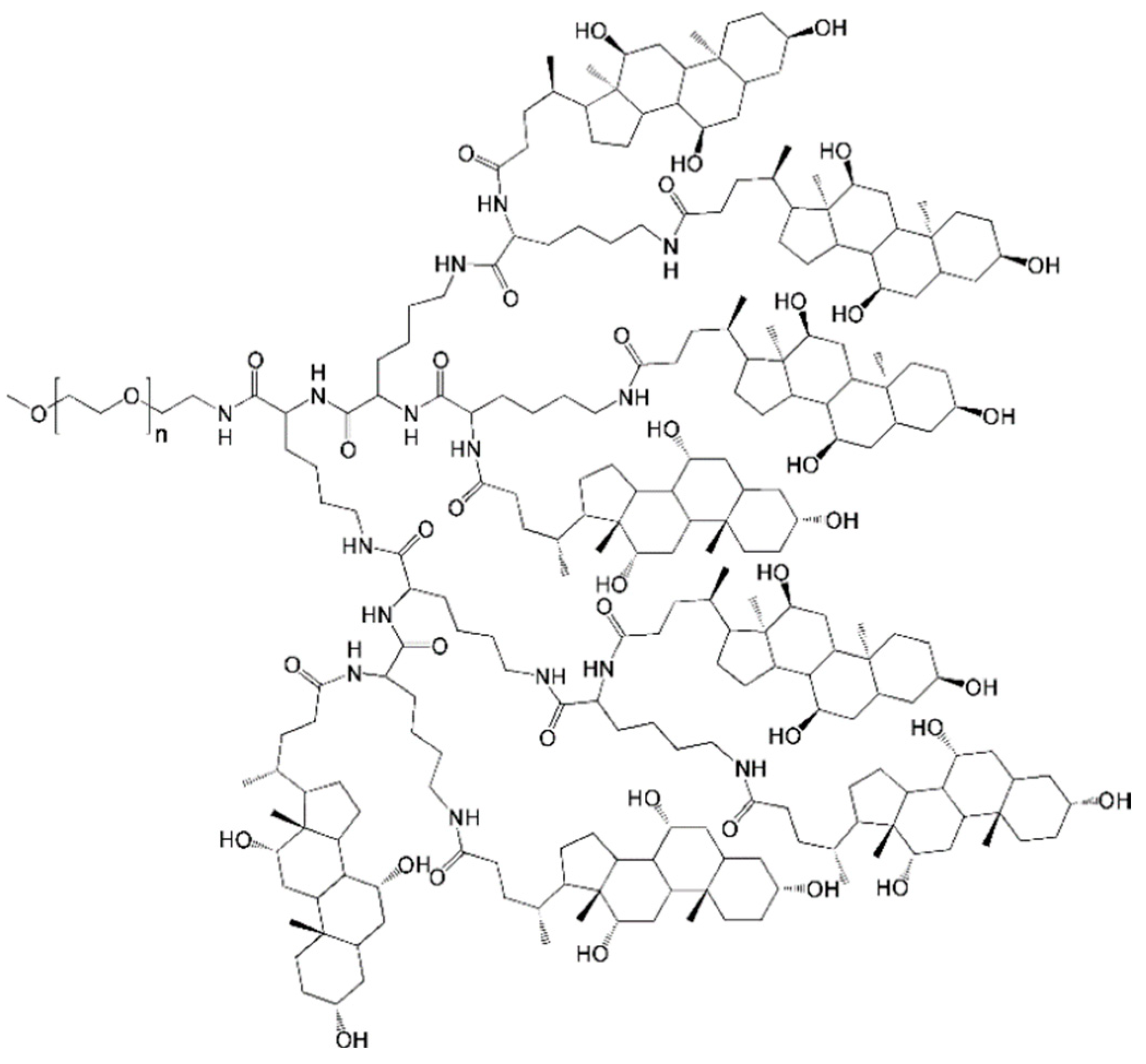
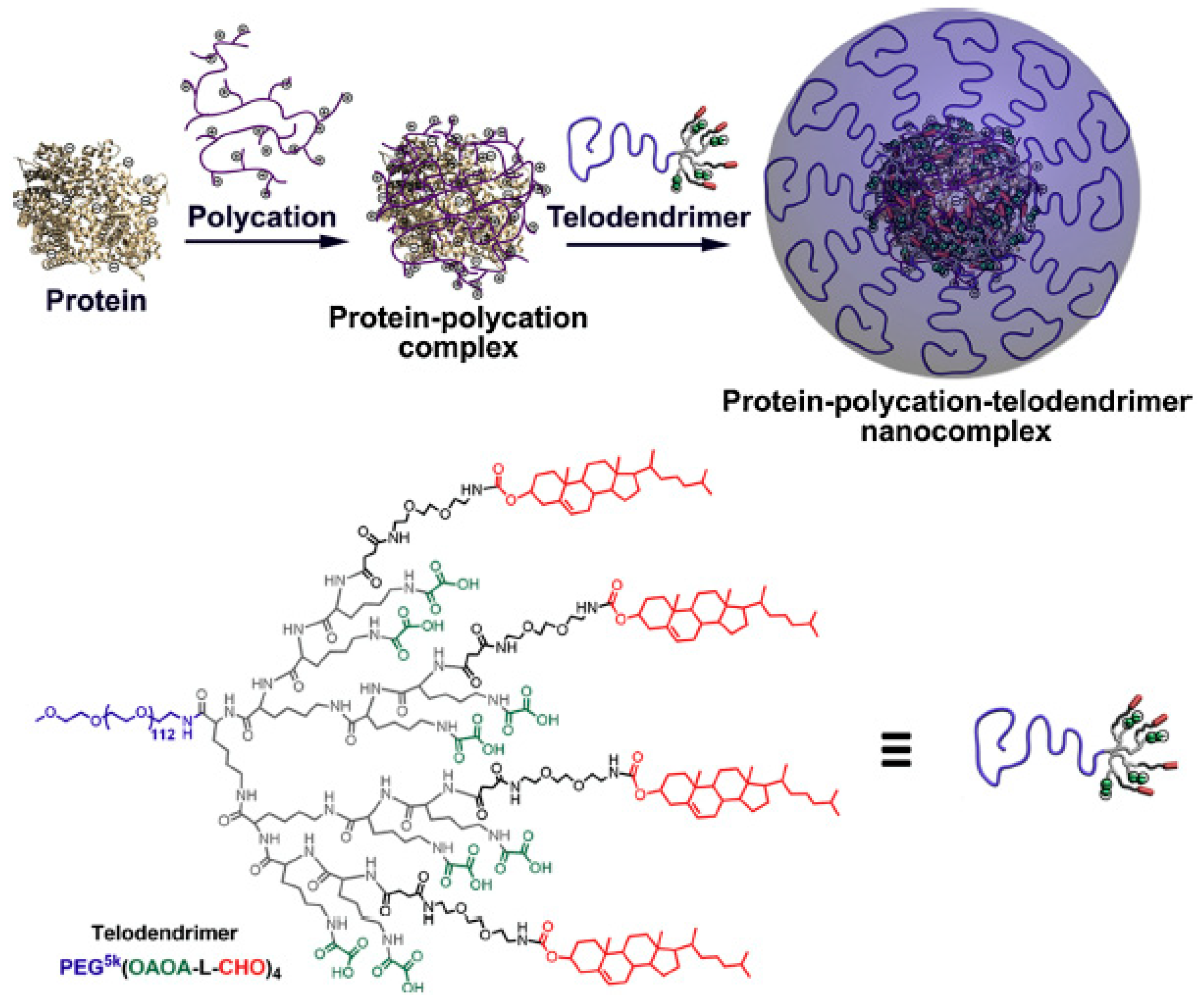
4.2. Telodendrimers in Protein Delivery and Ligand—Receptor Interactions
4.3. Passive Targeting: Accumulation Using the EPR Effect
4.4. Active Targeting Using Functional Moieties on the Telodendrimer
4.5. Telodendrimers in Controlled Release
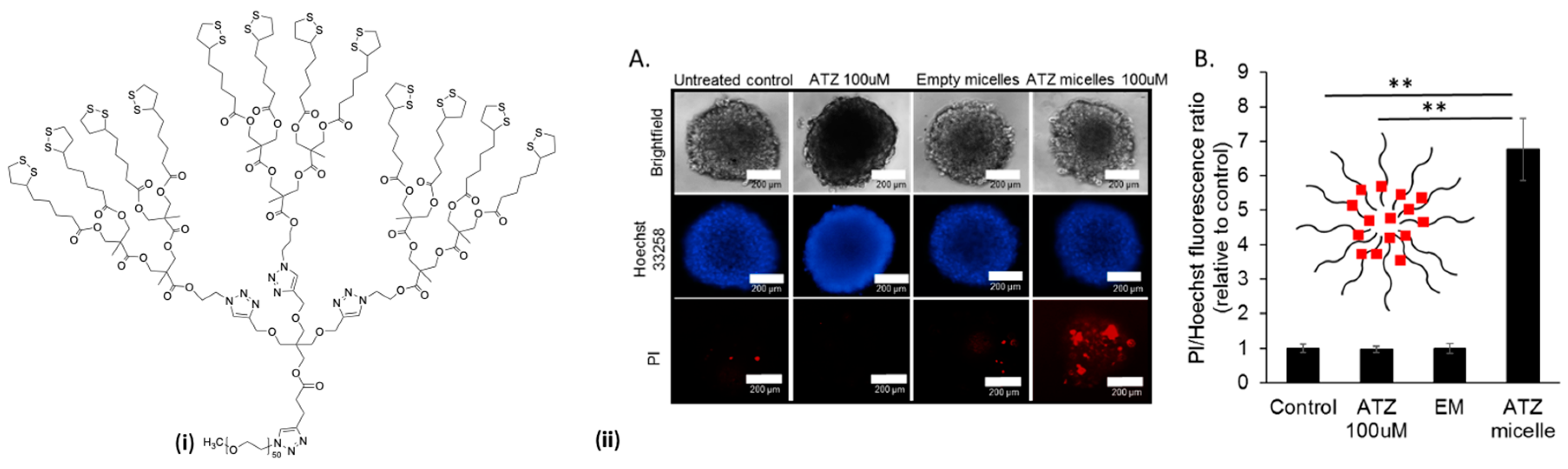
4.5.1. Disulfide Cross-Linking
4.5.2. pH-Responsive Nanocarriers
4.5.3. Enzyme-Responsive Telodendrimer Assemblies
4.6. Drugs for Combination Therapy
Telodendrimers in Drug Combination Therapy
4.7. Computer Modeling in Telodendrimer Design and Optimization
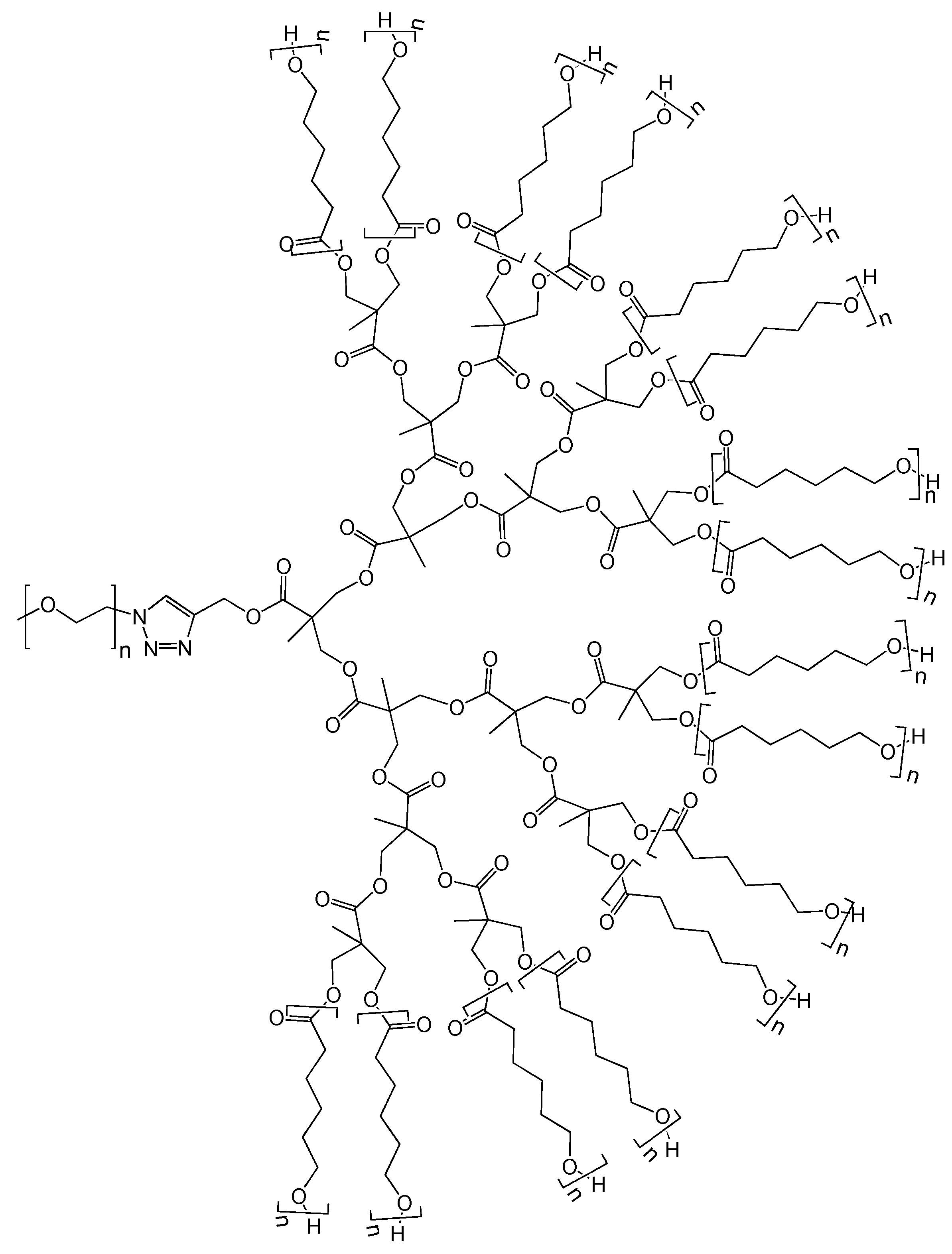
4.8. Unique Structures and Applications of Telodendrimers
5. Conclusions and Future Perspective
Funding
Conflicts of Interest
References
- Jazani, A.M.; Oh, J.K. Development and disassembly of single and multiple acid-cleavable block copolymer nanoassemblies for drug delivery. Polym. Chem. 2020, 11, 2934–2954. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, C.; Yong, T.; Li, Z.; Gan, L.; Yan, X. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem. Soc. Rev. 2020, 49, 2273–2290. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.; Traverso, G.; Farokhzad, O.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery. Nat. Rev. Chem. 2017, 1, 0063. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, H.Y.; Park, M.J. End-group chemistry and junction chemistry in polymer science: Past, present, and future. Macromolecules 2020, 53, 746–763. [Google Scholar] [CrossRef]
- Nishimori, K.; Ouchi, M. AB-alternating copolymers via chain-growth polymerization: Synthesis, characterization, self-assembly, and functions. Chem. Commun. 2020, 56, 3473–3483. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.C.; Choo, Q.; Ashtikar, M.; Rocha, T.C.; Brener-Hoffman, S.; Wacker, M.G. Nanomedicines—Tiny particles and big challenges. Adv. Drug Deliv. Rev. 2019, 151–152, 23–43. [Google Scholar] [CrossRef]
- Kakkar, A. Miktoarm Star Polymers: From basics of branched structure to synthesis, self-assembly and applications. In Polymer Chemistry Book Series; Royal Society of Chemistry: Cambridge, UK, 2017; ISBN 978-1-78262-575-9. [Google Scholar] [CrossRef]
- Khanna, K.; Varshney, S.; Kakkar, A.K. Miktoarm star polymers: Advances in synthesis, self-assembly, and applications. Polym. Chem. 2010, 1, 1171–1185. [Google Scholar] [CrossRef]
- Malkoch, M.; Gallego, S.G. Dendrimer Chemistry: Synthetic approaches towards complex architectures. In Monographs in Supramolecular Chemistry; Royal Society of Chemistry: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Moquin, A.; Sturn, J.; Zhang, I.; Ji, J.; von Celsing, R.; Vali, H.; Maysinger, D.; Kakkar, A. Unraveling aqueous self-assembly of telodendrimers to shed light on their efficacy in drug encapsulation. ACS Appl. Bio Mater. 2019, 2, 4515–4526. [Google Scholar] [CrossRef]
- Carraher, C.E., Jr. Carraher’s Polymer Chemistry, 10th ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–11. ISBN 9781498737388. [Google Scholar]
- Buhleier, E.; Wehner, W.; Vögtle, F. Cascade and nonskid-chain-like syntheses of molecular cavity topologies. Synth. Commun. 1978, 2, 155–158. [Google Scholar] [CrossRef]
- de Brabander-van den Berg, E.M.M.; Meijer, E.W. Poly(propylene imine) Dendrimers: Large-scale synthesis by heterogeneously catalyzed hydrogenations. Angew. Chem. Int. Ed. 1993, 32, 1308–1311. [Google Scholar] [CrossRef]
- Wörner, C.; Mülhaupt, R. Polynitrile- and polyamine-functional poly(trimethylene imine) dendrimers. Angew. Chem. Int. Ed. 1993, 32, 1306–1308. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic macromolecules: Synthesis of starburst dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Preparation of polymers with controlled molecular architecture. A New convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Fan, Y.; Qiu, J.; Cao, L.; Laurent, R.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P.; Shi, X. Revisiting cationic phosphorus dendrimers as a nonviral vector for optimized gene delivery towards cancer therapy applications. Biomacromolecules 2020, 21, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as pharmaceutical excipients: Synthesis, properties, toxicity and biomedical applications. Materials (Basel) 2020, 13, 65. [Google Scholar] [CrossRef]
- Zhong, D.; Wu, H.; Wu, Y.; Li, Y.; Xu, X.; Yang, J.; Gu, Z. Rational design and facile fabrication of biocompatible triple responsive dendrimeric nanocages for targeted drug delivery. Nanoscale 2019, 11, 15091–15103. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Zablocka, M.; Majoral, J.-P. Dendrimer-enabled therapeutic antisense delivery systems as innovation in medicine. Bioconjug. Chem. 2019, 30, 1938–1950. [Google Scholar] [CrossRef]
- Maciel, D.; Guerrero-Beltrán, C.; Ceña-Diez, R.; Tomás, H.; Muñoz-Fernández, M.; Rodrigues, J.M. New anionic poly(alkylideneamine) dendrimers as microbicide agents against HIV-1 infection. Nanoscale 2019, 11, 969–9690. [Google Scholar] [CrossRef]
- Ghaffari, M.; Dehghan, G.; Abedi-Gaballu, F.; Kashanian, S.; Baradaran, B.; Dolatabadi, J.E.N.; Losic, D. Surface functionalized dendrimers as controlled release delivery nanosystems for tumor targeting. Eur. J. Pharm. Sci. 2018, 122, 311–330. [Google Scholar] [CrossRef]
- Sharma, A.; Porterfield, J.E.; Smith, E.; Sharma, R.; Kannan, S.; Kannan, R.M. Effect of mannose targeting of hydroxyl PAMAM dendrimers on cellular and organ biodistribution in a neonatal brain injury model. J. Control. Release 2018, 283, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Palmerston, M.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 23, 1401. [Google Scholar] [CrossRef] [PubMed]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1–12. [Google Scholar]
- Singh, J.; Jain, K.; Mehra, N.K.; Jain, N.K. Dendrimers in anticancer drug delivery: Mechanism of interaction of drug and dendrimers. Artific. Cells Nanomed. Biotechnol. 2016, 44, 1626–1634. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Guyot, A.; Fetters, L.J. Star-branched polymers. 1. The synthesis of star polyisoprenes using octa- and dodecachlorosilanes as linking agents. Macromolecules 1978, 11, 668–672. [Google Scholar] [CrossRef]
- Denkewalter, R.G.; Kolc, J.; Lukasavage, W.J. Macromolecular Highly Branched Homogeneous Compound Based on Lysine Units. U.S. Patent 4,289,872, 15 September 1981. [Google Scholar]
- Newkome, G.R.; Yao, Z.-Q.; Baker, G.R.; Gupta, V.K. Cascade molecules: A new approach to micelles, A [27]-Arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Ihre, H.; Hult, A.; Söderlind, E. Synthesis, characterization, and 1H NMR self-diffusion studies of dendritic aliphatic polyesters based on 2,2-bis(hydroxymethyl)propionic acid and 1,1,1-tris(hydroxyphenyl)ethane. J. Am. Chem. Soc. 1996, 118, 6388–6395. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.-M.; Majoral, J.-P. Synthesis and reactivity of unusual phosphorus dendrimers. A useful divergent growth approach up to the seventh generation. J. Am. Chem. Soc. 1995, 117, 3282–3283. [Google Scholar] [CrossRef]
