Sustainable Development of Enhanced Luminescence Polymer-Carbon Dots Composite Film for Rapid Cd2+ Removal from Wastewater
Abstract
1. Introduction
2. Results and Discussion
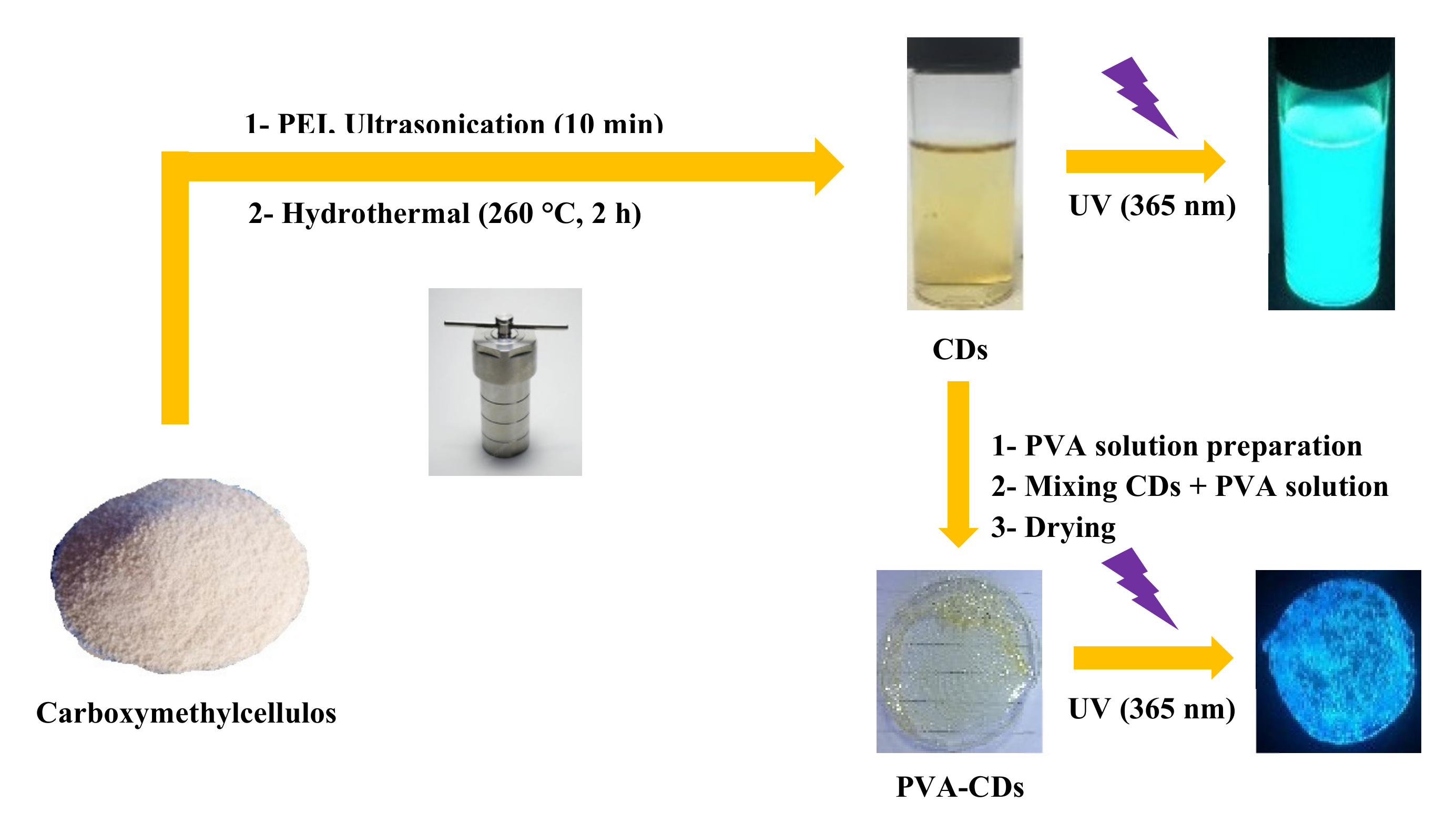
2.1. Synthesis of CDs and PVA-CDs
2.2. Physical Structure
2.3. Chemical Structure
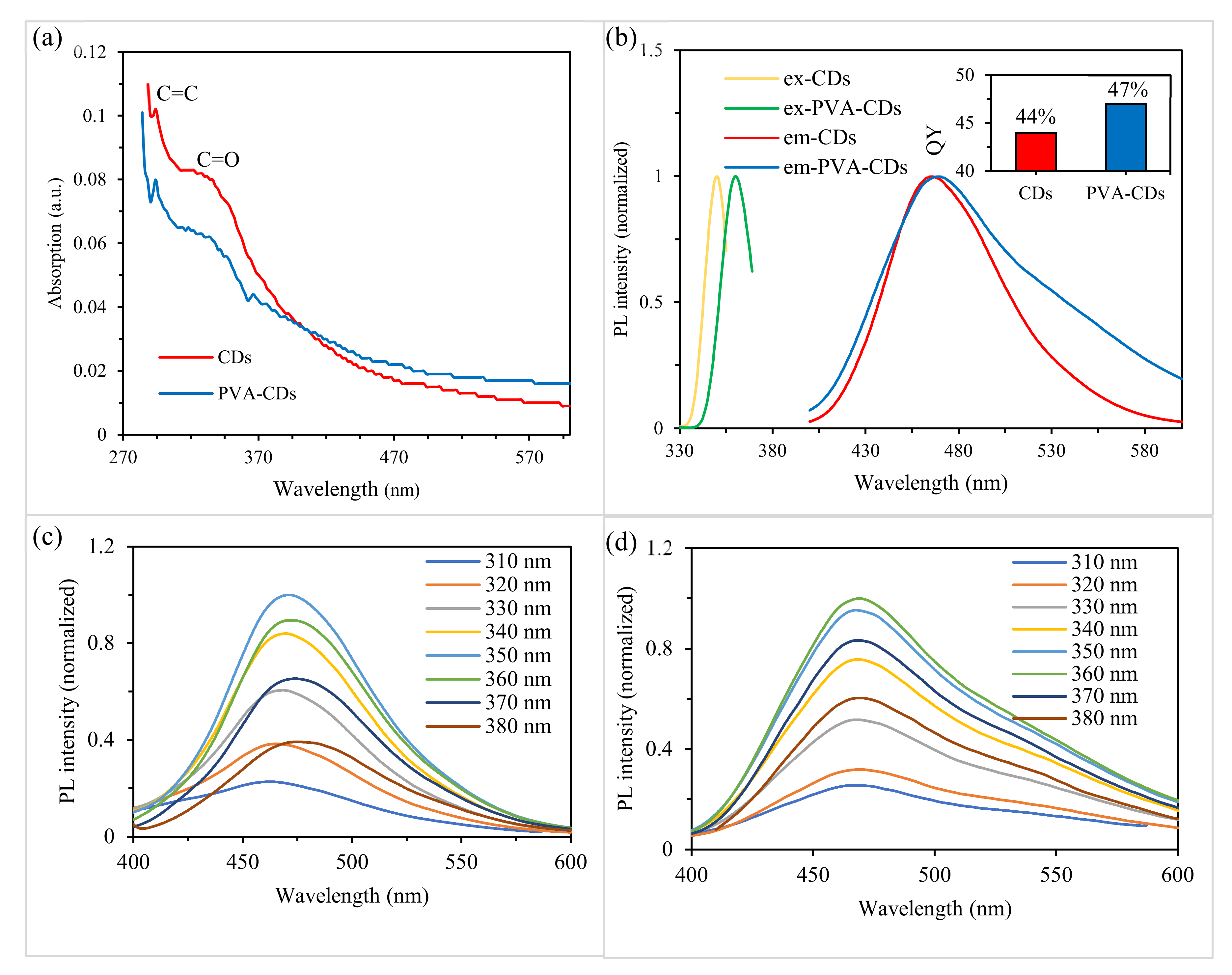
2.4. Optical Properties
2.5. Heavy Metal Complexation Test Using PVA-CDs Films
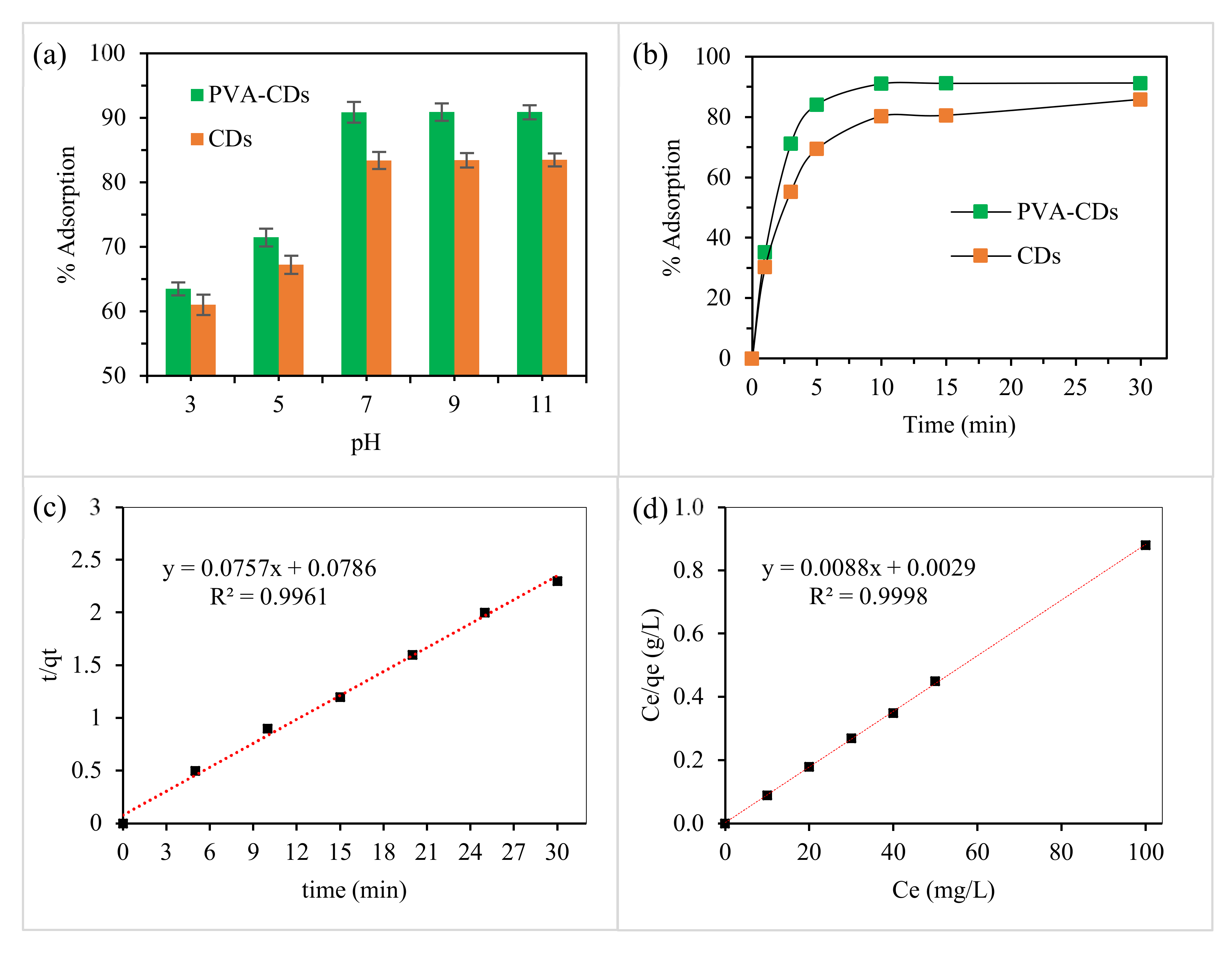
2.6. Effect of pH and Contact Time on Cd2+ Removal
2.7. Adsorption Kinetics and Isotherm
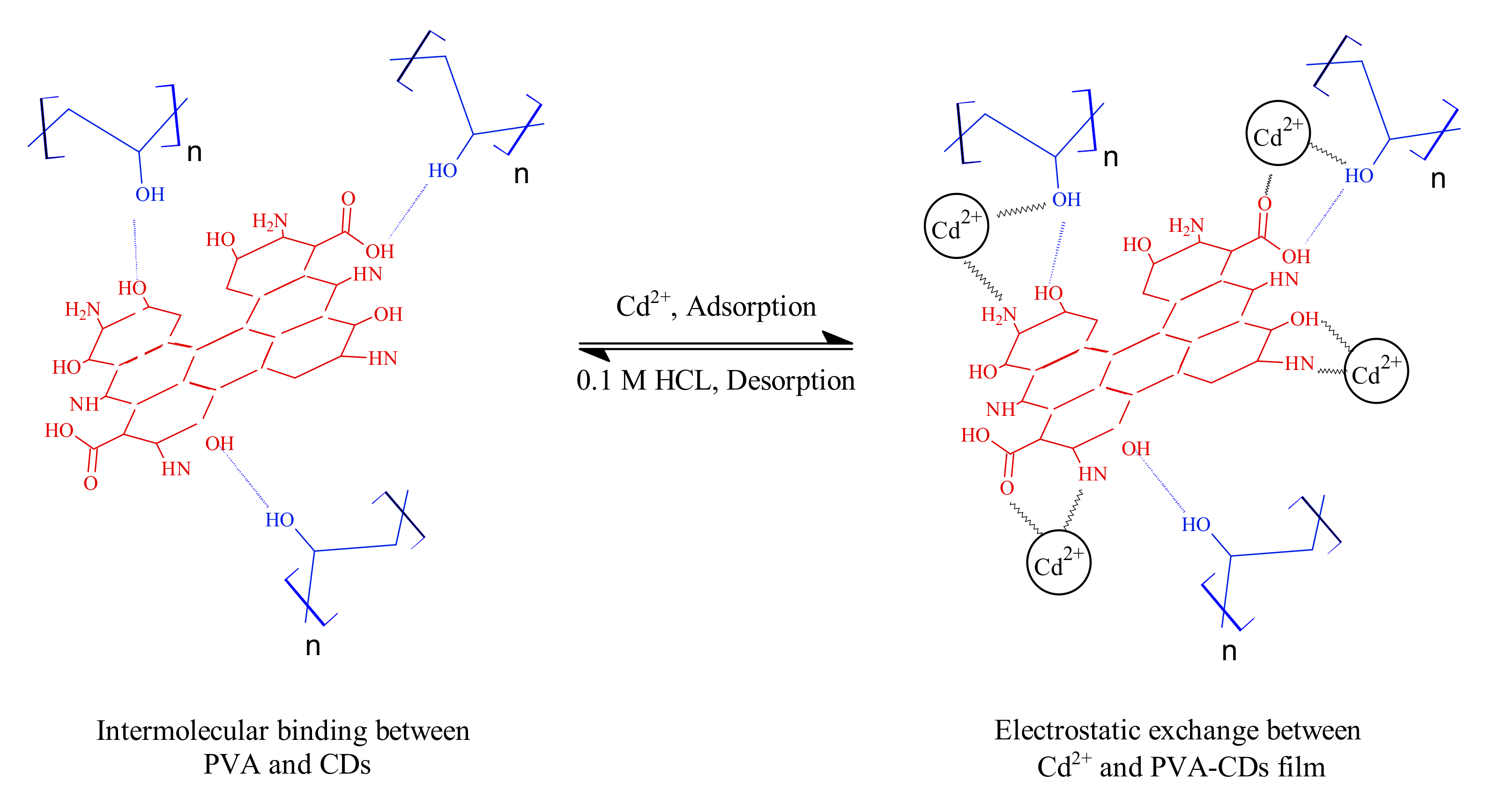
2.8. Adsorption Mechanism
2.9. Desorption and Recyclability
2.10. Analysis of Real Water Samples
3. Materials and Methods
3.1. Materials
3.2. Preparation of CDs and PVA-CDs
3.3. Characterizations
3.4. Quantum Yield Determinations
3.5. Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kahrizi, P.; Mohseni-Shahri, F.S.; Moeinpour, F. Adsorptive removal of cadmium from aqueous solutions using NiFe2O4/hydroxyapatite/graphene quantum dots as a novel nano-adsorbent. J. Nanostruct. Chem. 2018, 8, 441–452. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, D.; Wei, Q.; Wei, D.; Yan, T.; Yan, T.; Yan, L.; Hu, L.; Du, B. Rapid removal of Pb (II) from aqueous solution using branched polyethylenimine enhanced magnetic carboxymethyl chitosan optimized with response surface methodology. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Jlassi, K.; Eid, K.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. Rational synthesis, characterization, and application of environmentally friendly (polymer–carbon dot) hybrid composite film for fast and efficient UV-assisted Cd2+ removal from water. Environ. Sci. Eur. 2020, 32, 1. [Google Scholar] [CrossRef]
- Figueiredo, H.; Quintelas, C. Tailored zeolites for the removal of metal oxyanions: Overcoming intrinsic limitations of zeolites. J. Hazard. Mater. 2014, 274, 287–299. [Google Scholar] [CrossRef]
- Jlassi, K.; Abidi, R.; Benna, M.; Chehimi, M.M.; Kasak, P.; Krupa, I. Bentonite-decorated calix (4) arene: A new, promising hybrid material for heavy-metal removal. Appl. Clay Sci. 2018, 161, 15–22. [Google Scholar] [CrossRef]
- Ge, H.; Wang, J. Ear-like poly (acrylic acid)-activated carbon nanocomposite: A highly efficient adsorbent for removal of Cd(II) from aqueous solutions. Chemosphere 2017, 169, 443–449. [Google Scholar] [CrossRef]
- Tadesse, A.; Ramadevi, D.; Hagos, M.; Battu, G.; Basavaiah, K. Synthesis of nitrogen doped carbon quantum dots/magnetite nanocomposites for efficient removal of methyl blue dye pollutant from contaminated water. RSC Adv. 2018, 8, 8528–8536. [Google Scholar] [CrossRef]
