Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of the Best Performing Adsorbent
2.2. Response Surface Methodology (RSM): FCCCD
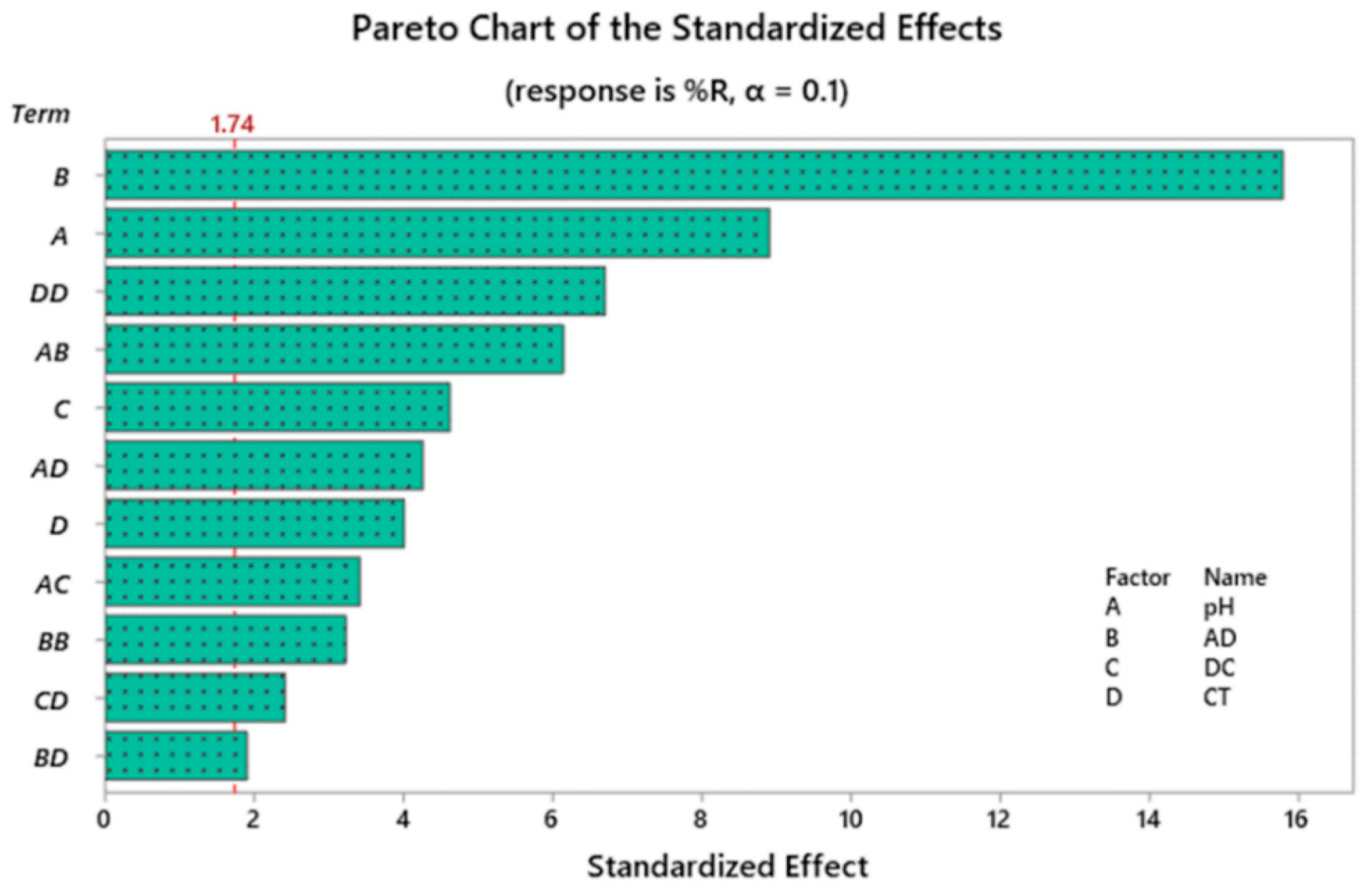
2.3. Investigation of Statistically Significant Variables
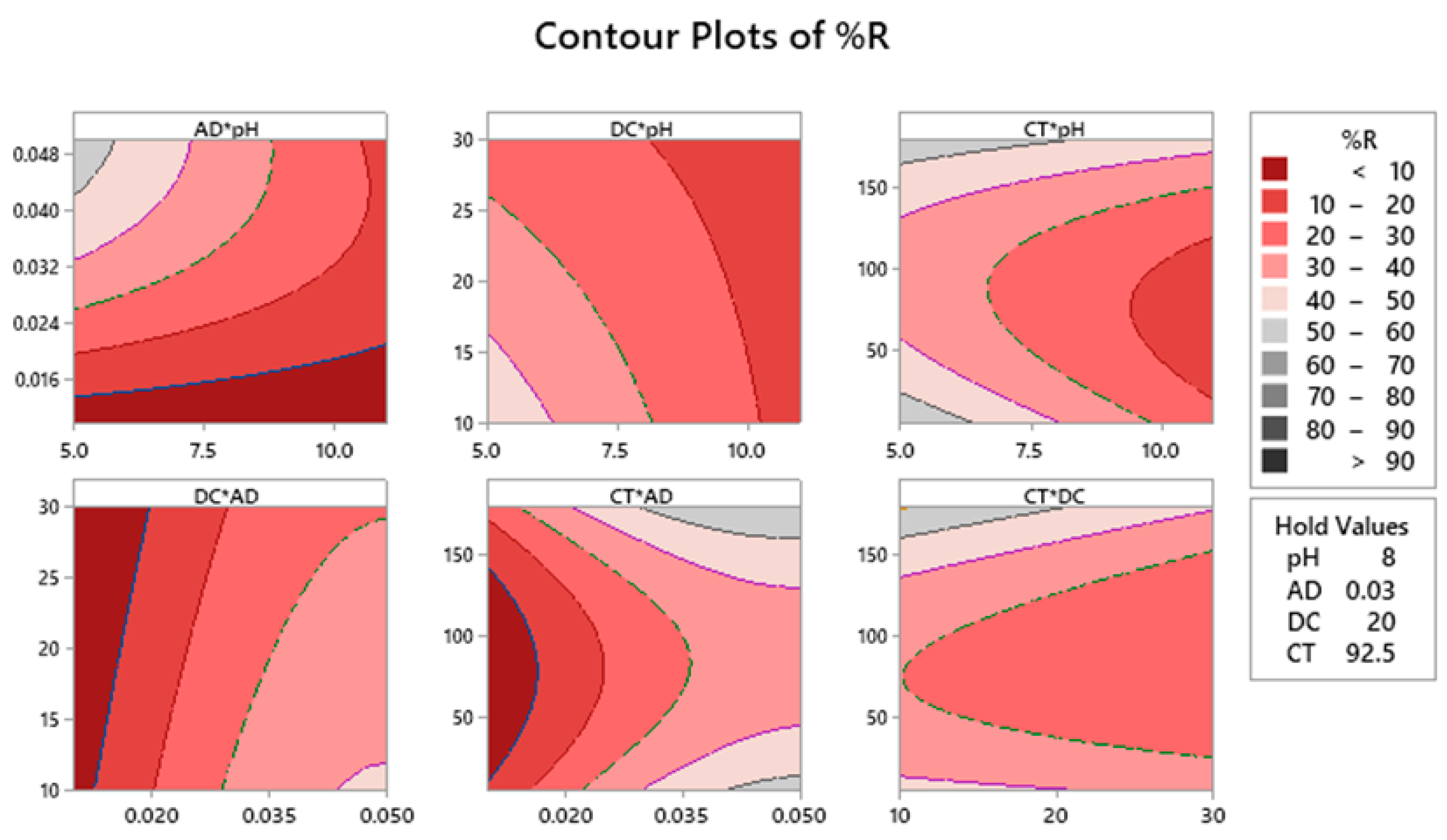
2.4. Contour Plots of %R and Surface Optimization
2.5. Adsorbent Characterization
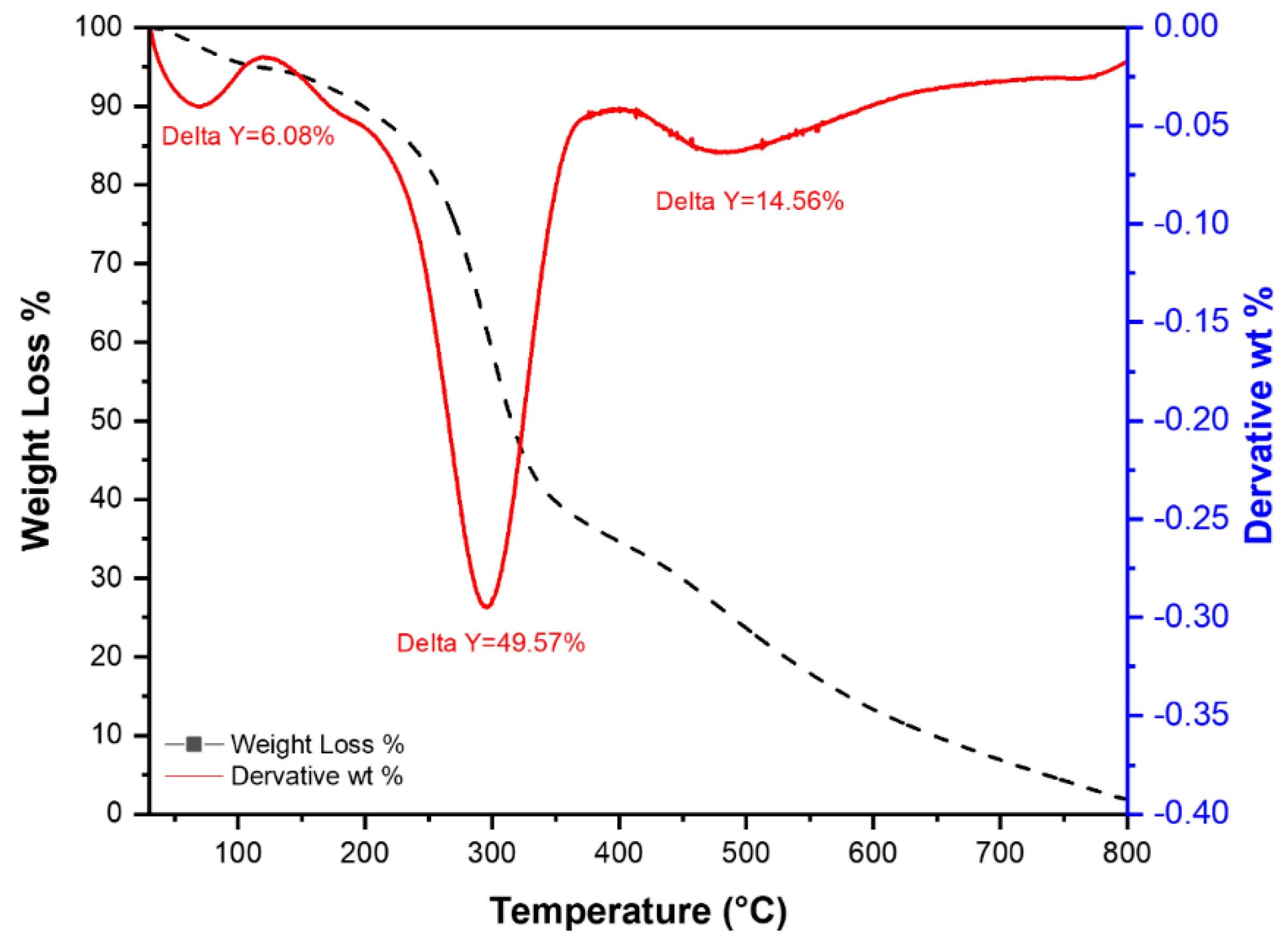
2.5.1. Thermogravimetric Analysis (TGA)
- (1)
- First step: loss of adsorbed water molecules at a temperature range of 25–100 °C and represents 6.08% of the sample,
- (2)
- In this step, >49% of the sample is decomposed between (200–500 °C) including the loss of crystalline water at ~200 °C and part of the organic matter, as represented by a major peak at 305 °C, which could be ascribed to the decomposition of the organic material in RPAL,
- (3)
- The last step at ~525 °C where 14.46% of the RPAL sample was lost at this stage and it could be related to the carbonization of PAL.