- Rengan, K.; Engel, R. Phosphonium cascade molecules. J. Chem. Soc. Chem. Commun. 1990, 16, 1084–1085. [Google Scholar] [CrossRef]
- Caminade, A.-M. Phosphorus dendrimers for nanomedicine. Chem. Commun. 2017, 53, 9830–9838. [Google Scholar] [CrossRef]
- Haag, R.; Sunder, A.; Stumbé, J.-F. An approach to glycerol dendrimers and pseudo-dendritic polyglycerols. J. Am. Chem. Soc. 2000, 122, 2954–2955. [Google Scholar] [CrossRef]
- Douat-Casassus, C.; Darbre, T.; Reymond, J.-L. Selective catalysis with peptide dendrimers. J. Am. Chem. Soc. 2004, 126, 7817–7826. [Google Scholar] [CrossRef]
- Aoi, K.; Itoh, K.; Okada, M. Divergent/convergent joint approach with a half-protected initiator core to synthesize surface-block dendrimers. Macromolecules 1997, 30, 8072–8074. [Google Scholar] [CrossRef]
- Antoni, P.; Hed, Y.; Nordberg, A.; Nyström, D.; von Holst, H.; Hult, A.; Malkoch, M. Bifunctional dendrimers: From robust synthesis and accelerated one-pot post-functionalization strategy to potential applications. Angew. Chem. Int. Ed. 2009, 48, 2126–2130. [Google Scholar] [CrossRef]
- Wu, P.; Feldman, A.K.; Nugent, A.K.; Hawker, C.J.; Scheel, A.; Voit, B.; Pyun, J.; Fréchet, J.M.J.; Sharpless, K.B.; Fokin, V.V. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(1)-catalyzed ligation of azides and alkynes. Angew. Chem. Int. Ed. 2004, 43, 3928–3932. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, J.H.; Kim, H.J.; Han, S.C.; Kim, J.H.; Shin, W.S.; Jin, S.-H. Synthesis of symmetrical and unsymmetrical PAMAM dendrimers by fusion between azide- and alkyne-functionalized PAMAM dendrons. Bioconjug. Chem. 2007, 18, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, B.-K.; Kim, J.H.; Shin, W.S.; Jin, S.-H. Facile approach for diblock codendrimers by fusion between Fréchet dendrons and PAMAM dendrons. J. Org. Chem. 2006, 71, 4988–4991. [Google Scholar] [CrossRef]
- Sharma, A.; Neibert, K.; Sharma, R.; Hourani, R.; Maysinger, D.; Kakkar, A. Facile construction of multifunctional nanocarriers using sequential click chemistry for applications in biology. Macromolecules 2011, 44, 521–529. [Google Scholar] [CrossRef]
- Arseneault, M.; Dufour, P.; Levesque, I.; Morin, J.-F. Synthesis of a controlled three-Faced PAMAM particle. Polym. Chem. 2011, 2, 2293–2298. [Google Scholar] [CrossRef]
- Chatani, S.; Podgórski, M.; Wang, C.; Bowman, C.N. Facile and efficient synthesis of dendrimers and one-pot preparation of dendritic-linear polymer conjugates via a single chemistry: Utilization of kinetically selective thiol-Michael addition reactions. Macromolecules 2014, 47, 4894–4900. [Google Scholar] [CrossRef]
- Ghosh, P.S.; Hamilton, A.D. Supramolecular dendrimers: Convenient synthesis by programmed self-assembly and tunable thermoresponsivity. Chem. Eur. J. 2012, 18, 2361–2365. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kim, S.-Y.; Sharma, A.; Zhang, Z.; Kambhampati, S.P.; Kannan, S.; Kannan, R.M. Activated microglia targeting dendrimer-minocycline conjugate as therapeutics for neuroinflammation. Bioconjug. Chem. 2017, 28, 2874–2886. [Google Scholar] [CrossRef]
- Anandkumar, D.; Rajakumar, P. Synthesis and anticancer activity of bile acid dendrimers with triazole as bridging unit through click chemistry. Steroids 2017, 125, 37–46. [Google Scholar] [CrossRef]
- Zolotarskaya, O.Y.; Xu, L.; Valerie, K.; Yang, H. Click Synthesis of a Polyamidoamine dendrimer-based camptothecin prodrug. RCS Adv. 2015, 5, 58600–58608. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.G.; Singh, Y.; Vamvounis, G.; Wyall, M.F.; Burn, P.L.; Blanchfield, J.T. Carbohydrate globules: Molecular asterisk-cored dendrimers for carbohydrate presentation. Polym. Chem. 2014, 5, 1173–1179. [Google Scholar] [CrossRef]
- Liu, H.; Tørring, T.; Dong, M.; Rosen, C.B.; Besenbacher, F.; Gothelf, K.V. DNA-templated covalent coupling of G4 PAMAM dendrimers. J. Am. Chem. Soc. 2010, 132, 18054–18056. [Google Scholar] [CrossRef]
- Malkoch, M.; Schleicher, K.; Drockenmuller, E.; Hawker, C.J.; Russell, T.P.; Wu, P.; Fokin, V.V. Structurally diverse dendritic libraries: A highly efficient functionalization approach using click chemistry. Macromolecules 2005, 38, 3663–3678. [Google Scholar] [CrossRef]
- García-Gallego, S.; Andrén, O.C.J.; Malkoch, M. Accelerated chemoselective reactions to sequence-controlled heterolayered dendrimers. J. Am. Chem. Soc. 2020, 142, 1501–1509. [Google Scholar] [CrossRef]
- Blomquist, A.T.; Liu, L.H. Many-membered carbon rings. VII. Cycloöctyne. J. Am. Chem. Soc. 1953, 75, 2153–2154. [Google Scholar] [CrossRef]
- Wittig, G.; Krebs, A. Zur Existenz niedergliedriger cycloalkine, I. Chem. Ber. 1961, 94, 3260–3275. [Google Scholar] [CrossRef]
- Ledin, P.A.; Friscourt, F.; Guo, J.; Boons, G.-J. Convergent assembly and surface modification of multifunctional dendrimers by three consecutive click reactions. Chem. Eur. J. 2011, 17, 839–846. [Google Scholar] [CrossRef]
- Ornelas, C.; Broichhagen, J.; Weck, M. Strain-promoted alkyne azide cycloaddition for the functionalization of poly(amide)-based dendrons and dendrimers. J. Am. Chem. Soc. 2010, 132, 3923–3931. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, M.A.; Rattan, R.; Silpe, J.; Dougherty, C.; Michmerhuizen, N.L.; van Winkle, M.; Huang, B.; Choi, S.K.; Sinniah, K.; Orr, B.G.; et al. Poly(amidoamine) dendrimer-methotrexate conjugates: The mechanism of interaction with folate binding protein. Mol. Pharm. 2014, 11, 4049–4058. [Google Scholar] [CrossRef]
- Gonzaga, F.; Sadowski, L.P.; Rambarran, T.; Grande, J.; Adronov, A.; Brook, M.A. Highly efficient divergent synthesis of dendrimers via metal-free “click” chemistry. J. Polym. Sci. A Polym. Chem. 2013, 51, 1272–1277. [Google Scholar] [CrossRef]
- Arseneault, M.; Levesque, I.; Morin, J.-F. Efficient and rapid divergent synthesis of ethylene oxide-containing dendrimers through catalyst-free chemistry. Macromolecules 2012, 45, 3687–3694. [Google Scholar] [CrossRef]
- Paolucci, V.; Mejlsøe, S.L.; Ficker, M.; Vosch, T.; Christensen, J.B. Photophysical properties of fluorescent core dendrimers controlled by size. J. Phys. Chem. B 2016, 120, 9576–9580. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ficker, M.; Mejlsøe, S.L.; Hall, A.; Paolucci, V.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Poly-(amidoamine) dendrimers with a precisely core positioned sulforhodamine B molecule for comparative biological tracing and profiling. J. Control. Release 2017, 246, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N.K. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 2003, 257, 111–124. [Google Scholar] [CrossRef]
