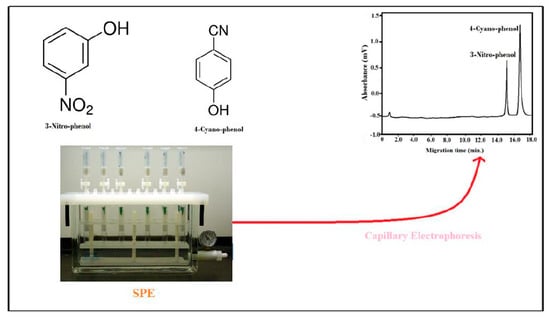
Multi-Walled Carbon Nanotubes Solid-Phase Extraction and Capillary Electrophoresis Methods for the Analysis of 4-Cyanophenol and 3-Nitrophenol in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments Used
2.3. Synthesis of MWCNTs
2.4. Fabrication of SPE Cartridge
2.5. Capillary Electrophoretic Conditions
2.6. Phenols Extraction From Water by SPE
3. Results and Discussion
3.1. Preparation and Characterization of MWCNTs
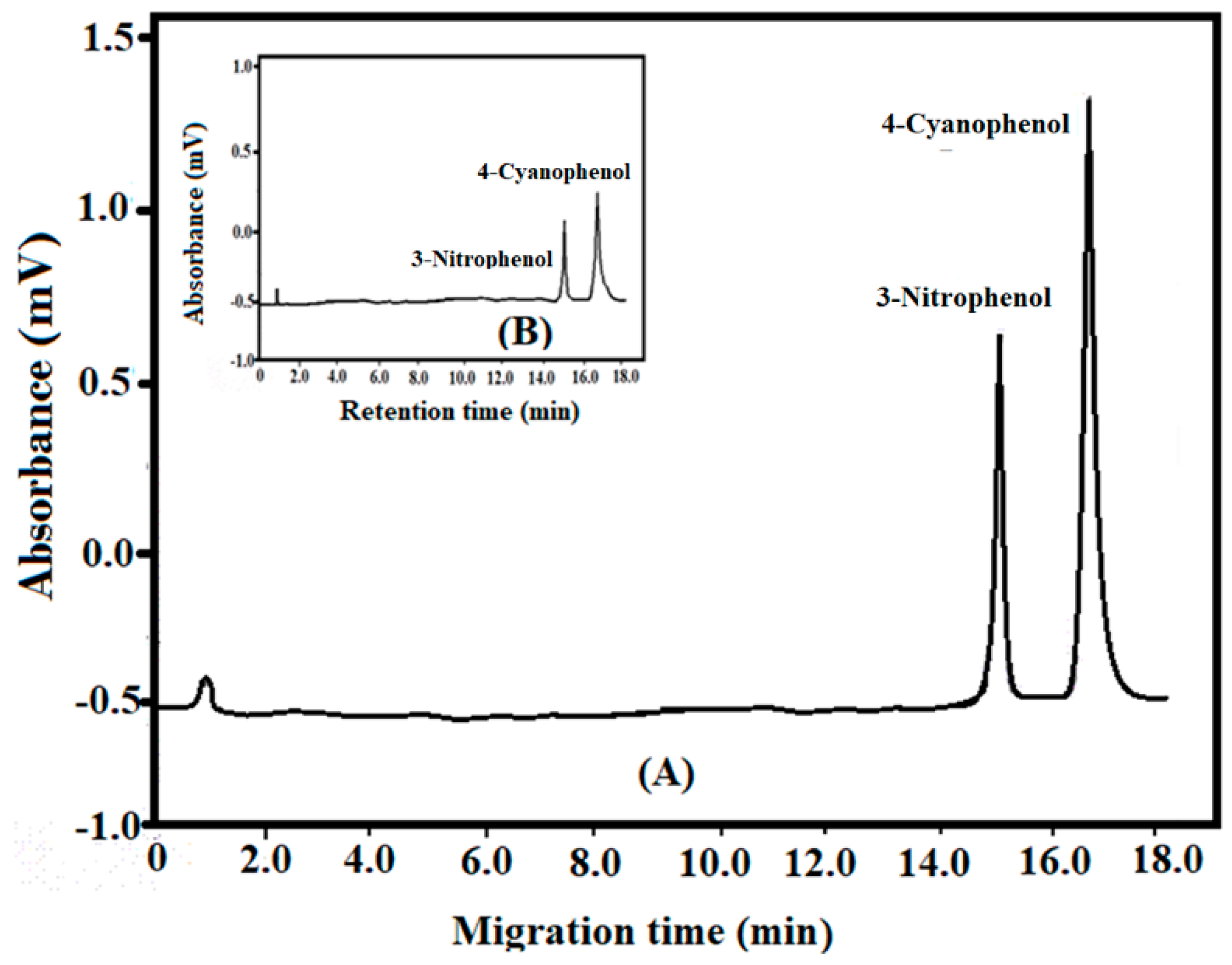
3.2. Capillary Electrophoresis of Phenols Method
3.3. Capillary Electrophoresis Validation
3.4. SPE Phenols Extraction
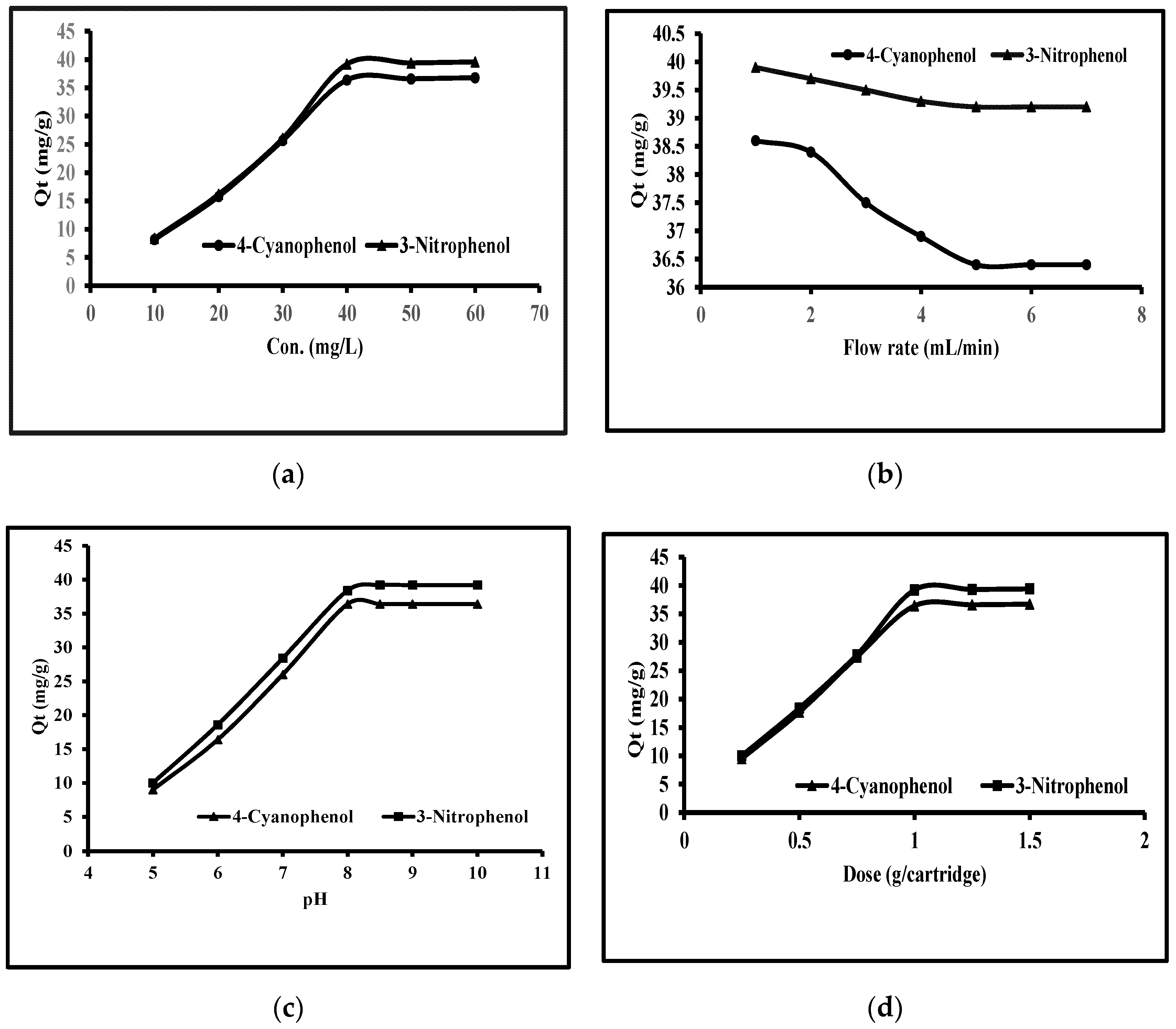
3.4.1. Phenols Concentrations
3.4.2. Extraction Flow Rate
3.4.3. pH Effect
3.4.4. MWCNTs Dose
3.4.5. Desorption of Phenols
4. Application of SPE and CE Methods in Spiked Real Water Samples
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bichai, F.; Ashbolt, N. Public health and water quality management in low-exposure stormwater schemes: A critical review of regulatory frameworks and path forward. Sustain. Cities Soc. 2017, 28, 453–465. [Google Scholar] [CrossRef]
- Gwenzi, W.; Dunjana, N.; Pisa, C.; Tauro, T.; Nyamadzawo, G. Water quality and public health risks associated with roof rainwater harvesting systems for potable supply: Review and perspectives. Sustain. Water Qual. Ecol. 2015, 6, 107–118. [Google Scholar] [CrossRef]
- Prathumratana, L.; Sthiannopkao, S.; Kim, K.W. The relationship of climatic and hydrological parameters to surface water quality in the lower Mekong river. Environ. Int. 2008, 34, 860–866. [Google Scholar] [CrossRef]
- Jeevanantham, S.; Saravanan, A.; Hemavathy, R.V.; Kumar, P.S.; Yaashikaa, P.R.; Yuvaraj, D. Removal of toxic pollutants from water environment by phytoremediation: A survey on application and future prospects. Environ. Technol. Innov. 2019, 13, 264–276. [Google Scholar] [CrossRef]
- Ali, I. Nano anti-cancer drugs: Pros and cons and future perspectives. Curr. Cancer Drug Targets 2011, 11, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Budavari, S. (Ed.) The Merck Index: An Encyclopedia of Chemical, Drugs, and Biologicals; Merck: Whitehouse Station, NJ, USA, 1996. [Google Scholar]