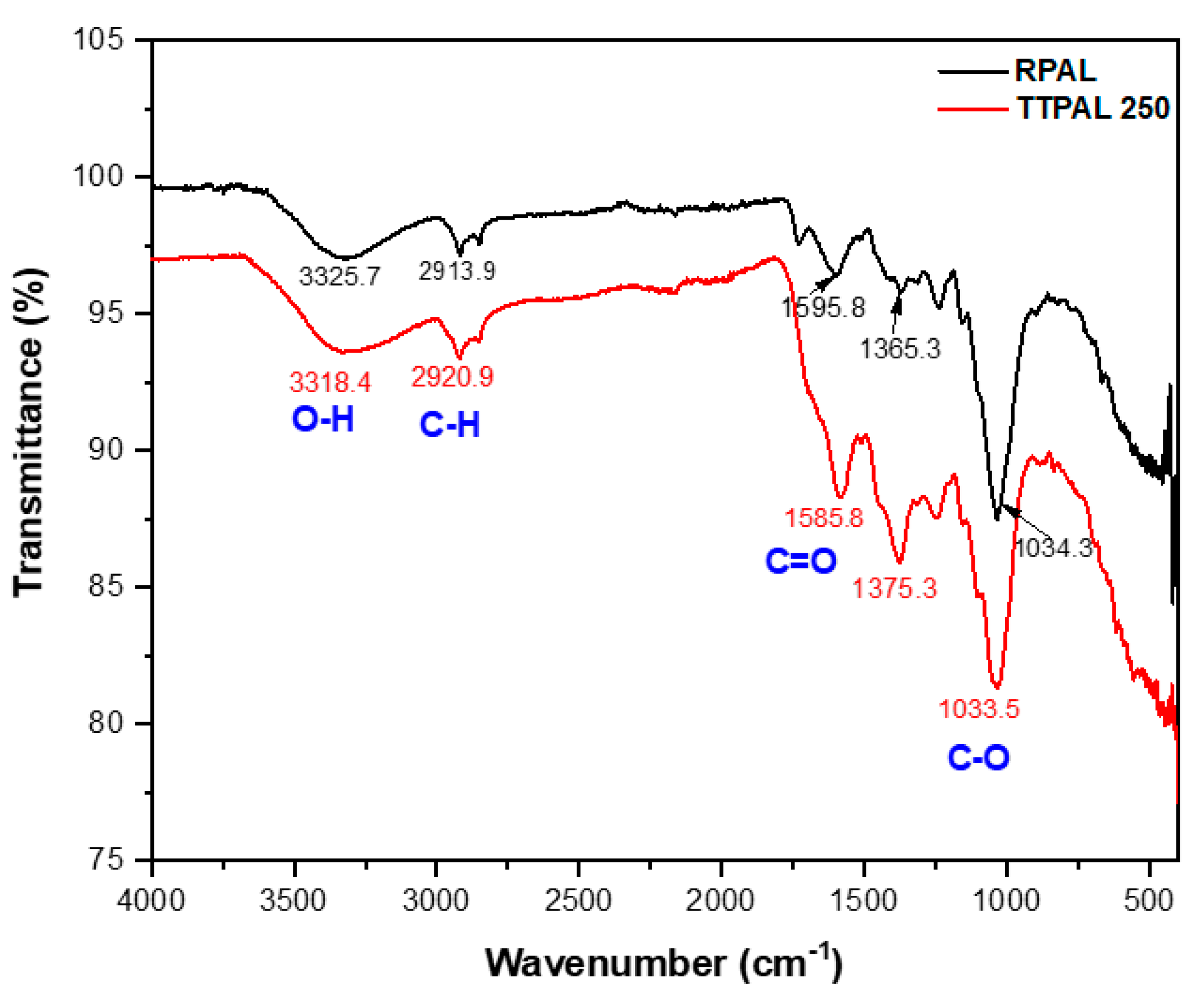
2.5.2. Fourier Transform Infrared Spectroscopic Analysis (FT-IR)
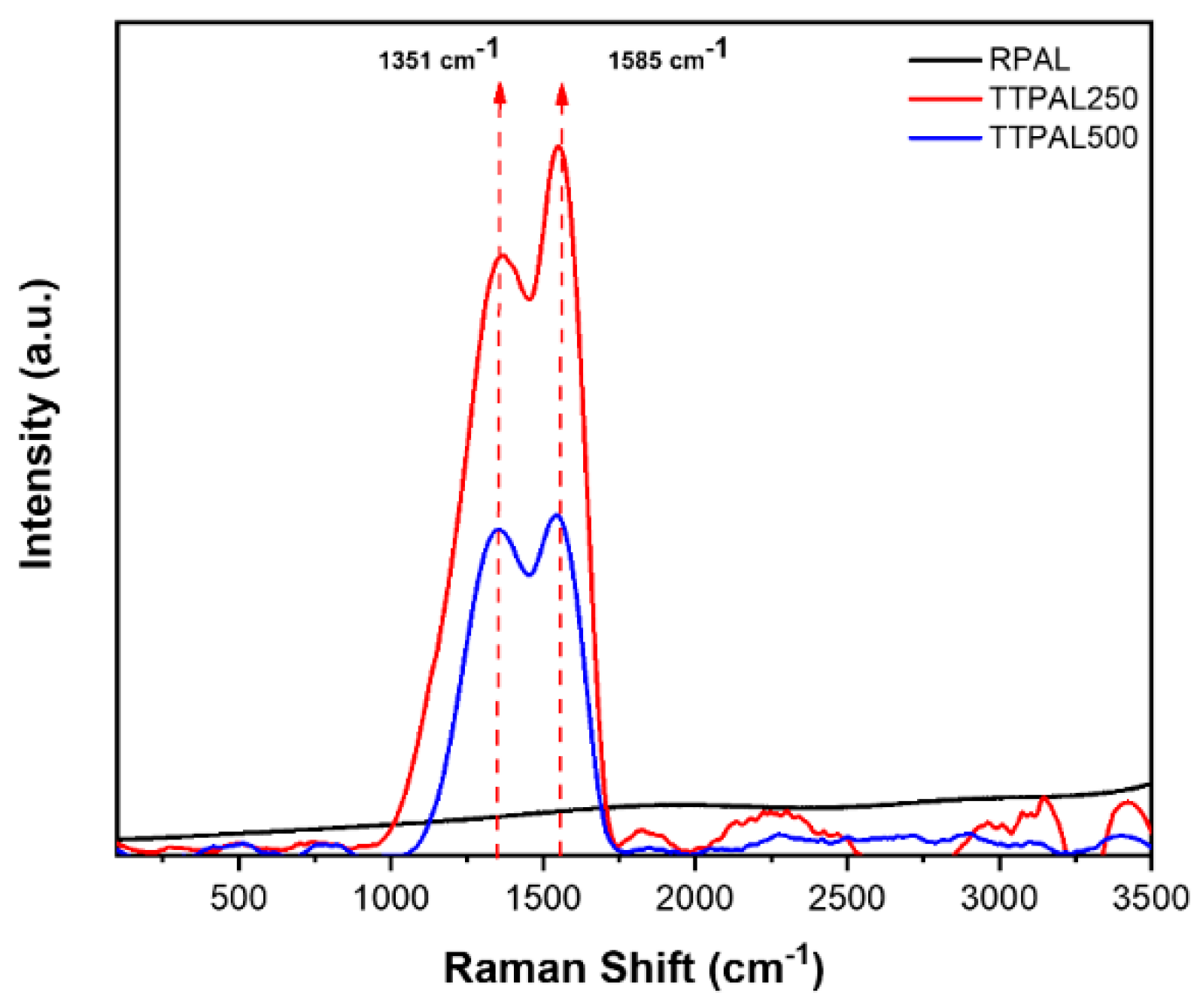
2.5.3. Raman Analysis
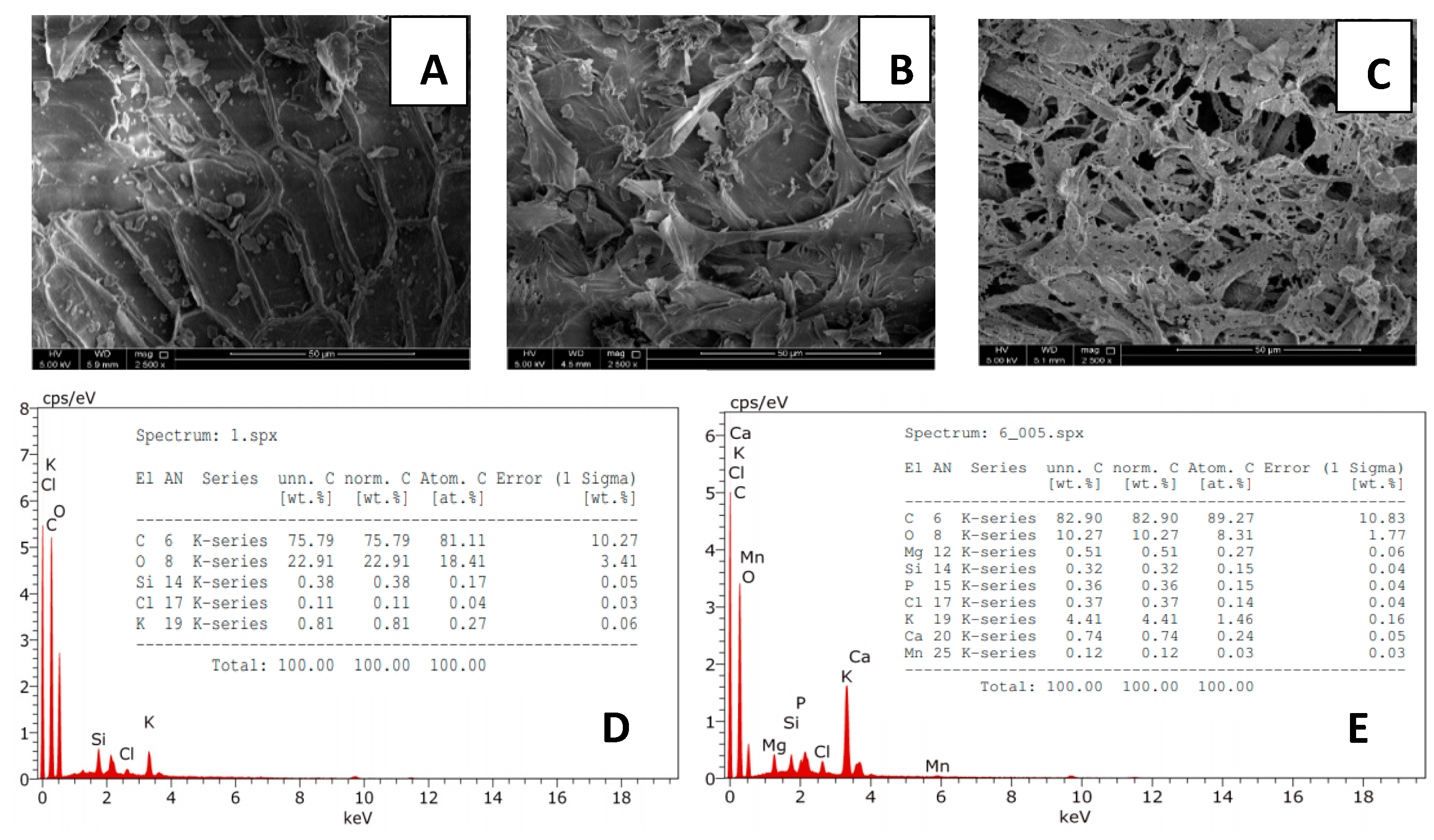
2.5.4. Scanning Electron Microscopy Analysis (SEM)
2.5.5. Carbon, Hydrogen, and Nitrogen Analysis (CHN)
2.5.6. Brunauer–Emmett–Teller (BET) Surface Area Analysis
2.6. Equilibrium and Kinetics Studies of the Adsorption of RB onto PAL
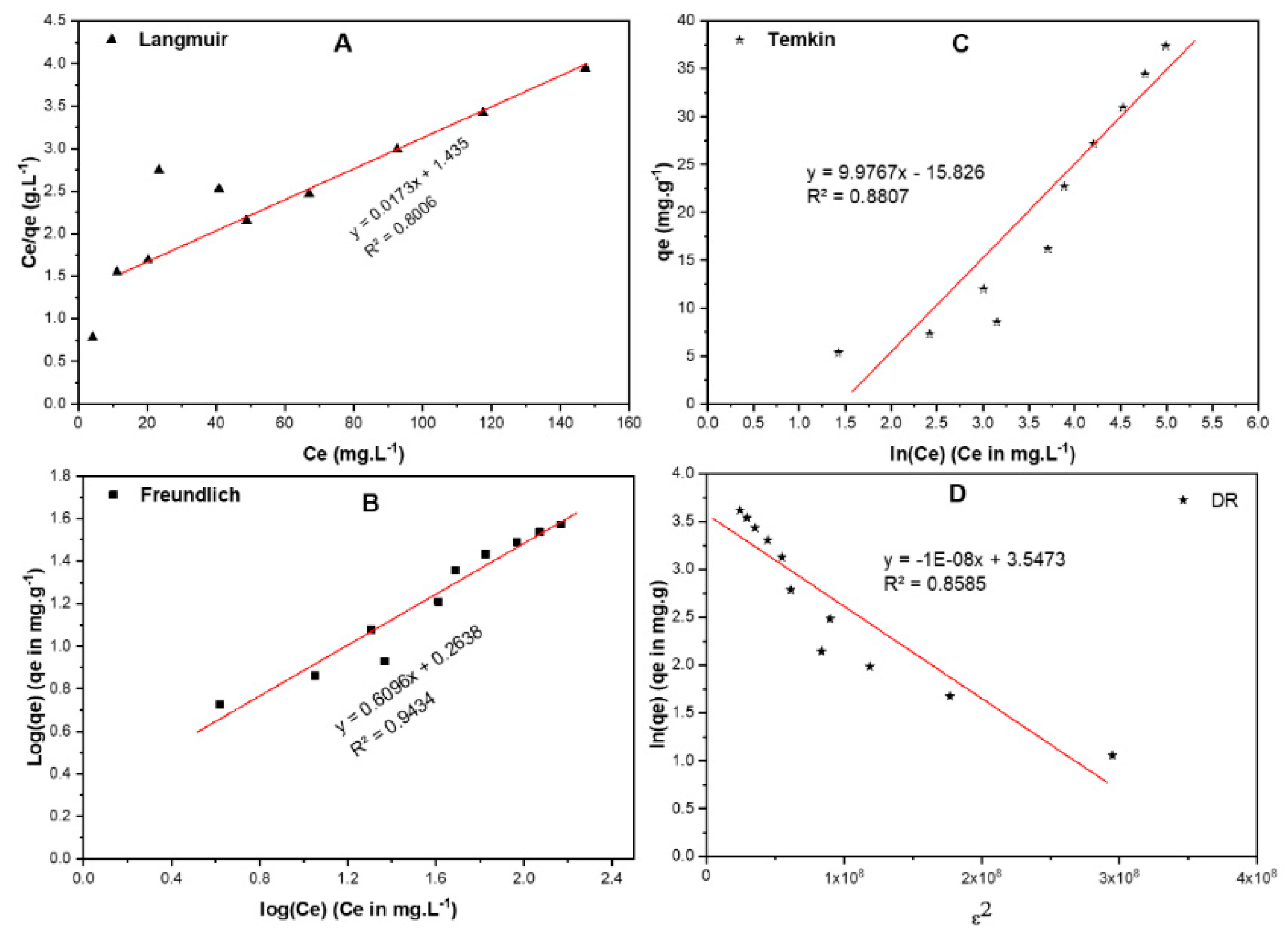
