Competitive Interaction of Phosphate with Selected Toxic Metals Ions in the Adsorption from Effluent of Sewage Sludge by Iron/Alginate Beads
Abstract
1. Introduction
2. Results
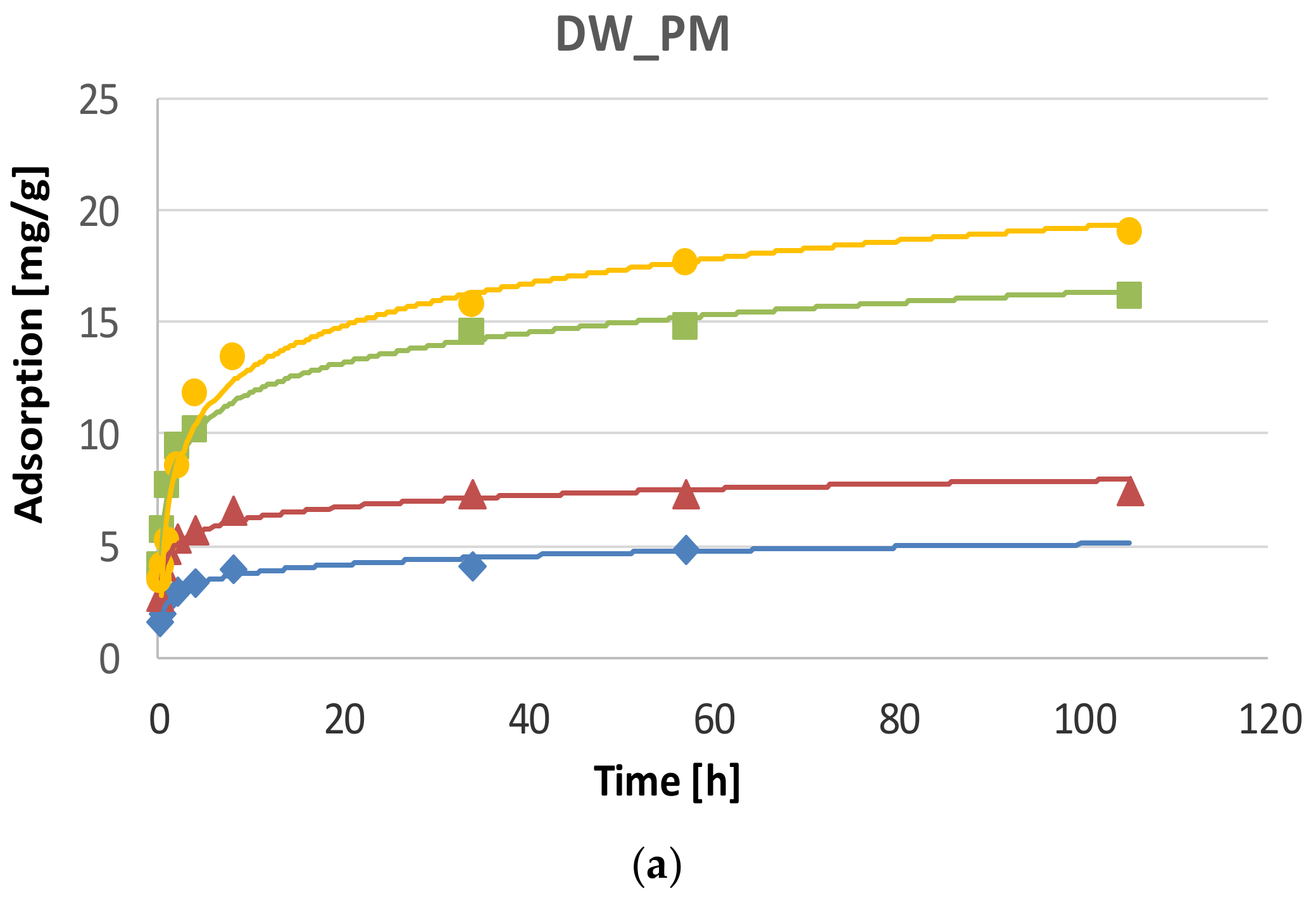
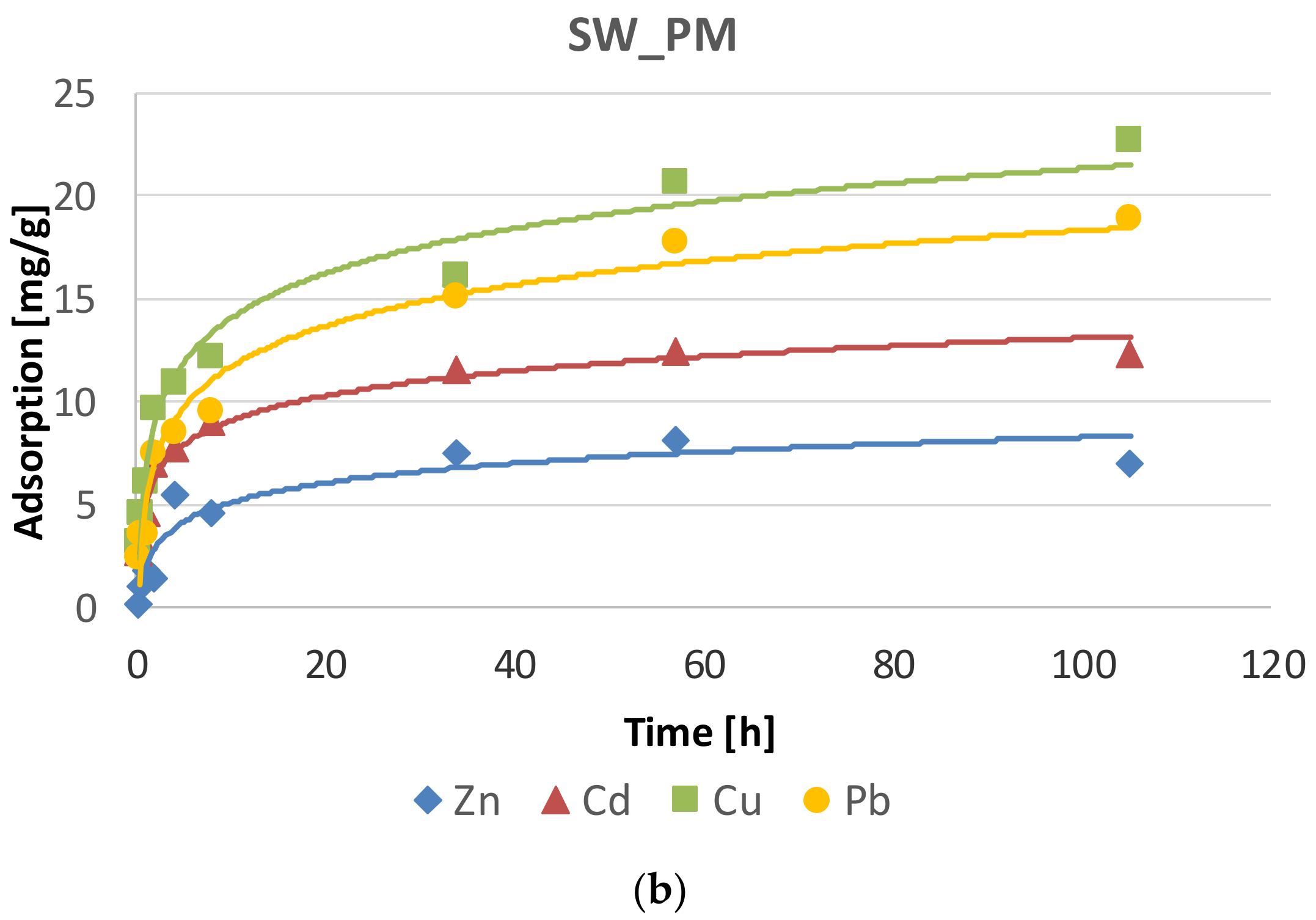
2.1. Phosphate Adsorption Kinetics
2.2. Phosphate Adsorption Isotherm
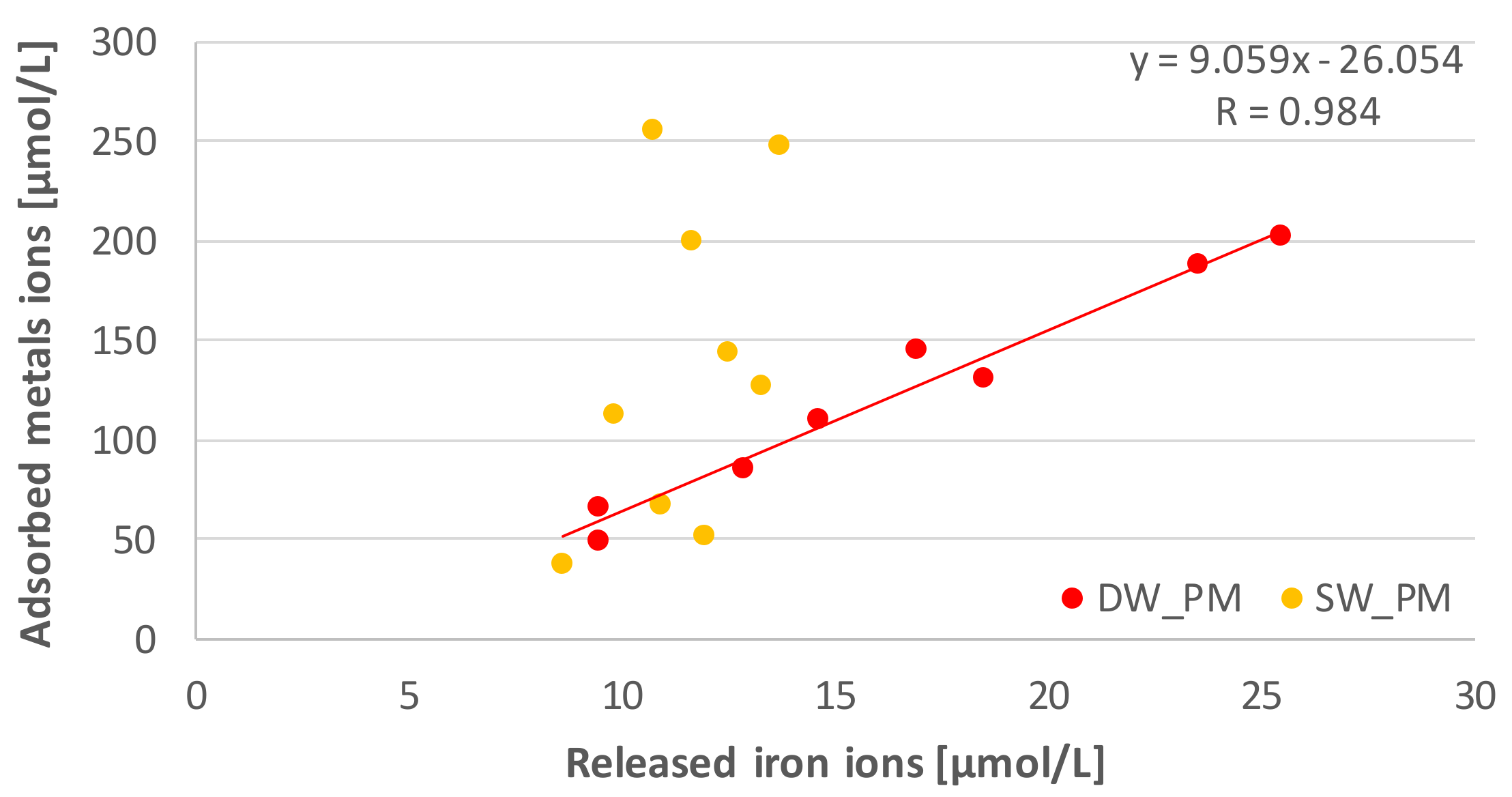
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Analytical Methods
4.3. Adsorption Kinetic Measurements
4.4. Phosphate Adsorption Experiments
4.5. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Cornel, P.; Schaum, C. Phosphorus recovery from wastewater: Needs, technologies and costs. Water Sci. Technol. 2009, 59, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.; Smith, J.A. Phosphorus Removal and Recovery from Municipal Wastewaters. Elements 2008, 4, 109–112. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef]
- Wzorek, Z.; Lenik, E.; Gorazda, K.; Wilkosz, A. Popioły ze spalania dpadów z przemysłu mięsnego i osadów ściekowych jako źródło fosforu. Arch. Gospod. Odpadami Ochr. Środowiska 2006, 3, 83–90. [Google Scholar]
- Kasprzyk, M.; Gajewska, M.; Molendowska, S. Możliwość odzysku fosforu z odcieków, osadów ściekowych i popiołów po termicznym przekształcaniu osadów ściekowych. Possibilities of phosphorus recovery from effluents, sewage sludge and ashes from sewage sludge thermal processing. Ecol. Eng. 2017, 18, 65–78. (In Polish) [Google Scholar] [CrossRef]
- Krüger, O.; Adam, C. Phosphorus in recycling fertilizers—Analytical challenges. Environ. Res. 2017, 155, 353–358. [Google Scholar] [CrossRef]
- Gibbs, M.M.; Hickey, C.W.; Ozkundakci, D. Ocena trwałości i porównanie skuteczności czterech czynników inaktywujących P w zarządzaniu wewnętrznymi ładunkami fosforu w jeziorach: Inkubacje osadów. Hydrobiologia 2011, 658, 253–275. [Google Scholar] [CrossRef]