- Liu, M.; Kono, K.; Fréchet, J.M.J. Water-soluble dendritic unimolecular micelles: Their potential as drug delivery agents. J. Control. Release 2000, 65, 121–131. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Muliawan, E.B.; Hatzikiriakos, S.G.; Brooks, D.E. Synthesis, characterization, and viscoelastic properties of high molecular weight hyperbranched polyglycerols. Macromolecules 2006, 39, 7708–7717. [Google Scholar] [CrossRef]
- Shukla, R.; Thomas, T.P.; Peters, J.; Kotlyar, A.; Myc, A.; Baker, J.R., Jr. Tumor angiogenic vasculature targeting with PAMAM dendrimer-RGD conjugates. Chem. Commun. 2005, 46, 5739–5741. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, P.; Seiler, P.; Diederich, F. Dendrophanes: Novel steroid-recognizing dendritic receptors. Preliminary communication. Helv. Chim. Acta 1996, 79, 779–788. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.C.I.M.; Gupta, U.; Greish, K.; Iyer, A. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. Positively charged polymers as promising devices against multidrug resistant gram-negative bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Oliveri, P.; Malegori, C. Assessment of the efficiency of a nanospherical gallic acid dendrimer for long-term preservation of essential oils: An integrated chemometric assisted FTIR study. ChemistrySelect 2019, 4, 8891–8901. [Google Scholar] [CrossRef]
- Huang, D.; Wu, D. Biodegradable dendrimers for drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 713–727. [Google Scholar] [CrossRef]
- Heyder, R.S.; Zhong, Q.; Bazito, R.C.; da Rocha, S.R.P. Cellular internalization and transport of biodegradable polyester dendrimers on a model of the pulmonary epithelium and their formulation in pressurized metered-dose inhalers. Int. J. Pharm. 2017, 520, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yan, S.; Osako, T.; Uozumi, Y. Batch and continuous-flow Huisgen 1,3-dipolar cycloadditions with an amphiphilic resin-supported triazine-based polyethyleneamine dendrimer copper catalyst. ACS Sustain. Chem. Eng. 2017, 5, 10722–10734. [Google Scholar] [CrossRef]
- Niu, Y.; Yeung, L.K.; Crooks, R.M. Size-selective hydrogenation of olefins by dendrimer-encapsulated palladium nanoparticles. J. Am. Chem. Soc. 2001, 123, 6840–6846. [Google Scholar] [CrossRef]
- Xu, L.; Cooper, R.C.; Wang, J.; Yeudall, W.A.; Yang, H. Synthesis and application of injectable bioorthogonal dendrimer hydrogels for local drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 1641–1653. [Google Scholar] [CrossRef]
- Yu-Juan, J.; You-Jun, L.; Guo-Ping, L.; Jie, L.; Yuan-Feng, W.; Rui-Qin, Y.; Wen-Ting, L. Application of photoluminescent CdS/PAMAM nanocomposites in fingerprint detection. Forensic Sci. Int. 2008, 179, 34–38. [Google Scholar]
- Lohse, B.; Vestberg, R.; Ivanov, M.T.; Hvilsted, S.; Berg, R.H.; Hawker, C.J.; Ramanujam, P.S. Acridizinium-substituted dendrimers as a new potential rewritable optical data storage material for blu-ray. Chem. Mater. 2008, 20, 6715–6720. [Google Scholar] [CrossRef]
- Liao, Y.; Gao, J.; Zhang, Y.; Zhou, Y.; Yuan, R.; Xu, W. Proximity ligation-responsive catalytic hairpin assembly-guided DNA dendrimers for synergistically amplified electrochemical biosensing. Sens. Actuators B Chem. 2020, 322, 128566. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.M.; Syed Putra, S.S.; Hayyan, M.; Hayyan, A.; Basirun, W.J.; Mirghani, M.E.S.; Majrashi, A.A.; Nawawi, W.M.F.W.; Alias, Y.; Mohd Salleh, H.; et al. Polyamidoamine dendrimers: Favorable polymeric nanomaterials for lipase activation. Mater. Today Commun. 2020, 25, 101492. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhao, X.; Chen, L.-J.; Yang, C.-X.; Yan, X.-P. Dendrimer grafted persistent luminescent nanoplatform for aptamer guided tumor imaging and acid-responsive drug delivery. Talanta 2020, 219, 121209. [Google Scholar] [CrossRef]
- Song, C.; Shen, M.; Rodrigues, J.; Mignani, S.; Majoral, J.-P.; Shi, X. Superstructured poly(amidoamine) dendrimer-based nanoconstructs as platforms for cancer nanomedicine: A concise review. Coord. Chem. Rev. 2020, 421, 213463. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H.; Salehi, R. Synthesis of photoluminescent glycodendrimer with terminal β-cyclodextrin molecules as a biocompatible pH-sensitive carrier for doxorubicin delivery. Carbohydr. Polym. 2020, 246, 116658. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, W.; Ding, B.; Chen, D.; Cheng, L. cRGD mediated redox and pH dual responsive poly(amidoamine) dendrimer-poly(ethylene glycol) conjugates for efficiently intracellular antitumor drug delivery. Colloid. Surfaces B 2020, 194, 111195. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Liu, S.; Wei, T.; Cheng, Q.; Siegwart, D.J. Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo. J. Control. Release 2020, 325, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Srinivasa Reddy, T.; Kulhari, H.; Kadari, A.; Adams, D.J.; Bansal, V.; Sistla, R. N-acetyl-d-glucosamine-conjugated PAMAM dendrimers as dual receptor-targeting nanocarriers for anticancer drug delivery. Eur. J. Pharm. Biopharm. 2020, 154, 377–386. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.; Yao, M.; Palmerston, M.; Sarisozen, C.; Mao, S.; Torchilin, V.P. Charge reversible hyaluronic acid-modified dendrimer-based nanoparticles for siMDR-1 and doxorubicin co-delivery. Eur. J. Pharm. Biopharm. 2020, 154, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kambhampati, S.P.; Zhang, Z.; Sharma, A.; Chen, C.; Duh, E.I.; Kannan, S.; Tso, M.O.M.; Kannan, R.M. Dendrimer mediated targeted delivery of sinomenine for the treatment of acute neuroinflammation in traumatic brain injury. J. Control. Release 2020, 325, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.C.; Maier, J.D.; Vetter, J.; Podvalnyy, N.; Khanzhin, N.; Hennet, T.; Stertz, S. Antiviral potential of 3′-sialyllactose- and 6′-sialyllactose-conjugated dendritic polymers against human and avian influenza viruses. Sci. Rep. 2020, 10, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Lin, X.; Valle, R.P.; Zuo, Y.Y.; Gu, N. Poly(amidoamine) dendrimer as a respiratory nanocarrier: Insights from experiments and molecular dynamics simulations. Langmuir 2019, 35, 5364–5371. [Google Scholar] [CrossRef]
- Juárez-Chávez, L.; Pina-Canseco, S.; Soto-Castro, D.; Santillan, R.; Magaña-Vergara, N.E.; Salazar-Schettino, P.M.; Cabrera-Bravo, M.; Pérez-Campos, E. In vitro activity of steroidal dendrimers on trypanosoma cruzi epimastigote form with PAMAM dendrons modified by “click” chemistry. Bioorg. Chem. 2019, 86, 452–458. [Google Scholar] [CrossRef]
