Experimental and Density Functional Theory Studies on 1,1,1,4,4,4-Hexafluoro-2-Butene Pyrolysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental Results and Discussion
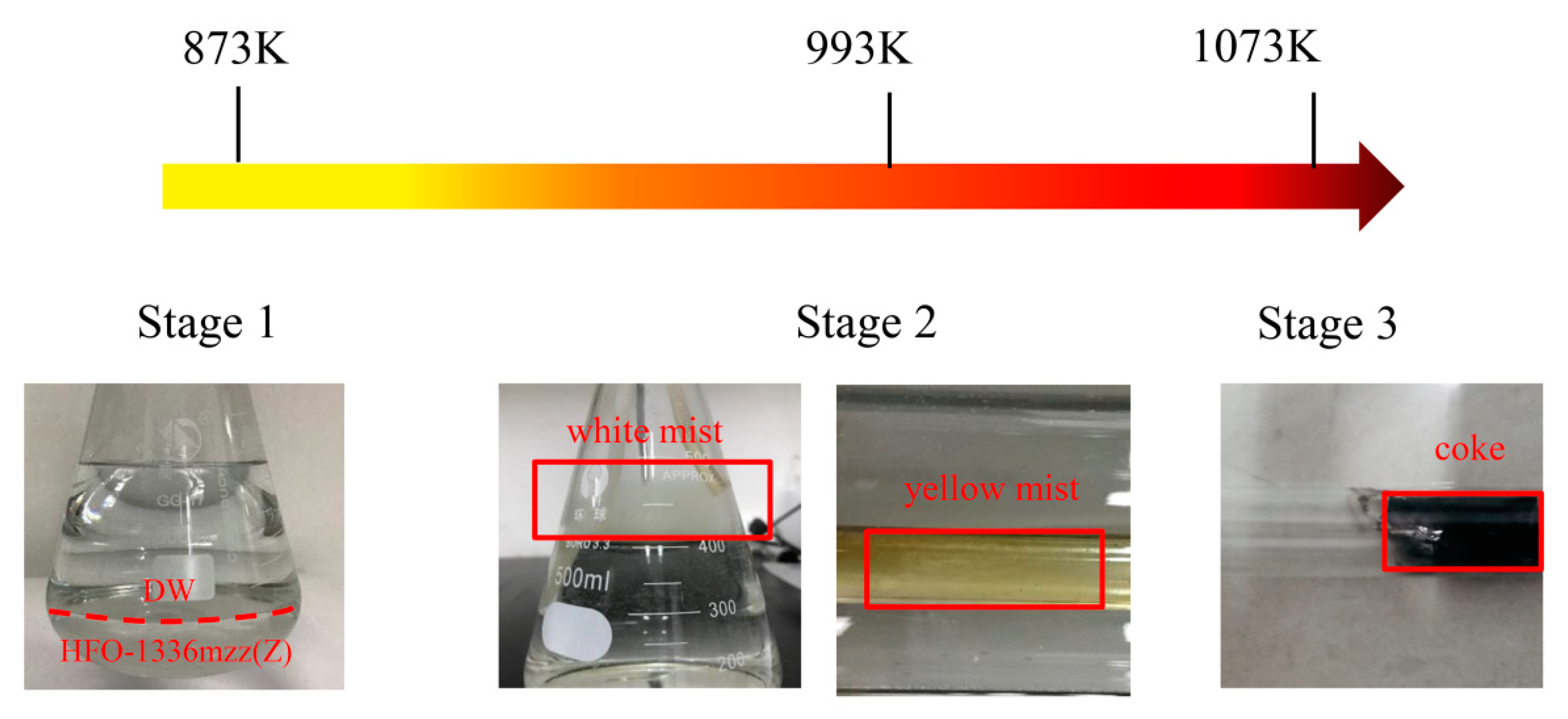
2.1.1. Thermal Decomposition Temperature and Products
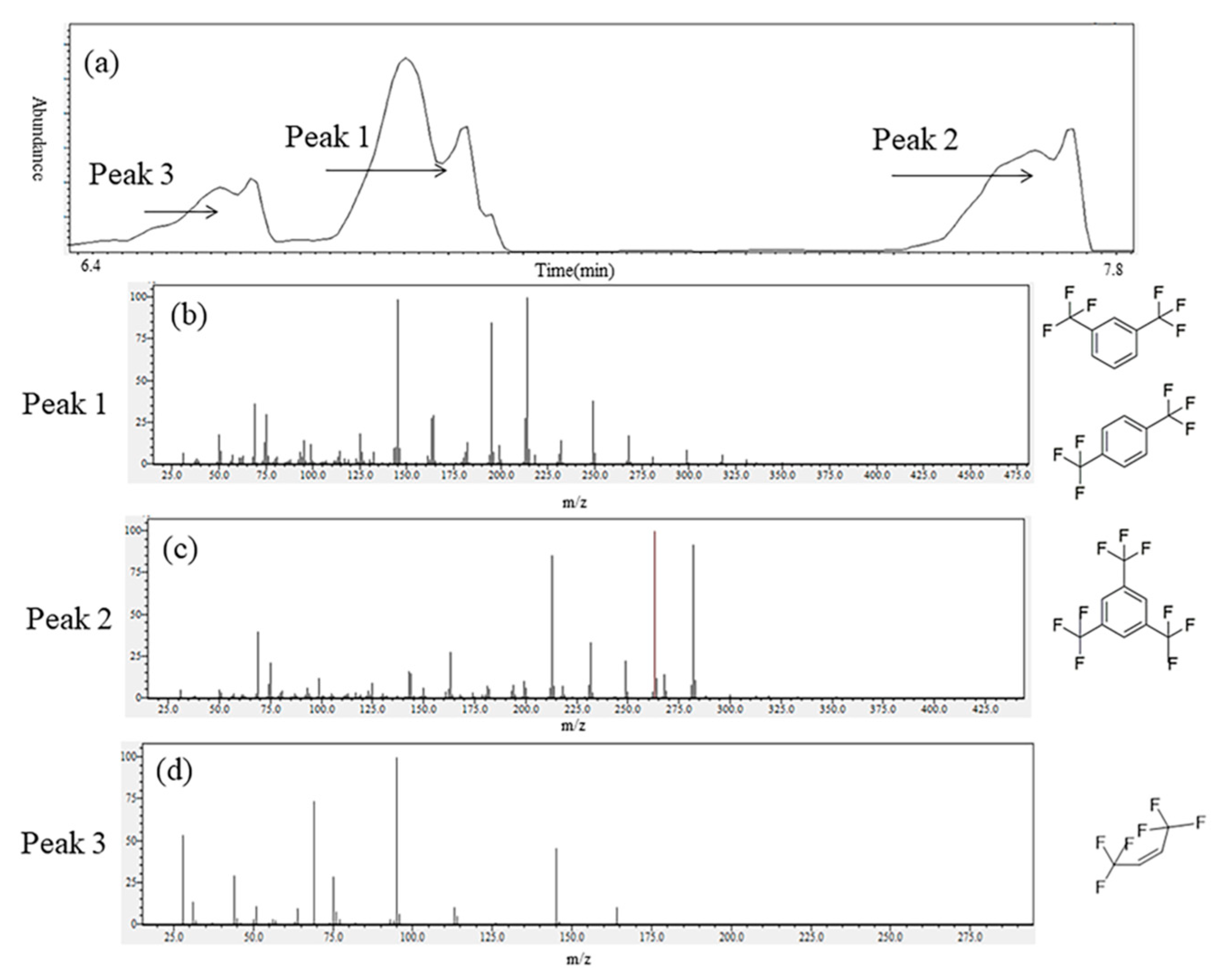
2.1.2. GC–MS Results
Gas Products
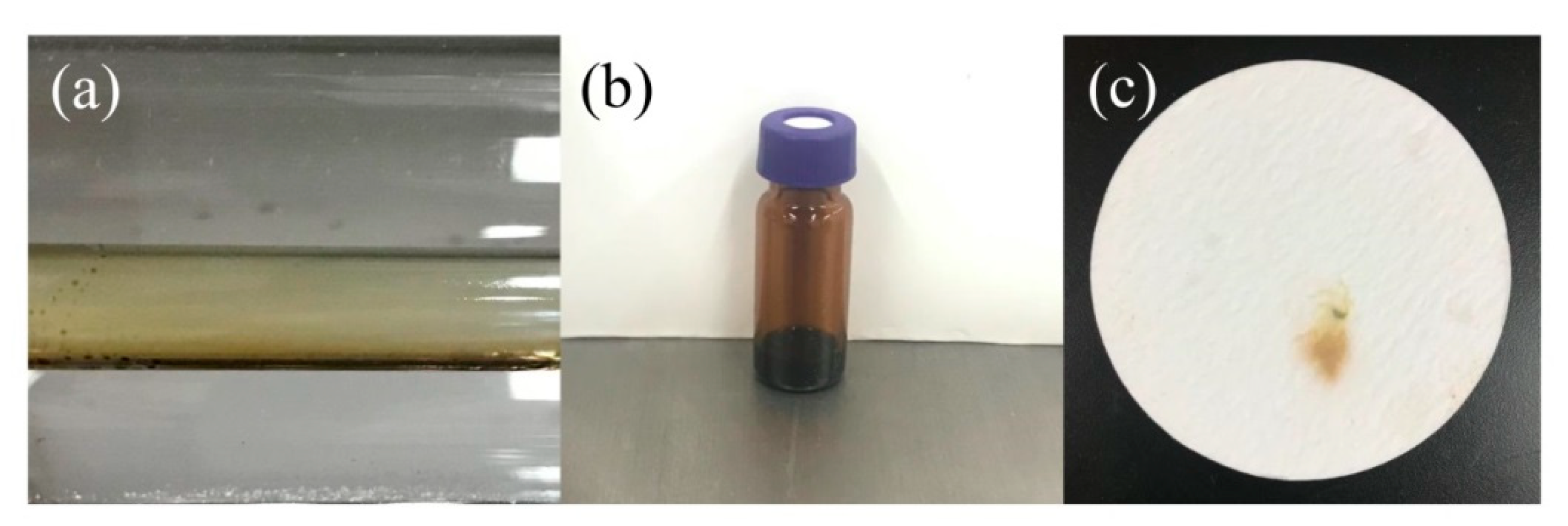
Oily Liquids Products
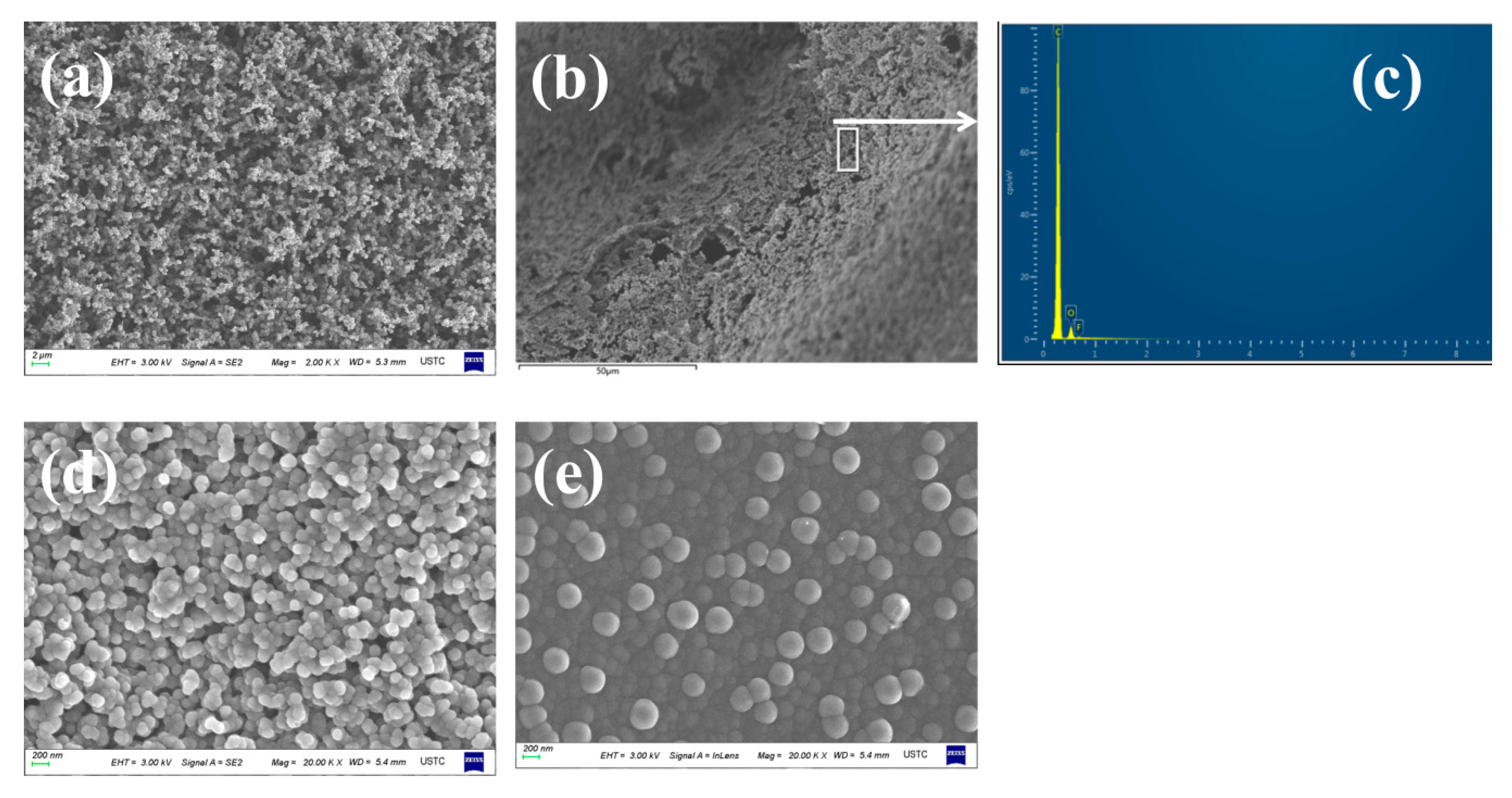
2.1.3. Coke Collected Form the Inner Wall of the Quartz Tube
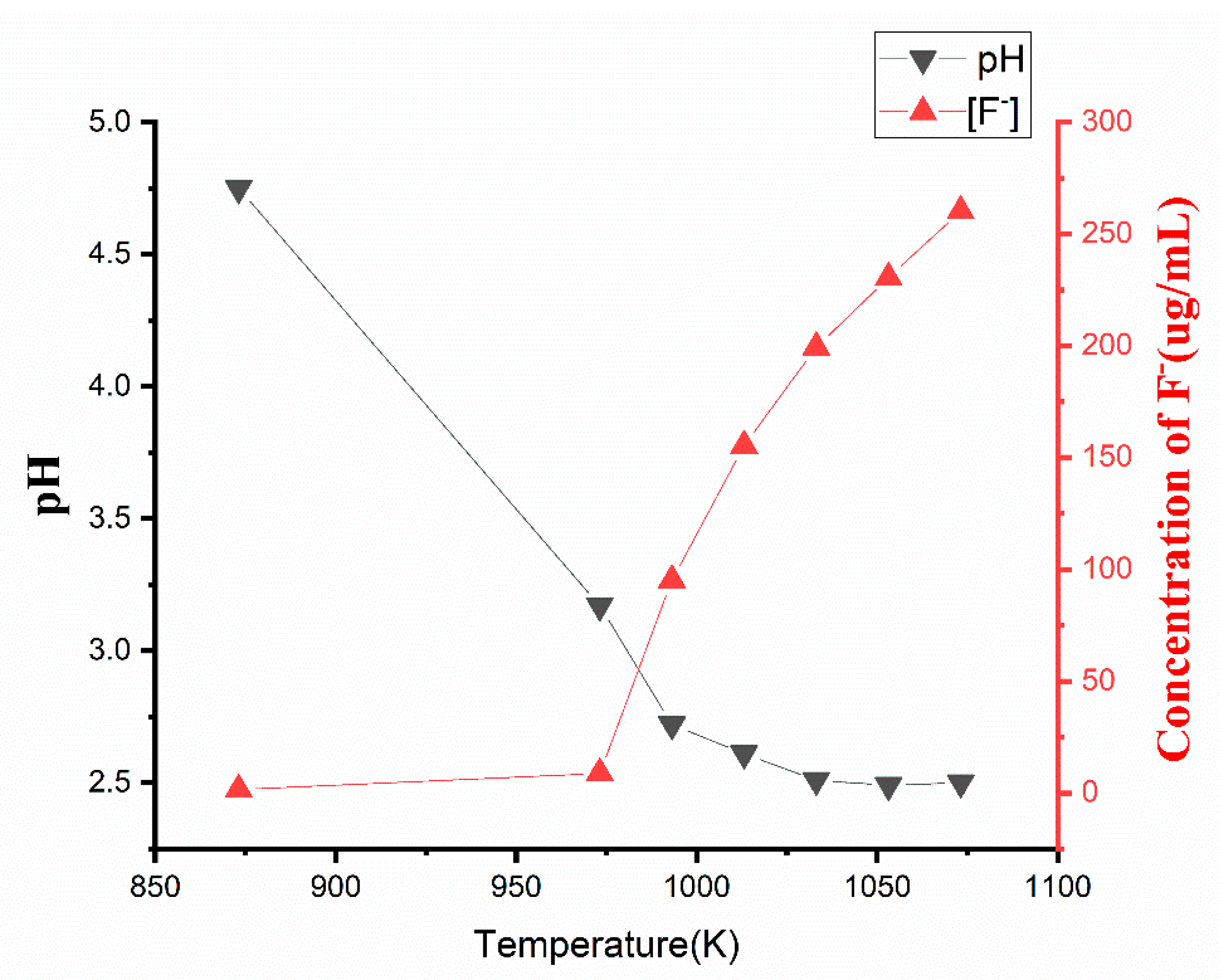
2.1.4. The Trend of Hydrogen Fluoride Concentration
2.2. Theoretical Calculation
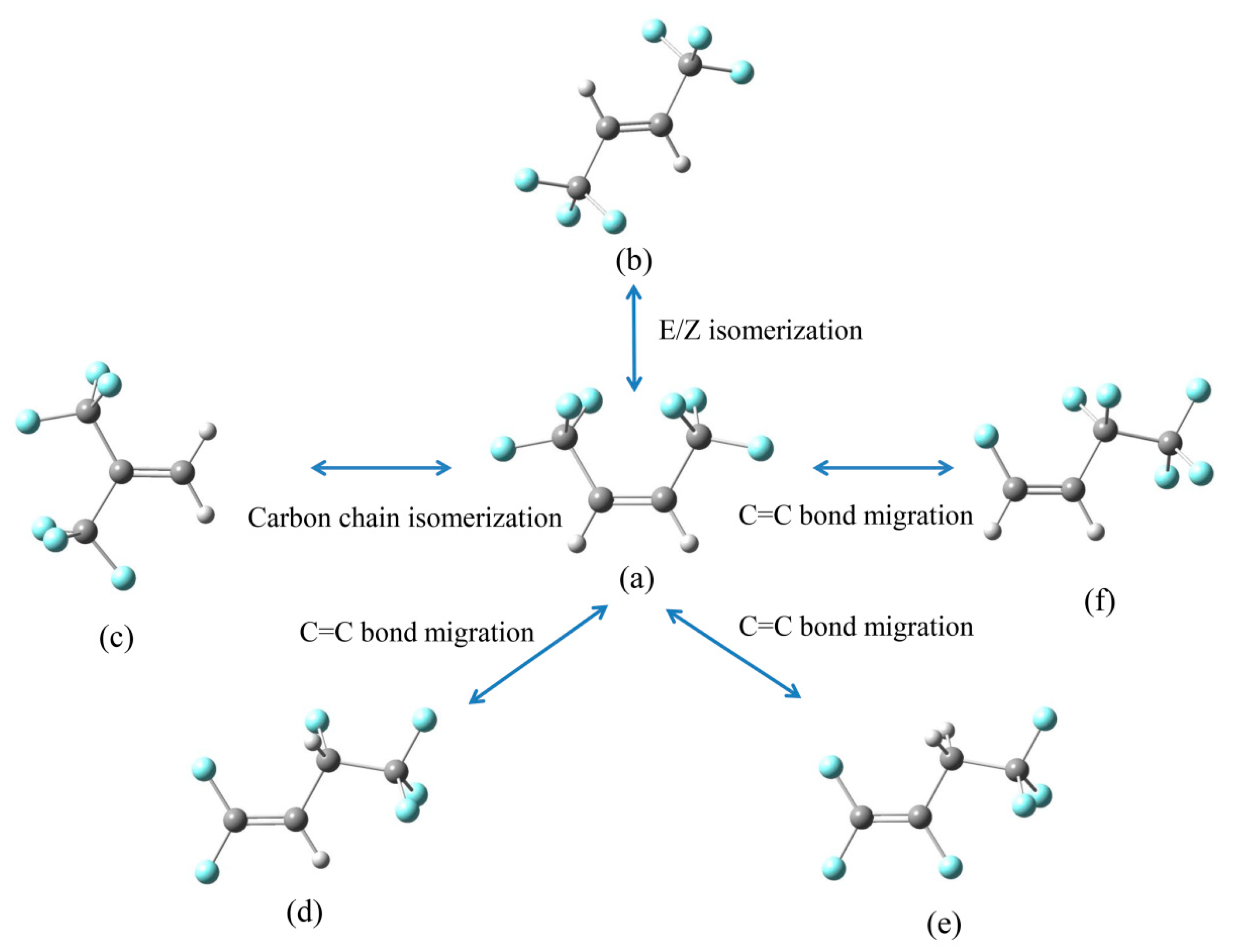
2.2.1. The Homolytic Cleavage Reactions of HFO-1336mzz(Z)
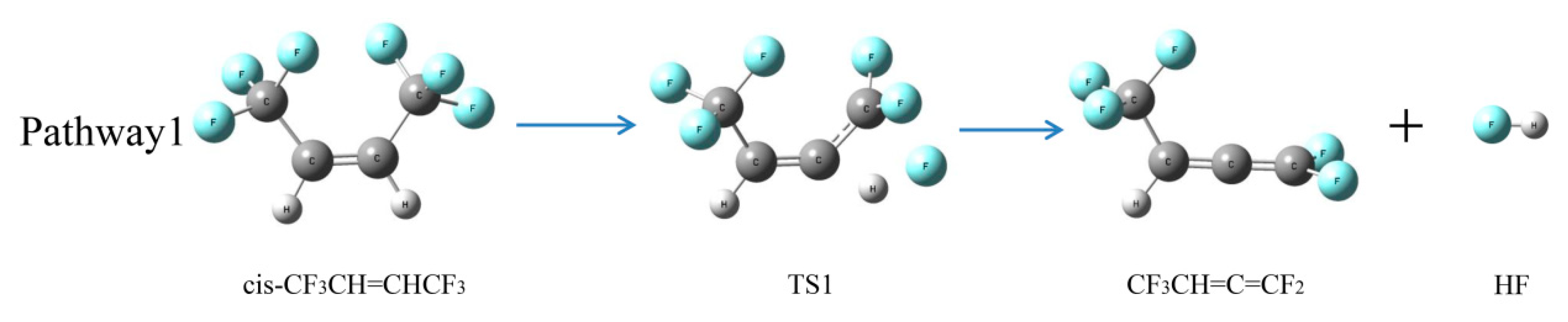
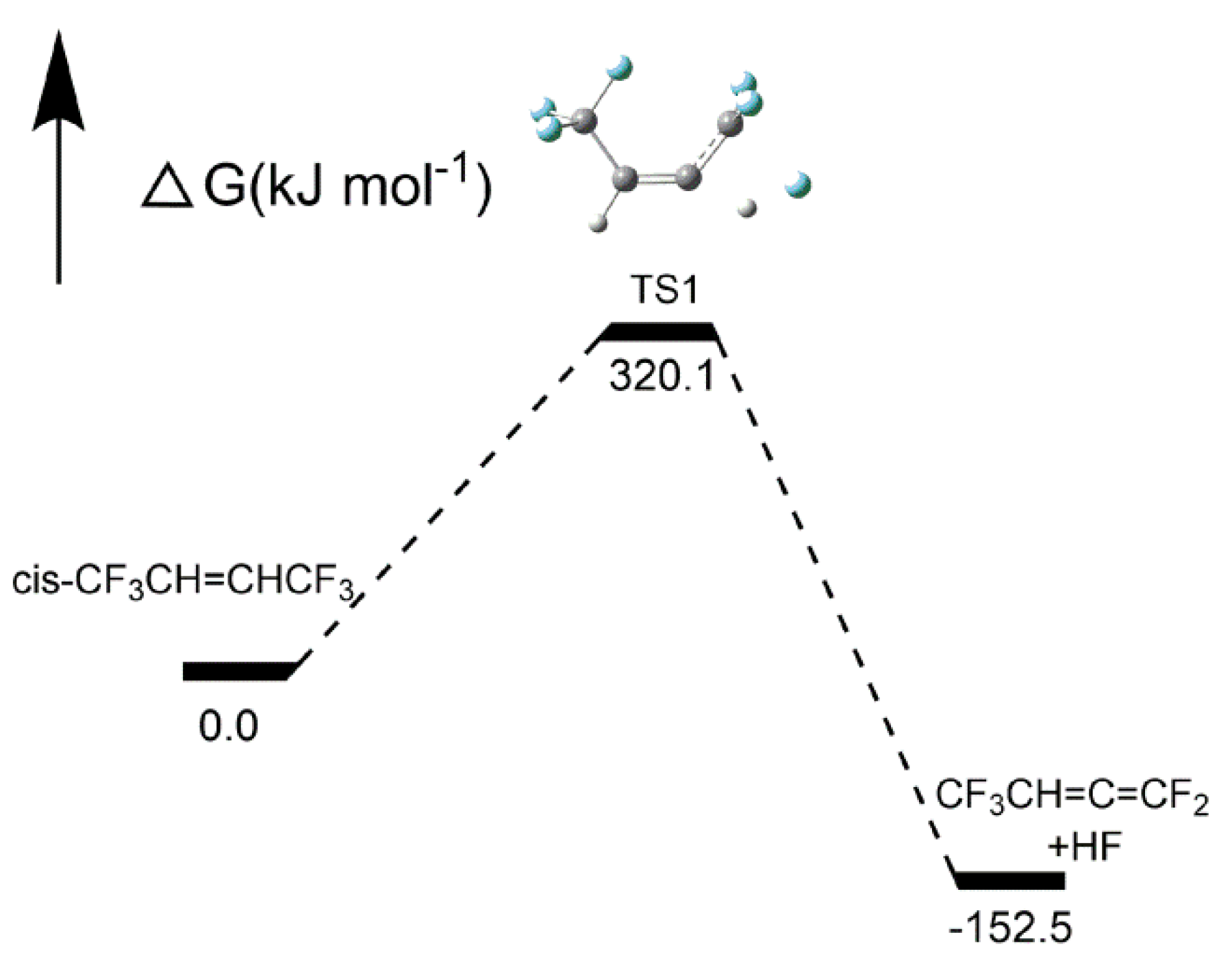
2.2.2. Intramolecular Elimination
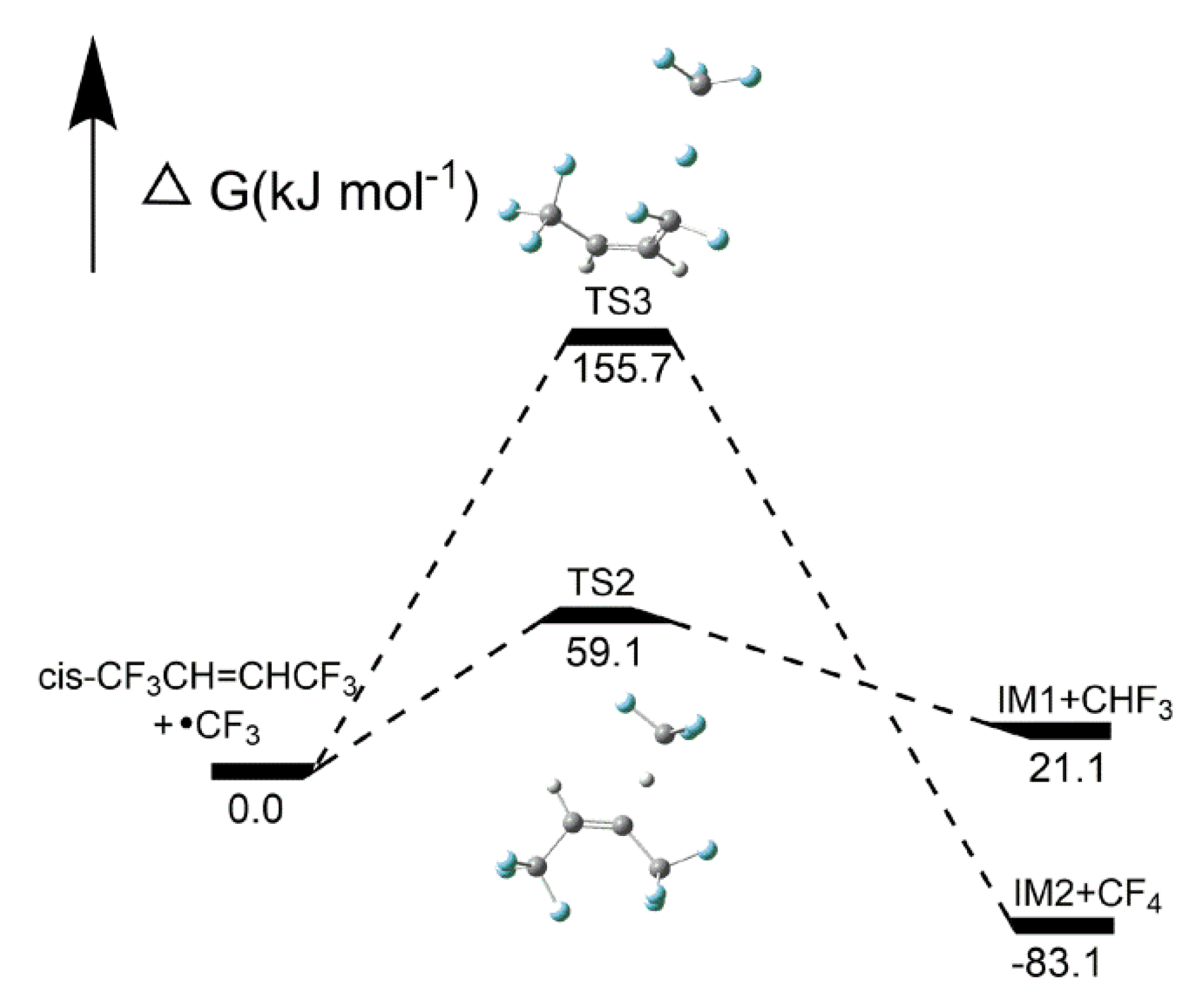
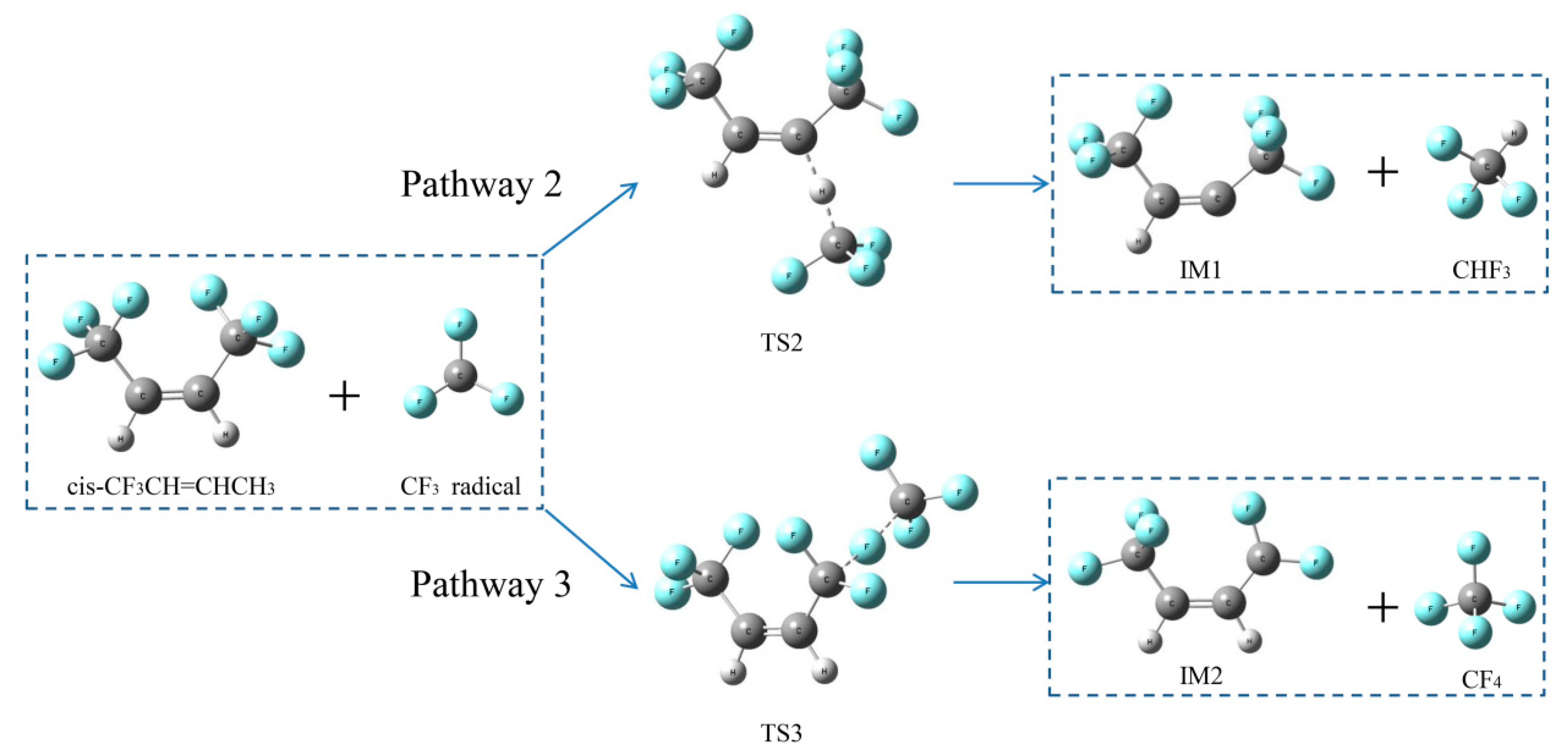
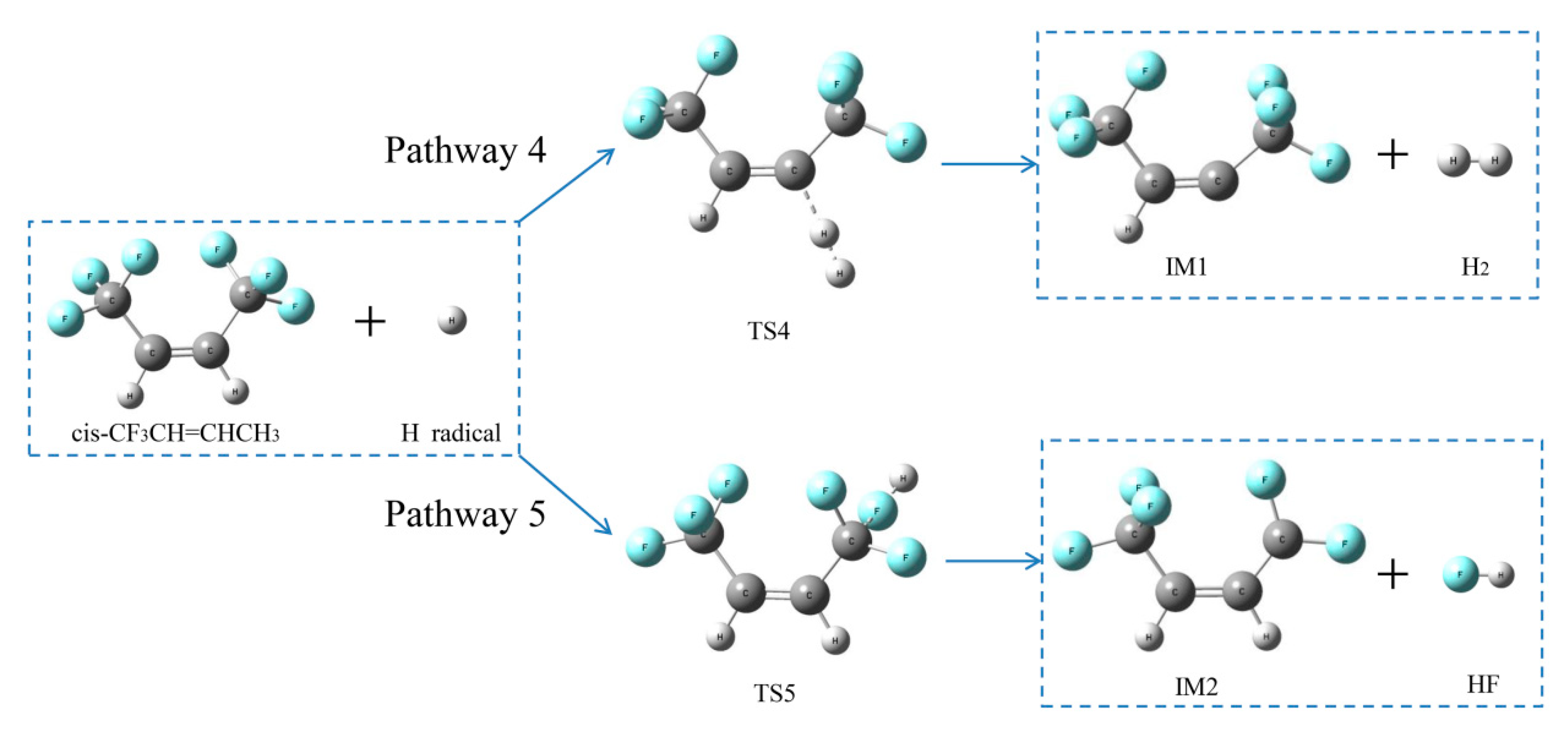
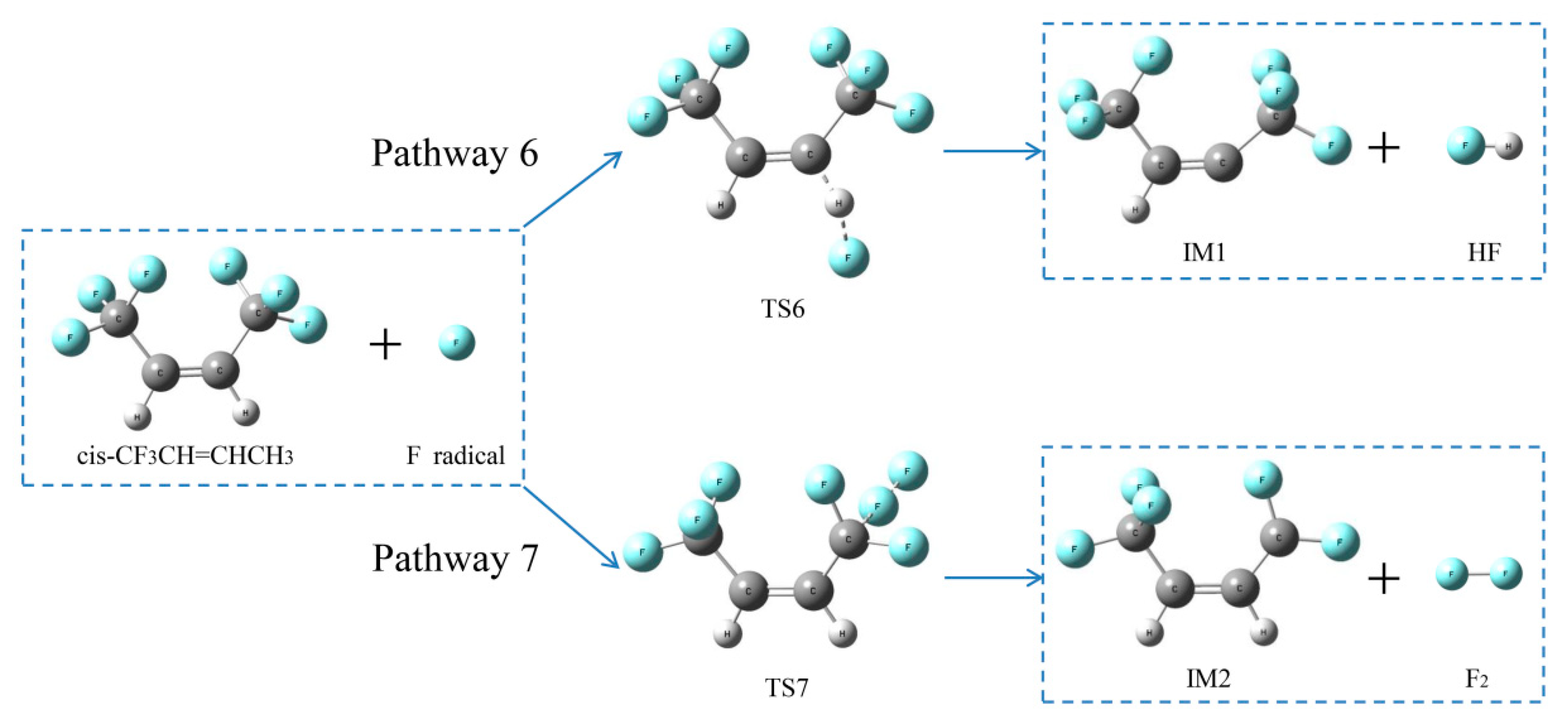
2.2.3. H- and F-abstraction Reactions
Reaction pathways of cis-CF3CH=CHCF3 + CF3•
Reaction pathways of cis-CF3CH=CHCF3 + H•
Reaction pathways of cis-CF3CH=CHCF3 + F•
2.2.4. Formation Pathways of HF and Cyclic Products
3. Methodologies
3.1. Materials
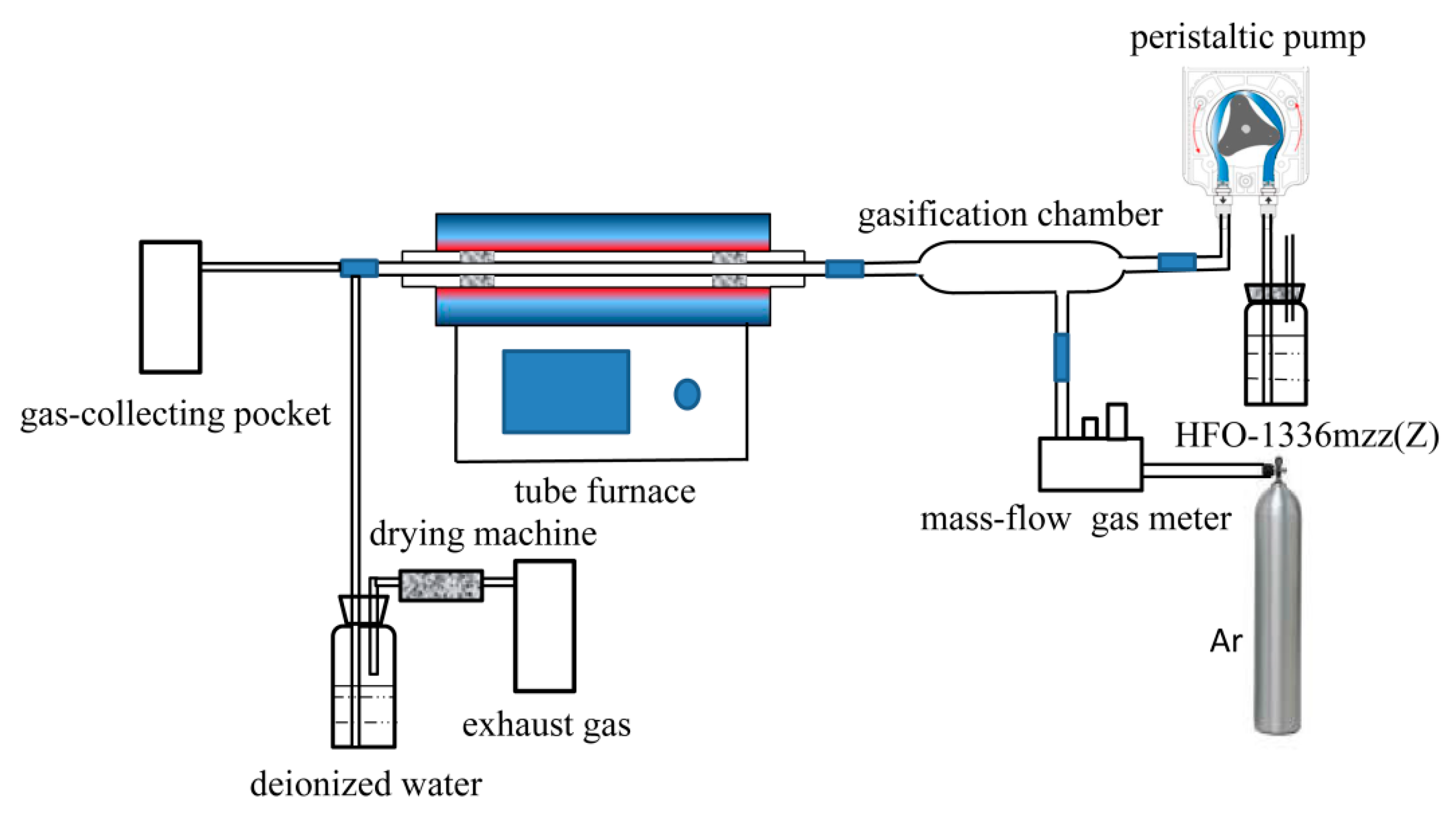