- Wang, J.; Chu, Y.; Qiu, F.; Li, X.; Wu, H.; Pan, J.; Niu, X.; Zhang, T.; Yang, D. One-pot simple green synthesis of water-soluble cleaner fluorescent carbon dots from cellulose and its sensitive detection of iron ion. J. Clean. Prod. 2017. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Pudza, M.Y.; Zentou, H. Efficient removal of Cu(II) from aqueous systems using enhanced quantum yield nitrogen-doped carbon nanodots. RSC Adv. 2020, 10, 14979–14990. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, Q.; Li, S.; Fu, S.; Chai, F.; Wang, C.; Qu, F. One pot synthesis of highly fluorescent N doped C-dots and used as fluorescent probe detection for Hg2+ and Ag+ in aqueous solution. Sens. Actuators B Chem. 2017, 243, 244–253. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Photoluminescence Tuning in Carbon Dots: Surface Passivation or/and Functionalization, Heteroatom Doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Ortega-Liebana, M.C.; Chung, N.X.; Limpens, R.; Gomez, L.; Hueso, J.L.; Santamaria, J.; Gregorkiewicz, T. Uniform luminescent carbon nanodots prepared by rapid pyrolysis of organic precursors confined within nanoporous templating structures. Carbon 2017, 117, 437–446. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; et al. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Purbia, R.; Paria, S. A simple turn on fluorescent sensor for the selective detection of thiamine using coconut water derived luminescent carbon dots. Biosens. Bioelectron. 2016, 79, 467–475. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Basu, H.; Singhal, R.K.; Kailasa, S.K. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens. Actuators B Chem. 2015, 213, 434–443. [Google Scholar] [CrossRef]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Microwave-assisted rapid green synthesis of photoluminescent carbon nanodots from flour and their applications for sensitive and selective detection of mercury(II) ions. Sens. Actuators B Chem. 2013, 184, 156–162. [Google Scholar] [CrossRef]
- Xu, J.; Lai, T.; Feng, Z.; Weng, X. Formation of fluorescent carbon nanodots from kitchen wastes and their application for detection of Fe3+. Luminescence 2014, 30, 420–424. [Google Scholar] [CrossRef]
- Du, F.; Zhang, M.; Li, X.; Li, J.; Jiang, X. Economical and green synthesis of bagasse-derived fluorescent carbon dots for biomedical applications. Nanotechnology 2014, 25, 315702. [Google Scholar] [CrossRef]