2.6.1. Equilibrium Isotherms
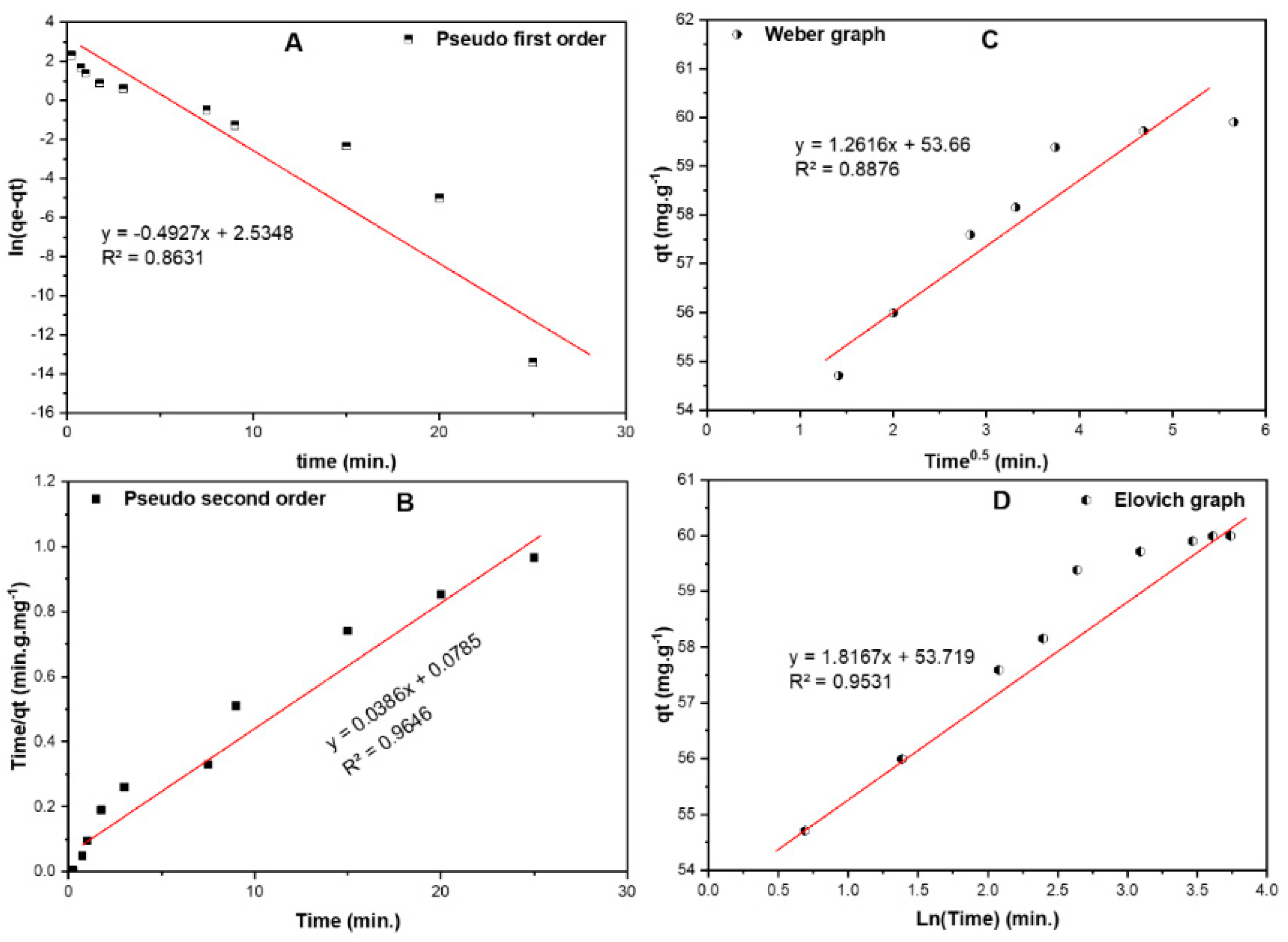
2.6.2. Biosorption Kinetics
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instrumentation and Software
3.3. Face—Centered Central Composite Design (FCCCD)
3.4. Preparation of RB
3.5. Adsorbent Preparation
3.5.1. Air–Dried Raw Pineapple Leaves (RPAL)
3.5.2. Thermal Treatment of Pineapple Leaves
3.6. Evaluation of the Adsorption Perfomance of the Prepared Adsorbents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hinrichsen, D.; Tacio, H.D. Finding the source: The Coming Freshwater Crisis is Already Here. Available online: http://www.wilsoncenter.org/topics/pubs/popwawa2 (accessed on 26 April 2020).
- Global Water Supply and Sanitation Assessment 2000 Report; World Health Organization: Geneva, Switzerland, 2000. Available online: http://www.who.int/water_sanitation_health/monitoring/jmp2000 (accessed on 4 May 2020).