- Dean-Ross, D.; Rahimi, M. Toxicity of phenolic compounds to sediment bacteria. Bull. Environ. Contam. Toxicol. 1995, 55, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.M.; Lee, S.S.; Lai, C.S.; Lin, S.D. Phenol burn. J. Int. Soc. Burn Inj. 2006, 32, 517–521. [Google Scholar] [CrossRef]
- Greca, M.D.; Monaco, P.; Pinto, G.; Pollio, A. Phytotoxicity of low-molecular-weight phenols from olive mill waste waters. Bull. Environ. Contam. Toxicol. 2001, 67, 352–359. [Google Scholar]
- Hirose, M.; Takesada, Y.; Tanaka, H.; Tamano, S.; Kato, T.; Shirai, T. Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4-methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multi-organ carcinogenesis model. Carcinogenesis 1998, 19, 207–212. [Google Scholar] [CrossRef]
- Hooiveld, M.; Heederik, D.J.; Kogevinas, M.; Boffetta, P.; Needham, L.L., Jr.; Patterson, D.G.; Bueno-de-Mesquita, H.B. Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenols, and contaminants. Am. J. Epidemiol. 1998, 147, 891–901. [Google Scholar] [CrossRef]
- Michałowicz, J.; Duda, W. Phenols—Sources and Toxicity. Polish J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Schweigert, N.; ZehnderL, A.J.B.; Eggen, R.I.L. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds—Natural Sources, Importance and Applications; IntechOpen-Open Science Open Minds: London, UK, 2017. [Google Scholar]
- Bigley, F.P.; Grob, R.L. Determination of phenols in water and wastewater by post-column reaction detection high-performance liquid chromatography. J. Chromatogr. A 1985, 350, 407–416. [Google Scholar] [CrossRef]
- Fiehn, O.; Jekel, M. Analysis of phenolic compounds in industrial wastewater with high performance liquid chromatography and post-column reaction detection. J. Chromatogr. A 1997, 769, 189–200. [Google Scholar] [CrossRef]
- Jinyi, L.; Plaisier, A. Study on treatment of wastewater containing nitrophenol compounds by liquid membrane process. J. Membr. Sci. 2004, 229, 235–239. [Google Scholar]
- Merlin, F.; Tchieno, M.; Tonle, I.K. p-Nitrophenol determination and remediation: An overview. Rev. Anal. Chem. 2008, 37, 20170019. [Google Scholar]