- Lu, W.; Quin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 76, 72423–72432. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Abdul-Rashid, S.; Mahdi, M.A.; Ibrahim, N.A.; Pudza, M.Y. Fabrication, characterization and response surface method optimization for quantum efficiency of fluorescent nitrogen-doped carbon dots obtained from carboxymethylcellulose of oil palms empty fruit bunch. Chin. J. Chem. Eng. 2019, 28, 584–592. [Google Scholar] [CrossRef]
- Jayaweera, S.; Yin, K.; Hu, X.; Ng, W.J. Facile preparation of fluorescent carbon dots for label-free detection of Fe3+. J. Photochem. Photobiol. A Chem. 2019, 370, 156–163. [Google Scholar] [CrossRef]
- Shen, P.; Gao, J.; Cong, J.; Liu, Z.; Li, C.; Yao, J. Synthesis of Cellulose-Based Carbon Dots for Bioimaging. ChemistrySelect 2016, 1, 1314–1317. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Tan, J.; Wu, Y.; Liu, S. Hydrothermal carbonization of carboxymethylcellulose: One-pot preparation of conductive carbon microspheres and water-soluble fluorescent carbon nanodots. Chem. Eng. J. 2015, 266, 112–120. [Google Scholar] [CrossRef]
- Wu, P.; Li, W.; Wu, Q.; Liu, Y.; Liu, S. Hydrothermal synthesis of nitrogen-doped carbon quantum dots from microcrystalline cellulose for the detection of Fe 3+ ions in an acidic environment. RSC Adv. 2017, 7, 44144–44153. [Google Scholar] [CrossRef]
- Jayaweera, S.; Yin, K.; Hu, X.; Ng, W.J. Fluorescent N/Al Co-Doped Carbon Dots from Cellulose Biomass for Sensitive Detection of Manganese (VII). J. Fluoresc. 2019, 29, 1291–1300. [Google Scholar] [CrossRef]
- Yang, G.; Wan, X.; Su, Y.; Zeng, X.; Tang, J. Acidophilic S-doped carbon quantum dots derived from cellulose fibers and their fluorescence sensing performance for metal ions in an extremely strong acid environment. J. Mater. Chem. A 2016, 4, 12841–12849. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, M.J. Removal of Heavy Metal Ions by Poly(vinyl alcohol) and Carboxymethyl Cellulose Composite Hydrogels Prepared by a Freeze-Thaw Method. ACS Sustain. Chem. Eng. 2016, 4, 2830–2837. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-Convertible Carbon Dots for Imaging-Guided Drug Delivery with Enhanced in Vivo Cancer Therapeutic Efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Kumar, H.; Mishra, G.; Gothwal, A.; Kesharwani, P.; Gupta, U. Polymeric Nanocarriers: A New Horizon for the Effective Management of Breast Cancer. Curr. Pharm. Des. 2017, 23, 5315–5326. [Google Scholar] [CrossRef]
- Wang, W.; Lai, H.; Cheng, Z.; Kang, H.; Wang, Y.; Zhang, H.; Wang, J.; Liu, Y. Water-induced poly(vinyl alcohol)/carbon quantum dot nanocomposites with tunable shape recovery performance and fluorescence. J. Mater. Chem. B 2018, 6, 7444–7450. [Google Scholar] [CrossRef]