3.2. Experimental Equipment and Procedure
3.3. Theoretical Methodology
3.4. GC–MS and IC Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molés, F.; Navarro-Esbrí, J.; Peris, B.; Mota-Babiloni, A.; Barragán-Cervera, Á.; Kontomaris, K.K. Thermo-economic evaluation of low global warming potential alternatives to HFC-245fa in Organic Rankine Cycles. Energy Procedia 2017, 142, 1199–1205. [Google Scholar] [CrossRef]
- Minor, B.; Kontomaris, K.; Hydutsky, B. Nonflammable Low GWP Working Fluid for Organic Rankine Cycles. In Proceedings of the ASME Turbo Expo 2014: Turbine Technical Conference and Exposition, Düsseldorf, Germany, 16–20 June 2014. [Google Scholar] [CrossRef]
- Cis-1,1,1,4,4,4-hexafluoro-2-butene (HFO-1336mzz-Z) (2018). Toxicol. Ind. Health 2019, 35, 180–188. [CrossRef] [PubMed]
- Gil, B.; Kasperski, J. Efficiency Evaluation of the Ejector Cooling Cycle using a New Generation of HFO/HCFO Refrigerant as a R134a Replacement. Energies 2018, 11, 2136. [Google Scholar] [CrossRef]
- Molés, F.; Navarro-Esbrí, J.; Peris, B.; Mota-Babiloni, A.; Barragán-Cervera, Á.; Kontomaris, K.K. Low GWP alternatives to HFC-245fa in Organic Rankine Cycles for low temperature heat recovery: HCFO-1233zd-E and HFO-1336mzz-Z. Appl. Therm. Eng. 2014, 71, 204–212. [Google Scholar] [CrossRef]