- Bolsl, N.C.; Boliska, S.A.; Dixon, D.G.; Hodson, P.V.; Kaiser, K.L.E. The use of fish cell cultures as an indication of contaminant toxicity to fish. Aquat. Toxicol. 1985, 6, 147–155. [Google Scholar] [CrossRef]
- Keith, L.H.; Telliard, W.A. Priority pollutants: A perspective review. Environ. Sci. Technol. 1979, 13, 416–423. [Google Scholar] [CrossRef]
- Megharaj, M.; Pearson, H.W.; Venkateswarlu, K. Toxicity of p-amino-phenol and p-nitro-phenol to Chlorella vulgaris and two spcies of nostoc isolated from soil. Pest. Biochem. Physiol. 1991, 40, 266–273. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K.; Aboul-Enein, H.Y.; Hussain, H.Y. Hyphenation in sample preparation: Advancement from micro to nanoworld. J. Sep. Sci. 2008, 31, 2040–2053. [Google Scholar] [CrossRef]
- Sanagi, M.M.; Loh, S.H.; Nazihah, W.; Ibrahim, W.A.W.; Pourmand, N.; Salisu, A.; Aini, W. Agarose and alginate-based biopolymers for sample preparation: Excellent green extraction tools for this century. J. Sep. Sci. 2016, 39, 1152–1159. [Google Scholar] [CrossRef]
- Sanagi, M.M.; Muhammad, S.S.; Hussain, I.; Ibrahim, W.A.W. Novel solid-phase membrane tip extraction and gas chromatography-mass spectrometry methods for rapid analyses of triazine herbicides in real waters. J. Sep. Sci. 2015, 38, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Thurman, E.M.; Mills, M.S. Solid-Phase Extraction: Principles and Practice, 1st ed.; Wiley-Interscience: Hoboken, NJ, USA, 1998. [Google Scholar]
- Colin, P. (Ed.) Solid-Phase Extraction, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Al-Shaalan, N.H.; Ali, I.; ALOthman, Z.A.; Al-Wahaibi, L.H.; Alabdulmonem, H. Application of composite nanomaterial to determine phenols in wastewater by solid-phase micro membrane tip extraction and capillary electrophoresis. Molecules 2019, 24, 3443. [Google Scholar] [CrossRef] [PubMed]
- Burakova, E.A.; Dyachkova, T.P.; Rukhov, A.V.; Tugolukov, E.N.; Galunin, E.V.; Tkachev, A.G.; Ali, I. Novel and economic method of carbon nanotubes synthesis on a nickel magnesium oxide catalyst using microwave radiation. J. Mol. Liq. 2018, 253, 340–346. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y. Determination of phenol and its derivatives in wastewater by capillary electrophoresis. Fresenius Environ. Bull. 2002, 11, 36–39. [Google Scholar]