- Patil, N.G.; Augustine, R.; Zhang, Y.; Hong, S.C.; Kim, I. Synthesis of stimuli-responsive heterofunctional dendrimer by Passerini multicomponent reaction. ACS Omega 2019, 4, 6660–6668. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Davis, T.P.; Ke, P.C.; Wu, Y.; Ding, F. Understanding effects of PAMAM dendrimer size and surface chemistry on serum protein binding with discrete molecular dynamics simulations. ACS Sustain. Chem. Eng. 2018, 6, 11704–11715. [Google Scholar] [CrossRef]
- Diaz, C.; Benitez, C.; Vidal, F.; Barraza, L.F.; Jiménez, V.A.; Guzman, L.; Fuentealba, J.; Yevenes, G.E.; Alderete, J.B. Cytotoxicity and in vivo plasma kinetic behavior of surface-functionalized PAMAM dendrimers. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2227–2234. [Google Scholar] [CrossRef]
- Najlah, M.; Freeman, S.; Khoder, M.; Attwood, D.; D’Emanuele, A. In vitro evaluation of third generation PAMAM dendrimer conjugates. Molecules 2017, 22, 1661. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. Coordination chemistry with phosphorus dendrimers. Applications as catalysts, for materials, and in biology. Coord. Chem. Rev. 2016, 308, 478–497. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015, 44, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Yañez, C.; Rodriguez, C.C. Dendrimers: Amazing platforms for bioactive molecule delivery. Materials 2020, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Ekladious, I.; Colson, Y.L.; Grinstaf, M.W. Polymer-drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and characterization of versatile amphiphilic dendrimers peripherally decorated with positively charged amino acids. Polym. Int. 2018, 11, 1572–1584. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and characterization of fourth generation polyester-based dendrimers with cationic amino acids-modified crown as promising water soluble biomedical devices. Polym. Adv. Technol. 2018, 29, 2735–2749. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Ponassi, M.; Rosano, C.; Zoppi, V.; Spallarossa, A. Hydrophilic and amphiphilic water-soluble dendrimer prodrugs suitable for parenteral administration of a non-soluble non-nucleoside HIV-1 reverse transcriptase inhibitor thiocarbamate derivative. Eur. J. Pharm. Sci. 2018, 124, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Castellaro, S. Synthesis and characterization of polyester-based dendrimers containing peripheral arginine or mixed amino acids as potential vectors for gene and drug delivery. Macromol. Res. 2017, 25, 1172–1186. [Google Scholar] [CrossRef]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of ellagic acid micro and nano formulations with amazingly increased water solubility by its entrapment in pectin or non-PAMAM dendrimers suitable for clinical applications. New J. Chem. 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-based dendrimer nanoparticles combined with etoposide have an improved cytotoxic and pro-oxidant effect on human neuroblastoma cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress”. Drug Deliv. Transl. Res. 2020, 10, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1997, 252, 3582–3586. [Google Scholar]
- Wu, B.; Yu, P.; Cui, C.; Wu, M.; Zhang, Y.; Liu, L.; Wang, C.-X.; Zhuo, R.-X.; Huang, S.-W. Folate-containing reduction-sensitive lipid-polymer hybrid nanoparticles for targeted delivery of doxorubicin. Biomater. Sci. 2015, 3, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Eto, Y.; Yasuo, Y.; Yohei, M.; Naoki, O.; Shinsaku, N. Development of PEGylated adenovirus vector with targeting ligand. Int. J. Pharm. 2008, 354, 3–8. [Google Scholar] [CrossRef]
- Thakur, S.; Kesharwani, P.; Tekade, R.K.; Jain, N.K. Impact of PEGylation on biopharmaceutical properties of dendrimers. Polymer 2015, 59, 67–92. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Verhoef, J.J.F.; Anchordoquy, T.J. Questioning the use of PEGylation for drug delivery. Drug Deliv. Transl. Res. 2013, 3, 499–503. [Google Scholar] [CrossRef]
- Shiraishi, K.; Yokoyama, M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: A review. Sci. Technol. Adv. Mater. 2019, 20, 324–336. [Google Scholar] [CrossRef]
- Neun, B.W.; Barenholz, Y.; Szebeni, J.; Dobrovolskaia, M.A. Understanding the role of anti-PEG antibodies in the complement activation by Doxil in vitro. Molecules 2018, 23, 1700–1719. [Google Scholar] [CrossRef]
- Armstrong, J.K.; Hempel, G.; Koling, S.; Chan, L.S.; Fisher, T.; Meiselman, H.J.; Garratty, G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 2007, 110, 103–111. [Google Scholar] [CrossRef]
- Silva, D.M.; Paleco, R.; Traini, D.; Sencadas, V. Development of ciprofloxacin-loaded poly(vinyl alcohol) dry powder formulations for lung delivery. Int. J. Pharm. 2018, 547, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.N.; Lee, H.J.; Kim, S.E.; Chang, J.H.; Park, C.; Kim, C.; Park, J.H.; Lee, S.C. Disulfide-cross-linked PEG-poly(amino acid)s copolymer micelles for glutathione-mediated intracellular drug delivery. Chem. Commun. 2008, 48, 6570–6572. [Google Scholar] [CrossRef]
- Kopeček, J.; Kopečková, P. HPMA Copolymers: Origins, early developments, present, and future. Adv. Drug Deliv. Rev. 2010, 62, 122–149. [Google Scholar] [CrossRef] [PubMed]
- Luss, A.L.; Kulikov, P.P.; Romme, S.B.; Andersen, C.L.; Pennisi, C.P.; Docea, A.O.; Kuskov, A.N.; Velonia, K.; Mezhuev, Y.O.; Shtilman, M.I.; et al. Nanosized carriers based on amphiphilic poly-N-vinyl-2-pyrrolidone for intranuclear drug delivery. Nanomedicine (Lond. Engl.) 2018, 13, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Li, L.; Bai, Z.; Levkin, P.A. Boronate-dextran: An acid-responsive biodegradable polymer for drug delivery. Biomaterials 2013, 34, 8504–8510. [Google Scholar] [CrossRef]
- Sun, H.; Guo, B.; Li, X.; Cheng, R.; Meng, F.; Liu, H.; Zhong, Z. Shell-sheddable micelles based on dextran-SS-poly(ε-caprolactone) diblock copolymer for efficient intracellular release of doxorubicin. Biomacromolecules 2010, 11, 848–854. [Google Scholar] [CrossRef]
- Li, B.; Wang, Q.; Wang, X.; Wang, C.; Jiang, X. Preparation, drug release and cellular uptake of doxorubicin-loaded dextran-b-poly(ε-caprolactone) nanoparticles. Carbohydr. Polym. 2013, 93, 430–437. [Google Scholar] [CrossRef]
- Schatz, C.; Louguet, S.; Le Meins, J.-F.; Lecommendoux, S. Polysaccharide-block-polypeptide copolymer vesicles: Towards synthetic viral capsids. Angew. Chem. Int. Ed. 2009, 48, 2572–2575. [Google Scholar] [CrossRef]
- Ossipov, D.A. Nanostructured hyaluronic acid-based material for active delivery to cancer. Expert Opinion Drug Deliv. 2010, 7, 681–703. [Google Scholar] [CrossRef]