- Kontomaris, K. HFO-1336mzz-Z: High Temperature Chemical Stability and Use as A Working Fluid in Organic Rankine Cycles. In Proceedings of the 15th International Refrigeration and Air Conditioning Conference, Purdue University, West Lafayette, IN, USA, 14–17 July 2014; p. 1525. [Google Scholar]
- Molés, F.; Navarro-Esbrí, J.; Peris, B.; Mota-Babiloni, A.; Kontomaris, K. Thermodynamic analysis of a combined organic Rankine cycle and vapor compression cycle system activated with low temperature heat sources using low GWP fluids. Appl. Therm. Eng. 2015, 87, 444–453. [Google Scholar] [CrossRef]
- Wang, X.; Wu, R.; Cheng, L.; Zhang, X.; Zhou, X. Suppression of propane cup-burner flame with HFO-1336mzz(Z) and its thermal stability study. Thermochim. Acta 2020, 683, 178463. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, X.; Fu, H.; Tan, Z.; Zhang, H. Theoretical and experimental studies on the thermal decomposition and fire-extinguishing performance of cis-1,1,1,4,4,4-hexafluoro-2-butene. Int. J. Quantum Chem. 2020, 120, e26160. [Google Scholar] [CrossRef]
- Huo, E.; Liu, C.; Xin, L.; Li, X.; Xu, X.; Li, Q.; Wang, S.; Dang, C. Thermal stability and decomposition mechanism of HFO-1336mzz(Z) as an environmental friendly working fluid: Experimental and theoretical study. Int. J. Energy Res. 2019, 43, 4630–4643. [Google Scholar] [CrossRef]