- Ullah, R.; Malik, R.; Qadir, A. Assessment of groundwater contamination in an industrial city, Sialkot, Pakistan. Afr. J. Environ. Sci. Technol. 2009, 3, 429–446. [Google Scholar] [CrossRef]
- Owa, F.D. Water pollution: Sources, effects, control and management. Mediterr. J. Soc. Sci. 2013, 4, 65–68. [Google Scholar] [CrossRef]
- El-Azazy, M.; Kalla, R.N.; Issa, A.A.; Al-Sulaiti, M.; El-Shafie, A.S.; Shomar, B.; Al-Saad, K. Pomegranate peels as versatile adsorbents for water purification: Application of Box–Behnken design as a methodological optimization approach. Environ. Prog. Sustain. Energy 2019, 38, 13223. [Google Scholar] [CrossRef]
- Al-Saad, K.; El-Azazy, M.; Issa, A.A.; Al-Yafei, A.; El-Shafie, A.S.; Al-Sulaiti, M.; Shomar, B. Recycling of date pits into a green adsorbent for removal of heavy metals: A fractional factorial design-based approach. Front. Chem. 2019, 7, 552. [Google Scholar] [CrossRef] [Green Version]
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato peels as an adsorbent for heavy metals from aqueous solutions: Eco-structuring of a green adsorbent operating Plackett-Burman Design. J. Chem. 2019, 2019, 4926240. [Google Scholar] [CrossRef] [Green Version]
- El-Azazy, M.; Dimassi, S.; El-Shafie, A.S.; Issa, A.A. Bio-Waste Aloe vera Leaves as an efficient adsorbent for Titan Yellow from Wastewater: Structuring of a novel adsorbent using Plackett-Burman factorial design. J. Appl. Sci. 2019, 9, 4856. [Google Scholar] [CrossRef] [Green Version]
- El-Azazy, M.; El-Shafie, A.S.; Ashraf, A.; Issa, A.A. Eco-structured biosorptive removal of basic fuchsin using pistachio nutshells: A definitive screening design—Based approach. J. Appl. Sci. 2019, 9, 4855. [Google Scholar] [CrossRef] [Green Version]
- Avci, A.; Inci, I.; Baylan, N. Adsorption of ciprofloxacin hydrochloride on multiwall carbon nanotube. J. Mol. Struct. 2020, 1206, 127711. [Google Scholar] [CrossRef]
- Hao, O.J.; Kim, H.; Chiang, P.-C. Decolorization of wastewater. Crit. Rev. Environ. Sci. Technol. 2000, 30, 449–505. [Google Scholar] [CrossRef]
- Benkhaya, S.; Harfi, S.; El Harfi, A. Classifications, properties and applications of textile dyes: A review. Appl. J. Environ. Eng. Sci. 2017, 3, 311–320. [Google Scholar] [CrossRef]
- Shabir, G.; Saeed, A.; Channar, P.A. A review on the recent trends in synthetic strategies and applications of xanthene dyes. Mini Rev. Org. Chem. 2018, 14. [Google Scholar] [CrossRef]
- Tabery, H.M. Toxic effect of rose bengal dye on the living human corneal epithelium. Acta Ophthalmol. Scand. 1998, 76, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, K.F.; Reed, R.H.; Perry, J.D. Phototoxicity of rose bengal in mycological media-implications for laboratory practice. Lett. Appl. Microbiol. 2008, 28, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Vinuth, M.; Bhojya, N.; Vinoda, B.M.; Pradeepa, S.M.; Kumar, A.; Chandra, S. Rapid removal of hazardous rose bengal dye using Fe(III)—Montmorillonite as an effective adsorbent in aqueous solution. J. Environ. Anal. Toxicol. 2016, 6, 2. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdelbar, N.M.; Mohamed, A.A. Molecular imprinted chitosan-TiO2 nanocomposite for the selective removal of Rose Bengal from wastewater. Int. J. Biol. Macromol. 2018, 107, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Maji, S.K.; Adhikary, B. γ-Fe2O3 nanoparticles: An easily recoverable effective photo-catalyst for the degradation of rose Bengal and methylene blue dyes in the waste-water. Mater. Res. Bull. 2014, 49, 28–34. [Google Scholar] [CrossRef]
- Elfeky, A.S.; Salem, S.S.; Elzaref, A.S.; Owda, M.E.; Eladawy, H.A.; Saeed, A.M.; Awad, M.A.; Abou-Zeid, R.E.; Fouda, A. Multifunctional cellulose nanocrystal /metal oxide hybrid, photodegradation, antibacterial and larvicidal activities. Carbohydr. Polym. 2020, 230, 115711. [Google Scholar] [CrossRef]
- Rasool, M.A.; Goethem, V.C.; Vankelecom, I.F.J. Green preparation process using methyl lactate for cellulose-acetate-based nanofiltration membranes. Sep. Purif. Technol. 2019, 232, 115903. [Google Scholar] [CrossRef]
- Karimi, A.; Khataee, A.; Safarpour, M.; Vatanpour, V. Development of mixed matrix ZIF-8/polyvinylidene fluoride membrane with improved performance in solvent resistant nanofiltration. Sep. Purif. Technol. 2019, 237, 116358. [Google Scholar] [CrossRef]
- Shen, G.; Pan, L.; Zhang, R.; Sun, S.; Hou, F.; Zhang, X.; Zou, J. Low-spin-state hematite with superior adsorption of anionic contaminations for water purification. Adv. Mater. 2020, 32, 1905988. [Google Scholar] [CrossRef]
- Sivamani, S.; Leena, G.B. Removal of dyes from wastewater using adsorption—A review. Int. J. Biosci. Technol. 2009, 2, 47–51. [Google Scholar] [CrossRef]
- Jiahao, M.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Asim, M.; Abdan, K.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.R.; Hoque, M.E. A review on pineapple leaves fibre and its composites. Int. J. Polym. Sci. 2015, 2015, 4–5. [Google Scholar] [CrossRef] [Green Version]
- Bartholomew, D.P.; Paull, R.E.; Rohrbach, K.G. The Pineapple: Botany, Production, and Uses, 1st ed.; CAB International: New York, NY, USA, 2003; pp. 3–5. [Google Scholar]
- Nanthaya, K.; Taweechai, A. A new approach to ‘‘Greening’’ plastic composites using pineapple leaf waste for performance and cost effectiveness. Mater. Des. 2014, 55, 292–299. [Google Scholar]
- Mishra, S.; Misra, M.; Tripathy, S.S.; Nayak, S.K.; Mohanty, A.K. Potentiality of pineapple leaf fibre as reinforcement in PALF-polyester composite: Surface modification and mechanical performance. J. Reinf. Plast. Comp. 2001, 20, 321–334. [Google Scholar] [CrossRef]
- Weng, C.H.; Wu, Y.C. Potential low-cost biosorbent for copper removal: Pineapple leaf powder. J. Environ. Eng. 2012, 138, 286–292. [Google Scholar] [CrossRef]