- The United State Pharmacopeia, 24th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2000; pp. 2150–2151.
Sample Availability: Samples of the compounds are available from the authors under request. |

| Sl. No. | Validated Parameters | 3-Nitrophenol | 4-Cyanophenol | ||||
|---|---|---|---|---|---|---|---|
| % RSD | Correlation Coefficients | Confidence Levels | % RSD | Correlation Coefficients | Confidence Levels | ||
| 1. | Precision | 0.61 | 0.9998 | 97.7 | 0.76 | 0.9997 | 96.6 |
| 2. | Linearity | 0.56 | 0.9998 | 97.5 | 1.21 | 0.9997 | 96.5 |
| 3. | LOD | 0.84 | 0.9998 | 96.6 | 1.31 | 0.9996 | 95.8 |
| 4. | LOQ | 0.84 | 0.9997 | 96.5 | 1.06 | 0.9996 | 96.9 |
| 5. | Ruggedness | 0.91 | 0.9998 | 96.7 | 1.43 | 0.9996 | 96.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
ALOthman, Z.A.; Badjah, A.Y.; Locatelli, M.; Ali, I. Multi-Walled Carbon Nanotubes Solid-Phase Extraction and Capillary Electrophoresis Methods for the Analysis of 4-Cyanophenol and 3-Nitrophenol in Water. Molecules 2020, 25, 3893. https://doi.org/10.3390/molecules25173893
ALOthman ZA, Badjah AY, Locatelli M, Ali I. Multi-Walled Carbon Nanotubes Solid-Phase Extraction and Capillary Electrophoresis Methods for the Analysis of 4-Cyanophenol and 3-Nitrophenol in Water. Molecules. 2020; 25(17):3893. https://doi.org/10.3390/molecules25173893
Chicago/Turabian StyleALOthman, Zeid A., Ahmad Yacine Badjah, Marcello Locatelli, and Imran Ali. 2020. "Multi-Walled Carbon Nanotubes Solid-Phase Extraction and Capillary Electrophoresis Methods for the Analysis of 4-Cyanophenol and 3-Nitrophenol in Water" Molecules 25, no. 17: 3893. https://doi.org/10.3390/molecules25173893
APA StyleALOthman, Z. A., Badjah, A. Y., Locatelli, M., & Ali, I. (2020). Multi-Walled Carbon Nanotubes Solid-Phase Extraction and Capillary Electrophoresis Methods for the Analysis of 4-Cyanophenol and 3-Nitrophenol in Water. Molecules, 25(17), 3893. https://doi.org/10.3390/molecules25173893