- Huo, E.; Liu, C.; Xu, X.; Dang, C. A ReaxFF-based molecular dynamics study of the pyrolysis mechanism of HFO-1336mzz(Z). Int. J. Refrig. 2017, 83, 118–130. [Google Scholar] [CrossRef]
- Huo, E.; Liu, C.; Xu, X.; Li, Q.; Dang, C. A ReaxFF-based molecular dynamics study of the oxidation decomposition mechanism of HFO-1336mzz(Z). Int. J. Refrig. 2018, 93, 249–258. [Google Scholar] [CrossRef]
- Huo, E.; Liu, C.; Xu, X.; Li, Q.; Dang, C. Dissociation mechanisms of HFO-1336mzz(Z) on Cu(1 1 1), Cu(1 1 0) and Cu(1 0 0) surfaces: A density functional theory study. Appl. Surf. Sci. 2018, 443, 389–400. [Google Scholar] [CrossRef]
- Huo, E.; Liu, C.; Xu, X.; Li, Q.; Dang, C.; Wang, S.; Zhang, C. The oxidation decomposition mechanisms of HFO-1336mzz(Z) as an environmentally friendly refrigerant in O2/H2O environment. Energy 2019, 185, 1154–1162. [Google Scholar] [CrossRef]
- Huo, E.; Li, Q.; Liu, C.; Huang, Z.; Xin, L. Experimental and theoretical studies on the thermal stability and decomposition mechanism of HFO-1336mzz(Z) with POE lubricant. J. Anal. Appl. Pyrolysis 2020, 147, 104795. [Google Scholar] [CrossRef]
- Tanaka, K.; Akasaka, R.; Sakaue, E.; Ishikawa, J.; Kontomaris, K.K. Thermodynamic Properties of cis-1,1,1,4,4,4-Hexafluoro-2-butene (HFO-1336mzz(Z)): Measurements of the pρT Property and Determinations of Vapor Pressures, Saturated Liquid and Vapor Densities, and Critical Parameters. J. Chem. Eng. Data 2016, 61, 2467–2473. [Google Scholar] [CrossRef]