- Yue, Q.; Zhao, Y.; Li, Q.; Li, W.; Gao, B.; Han, S.; Qi, Y.; Yu, H. Research on the characteristics of red mud granular adsorbents (RMGA) for phosphate removal. J. Hazard. Mater. 2010, 176, 741–748. [Google Scholar] [CrossRef]
- Bus, A.; Baryła, A.; Karczmarczyk, A. Wybór materiału reaktywnego do usuwania fosforu z wód i ścieków na przykładzie kruszywa popiołoporytowego Pollytag. Inżynieria Ekologiczna 2014, 39, 33–41. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Phosphate adsorption on synthetic goethite and akaganeite. J. Colloid Interface Sci. 2006, 298, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, H.; Liu, R.; Qu, J. Removal of phosphate from water by a Fe–Mn binary oxide adsorbent. J. Colloid Interface Sci. 2009, 335, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Tanada, S.; Kabayama, M.; Kawasaki, N.; Sakiyama, T.; Nakamura, T.; Araki, M.; Tamura, T. Removal of phosphate by aluminum oxide hydroxide. J. Colloid Interface Sci. 2003, 257, 135–140. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Liu, J. Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res. 2004, 38, 1318–1326. [Google Scholar] [CrossRef]
- Haghseresht, F.; Wang, S.; Do, D. A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl. Clay Sci. 2009, 46, 369–375. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Min, S.-H.; Han, J.S. Phosphate removal by refined aspen wood fiber treated with carboxymethyl cellulose and ferrous chloride. Bioresour. Technol. 2006, 97, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, L.T. Biosorbents prepared from wood particles treated with anionic polymerand iron salt: Effect of particle size on phosphate adsorption. Bioresour. Technol. 2008, 99, 626–630. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Haridas, A. Removal of phosphate from aqueous solutions and sewage using natural and surface modified coir pith. J. Hazard. Mater. 2008, 152, 527–535. [Google Scholar] [CrossRef]