- Hoang, Q.B.; Mai, V.T.; Nguyen, D.K.; Truong, D.Q.; Mai, X.D. Crosslinking induced photoluminescence quenching in polyvinyl alcohol-carbon quantum dot composite. Mater. Today Chem. 2019, 12, 166–172. [Google Scholar] [CrossRef]
- Gamal El-Shamy, A. Novel hybrid nanocomposite based on Poly(vinyl alcohol)/carbon quantum dots/fullerene (PVA/CQDs/C60) for thermoelectric power applications. Compos. Part. B Eng. 2019, 174, 106993. [Google Scholar] [CrossRef]
- Taspika, M.; Permatasari, F.A.; Nuryadin, B.W.; Mayangsari, T.R.; Aimon, A.H.; Iskandar, F. Simultaneous ultraviolet and first near-infrared window absorption of luminescent carbon dots/PVA composite film. RSC Adv. 2019, 9, 7375–7381. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Pan, H.; Xu, N.; Mei, C.; Mao, H.; Zhang, W.; Cai, J.; Xu, C. Preparation and performance of radiata-pine-derived polyvinyl alcohol/carbon quantum dots fluorescent films. Materials 2020, 13, 67. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hassan, A.Q.; Mohammed, S.J.; Karim, W.O.; Kadir, M.F.Z.; Tajuddin, H.A.; Chan, N.N.M.Y. Structural and Optical Characteristics of PVA : C-Dot Composites : Tuning the Absorption of Ultra Violet (UV) Region. Nanomaterials 2019, 9, 216. [Google Scholar] [CrossRef]
- Luminescent poly (vinyl alcohol)/carbon quantum dots composites with tunable water-induced shape memory behavior in different pH and temperature environments. ACS Appl. Mater. Interfaces 2016, 8, 34744–34754. [CrossRef]
- Wang, Y.; Zhao, Y.; Zhang, F.; Chen, L.; Yang, O.; Liu, X. Fluorescent polyvinyl alcohol films based on nitrogen and sulfur co-doped carbon dots towards white light-emitting devices. New. J. Chem. 2016, 40, 8710–8716. [Google Scholar] [CrossRef]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar]
- Wang, L.; Cheng, C.; Tapas, S.; Lei, J.; Matsuoka, M.; Zhang, J.; Zhang, F. Carbon dots modified mesoporous organosilica as an adsorbent for the removal of 2,4-dichlorophenol and heavy metal ions. J. Mater. Chem. A 2015, 3, 13357–13364. [Google Scholar] [CrossRef]
- Yang, T.; He, R.; Nie, G.; Wang, W.; Zhang, G.; Hu, Y.; Wu, L. Creation of Hollow Calcite Single Crystals with CQDs: Synthesis, Characterization, and Fast and Efficient Decontamination of Cd(II). Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Dutta, D.; Thiyagarajan, S.; Bahadur, D. SnO2 quantum dots decorated reduced graphene oxide nanocomposites for efficient water remediation. Chem. Eng. J. 2016, 297, 55–65. [Google Scholar] [CrossRef]