- Mopoung, R.; Kengkhetkit, N. Lead and cadmium removal efficiency from aqueous solution by NaOH treated pineapple waste. Int. J. Appl. Chem. 2016, 12, 23–35. [Google Scholar]
- Gogoi, S.; Chakraborty, S.; Saikia, M.D. Surface modified pineapple crown leaf for adsorption of Cr(VI) and Cr(III) ions from aqueous solution. J. Environ. Chem. Eng. 2018, 6, 2492–2501. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, Y.T.; Tzeng, T.W. Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazard. Mater. 2009, 170, 417–424. [Google Scholar] [CrossRef]
- Mahamad, M.N.; Zaini, M.A.A.; Zakaria, Z.A. Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. Int. Biodeterior. Biodegrad. 2015, 102, 274–280. [Google Scholar] [CrossRef]
- Rahmat, N.A.; Ali, A.A.; Salmiati, H.N.; Muhamad, M.S.; Kristanti, R.A.; Hadibarata, T. Removal of Remazol Brilliant Blue R from aqueous solution by adsorption using pineapple leaf powder and lime peel powder. Water Air Soil Pollut. 2016, 227, 1–11. [Google Scholar] [CrossRef]
- Lutpi, N.A.; Yin, T.H.; Shian, W.Y. Removal of methylene blue using pineapple peel powder as adsorbent. In Proceedings of the 3rd CUTSE International Conference Miri, Sarawak, Malaysia, 8–9 November 2011; pp. 352–356. [Google Scholar]
- Elazazy, M.S. Factorial design and machine learning strategies: Impacts on pharmaceutical analysis. In Spectroscopic Analyses; Zafar, F., Ed.; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/books/spectroscopic-analyses-developments-and-applications/factorial-design-and-machine-learning-strategies-impacts-on-pharmaceutical-analysis (accessed on 1 May 2020). [CrossRef] [Green Version]
- Ho, L.H.; Feng, S.Y.; Yen, T.M. Using modified IPA to improve service quality of standard hotel in Taiwan, an analysis of transformations. J. Serv. Sci. Manag. 2014, 7, 211–252. [Google Scholar]
- Narayanaswamy, R.; Wolfbeis, O.S. Optical Sensors: Industrial Environmental and Diagnostic Applications; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar] [CrossRef]
- Sabnis, R.W. Handbook of Biological Dyes and Stains: Synthesis and Industrial Applications, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Jhare, D.; Mittal, J. Batch and bulk removal of hazardous colouring agent rose bengal by adsorption over bottom Ash. RSC Adv. 2012, 2, 8381–8389. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman spectroscopy of graphene and related materials. In New Developments in Photon and Materials Research, 1st ed.; Jang, J.I., Ed.; Nova Science Publishers, Inc.: Oxford, UK, 2013; pp. 403–418. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1052–1069. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. Adsorption of gases on glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wang, J. Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of chromium (VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Sarma, G.K.; Khan, A.; El-Toni, A.M.; Rashid, M.H. Shape-tunable CuO-Nd(OH)3 nanocomposites with excellent adsorption capacity in organic dye removal and regeneration of spent adsorbent to reduce secondary waste. J. Hazard. Mater. 2019, 380, 120838. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yuan, Y.; Li, H.; Li, X.; Zhang, C.; Guo, F.; Liu, X.; Wang, K.; Zhao, X. Waste-cellulose-derived porous carbon adsorbents for methyl orange removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar]


| Absorbent | Modification Method | Analytical Approach Used | Surface Area (m2/g) | Adsorbate | Adsorption Capacity (mg/g) | %Removal | References |
|---|---|---|---|---|---|---|---|
| Raw pineapple leaves (RPAL) | Please check the experimental part of this paper. | FCCCD | 4.79 | Rose Bengal | 58.8 | 92.53% | Current work |
| Pineapple leaf powder (PLP) | Leaves were washed, dried at 80 °C for 24 h, at pressure 70 kPa, pulverized, and grinded to fine PLP to be used. | Single variate analysis | ND * | Cu (II) | 9.28 | 90% | [29] |
| NaOH-treated pineapple waste | Leaves were washed several times, dried in oven at 105 °C for 24 h, grinded and screened by 60 mesh sieves to use. | Single variate analysis (Batch experiments) | ND * | Pb (II) and Cd (II) | ND * | <95% | [30] |
| Surface modified pineapple crown leaves (PCL) | Leaves were washed, dried at 70 °C for 48 h, pretreated by isopropyl alcohol and NaOH to produce (P)PCL, modified by acetic acid and hydrogen peroxide to produce (M)PCL. | Single variate analysis | (P)PCL: 32.90 | Cr (VI) and Cr (III) | Cr(VI) on (M)PCL: 3.91 Cr(VI) on (P)PCL: 2.69 Cr(III) on (M)PCL: 2.54 Cr(III) on (P)PCL: 1.82 | ND * | [31] |
| Pineapple leaf powder (PLP) | Leaves were washed several times, dried in oven at 80 °C for 48 h, grinded into powder for further use. | Single variate analysis | 5.24 | Methylene Blue | ND * | <95% | [32] |
| Pineapple crown activated carbon and ZnCl2 | Leave were washed with distilled water, dried at 110 °C, chopped into small pieces, and mixed at ratio 1:1 with zinc chloride. | Single variate analysis (Batch experiments) | 914.7 | Methylene Blue | 288.34 | * ND | [33] |
| Pineapple leaf powder | Leaves were washed several times, dried in oven at 105 °C for 24 h, grinded and sieved to fine powder to use. | Single variate analysis | ND * | Remazol Brilliant Blue R | 9.58 | <90% | [34] |
| Pineapple leaf powder | Leaves were washed with distilled water, dried in oven at 105 °C, crushed, and sieved. | Single variate analysis (Batch experiments) | ND * | Methylene Blue | 78.13 | * ND | [35] |
| Adsorbent Type | Percentage Removal (%R) | Adsorption Capacity (qe, mg/g) |
|---|---|---|
| RPAL | 42.96 | 6.44 |
| TTPAL250 | 18.99 | 2.85 |
| TTPAL500 | 18.53 | 2.78 |
| Factors | Low Level | Medium Level | High Level | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 5 | 8 | 11 | |||||||