- Pan, H.; Yang, J.; Kopečková, P.; Kopeček, J. Backbone degradable mulitblock N-(2-hydroxypropyl)methacrylamide copolymer conjugates via reversible addition-fragmentation chain transfer polymerization and thiol-ene coupling reaction. Biomacromolecules 2011, 12, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Cambón, A.; Rey-Rico, A.; Mistry, D.; Brea, J.; Loza, M.I.; Attwood, D.; Barbosa, S.; Alvarez-Lorenzo, C.; Concheiro, A.; Taboada, P.; et al. Doxorubicin-loaded micelles of reverse poly(butylene oxide)-poly(ethylene oxide)-poly(butylene oxide) block copolymers as efficient “active” chemotherapeutic agents. Int. J. Pharm. 2013, 10, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Pottanam Chali, S.; Ravoo, B.J. Polymer nanocontainers for intracellular delivery. Angew. Chem. Int. Ed. 2020, 59, 2962–2972. [Google Scholar] [CrossRef]
- Shunmugam, R.; Sathyan, A.; Mane, S.R. Biomedical applications of pH-responsive amphiphilic polymer nanoassemblies. ACS Appl. Nano Mater. 2020, 3, 2104–2117. [Google Scholar]
- Langer, R.; Cortinas, A.B.; Patel, A.K.; Delcassian, D. Drug delivery across length scales. J. Drug Target. 2019, 27, 229–243. [Google Scholar]
- Morris, M.A.; Fleury, G.; Ponsinet, V.; Walsh, J.J.; Lundy, R.; Cummins, C. Enabling future nanomanufacturing through block copolymer self-assembly: A review. Nano Today 2020, 35, 100936. [Google Scholar]
- Foster, J.C.; O’Reilly, R.K.; Arkinstall, L.A.; Lawrenson, S.B.; Varlas, S. Self-assembled nanostructures from amphiphilic block copolymers prepared by ring-opening metathesis polymerisation (ROMP). Prog. Polym. Sci. 2020, 107, 101278. [Google Scholar]
- Nottelet, B.; Garric, X.; Bakkour, Y.; Buwalda, S.J.; El Jundi, A. Double hydrophilic block copolymers self-assemblies in biomedical applications. Adv. Colloid Interface Sci. 2020, 283, 1002213. [Google Scholar]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. The synthesis and polymerization of a hyperbranched polyether macromonomer. Polymer 1992, 33, 1507. [Google Scholar] [CrossRef]
- Gitsov, I.; Wooley, K.L.; Fréchet, J.M.J. Novel polyether copolymers consisting of linear and dendritic blocks. Angew. Chem. Int. Ed. 1992, 31, 1200–1202. [Google Scholar] [CrossRef]
- Gitsov, I.; Fréchet, J.M.J. Solution and solid-state properties of hybrid linear-dendritic block copolymers. Macromolecules 1993, 26, 6536–6546. [Google Scholar] [CrossRef]
- Gitsov, I.; Wooley, K.L.; Hawker, C.J.; Ivanova, P.T.; Fréchet, J.M.J. Synthesis and properties of novel linear-dendritic block copolymers. Reactivity of dendritic macromolecules toward Linear polymers. Macromolecules 1993, 26, 5621–5627. [Google Scholar] [CrossRef]
- Gitsov, I.; Ivanova, P.T.; Fréchet, J.M.J. Dendrimers as macroinitiators for anionic ring-opening polymerization. Polymerization of ε-caprolactone. Macromol. Rapid Commun. 1994, 15, 387–393. [Google Scholar] [CrossRef]
- Gitsov, I.; Simonyan, A.; Vladimirov, N.G. Synthesis of novel asymmetric dendritic-linear-dendritic block copolymers via “living” anionic polymerization of ethylene oxide initiated by dendritic macroinitiators. J. Polym. Sci. A Polym. Chem. 2007, 45, 5136–5148. [Google Scholar] [CrossRef]
- Chapman, T.M.; Hillyer, G.L.; Mahan, E.J.; Shaffer, K.A. Hydraamphiphiles: Novel linear dendritic block copolymer surfactants. J. Am. Chem. Soc. 1994, 116, 11195–11196. [Google Scholar] [CrossRef]
- van Hest, J.C.M.; Delnoye, D.A.P.; Baars, M.W.P.L.; van Genderen, M.H.P.; Meijer, E.W. Polystyrene-dendrimer amphiphilic block copolymers with a generation-dependent aggregation. Science 1995, 268, 1592–1595. [Google Scholar] [CrossRef]
- van Hest, J.C.M.; Delnoye, D.A.P.; Baars, M.W.P.L.; Elissen-Román, C.; van Genderen, M.H.P.; Meijer, E.W. Polystyrene-poly(propylene imine) dendrimers: Synthesis, characterization, and association behavior of a new class of amphiphiles. Chem. Eur. J. 1996, 2, 1616–1626. [Google Scholar] [CrossRef]
- Wurm, F.; Nieberle, J.; Frey, H. Synthesis and characterization of poly(glyceryl glycerol) block copolymers. Macromolecules 2008, 41, 1909–1911. [Google Scholar] [CrossRef]
- Wurm, F.; Schüle, H.; Frey, H. Amphiphilic Linear-hyperbranched block copolymers with linear poly(ethylene oxide) and hyperbranched poly(carbosilane) block. Macromolecules 2008, 42, 9602–9611. [Google Scholar] [CrossRef]
- Guo, D.; Shi, C.; Wang, L.; Ji, X.; Zhang, S.; Luo, J. Rationally designed micellar nanocarriers for the delivery of hydrophilic methotrexate in Psoriasis treatment. ACS Appl. Bio Mater. 2020, 3, 4832–4846. [Google Scholar] [CrossRef]
- Shi, C.; Wang, X.; Wang, L.; Meng, Q.; Guo, D.; Chen, L.; Dai, M.; Wang, G.; Cooney, R.; Luo, J. A nanotrap improves survival in severe sepsis by attenuating hyperinflammation. Nat. Commun. 2020, 11, 3384–3397. [Google Scholar] [CrossRef] [PubMed]
- Calik, F.; Degirmenci, A.; Eceoglu, M.; Sanyal, A.; Sanyal, R. Dendron-polymer conjugate based cross-linked micelles: A robust and versatile nanosystem for targeted deliver. Bioconjug. Chem. 2019, 30, 1087–1097. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Zhu, Y.; Li, Y.; Zhang, H.; Ma, A.-H.; Long, Q.; Keck, J.; Lam, K.S.; Pan, C.-X.; Jonas, B.A. Daunorubicin-containing CLL1-targeting nanomicelles have anti-leukemia stem cell activity in acute myeloid leukemia. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102004. [Google Scholar] [CrossRef]
- Bolu, B.S.; Sanyal, R.; Sanyal, A. Drug delivery systems for self-assembly of dendron-polymer conjugates. Molecules 2018, 23, 1570. [Google Scholar] [CrossRef]
- Bolu, B.S.; Golba, B.; Boke, N.; Sanyal, A.; Sanyal, R. Designing dendron-polymer conjugate based targeted drug delivery platforms with a mix-and-match modularity. Bioconjug. Chem. 2017, 28, 2962–2975. [Google Scholar] [CrossRef]
- Franco, M.S.; Oliveira, M.C. Ratiometric drug delivery using non-liposomal nanocarriers as an approach to increase efficacy and safety of combination chemotherapy. Biomed. Pharmacother. 2017, 96, 58–595. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Zhang, L.; Lin, M.Y.; Guo, D.; Wang, L.; Yang, Y.; Duncan, T.M.; Luo, J. Structure-based nanocarrier design for protein delivery. ACS Macro Lett. 2017, 6, 267–271. [Google Scholar] [CrossRef]
- Kenyon, N.J.; Bratt, J.M.; Lee, J.; Luo, J.; Franzi, L.M.; Zeki, A.A.; Lam, K.S. Self-assembling nanoparticles containing dexamethasone as a novel therapy in allergic airways inflammation. PLoS ONE 2013, 8, e77730. [Google Scholar] [CrossRef]
- Wang, X.-S.; Kong, D.-J.; Lin, T.-Y.; Li, X.-C.; Izumiya, Y.; Ding, X.-Z.; Zhang, L.; Hu, X.-C.; Yang, J.-Q.; Gao, S.-G.; et al. A versatile nanoplatform for synergistic combination therapy to treat human esophageal cancer. Acta Pharmacol. Sin. 2017, 38, 931–942. [Google Scholar] [CrossRef]
- Xue, X.; Bo, R.; Qu, H.; Jia, B.; Xiao, W.; Yuan, Y.; Vapniarsky, N.; Lindstrom, A.; Wu, H.; Zhang, D.; et al. A nephrotoxicity-free, iron-based contrast agent for magnetic resonance imaging of tumors. Biomaterials 2020, 257, 120234. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, W.; Xiao, W.; Carney, R.P.; Men, Y.; Li, Y.; Quon, G.; Ajena, Y.; Lam, K.S.; Pan, T. A Plug-and-play, drug-on-pillar platform for combination drug screening implemented by microfluidic adaptive printing. Anal. Chem. 2018, 90, 13969–13977. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, X. Telodendrimers for the delivery of therapeutic proteins. Mater. China 2018, 37, 273–281. [Google Scholar]