- Tanaka, K.; Akasaka, R.; Sakaue, E.; Ishikawa, J.; Kontomaris, K.K. Measurements of the Critical Parameters for cis-1,1,1,4,4,4-Hexafluoro-2-butene. J. Chem. Eng. Data 2017, 62, 1135–1138. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Y.; Wang, T.; Wang, C.; Li, S. Experimental study on the thermal decomposition of 2H-heptafluoropropane. J. Anal. Appl. Pyrolysis 2011, 90, 27–32. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, W.; Chao, M.; Liao, G. The study of thermal decomposition of 2-bromo-3,3,3-trifluoropropene and its fire-extinguishing mechanism. J. Fluor. Chem. 2013, 153, 101–106. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Y.-J.; Zhang, P.; Pan, R.-M. Study on thermal decomposition properties and its decomposition mechanism of pentafluoroethane (HFC-125) fire extinguishing agent. J. Fluor. Chem. 2016, 190, 48–55. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Xu, X.; Li, Q. Mechanism of thermal decomposition of HFO-1234yf by DFT study. Int. J. Refrig. 2017, 74, 399–411. [Google Scholar] [CrossRef]
- Huang, J.B.; Liu, C.; Tong, H.; Li, W.; Wu, D. A density functional theory study on formation mechanism of CO, CO2 and CH4 in pyrolysis of lignin. Comput. Theor. Chem. 2014, 1045, 1–9. [Google Scholar] [CrossRef]