- Rahmanian, O.; Dinari, M.; Abdolmaleki, M.K. Carbon quantum dots/layered double hydroxide hybrid for fast and efficient decontamination of Cd(II): The adsorption kinetics and isotherms. Appl. Surf. Sci. 2018, 428, 272–279. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.; Mahdi, M.A.; Ibrahim, N.A.; Pudza, M.Y. Facile Synthesis of Nitrogen-Doped Carbon Dots from Lignocellulosic Waste. Nanomaterials 2019, 9, 1500. [Google Scholar] [CrossRef]
- Hu, M.; Gu, X.; Hu, Y.; Deng, Y.; Wang, C. PVA/Carbon Dot Nanocomposite Hydrogels for Simple Introduction of Ag Nanoparticles with Enhanced Antibacterial Activity. Macromol. Mater. Eng. 2016, 301, 1352–1362. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Sun, Y. One-step synthesis of self-doped carbon dots with highly photoluminescence as multifunctional biosensors for detection of iron ions and pH. Sens. Actuators B Chem. 2017, 241, 73–79. [Google Scholar] [CrossRef]
- Da Silva Souza, D.R.; Caminhas, D.C.; Mesquita, J.P.; Pereira, F.V. Luminescent carbon dots obtained from cellulose. Mater. Chem. Phys. 2018, 203, 148–155. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, H.; Tang, J.; Deng, S.; Yan, F.; Li, W.; Qu, M. Carbon dots doped with heteroatoms for fluorescent bioimaging: A review. Microchim. Acta 2017, 184, 343–368. [Google Scholar] [CrossRef]
- Wu, F.; Su, H.; Wang, K.; Wong, W.K.; Zhu, X. Facile synthesis of N-rich carbon quantum dots from porphyrins as efficient probes for bioimaging and biosensing in living cells. Int. J. Nanomed. 2017, 12, 7375–7391. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Yu, K.; Wang, J.; Song, K.; Wang, X.; Liang, C.; Dou, Y. Hydrothermal Synthesis of Cellulose-Derived Carbon Nanospheres from Corn Straw as Anode Materials for Lithium ion Batteries. Nanomaterials 2019, 9, 93. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Pires, E.; Fraile, J.M. Parametric study of the hydrothermal carbonization of cellulose and effect of acidic conditions. Carbon 2017, 123, 421–432. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Shahbazi, A. A Review of Hydrothermal Carbonization of Carbohydrates for Carbon Spheres Preparation. Trends Renew. Energy 2015, 1, 43–56. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Pires, N.R.; Santos, C.M.W.; Sousa, R.R.; de Paula, R.C.M.; Cunha, P.L.R.; Feitosa, J.P.A. Novel and fast microwave-assisted synthesis of carbon quantum dots from raw cashew gum. J. Braz. Chem. Soc. 2015, 26, 1274–1282. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ehrat, F.; Urban, P.; Teves, R.; Wyrwich, R.; Döblinger, M.; Feldmann, J.; Urban, A.S.; Stolarczyk, J.K. Effect of nitrogen atom positioning on the trade-off between emissive and photocatalytic properties of carbon dots. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Arul, V.; Sethuraman, M.G. Facile green synthesis of fluorescent N-doped carbon dots from Actinidia deliciosa and their catalytic activity and cytotoxicity applications. Opt. Mater. 2018, 78, 181–190. [Google Scholar] [CrossRef]