- Pan, A.; Zhang, H.; Li, Y.; Lin, T.-Y.; Wang, F.; Lee, J.; Cheng, M.; Dall’Era, M.; Li, T.; Devere White, R.; et al. Disulfide-crosslinked nanomicelles confer cancer-specific drug delivery and improve efficacy of paclitaxel in bladder cancer. Nanotechnology 2016, 27, 425103. [Google Scholar] [CrossRef]
- Shi, C.; Guo, D.; Xiao, K.; Wang, X.; Wang, L.; Luo, J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015, 6, 7449–7749. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Zhang, H.; Luo, J.; Li, Y.; Gao, T.; Lara, P.N., Jr.; White, R.V.; Lam, K.S.; Pan, C.-X. Multifunctional targeting micelle nanocarriers with both imaging and therapeutic potential for bladder cancer. Int. J. Nanomedicine 2012, 7, 2793–2804. [Google Scholar]
- Xiao, K.; Luo, J.; Li, Y.; Xiao, W.; Lee, J.S.; Gonik, A.M.; Lam, K.S. From Combinatorial Chemistry to Cancer Targeting Nanotherapeutics. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/7679/767909/From-combinatorial-chemistry-to-cancer-targeting-nanotherapeutics/10.1117/12.851931.short?SSO=1 (accessed on 30 August 2020).
- Xiao, K.; Luo, J.; Fowler, W.; Li, Y.; Lee, J.; Wang, L.; Lam, K.S. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials 2009, 30, 6006–6016. [Google Scholar] [CrossRef]
- Luo, J.; Xiao, K.; Li, Y.; Lee, J.S.; Shi, L.; Tan, Y.-H.; Xing, L.; Cheng, R.H.; Liu, G.-Y.; Lam, K.S. Well-defined, size-tunable, multi-functional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjug. Chem. 2010, 21, 1216–1224. [Google Scholar] [CrossRef]
- Bae, J.W.; Pearson, R.M.; Patra, N.; Sunoqrot, S.; Vuković, L.; Král, P.; Hong, S. Dendron-mediated self-assembly of highly PEGylated block copolymers: A modular nanocarrier platform. Chem. Commun. 2011, 47, 10302–10304. [Google Scholar] [CrossRef]
- Tian, L.; Hammond, P.T. Comb-dendritic block copolymers as tree-shaped macromolecular amphiphiles for nanoparticles self-Assembly. Chem. Mater. 2006, 18, 3976–3984. [Google Scholar] [CrossRef]
- Lundberg, P.; Walter, M.V.; Montañez, M.I.; Hult, D.; Hult, A.; Nyström, A.; Malkoch, M. Linear dendritic polymeric amphiphils with intrinsic biocompatibility: Synthesis and characterization to fabrication of micelles and honeycomb membranes. Polym. Chem. 2011, 2, 394–402. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, X.; Jin, E.; Tang, J.; Sui, M.; Shen, Y.; van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials 2013, 34, 5722–5735. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Moquin, A.; Bomal, E.; Na, L.; Maysinger, D.; Kakkar, A. Telodendrimers for physical encapsulation and covalent linking of individual or combined therapeutics. Mol. Pharm. 2017, 14, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Poon, Z.; Chen, S.; Engler, A.C.; Lee, H.; Atas, E.; von Maltzahn, G.; Bhatia, S.N.; Hammond, P.T. Ligand-clustered “patchy” nanoparticles for modulated cellular uptake and in vivo tumor targeting. Angew. Chem. Int. Ed. 2010, 49, 7266–7270. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Zhang, L.; Bodman, A.; Guo, D.; Wang, L.; Hall, W.A.; Wilkens, S.; Luo, J. Affinity-controlled protein encapsulation into sub-30 nm telodendrimer nanocarriers by multivalent and synergistic interactions. Biomaterials 2016, 101, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, C.; Wang, L.; Luo, J. Polycation-telodendrimers nanocomplexes for intracellular protein delivery. Colloid. Surf. B Biointerfaces 2018, 162, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Megia, E.; Correa, J.; Riguera, R. “Clickable” PEG-dendritic block copolymers. Biomacromolecules 2006, 7, 3104–3111. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar]
- Li, Y.; Xiao, K.; Luo, J.; Lee, J.; Pan, S.; Lam, K.S. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. J. Control. Release 2010, 144, 314–323. [Google Scholar] [CrossRef]
- Xiao, K.; Luo, J.; Li, Y.; Lee, J.S.; Fung, G.; Lam, K.S. PEG-oligocholic acid telodendrimer micelles for the targeted delivery of doxorubicin to B-Cell lymphoma. J. Control. Release 2011, 155, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Zhao, W.; Chen, Y.; Zhang, P.; Li, J.; Venkataramanan, R.; Li, S. PEG-farnesyl thiosalicylic acid telodendrimer micelles as an improved formulation for targeted delivery of paclitaxel. Mol. Pharm. 2014, 11, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
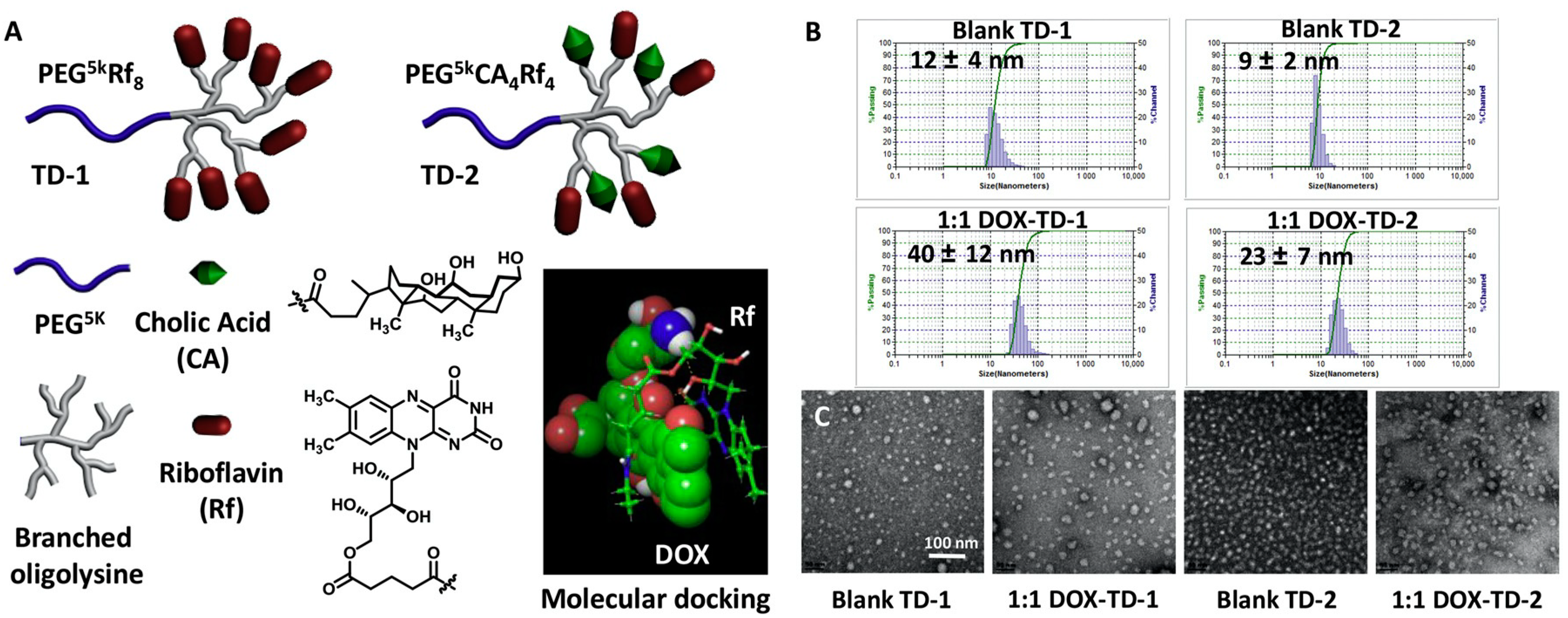
- Guo, D.; Shi, C.; Wang, X.; Wang, L.; Zhang, S.; Luo, J. Riboflavin-containing telodendrimer nanocarriers for efficient doxorubicin delivery: High loading capacity, increased stability, and improved anticancer efficacy. Biomaterials 2017, 141, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, J.; Li, Y.; Henderson, P.T.; Wang, Y.; Wachsmann-Hogiu, S.; Zhao, W.; Lam, K.S.; Pan, C.-X. Characterization of high-affinity peptides and their feasibility for use in nanotherapeutics targeting leukemia stem cells. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Li, Y.; Lee, J.S.; Gonik, A.M.; Dong, T.; Fung, G.; Sanchez, E.; Xing, L.; Cheng, H.R.; Luo, J.; et al. “OA02” Peptide facilitates the precise targeting of paclitaxel-loaded micellar nanoparticles to ovarian cancer in vivo. Cancer Res. 2012, 72, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Lin, T.-Y.; Xiao, K.; Haddad, A.S.; Henderson, P.T.; Jonas, B.A.; Chen, M.; Xiao, W.; Liu, R.; et al. Nanomicelle formulation modifies the pharmacokinetic profile and cardiac toxicity of daunorubicin. Nanomedicine (Lond.) 2014, 9, 1807–1820. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Li, Y.; Liu, Q.; Chen, J.-L.; Zhang, H.; Lac, D.; Zhang, H.; Ferrara, K.W.; Wachsmann-Hogiu, S.; Li, T.; et al. Novel theranostic nanoporphyrins for photodynamic diagnosis and trimodal therapy for bladder cancer. Biomaterials 2016, 104, 339–351. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Li, Y.-P.; Zhang, H.; Luo, J.; Goodwin, N.; Gao, T.; de Vere White, R.; Lam, K.S.; Pan, C.-X. Tumor-targeting multifunctional micelles for imaging and chemotherapy of advanced bladder cancer. Nanomedicine 2013, 8, 1239–1251. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, K.; Luo, J.; Xiao, W.; Lee, J.S.; Gonik, A.M.; Kato, J.; Dong, T.A.; Lam, K.S. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials 2011, 32, 6633–6645. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, Q.; Awwad, N.A.; Zhang, H.; Lai, L.; Luo, Y.; Lee, J.S.; Li, Y.; Lam, K.S. Reversible disulfide cross-linked micelles improve the pharmacokinetics and facilitate the target, on-demand delivery of doxorubicin in the treatment of B-cell lymphoma. Nanoscale 2018, 10, 8207–8216. [Google Scholar] [CrossRef]