- Lily, M.; Mishra, B.K.; Chandra, A.K. Kinetics, mechanism and thermochemistry of the gas phase reactions of CF3CH2OCH2CF3 with OH radicals: A theoretical study. J. Fluor. Chem. 2014, 161, 51–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Chen, X. Mechanism of glucose conversion in supercritical water by DFT study. J. Anal. Appl. Pyrolysis 2016, 119, 199–207. [Google Scholar] [CrossRef]
- Wang, M.; Liu, C. Theoretic studies on decomposition mechanism of o -methoxy phenethyl phenyl ether: Primary and secondary reactions. J. Anal. Appl. Pyrolysis 2016, 117, 325–333. [Google Scholar] [CrossRef]
- Mishra, B.K.; Lily, M.; Chakrabartty, A.K.; Bhattacharjee, D.; Deka, R.C.; Chandra, A.K. Theoretical investigation of atmospheric chemistry of volatile anaesthetic sevoflurane: Reactions with the OH radicals and atmospheric fate of the alkoxy radical (CF3)2CHOCHFO: Thermal decomposition vs. oxidation. New J. Chem. 2014, 38, 2813–2822. [Google Scholar] [CrossRef]
- Mishra, B.K.; Chakrabartty, A.K.; Deka, R.C. Theoretical investigation of the gas-phase reactions of CF2ClC(O)OCH3 with the hydroxyl radical and the chlorine atom at 298 K. J. Mol. Model. 2013, 19, 3263–3270. [Google Scholar] [CrossRef]