- Mezenner, N.Y.; Bensmaili, A. Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem. Eng. J. 2009, 147, 87–96. [Google Scholar] [CrossRef]
- Aryal, M.; Liakopoulou-Kyriakides, M. Equilibrium, kinetics and thermodynamic studies on phosphate biosorption from aqueous solutions by Fe(III)-treated Staphylococusxylosus biomass: Common ion effect. Colloids Surf. A Physicochem. Eng. Asp. 2011, 387, 43–49. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2018, 49, 318–356. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Hunkeler, D. Alginate−Oligochitosan Microcapsules: A Mechanistic Study Relating Membrane and Capsule Properties to Reaction Conditions. Chem. Mater. 1999, 11, 2486–2492. [Google Scholar] [CrossRef]
- Hill, C.B.; Khan, E. A Comparative Study of Immobilized Nitrifying and Co-Immobilized Nitrifying and Denitrifying Bacteria for Ammonia Removal from Sludge Digester Supernatant. Water Air Soil Pollut. 2008, 195, 23–33. [Google Scholar] [CrossRef]
- Min, J.H.; Hering, J.G. Arsenate sorption by Fe(III)-doped alginate gels. Water Res. 1998, 32, 1544–1552. [Google Scholar] [CrossRef]
- Hering, J.G.; Min, H.J. Removal of selenite and chromate using Fe(III)doped alginate gels. Water Environ. Res. 1999, 71, 169–175. [Google Scholar]
- Siwek, H.; Bartkowiak, A.; Włodarczyk, M.; Sobecka, K. Removal of Phosphate from Aqueous Solution Using Alginate/Iron (III) Chloride Capsules: A Laboratory Study. Water Air Soil Pollut. 2016, 227, 427. [Google Scholar] [CrossRef]
- Yeon, K.H.; Park, H.; Lee, S.H.; Park, Y.M.; Lee, S.H.; Iwamoto, M. Zirconium mesostructure immobilized in calcium alginate for phosphate removel. Korean J. Chem. Eng. 2008, 25, 1040–1046. [Google Scholar] [CrossRef]