- Xiao, K.; Lin, T.; Lam, K.S.; Li, Y. A facile strategy for fine-tuning the stability and drug release of stimuli-responsive cross-linked micellar nanoparticles towards precision drug delivery. Nanoscale 2017, 9, 7765–7770. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, D.; Wang, L.; Sun, Y.; Li, J.J.; Hill, B.; Yang, F.; Li, Y.; Lam, K.S. Unique photochemo-immuno-nanoplatform against orthotopic xenograft oral cancer and metastatic syngeneic breast cancer. Nano Lett. 2018, 18, 7092–7103. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Shi, C.; Xu, G.; Guo, D.; Luo, J. Photo and redox dual responsive reversibly cross-linked nanocarrier of efficient tumor-targeted drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 10381–10392. [Google Scholar] [CrossRef]
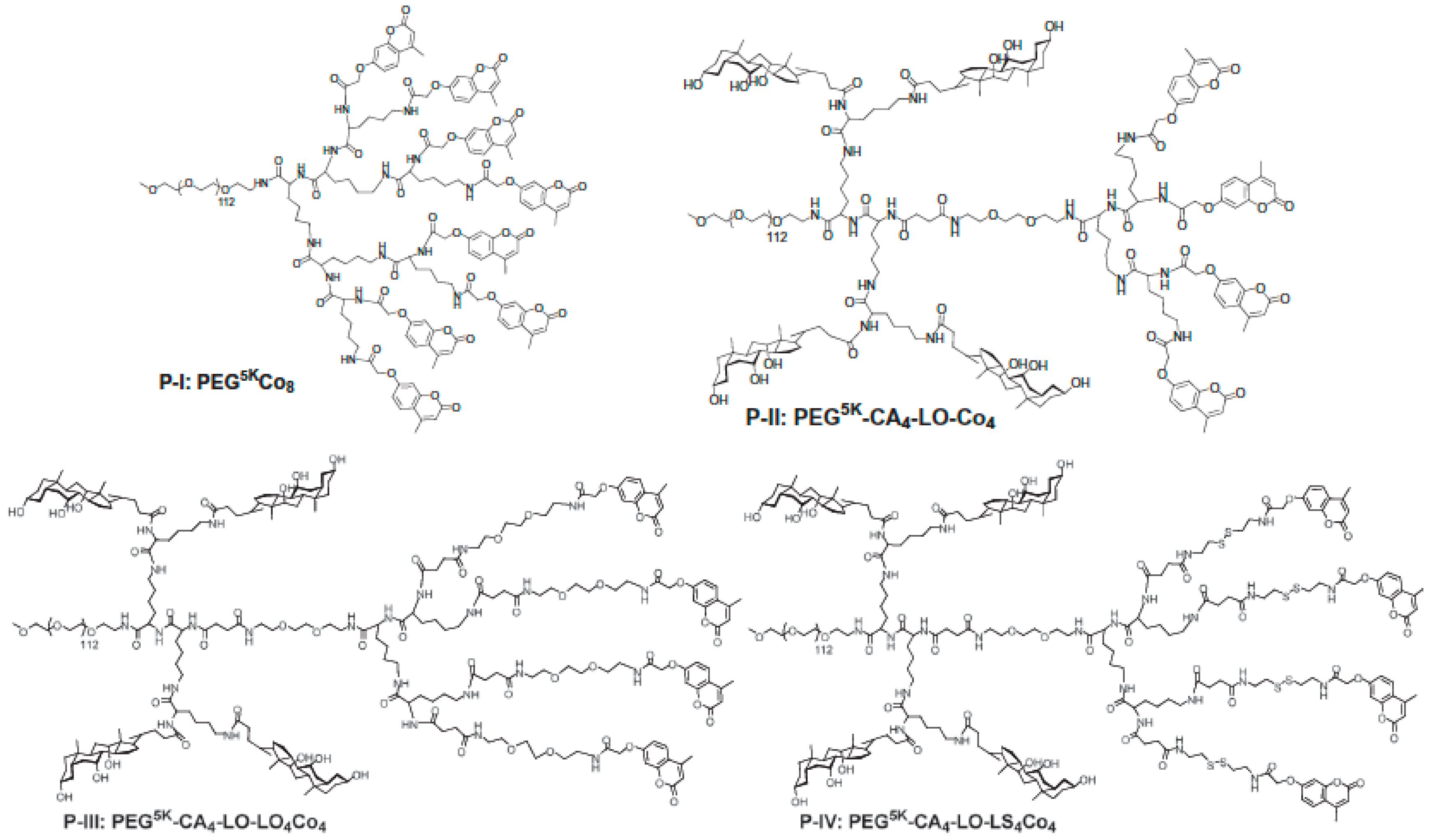
- Xu, G.; Shi, C.; Guo, D.; Wang, L.; Ling, Y.; Han, X.; Luo, J. Functional-segregated coumarin-containing telodendrimer nanocarriers for efficient delivery of SN-38 for colon cancer treatment. Acta Biomater. 2015, 21, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Jonsson, T.B.; Fréchet, J.M.J. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. J. Am. Chem. Soc. 2004, 126, 11936–11943. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, W.; Xiao, K.; Berti, L.; Luo, J.; Tseng, H.P.; Fung, G.; Lam, K.S. Well-defined, reversible boronate crosslinked nanocarriers for targeted drug delivery in response to acidic pH values and cis-diols. Angew. Chem. Int. Ed. 2012, 51, 2864–2869. [Google Scholar] [CrossRef]
- Harnoy, A.J.; Rosenbaum, I.; Tirosh, E.; Ebenstein, Y.; Shaharabani, R.; Beck, R.; Amir, R.J. Enzyme-respensive amphiphilic PEG-dendron hybrids and their assembly into smart micellar nanocarriers. J. Am. Chem. Soc. 2014, 136, 7531–7534. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Wright, F.A.; Guo, D.; Wang, X.; Wang, D.; Wojcikiewicz, R.J.H.; Luo, J. Multifunctional telodendrimer nanocarriers restore synergy of bortezomib and doxorubicin in ovarian cancer treatment. Cancer Res. 2017, 77, 3293–3305. [Google Scholar] [CrossRef]
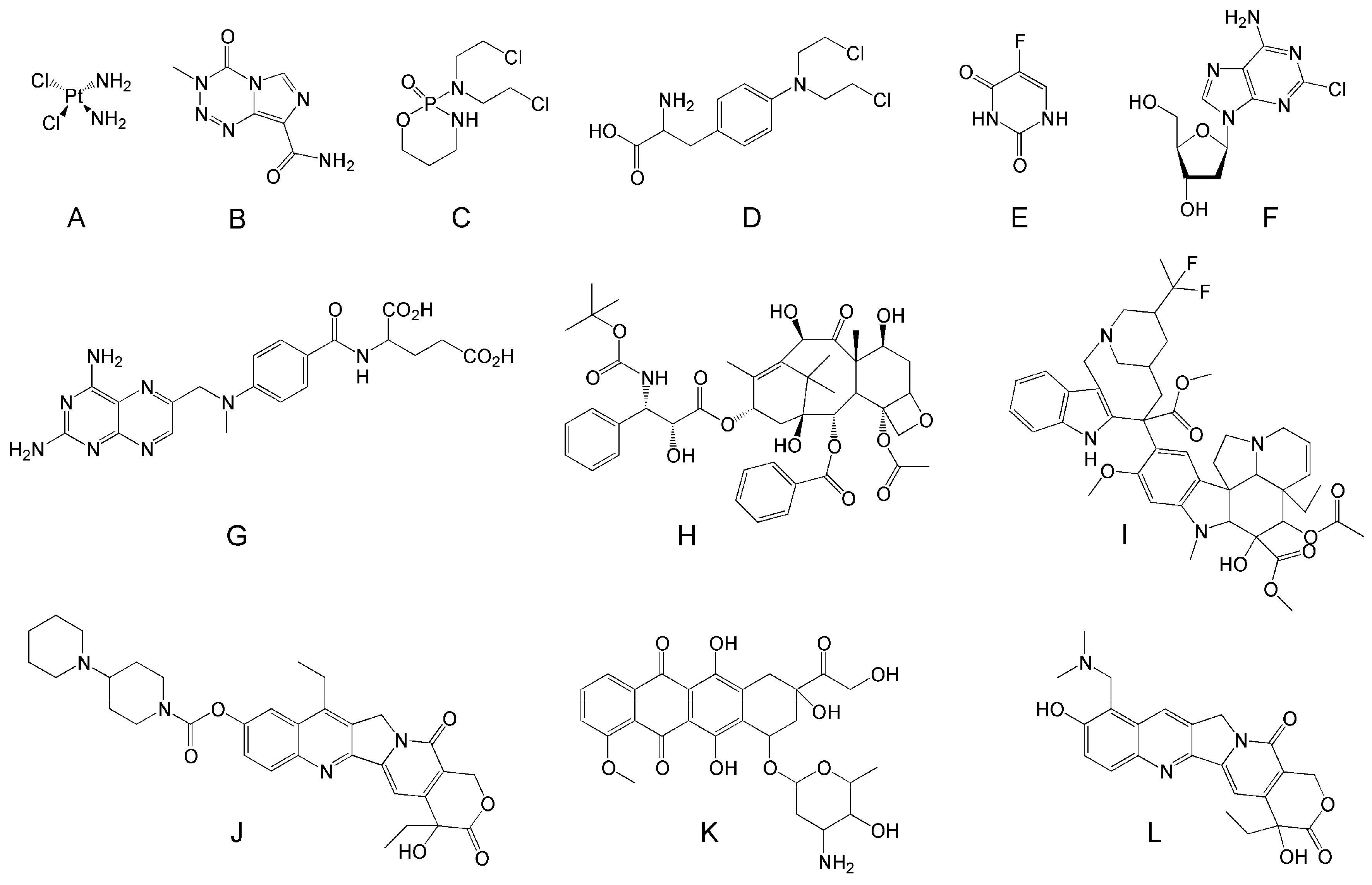
- Yu, Z.; ChunSheng, X.; MingQiang, L.; JianXun, D.; ChenQuang, Y.; XiuLi, Z.; XueSi, C. Co-delivery of doxorubicin and paclitaxel with linear-dendritic block copolymer for enhanced anti-cancer efficacy. Sci. China 2014, 57, 624–632. [Google Scholar]
- Cai, L.; Xu, G.; Shi, C.; Guo, D.; Wang, X.; Luo, J. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: A synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials 2015, 37, 456–468. [Google Scholar] [CrossRef]
- Li, W.-J.; Wang, Y.; Liu, Y.; Wu, T.; Cai, W.-L.; Shuai, X.-T.; Hong, G.-B. Preliminary study of MR and fluorescence dual-mode imaging: Combined macrophage-targeted and superparamagnetic polymeric micelles. Int. J. Med. Sci. 2018, 15, 129–141. [Google Scholar] [CrossRef]
- Xiao, W.; Luo, J.; Jain, T.; Riggs, J.W.; Tseng, H.P.; Henderson, P.T.; Cherry, S.R.; Rowland, D.; Lam, K.S. Biodistribution and pharmacokinetics of a telodendrimer micellar paclitaxel nanoformulation in a mouse xenograft model of ovarian cancer. Int. J. Nanomed. 2012, 7, 1587–1597. [Google Scholar] [CrossRef]
- Wang, X.-S.; Zhang, L.; Li, X.; Kong, D.-J.; Hu, X.-C.; Ding, X.-Z.; Yang, J.-Q.; Zhao, M.-Q.; He, Y.; Lam, K.S.; et al. Nanoformulated paclitaxel and AZD9291 synergistically eradicate non-small-cell lung cancers in vivo. Nanomedicine (Lond.) 2018, 13, 1107–1120. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, D.; Nangia, S.; Xu, G.; Lam, K.S.; Luo, J. A structure-property relationship study of the well-defined telodendrimers to improve hemocompatibility of nanocarriers for anticancer drug delivery. Langmuir 2014, 30, 6878–6888. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Luo, J.; Nangia, S. Multiscale approach to investigate self-Assembly of telodendrimer based nanocarriers for anticancer drug delivery. Langmuir 2015, 31, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, X.; Guo, D.; Luo, J.; Nangia, S. Drug-specific design of telodendrimer architecture for effective doxorubicin encapsulation. J. Phys. Chem. B 2016, 120, 9766–9777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content modulable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef] [PubMed]
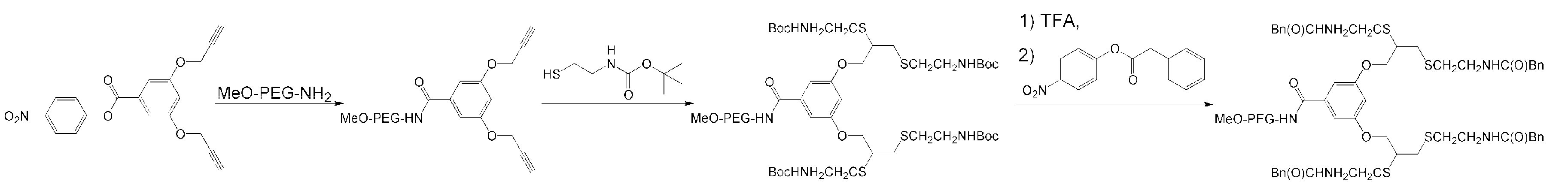
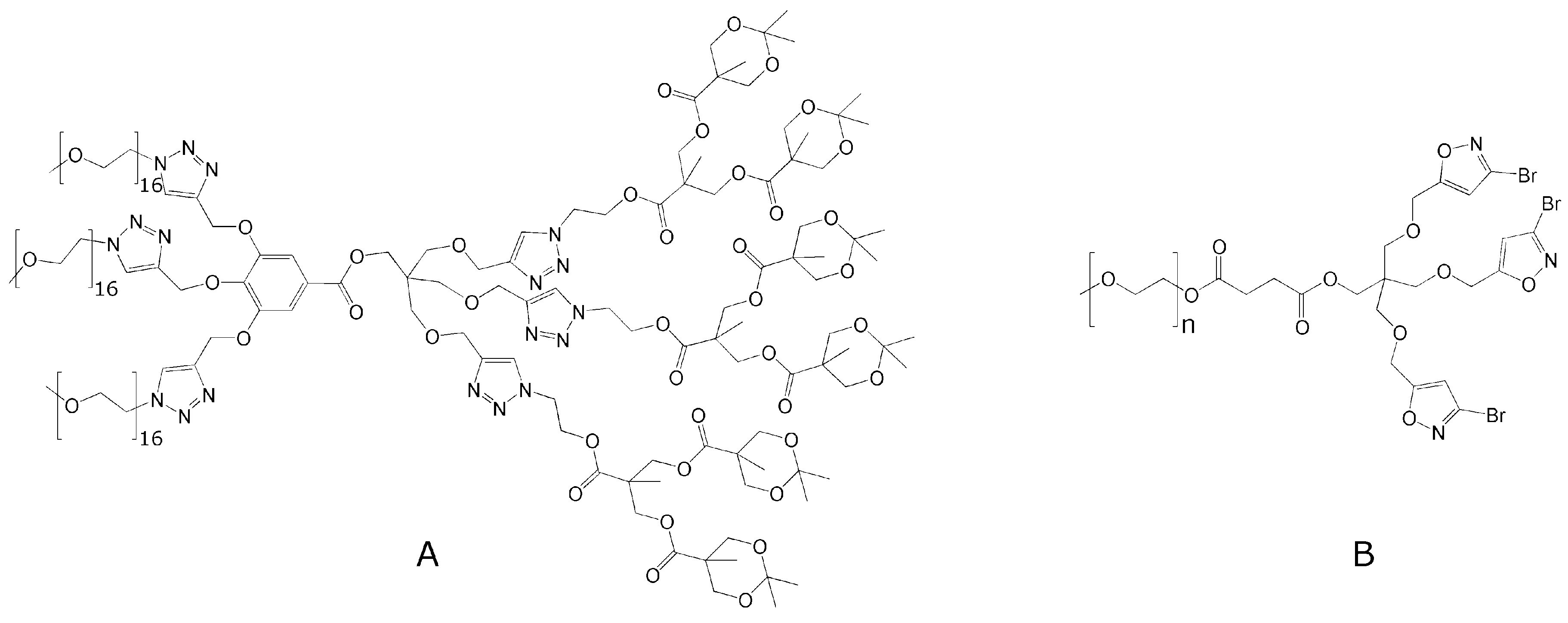
- Yazdani, H.; Kaul, E.; Bazgir, A.; Maysinger, D.; Kakkar, A. Telodendrimer-based macromolecular drug design using 1,3-dipolar cycloaddition for applications in biology. Molecules 2020, 25, 857. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Guo, Y.; An, S.; Kuang, Y.; Ma, H.; He, X.; Jiang, C. Linear-dendritic copolymer composed of polyethylene glycol and all-trans-retinoic acid as drug delivery platform for paclitaxel against breast cancer. Bioconjug. Chem. 2015, 26, 418–426. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Shi, C.; Guo, D.; Xu, G.; Wang, L.; Bodman, A.; Luo, J. Fine-tuning vitamin E-containing telodendrimers for efficient delivery of gambogic acid in colon cancer treatment. Mol. Pharm. 2015, 12, 1216–1229. [Google Scholar] [CrossRef]
- He, W.; Felderman, M.; Evans, A.C.; Geng, J.; Homan, D.; Bourguet, F.; Fischer, N.O.; Li, Y.; Lam, K.S.; Noy, A.; et al. Cell-free production of a functional oligomeric form of a Chlamydia major outer-membrane protein (MOMP) for vaccine development. J. Biol. Chem. 2017, 292, 15121–15132. [Google Scholar] [CrossRef]
- He, W.; Luo, J.; Bourguet, F.; Xing, L.; Yi, S.K.; Gao, T.; Blanchette, C.; Henderson, P.T.; Kuhn, E.; Malfatti, M.; et al. Controlling the diameter, monodispersity, and solubility of ApoA1 nanolipoprotein particles using telodendrimer chemistry. Protein Sci. 2013, 22, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bodman, A.; Shi, C.; Guo, D.; Wang, L.; Luo, J.; Hall, W.A. Tunable Lipidoid-telodendrimer hybrid nanoparticles for intracellular protein delivery in brain tumor treatment. Small 2016, 12, 4185–4192. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef]
- Li, Y.; Lin, T.-Y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachsmann-Hogiu, S.; et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nature 2014, 5, 4712–4727. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Guo, W.; Long, Q.; Ma, A.; Liu, Q.; Zhang, H.; Huang, Y.; Chandrasekaran, S.; Pan, C.; Lam, K.S.; et al. Nanoparticles for synergistic combination cancer therapy. Theranostics 2016, 6, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
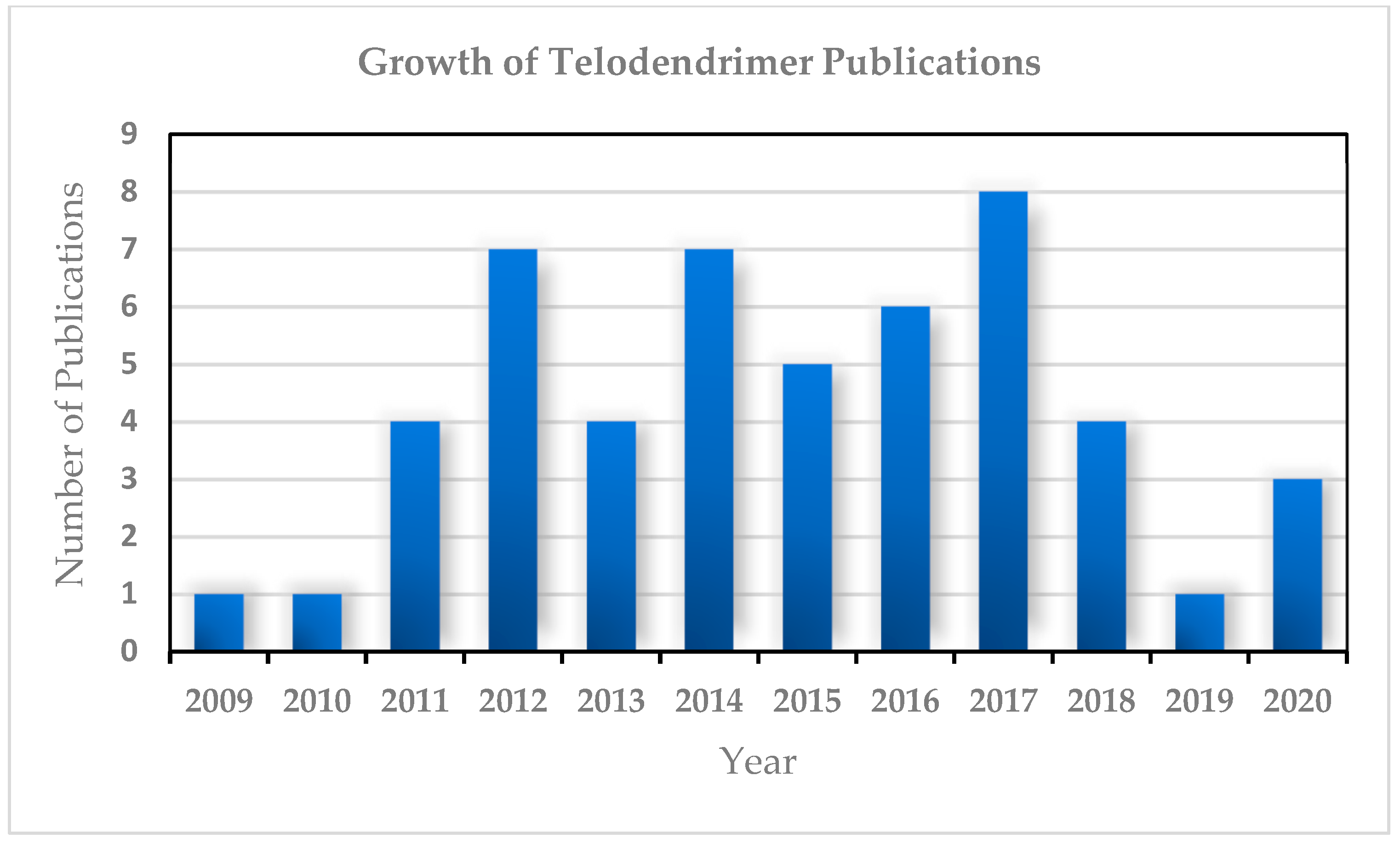
- Data Obtained Using Royal Danish Library (Library Catalogue REX) and SciFinderScholar. Grand Total Hits on Telodendrimers from 2009–2020 Were 51. Available online: https://www.kb.dk;https://scifinder.cas.org (accessed on 26 August 2020).