- Gour, N.K.; Deka, R.C.; Singh, H.J.; Mishra, B.K. Theoretical study on the gas-phase reactions of ethyl butyrate with OH radicals at 298 K. Mon. Chem. Chem. Mon. 2014, 145, 1759–1767. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, X.; Zhou, S.; Yu, R.; Liao, Y.; Li, J.; Tan, Z. Thermal Decomposition Mechanism and Fire-Extinguishing Performance of trans-1,1,1,4,4,4-Hexafluoro-2-butene: A Potential Candidate for Halon Substitutes. J. Phys. Chem. A 2020, 124, 5944–5953. [Google Scholar] [CrossRef]
- Richter, H.; Howard, J. Formation of polycyclic aromatic hydrocarbons and their growth to soot—a review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Johansson, K.O.; Head-Gordon, M.; Schrader, P.E.; Wilson, K.R.; Michelsen, H.A. Resonance-stabilized hydrocarbon-radical chain reactions may explain soot inception and growth. Science 2018, 361, 997–1000. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Exploring the Limit of Accuracy of the Global Hybrid Meta Density Functional for Main-Group Thermochemistry, Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2008, 4, 1849–1868. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Accounts 2007, 120, 215–241. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Available online: https://gaussian.com/citation/ (accessed on 20 August 2020).