- Chiban, M.; Soudani, A.; Sinan, F.; Persin, M. Wastewater treatment by batch adsorption method onto micro-particles of dried Withania frutescens plant as a new adsorbent. J. Environ. Manag. 2012, 95, S61–S65. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Zhang, X.-F.; Zhang, X.; Jiang, J.; Yao, J. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals. J. Colloid Interface Sci. 2018, 514, 190–198. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, Q.L.; Hu, S.R.; Ni, J.C.; He, Y.S. Adsorption mechanism of Cu(II) ions from aqueous solution by glutaraldehyde crosslinked humic acid-immobilized sodium alginate porous membrane adsorbent. Chem. Eng. J. 2011, 173, 511–519. [Google Scholar] [CrossRef]
- Benettayeb, A.; Guibal, E.; Morsli, A.; Kessas, R. Chemical modification of alginate for enhanced sorption of Cd(II), Cu(II) and Pb(II). Chem. Eng. J. 2017, 316, 704–714. [Google Scholar] [CrossRef]
- Do, X.-H.; Lee, B.-K. Removal of Pb2+ using a biochar–alginate capsule in aqueous solution and capsule regeneration. J. Environ. Manag. 2013, 131, 375–382. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Ávila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar] [CrossRef]
- Dionisiou, N.S.; Matsi, T.; Misopolinos, N.D. Phosphorus Adsorption–Desorption on a Surfactant-Modified Natural Zeolite: A Laboratory Study. Water Air Soil Pollut. 2013, 224, 1362–1363. [Google Scholar] [CrossRef]
- Siwek, H.; Bartkowiak, A.; Włodarczyk, M. Adsorption of Phosphates from Aqueous Solutions on Alginate/Goethite Hydrogel Composite. Water 2019, 11, 633. [Google Scholar] [CrossRef]
- Tovar-Gómez, R.; Moreno-Virgen, M.D.R.; Moreno-Pérez, J.; Bonilla-Petriciolet, A.; Hernández-Montoya, V.; Duran-Valle, C. Analysis of synergistic and antagonistic adsorption of heavy metals and acid blue 25 on activated carbon from ternary systems. Chem. Eng. Res. Des. 2015, 93, 755–772. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.; Mendoza-Castillo, D.; Silvestre-Albero, J.; Bonilla-Petriciolet, A. Tailoring the adsorption behavior of bone char for heavy metal removal from aqueous solution. Adsorpt. Sci. Technol. 2016, 34, 368–387. [Google Scholar] [CrossRef]
- Sousa, F.W.; Oliveira, A.G.; Ribeiro, J.P.; Rosa, M.D.F.; Keukeleire, D.; Nascimento, R.F.D. Green coconut shells applied as adsorbent for removal of toxic metal ions using fixed-bed column technology. J. Environ. Manag. 2010, 91, 1634–1640. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef]
- Chen, J.P.; Hong, L.; Wu, A.S.; Wang, L. Elucidation of Interactions between Metal Ions and Ca Alginate-Based Ion-Exchange Resin by Spectroscopic Analysis and Modeling Simulation. Langmuir 2002, 18, 9413–9421. [Google Scholar] [CrossRef]
- Ngomsik, A.-F.; Bée, A.; Siaugue, J.-M.; Cabuil, V.; Cote, G. Nickel adsorption by magnetic alginate microcapsules containing an extractant. Water Res. 2006, 40, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Jeon, B.-H.; Cho, D.-W.; Roh, H.-S.; Cho, Y.; Kim, S.-J.; Lee, D.S. Sorptive removal of heavy metals with nano-sized carbon immobilized alginate beads. J. Ind. Eng. Chem. 2015, 26, 364–369. [Google Scholar] [CrossRef]
- Gotoh, T.; Matsushima, K.; Kikuchi, K.-I. Adsorption of Cu and Mn on covalently cross-linked alginate gel beads. Chemosphere 2004, 55, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.W.; Karthikeyan, K.G.; Tshabalala, M.A. Orthophosphate Sorption onto Lanthanum-Treated Lignocellulosic Sorbents. Environ. Sci. Technol. 2005, 39, 6273–6279. [Google Scholar] [CrossRef]
- Idota, Y.; Kogure, Y.; Kato, T.; Yano, K.; Arakawa, H.; Miyajima, C.; Kasahara, F.; Ogihara, T. Relationship between Physical Parameters of Various Metal Ions and Binding Affinity for Alginate. Boil. Pharm. Bull. 2016, 39, 1893–1896. [Google Scholar] [CrossRef]