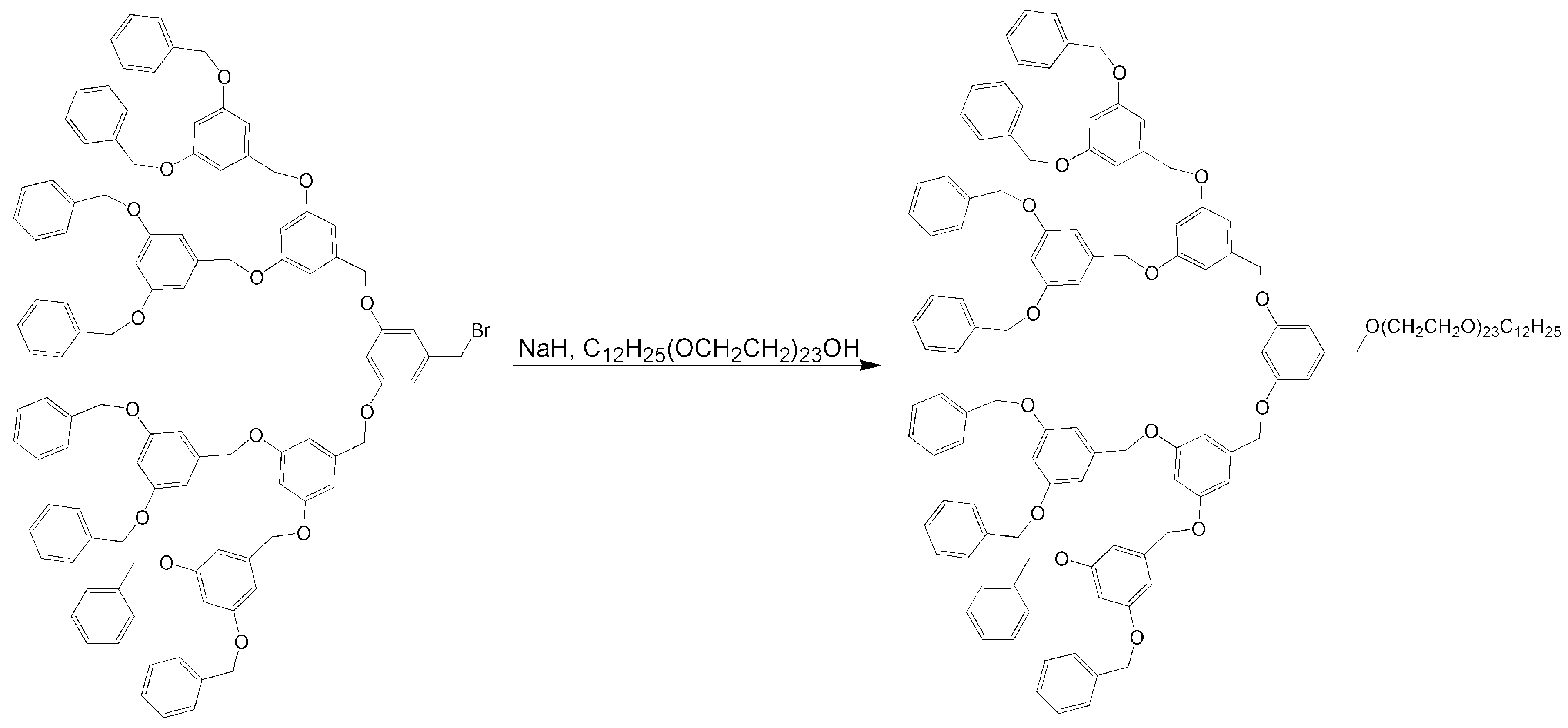
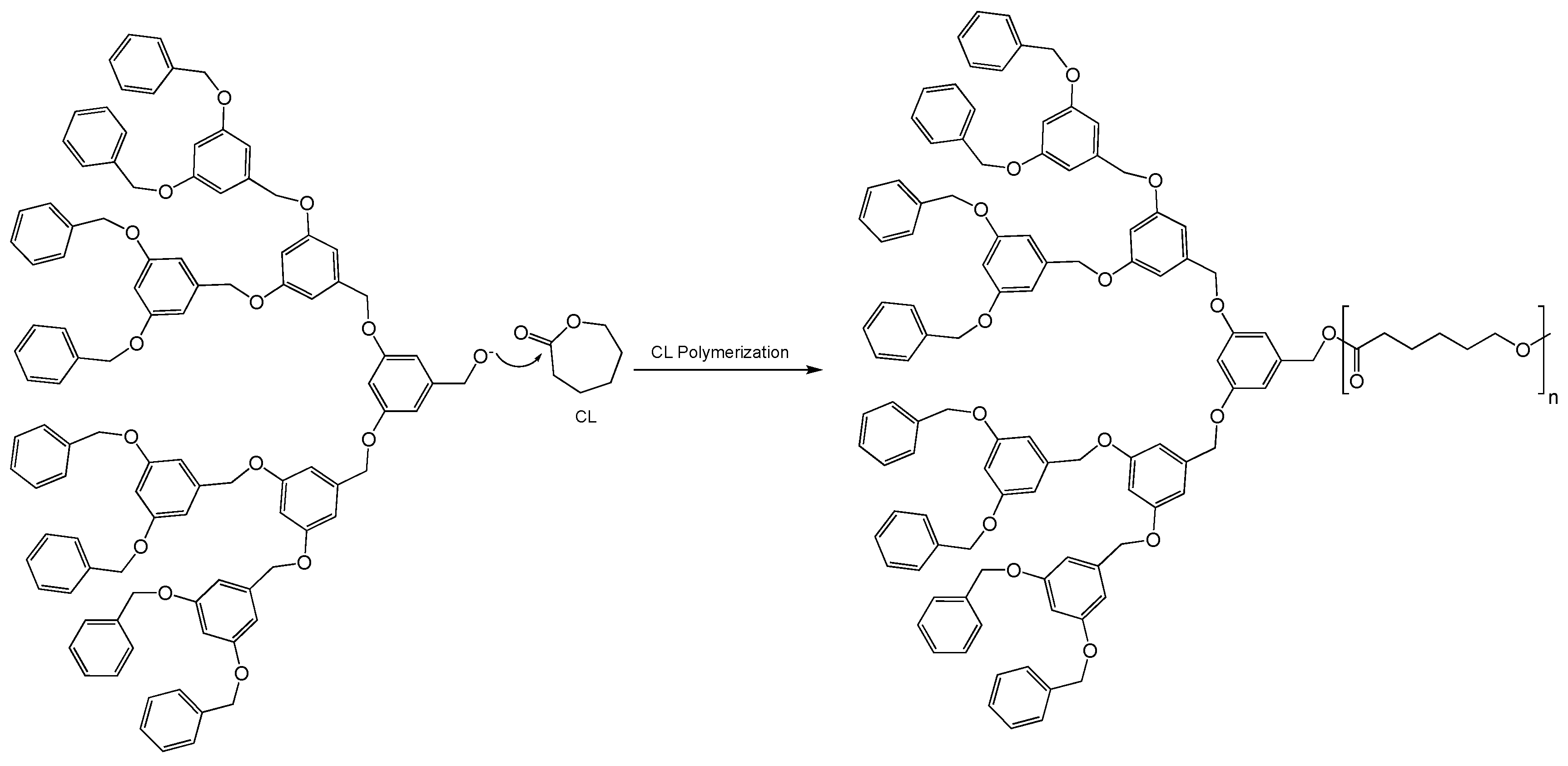
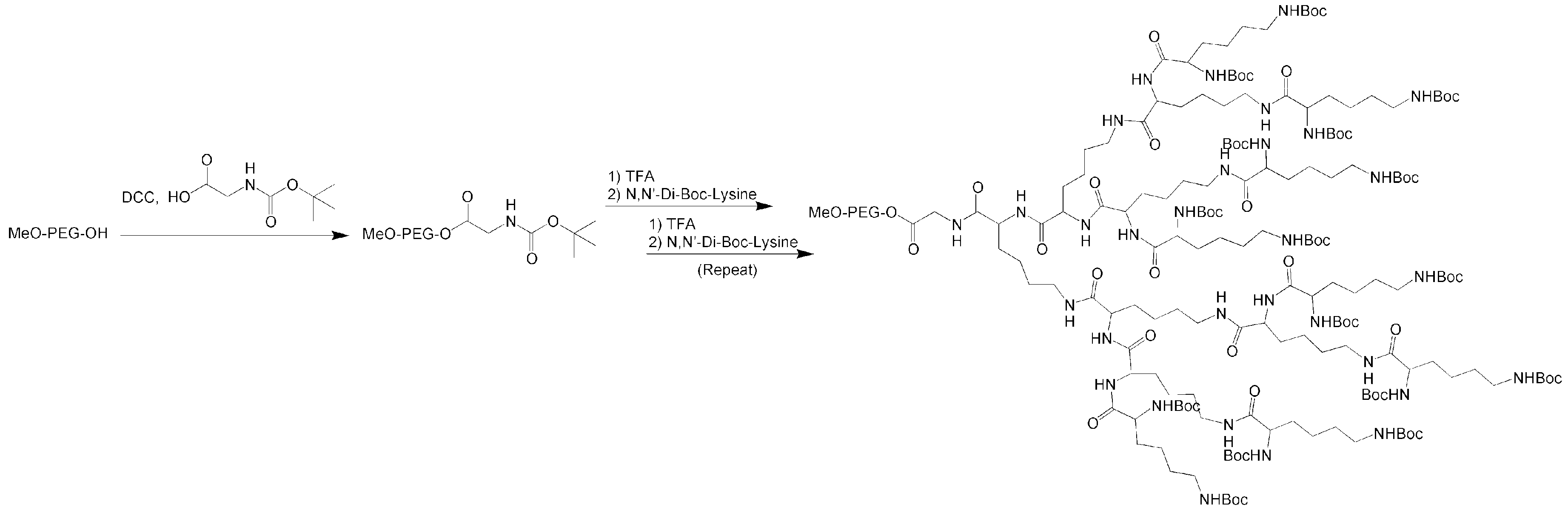
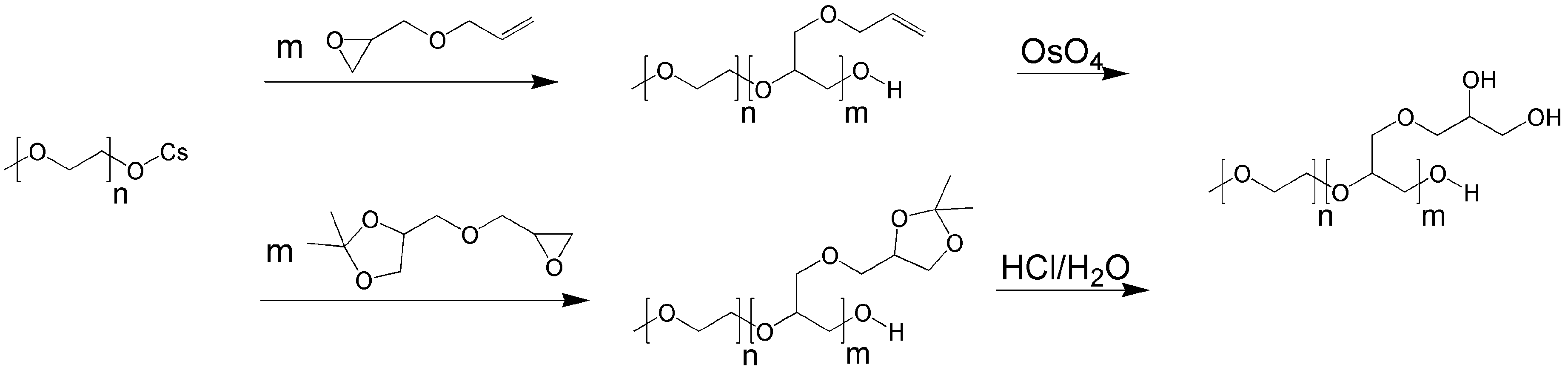
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejlsøe, S.; Kakkar, A. Telodendrimers: Promising Architectural Polymers for Drug Delivery. Molecules 2020, 25, 3995. https://doi.org/10.3390/molecules25173995
Mejlsøe S, Kakkar A. Telodendrimers: Promising Architectural Polymers for Drug Delivery. Molecules. 2020; 25(17):3995. https://doi.org/10.3390/molecules25173995
Chicago/Turabian StyleMejlsøe, Søren, and Ashok Kakkar. 2020. "Telodendrimers: Promising Architectural Polymers for Drug Delivery" Molecules 25, no. 17: 3995. https://doi.org/10.3390/molecules25173995
APA StyleMejlsøe, S., & Kakkar, A. (2020). Telodendrimers: Promising Architectural Polymers for Drug Delivery. Molecules, 25(17), 3995. https://doi.org/10.3390/molecules25173995