Sample Availability: Samples of the compounds are available from the authors. |






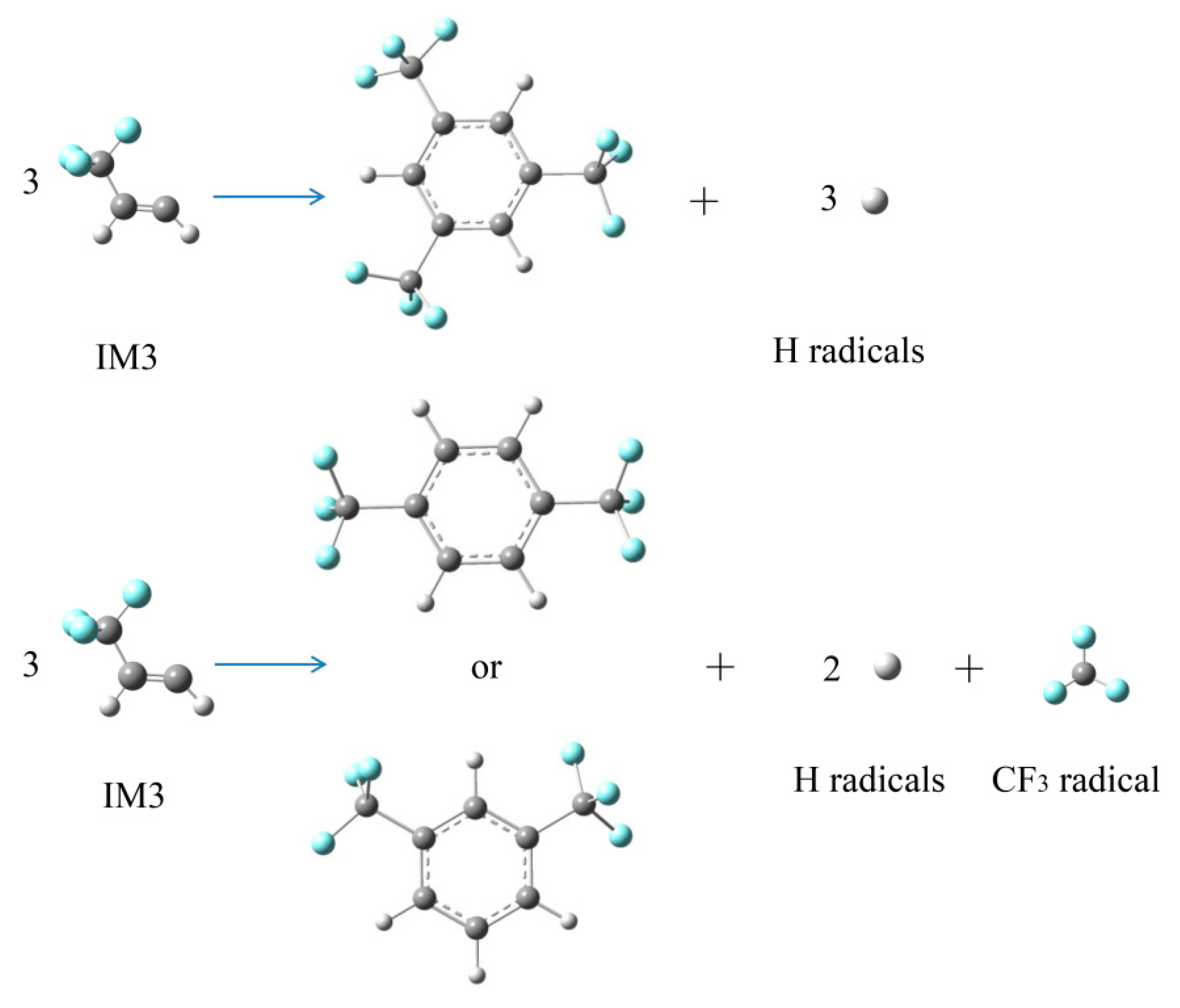
| Bonds | Bond Distance (Å) | Reaction Equations | BDEs (kJ · mol−1) | BDEs (298 K) (kJ · mol−1) [11] |
|---|---|---|---|---|
| C–H | 1.08 | CF3CH=CHCF3→CF3CH(C·)CF3 + H (2) | 446.3 | 460.8 |
| C–F | 1.34 | CF3CH=CHCF3→CF3CHCH(C·)CF2 + F (3) | 444.0 | 454.5 |
| C–C | 1.51 | CF3CH=CHCF3→CF3CHCH(C·) + CF3 (4) | 427.5 | 431.2 |
| C=C | 1.32 | CF3CH=CHCF3→CF3(C·)H(C·)HCF3 (1) | 240.7 | 238.6 |
| CF3CH=CHCF3→2CF3(C·)H (5) | 685.7 |
| Pathway | Reaction | Energy Barrier (kJ · mol−1) |
|---|---|---|
| Pathway 1 | cis-CF3CH=CHCF3→CF3CH=C=CF2 + HF | 320.1 |
| Pathway 5 | cis-CF3CH=CHCF3+H•→IM2 + HF | 4.7 |
| Pathway 6 | cis-CF3CH=CHCF3+F•→IM1 + HF | 147.3 |
| Parameters | GC–MS |
|---|---|
| Instrument | Shimadzu GCMS-QP2010 Plus, Ultra |
| Column | BD-624,30.0 m, 0.25 mm, 1.4 μm BD-5,60.0 m, 0.25 mm, 1.4 μm |
| Carrier gas flow rate | 3.0 mL/min |
| Column temperature | 323 K |
| Split ratio | 1:20 |
| Injection temperature | 513 K |
| Ion source temperature | 503 K |
| Ionization method | Electron impact |
| Ionization energy | 70 eV |
| Scanning range | m/z 28.0–400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, N.; Liu, C.; Xing, H.; Lu, S.; Lo, S.; Zhang, H. Experimental and Density Functional Theory Studies on 1,1,1,4,4,4-Hexafluoro-2-Butene Pyrolysis. Molecules 2020, 25, 3799. https://doi.org/10.3390/molecules25173799
Tao N, Liu C, Xing H, Lu S, Lo S, Zhang H. Experimental and Density Functional Theory Studies on 1,1,1,4,4,4-Hexafluoro-2-Butene Pyrolysis. Molecules. 2020; 25(17):3799. https://doi.org/10.3390/molecules25173799
Chicago/Turabian StyleTao, Neng, Changcheng Liu, Haoran Xing, Song Lu, Siuming Lo, and Heping Zhang. 2020. "Experimental and Density Functional Theory Studies on 1,1,1,4,4,4-Hexafluoro-2-Butene Pyrolysis" Molecules 25, no. 17: 3799. https://doi.org/10.3390/molecules25173799
APA StyleTao, N., Liu, C., Xing, H., Lu, S., Lo, S., & Zhang, H. (2020). Experimental and Density Functional Theory Studies on 1,1,1,4,4,4-Hexafluoro-2-Butene Pyrolysis. Molecules, 25(17), 3799. https://doi.org/10.3390/molecules25173799