- Karagündüz, A.; Unal, D. New method for evaluation of heavy metal binding to alginate beads using pH and conductivity data. Adsorption 2006, 12, 175–184. [Google Scholar] [CrossRef]
- Plazinski, W. Sorption of lead, copper, and cadmium by calcium alginate. Metal binding stoichiometry and the pH effect. Environ. Sci. Pollut. Res. 2012, 19, 3516–3524. [Google Scholar] [CrossRef]
- Yang, N.; Wang, R.; Rao, P.; Yan, L.; Zhang, W.; Wang, J.; Chai, F. The Fabrication of Calcium Alginate Beads as a Green Sorbent for Selective Recovery of Cu(II) from Metal Mixtures. Crystals 2019, 9, 255. [Google Scholar] [CrossRef]
- Park, H.G.; Kim, T.W.; Yoo, I.K. Activated carbon-containing alginate adsorbent for the simultaneous removal of heavy metals and toxicorganics. Process Biochem. 2007, 42, 1371–1377. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of Cross-Linking with Calcium Ions on the Physical Properties of Alginate Films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; An, Q.; Xiao, Z.-Y.; Zhai, S.-R.; Shi, Z. Versatile core/shell-like alginate@polyethylenimine composites for efficient removal of multiple heavy metal ions (Pb2+, Cu2+, CrO42−): Batch and fixed-bed studies. Mater. Res. Bull. 2019, 118, 110526. [Google Scholar] [CrossRef]
- Nieboer, E.; Richardson, D.H. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ. Pollut. Ser. B Chem. Phys. 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Zhou, Q.; Lin, X.; Qian, J.; Wang, J.; Luo, X. Porous zirconium alginate beads adsorbent for fluoride adsorption from aqueous solutions. RSC Adv. 2015, 5, 2100–2112. [Google Scholar] [CrossRef]
- Helrich, K. Official methods of analysis. Assoc. Off. Anal. Chem. 1990, 1, 1–1230. [Google Scholar]