| Adsorbent Dose (AD, B, g/50 mL) | 0.01 | 0.03 | 0.05 | |||||||
| Dye Concentration (DC, C, ppm) | 10 | 20 | 30 | |||||||
| Contact Time (CT, D, min) | 5 | 92.5 | 180 | |||||||
| Experimental Runs, Observed and Predicted Responses | ||||||||||
| Expt No | Blk * | pH | AD | DC | CT | %R Obs. ** | %R Pred. ** | RE *** | ||
| 01 | 1 | 5(−) | 0.03(0) | 20(0) | 92.5(0) | 38.19 | 36.20 | 0.05 | ||
| 02 | 1 | 8(0) | 0.01(−) | 20(0) | 92.5(0) | 1.43 | 2.83 | 0.49 | ||
| 03 | 1 | 8(0) | 0.05(+) | 20(0) | 92.5(0) | 47.06 | 35.19 | 0.34 | ||
| 04 | 1 | 8(0) | 0.03(0) | 20(0) | 92.5(0) | 33.00 | 25.36 | 0.30 | ||
| 05 | 1 | 8(0) | 0.03(0) | 20(0) | 5(−) | 38.19 | 40.15 | 0.05 | ||
| 06 | 1 | 8(0) | 0.03(0) | 10(−) | 92.5(0) | 34.72 | 30.87 | 0.12 | ||
| 07 | 1 | 11(+) | 0.03(0) | 20(0) | 92.5(0) | 23.41 | 15.59 | 0.50 | ||
| 08 | 1 | 8(0) | 0.03(0) | 30(+) | 92.5(0) | 28.15 | 20.14 | 0.40 | ||
| 09 | 1 | 8(0) | 0.03(0) | 20(0) | 180(+) | 62.73 | 50.91 | 0.23 | ||
| 10 | 1 | 8(0) | 0.03(0) | 20(0) | 92.5(0) | 19.32 | 25.36 | 0.24 | ||
| 11 | 1 | 5(−) | 0.01(−) | 10(−) | 5(−) | 25.46 | 24.28 | 0.05 | ||
| 12 | 1 | 5(−) | 0.01(−) | 30(+) | 5(−) | 7.84 | 12.17 | 0.36 | ||
| 13 | 1 | 11(+) | 0.05(+) | 10(−) | 5(−) | 21.14 | 26.06 | 0.19 | ||
| 14 | 1 | 8(0) | 0.03(0) | 20(0) | 92.5(0) | 19.24 | 25.36 | 0.24 | ||
| 15 | 1 | 5(−) | 0.01(−) | 30(+) | 180(+) | 10.40 | 10.18 | 0.02 | ||
| 16 | 2 | 5(−) | 0.05(+) | 10(−) | 5(−) | 86.64 | 92.53 | 0.06 | ||
| 17 | 2 | 11(+) | 0.01(−) | 10(−) | 180(+) | 26.86 | 30.36 | 0.11 | ||
| 18 | 2 | 5(−) | 0.05(+) | 10(−) | 180(+) | 88.40 | 92.60 | 0.04 | ||
| 19 | 2 | 5(−) | 0.01(−) | 10(−) | 180(+) | 27.07 | 34.02 | 0.20 | ||
| 20 | 2 | 11(+) | 0.01(−) | 10(−) | 5(−) | 1.88 | 3.88 | 0.51 | ||
| 21 | 2 | 5(−) | 0.05(+) | 30(+) | 5(−) | 71.46 | 74.71 | 0.04 | ||
| 22 | 2 | 8(0) | 0.03(0) | 20(0) | 92.5(0) | 23.71 | 25.36 | 0.06 | ||
| 23 | 2 | 11(+) | 0.01(−) | 30(+) | 180(+) | 17.04 | 22.04 | 0.23 | ||
| 24 | 2 | 8(0) | 0.03(0) | 20(0) | 92.5(0) | 20.07 | 25.36 | 0.21 | ||
| 25 | 2 | 11(+) | 0.01(−) | 30(+) | 5(−) | 5.14 | 6.29 | 0.18 | ||
| 26 | 2 | 5(−) | 0.05(+) | 30(+) | 180(+) | 50.70 | 59.65 | 0.15 | ||
| 27 | 2 | 8(0) | 0.03(0) | 20(0) | 92.5(0) | 23.41 | 25.36 | 0.08 | ||
| 28 | 2 | 11(+) | 0.05(+) | 10(−) | 180(+) | 44.26 | 49.17 | 0.10 | ||
| 29 | 2 | 11(+) | 0.05(+) | 30(+) | 180(+) | 31.96 | 39.67 | 0.19 | ||
| 30 | 2 | 11(+) | 0.05(+) | 30(+) | 5(−) | 25.45 | 29.75 | 0.14 | ||
| DF * | Adj SS | Adj MS | F-Value | p-Value | |
|---|---|---|---|---|---|
| Model | 12 | 1329.53 | 110.794 | 40.79 | 0 |
| Blocks | 1 | 27.58 | 27.583 | 10.15 | 0.005 |
| Linear | 4 | 993.74 | 248.436 | 91.45 | 0 |
| pH | 1 | 215.12 | 215.12 | 79.19 | 0 |
| AD | 1 | 677.08 | 677.082 | 249.24 | 0 |
| DC | 1 | 58.02 | 58.024 | 21.36 | 0 |
| CT | 1 | 43.52 | 43.518 | 16.02 | 0.001 |
| 2–Way Interactions | 5 | 209.07 | 41.813 | 15.39 | 0 |
| pH × AD | 1 | 102.61 | 102.609 | 37.77 | 0 |
| pH × DC | 1 | 31.67 | 31.665 | 11.66 | 0.003 |
| pH × CT | 1 | 49.25 | 49.254 | 18.13 | 0.001 |
| AD × CT | 1 | 9.81 | 9.814 | 3.61 | 0.074 |
| DC × CT | 1 | 15.72 | 15.724 | 5.79 | 0.028 |
| Squared Interactions | 2 | 123.74 | 61.872 | 22.78 | 0 |
| AD × AD | 1 | 28.23 | 28.234 | 10.39 | 0.005 |
| CT × CT | 1 | 121.83 | 121.825 | 44.85 | 0 |
| Error | 17 | 46.18 | 2.717 | 0.85 | 0.633 |
| Lack–of–Fit | 13 | 33.92 | 2.609 | ||
| Pure Error | 4 | 12.27 | 3.066 | ||
| Total | 29 | 1375.71 |
| Adsorbent | %C | %H | %N |
|---|---|---|---|
| RPAL | 39.555 | 4.991 | 2.447 |
| TTPAL250 | 52.140 | 4.942 | 3.117 |
| TTPAL500 | 50.353 | 2.529 | 2.438 |
| Parameters | RPAL | TTPAL250 | TTPAL500 |
|---|---|---|---|
| Langmuir SA (m2/g) | 4.59 | 8.43 | 9.81 |
| Total pore volume (cm3/g) | 0.016081 | 0.02674 | 0.040636 |
| Average pore radius (°A) | 105.5 | 81.4 | 96 |
| Isotherm | Equations (Generalized/Linearized Forms) | Parameters | Value |
|---|---|---|---|
| Langmuir | (mg/g) | 58.80 | |
| (L·mole−1) | 0.012 | ||
| R2 | 0.801 | ||
| Freundlich | 0.609 | ||
| (mole/g) (L/mole)1/n | 1.835 | ||
| R2 | 0.943 | ||
| Temkin | (J/mole) | 248.4 | |
| (L/mole) | 0.205 | ||
| R2 | 0.881 | ||
| DR | 1 × 10−8 | ||
| (kJ/mole) | 7.07 | ||
| (mg·g) | 34.72 | ||
| R2 | 0.858 |
| Model | Parameter | Value |
|---|---|---|
| Pseudo–first order (PFO) | K1 (min−1) | 0.493 |
| qe (mg/g) | 12.61 | |
| R2 | 0.863 | |
| Pseudo–second order (PSO) t where K2 is rate constant (g·mg−1·min−1) | K2 (g·mg−1·min−1) | 0.019 |
| qe (mg/g) | 25.91 | |
| R2 | 0.965 | |
| Elovich model where qt is adsorbed quantity at time t, α is initial sorption concentration rate (mg·g−1·min−1), and β is desorption constant (g/mg). | A | 3.79 × 1012 |
| Β | 1.817 | |
| R2 | 0.953 | |
| Weber–Morris intra–particle diffusion model where KI is intra–particle diffusion rate constant (mg·g−1·min−0.5), and C is boundary thickness effect. | KI | 1.262 |
| C | 53.66 | |
| R2 | 0.888 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.S.; El-Shafie, A.S.; Zaher, N.; El-Azazy, M. Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD). Molecules 2020, 25, 3752. https://doi.org/10.3390/molecules25163752
Hassan SS, El-Shafie AS, Zaher N, El-Azazy M. Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD). Molecules. 2020; 25(16):3752. https://doi.org/10.3390/molecules25163752
Chicago/Turabian StyleHassan, Siham S., Ahmed S. El-Shafie, Nourhan Zaher, and Marwa El-Azazy. 2020. "Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD)" Molecules 25, no. 16: 3752. https://doi.org/10.3390/molecules25163752
APA StyleHassan, S. S., El-Shafie, A. S., Zaher, N., & El-Azazy, M. (2020). Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD). Molecules, 25(16), 3752. https://doi.org/10.3390/molecules25163752