- Hao, Y.; Gan, Z.; Xu, J.; Wu, X.; Chu, P.K. Applied Surface Science Poly (ethylene glycol)/carbon quantum dot composite solid films exhibiting intense and tunable blue-red emission. Appl. Surf. Sci. 2014, 311, 490–497. [Google Scholar] [CrossRef]
- Bai, J.; Ren, W.; Wang, Y.; Li, X. High-performance thermoplastic polyurethane elastomer/carbon dots bulk nanocomposites with strong luminescence. High Perform. Polym. 2020, 1–11. [Google Scholar] [CrossRef]
- Bandi, R.; Devulapalli, N.P.; Dadigala, R.; Gangapuram, B.R.; Guttena, V. Facile Conversion of Toxic Cigarette Butts to N,S-Codoped Carbon Dots and Their Application in Fluorescent Film, Security Ink, Bioimaging, Sensing and Logic Gate Operation. ACS Omega 2018, 3, 13454–13466. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.H.; Cui, P.P.; Feng, X.T.; Chen, L.; Yang, Y.Z.; Liu, X.G. Water-soluble, nitrogen-doped fluorescent carbon dots for highly sensitive and selective detection of Hg2+ in aqueous solution. RSC Adv. 2015, 5, 40393–40401. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.S.; Zhang, P.; Zhou, Z.Y.; Gao, Q.Y.; Xiong, H.M. Solvent-Controlled Synthesis of Highly Luminescent Carbon Dots with a Wide Color Gamut and Narrowed Emission Peak Widths. Small 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, L.; Yang, R. Solid pyrolysis synthesis of excitation-independent emission carbon dots and its application to isoniazid detection. J. Nanoparticle Res. 2019, 21. [Google Scholar] [CrossRef]
- Patir, K.; Gogoi, S.K. Nitrogen-doped carbon dots as fluorescence ON-OFF-ON sensor for parallel detection of copper(II) and mercury(II) ions in solutions as well as in filter paper-based microfluidic device †. Nanoscale Adv. 2018, 1, 592–601. [Google Scholar] [CrossRef]
- Biswas, S.; Meikap, B.C.; Sen, T.K. Adsorptive Removal of Aqueous Phase Copper (Cu2+) and Nickel (Ni2+) Metal Ions by Synthesized Biochar–Biopolymeric Hybrid Adsorbents and Process Optimization by Response Surface Methodology (RSM). Water. Air. Soil Pollut. 2019, 230. [Google Scholar] [CrossRef]
- Alsuhybani, M.; Alshahrani, A.; Algamdi, M.; Al-Kahtani, A.A.; Alqadami, A.A. Highly efficient removal of Pb(II) from aqueous systems using a new nanocomposite: Adsorption, isotherm, kinetic and mechanism studies. J. Mol. Liq. 2020, 301, 112393. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Wang, Y.; Wang, X.; Liu, M.; Yin, D.; Xia, S.; Zhao, J.; Zhang, Y. Adsorption of Copper (II) onto activated carbons from sewage sludge by microwave-induced phosphoric acid and zinc chloride activation. Desalination 2011, 278, 231–237. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Microwave assisted multiwall carbon nanotubes enhancing Cd(II) adsorption capacity in aqueous media. J. Ind. Eng. Chem. 2015, 24, 24–33. [Google Scholar] [CrossRef]