- European Standard EN1189. Water Quality-Determination of Phosphorus-Ammonium Molybdate Specrtometric Method; European Committee for Standardization: Brussels, Belgium, 1996. [Google Scholar]
- Nair, P.S.; Logan, T.J.; Sharpley, A.N.; Sommers, L.E.; Tabatabai, M.A.; Yuan, T.L. Interlaboratory Comparison of a Standardized Phosphorus Adsorption Procedure. J. Environ. Qual. 1984, 13, 591–595. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Kumar, M.M.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
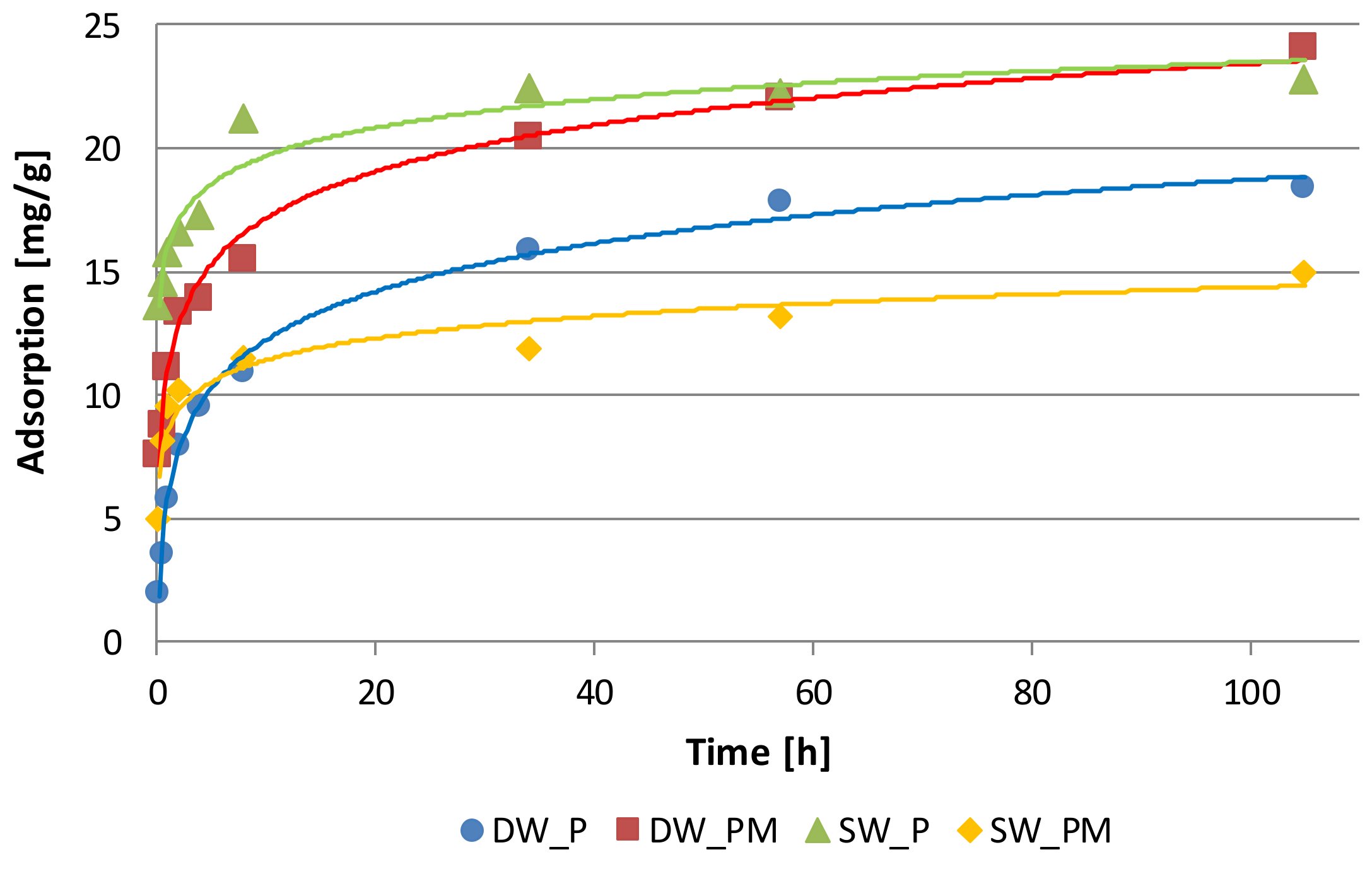


| Solution | Adsorption at Equilibrium State | Rate Constant of Adsorption | Initial Adsorption Rate | Adjusted Coefficient of Determination |
|---|---|---|---|---|
| aeq (mgPO4/g) | k2 (g/mg h) | Vo (mg/g h) | Radj2 | |
| DW_P | 18.83 ± 0.32 | 0.011 ± 0.002 | 4.17 ± 0.85 | 0.998 |
| DW_PM | 22.27 ± 0.51 | 0.028 ± 0.011 | 13.79 ± 5.28 | 0.996 |
| SW_P | 22.83 ± 0.12 | 0.047 ± 0.015 | 24.39 ± 7.79 | 0.999 |
| SW_PM | 15.11 ± 0.12 | 0.067 ± 0.022 | 15.41 ± 5.12 | 0.999 |
| System | Metal | Adsorption at Equilibrium State | Rate Constant of Adsorption | Initial Adsorption Rate | Adjusted Coefficient of Determination |
|---|---|---|---|---|---|
| aeq (mgPO4/g) | k2 (g/mg h) | Vo (mg/g h) | Radj2 | ||
| DW_PM | Pb | 19.12 ± 0.39 | 0.018 ± 0.005 | 6.49 ± 1.89 | 0.997 |
| Zn | 4.13 ± 0.03 | 0.422 ± 0.075 | 7.18 ± 1.28 | 0.999 | |
| Cd | 7.50 ± 0.03 | 0.166 ± 0.035 | 9.35 ±1.98 | 0.997 | |
| Cu | 15.08 ± 0.12 | 0.059 ± 0.011 | 13.48 ± 2.50 | 0.998 | |
| SW_PM | Pb | 19.42 ± 0.56 | 0.011 ± 0.003 | 4.06 ± 1.02 | 0.993 |
| Zn | 7.23 ± 0.16 | 0.059 ± 0.022 | 3.10 ± 1.15 | 0.999 | |
| Cd | 12.52 ± 0.19 | 0.042 ± 0.010 | 6.58 ± 1.59 | 0.997 | |
| Cu | 22.94 ± 1.03 | 0.010 ± 0.003 | 5.07 ± 1.63 | 0.982 |
| Solution | Freundlich Model * | Langmuir Model ** | ||||
|---|---|---|---|---|---|---|
| 1/nF | kF | Radj2 | qm (mgPO4 g−1) | kL (L mgPO4−1) | Radj2 | |
| DW_P | 0.49 ± 0.06 | 9.98 ± 0.89 | 0.968 | 89.3 ± 21.9 | 0.055 ± 0.008 | 0.913 |
| DW_PM | 0.44 ± 0.01 | 14.5 ± 0.34 | 0.998 | 112 ± 32.0 | 0.063 ± 0.007 | 0.947 |
| SW_P | 0.42 ± 0.08 | 19.8 ± 3.26 | 0.898 | 100 ± 11.8 | 0.135 ± 0.005 | 0.994 |
| SW_PM | 0.48 ± 0.05 | 11.0 ± 0.95 | 0.964 | 83.5 ± 8.01 | 0.083 ± 0.003 | 0.993 |
| Water Quality Indicators | Value | Water Quality Indicators | Value |
|---|---|---|---|
| pH | 7 | N_Ammonia, mg/L | 12.19 |
| RedOx Potential Eh, mV | −36.6 | N_Nitrite, mg/L | 0.03 |
| Color, mgPt/L | 19 s | N_Nitrate, mg/L | 7.43 |
| turbidity, (Nephelometric Turbidity Units) NTU | 65.8 | Cu2+, mg/L | 0.0065 |
| Hardness, CaCO3 mg/L | 220.4 | Pb2+, mg/L | 0.0341 |
| Alkalinity, mval/L | 4.1 | Zn2+, mg/L | 0.0592 |
| Phosphate, mg/L | 8.06 | Cd2+, mg/L | 0.0025 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwek, H.; Pawelec, K. Competitive Interaction of Phosphate with Selected Toxic Metals Ions in the Adsorption from Effluent of Sewage Sludge by Iron/Alginate Beads. Molecules 2020, 25, 3962. https://doi.org/10.3390/molecules25173962
Siwek H, Pawelec K. Competitive Interaction of Phosphate with Selected Toxic Metals Ions in the Adsorption from Effluent of Sewage Sludge by Iron/Alginate Beads. Molecules. 2020; 25(17):3962. https://doi.org/10.3390/molecules25173962
Chicago/Turabian StyleSiwek, Hanna, and Krzysztof Pawelec. 2020. "Competitive Interaction of Phosphate with Selected Toxic Metals Ions in the Adsorption from Effluent of Sewage Sludge by Iron/Alginate Beads" Molecules 25, no. 17: 3962. https://doi.org/10.3390/molecules25173962
APA StyleSiwek, H., & Pawelec, K. (2020). Competitive Interaction of Phosphate with Selected Toxic Metals Ions in the Adsorption from Effluent of Sewage Sludge by Iron/Alginate Beads. Molecules, 25(17), 3962. https://doi.org/10.3390/molecules25173962