- Khorzughy, S.H.; Eslamkish, T.; Ardejani, F.D.; Heydartaemeh, M.R. Cadmium removal from aqueous solutions by pumice and nano-pumice. Korean J. Chem. Eng. 2014, 32, 88–96. [Google Scholar] [CrossRef]
- Zhou, D.-M.; Wang, Y.-J.; Wang, H.-W.; Wang, S.-Q.; Cheng, J.-M. Surface-modified nanoscale carbon black used as sorbents for Cu(II) and Cd(II). J. Hazard. Mater. 2010, 174, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Dolgormaa, A.; Lv, C.-J.; Li, Y.; Yang, J.; Yang, J.-X.; Chen, P.; Wang, H.-P.; Huang, J. Adsorption of Cu(II) and Zn(II) ions from aqueous solution by gel/PVA-modified super-paramagnetic iron oxide nanoparticles. Molecules 2018, 23, 2982. [Google Scholar] [CrossRef]
- Pour, Z.S.; Ghaemy, M. Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly(vinyl alcohol)/carboxymethyl starch-g-poly(vinyl imidazole). RSC Adv. 2015, 5, 64106–64118. [Google Scholar] [CrossRef]
- Al-qudah, Y.H.F.; Mahmoud, G.A.; Khalek, M.A.A. Radiation crosslinked poly (vinyl alcohol)/acrylic acid copolymer for removal of heavy metal ions from aqueous solutions. J. Radiat. Res. Appl. Sci. 2014, 7, 135–145. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Zhou, L.; Zhang, Y.; Cao, Q.; Wang, R.; Xiao, H. Fluorescence-sensitive adsorbent based on cellulose using for mercury detection and removal from aqueous solution with selective ‘on-off’ response. Int. J. Biol. Macromol. 2019, 132, 1185–1192. [Google Scholar] [CrossRef]
- Abdullah, M.; Abidin, Z.Z. Coagulation Treatment of Palm Oil Mill Effluent using Plant-Based Tannin. Pollut. Res. 2018, 37, 788–793. [Google Scholar]
- Wiśniewski, M.; Gauden, P.A. Pearson’s hard-soft acid-base principle as a means of interpreting the reactivity of carbon materials. Adsorpt. Sci. Technol. 2006, 24, 389–402. [Google Scholar] [CrossRef]
- Nair, R.V.; Thomas, R.T.; Sankar, V.; Muhammad, H.; Dong, M.; Pillai, S. Rapid, Acid-Free Synthesis of High-Quality Graphene Quantum Dots for Aggregation Induced Sensing of Metal Ions and Bioimaging. ACS Omega 2017, 2, 8051–8061. [Google Scholar] [CrossRef] [PubMed]
- Minh, L.; Phan, T.; Hoon, S.; Phan, T.; Yeol, K.; Ha, S. Synthesis of fluorescent silicon quantum dots for ultra-rapid and selective sensing of Cr(VI) ion and biomonitoring of cancer cells. Mater. Sci. Eng. C J. 2018, 93, 429–436. [Google Scholar]
- Stafiej, A.; Pyrzynska, K. Adsorption of heavy metal ions with carbon nanotubes. Seperation Purif. Technol. 2007, 58, 49–52. [Google Scholar] [CrossRef]
- Cui, Y.W.; Li, J.; Du, Z.F.; Peng, Y.Z. Cr(VI) adsorption on red mud modified by lanthanum: Performance, kinetics and mechanisms. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Anand, S.R.; Bhati, A.; Saini, D.; Gunture Chauhan, N.; Khare, P.; Sonkar, S.K. Antibacterial Nitrogen-doped Carbon Dots as a Reversible ‘fluorescent Nanoswitch’ and Fluorescent Ink. ACS Omega 2019, 4, 1581–1591. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Pseudo-First Order | Pseudo-Second Order | Langmuir | Freundlich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 | qe | R2 | qe | KL | R2 | qe | KL | R2 | Kf | n | R2 |
| 0.1 | 5.4 | 0.9853 | 13.2 | 0.075 | 0.9961 | 113.6 | 0.016 | 0.9998 | 4.64 | 3.1 | 0.9853 |
| Adsorbent | Time (min) | qm (mg/g) | Ref. |
|---|---|---|---|
| NiFe2O4/hydroxyapatite/graphene quantum dots | 10 | 344.83 | [1] |
| Chitosan-carbon dots | 3 | 112.4 | [4] |
| Poly (acrylic acid)-activated carbon nanocomposite | 120 | 473.2 | [7] |
| SnO2 quantum dots decorated reduced graphene oxide | 20 | 40.81 | [46] |
| Carbon dots modified mesoporous organosilica | 500 | 105.5 | [44] |
| Carbon quantum dots/layered double hydroxide hybrid | 20 | 12.6 | [47] |
| Hollow calcite single crystals with CQDs | 90 | 66.68 | [45] |
| Multiwall carbon nanotubes | 120 | 88.62 | [72] |
| Nano-pumice | 120 | 200 | [73] |
| Nanoscale carbon black | 120 | 31.7 | [74] |
| N doped carbon dots integrated with PVA films | 10 | 113.6 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah Issa, M.; Z. Abidin, Z. Sustainable Development of Enhanced Luminescence Polymer-Carbon Dots Composite Film for Rapid Cd2+ Removal from Wastewater. Molecules 2020, 25, 3541. https://doi.org/10.3390/molecules25153541
Abdullah Issa M, Z. Abidin Z. Sustainable Development of Enhanced Luminescence Polymer-Carbon Dots Composite Film for Rapid Cd2+ Removal from Wastewater. Molecules. 2020; 25(15):3541. https://doi.org/10.3390/molecules25153541
Chicago/Turabian StyleAbdullah Issa, Mohammed, and Zurina Z. Abidin. 2020. "Sustainable Development of Enhanced Luminescence Polymer-Carbon Dots Composite Film for Rapid Cd2+ Removal from Wastewater" Molecules 25, no. 15: 3541. https://doi.org/10.3390/molecules25153541
APA StyleAbdullah Issa, M., & Z. Abidin, Z. (2020). Sustainable Development of Enhanced Luminescence Polymer-Carbon Dots Composite Film for Rapid Cd2+ Removal from Wastewater. Molecules, 25(15), 3541. https://doi.org/10.3390/molecules25153541








