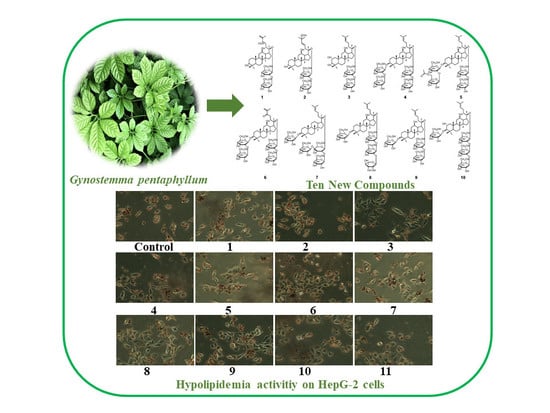
Ten New Dammarane-Type Saponins with Hypolipidemia Activity from a Functional Herbal Tea—Gynostemma pentaphyllum
Abstract
1. Introduction
2. Results and Discussion
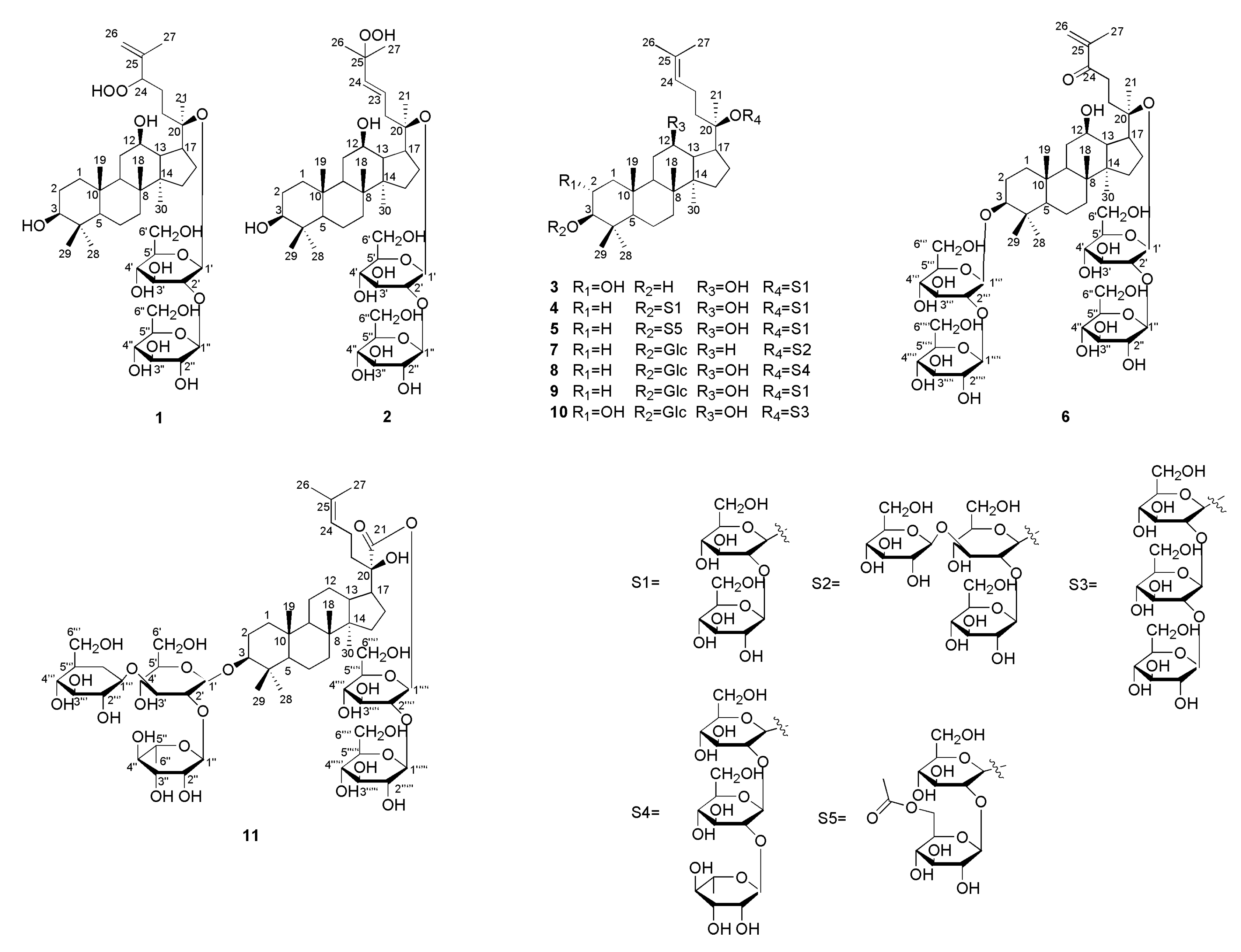
2.1. Structural Elucidation
2.2. Bioactivity Evaluation
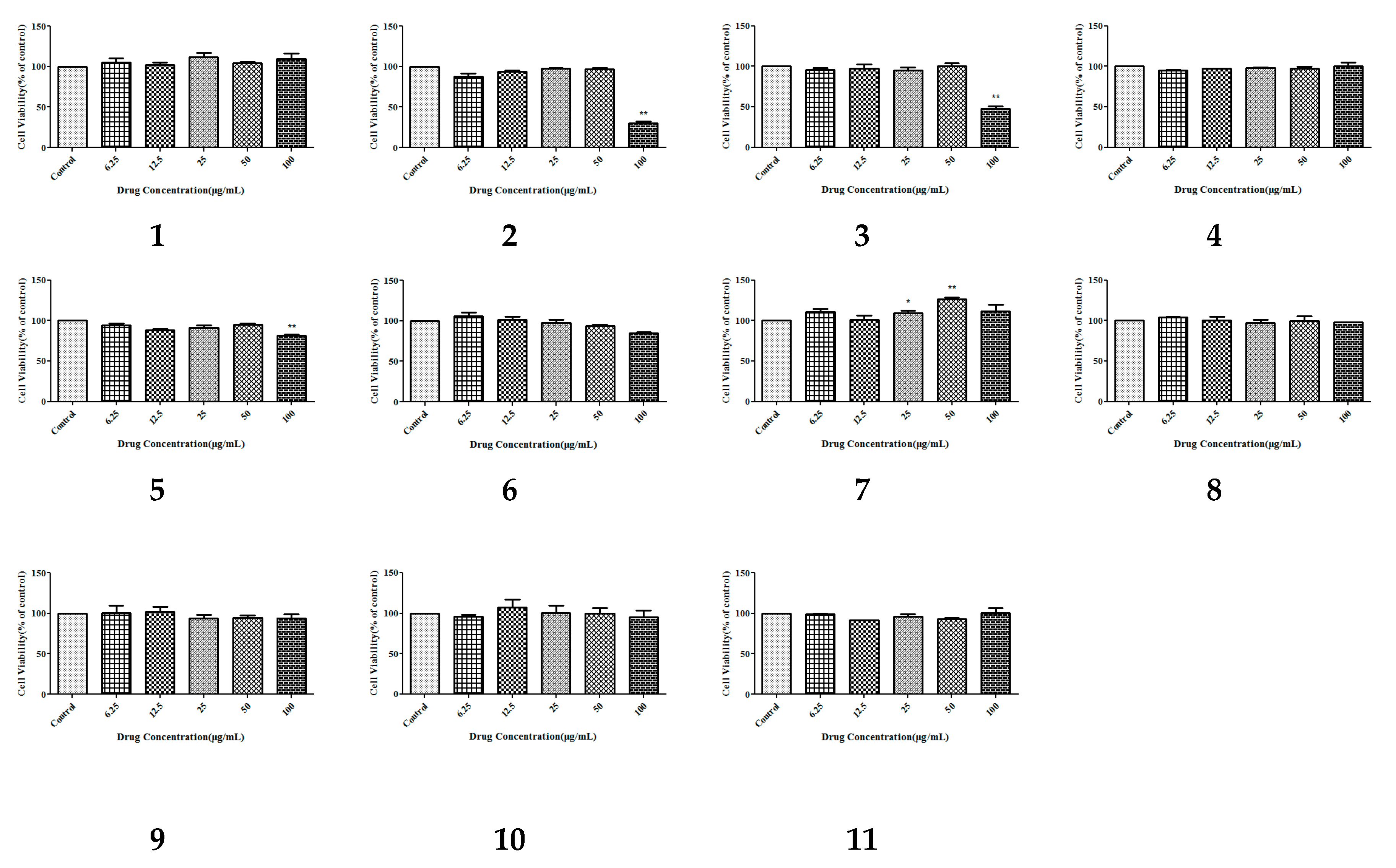
2.2.1. Cytotoxic Activity Assay
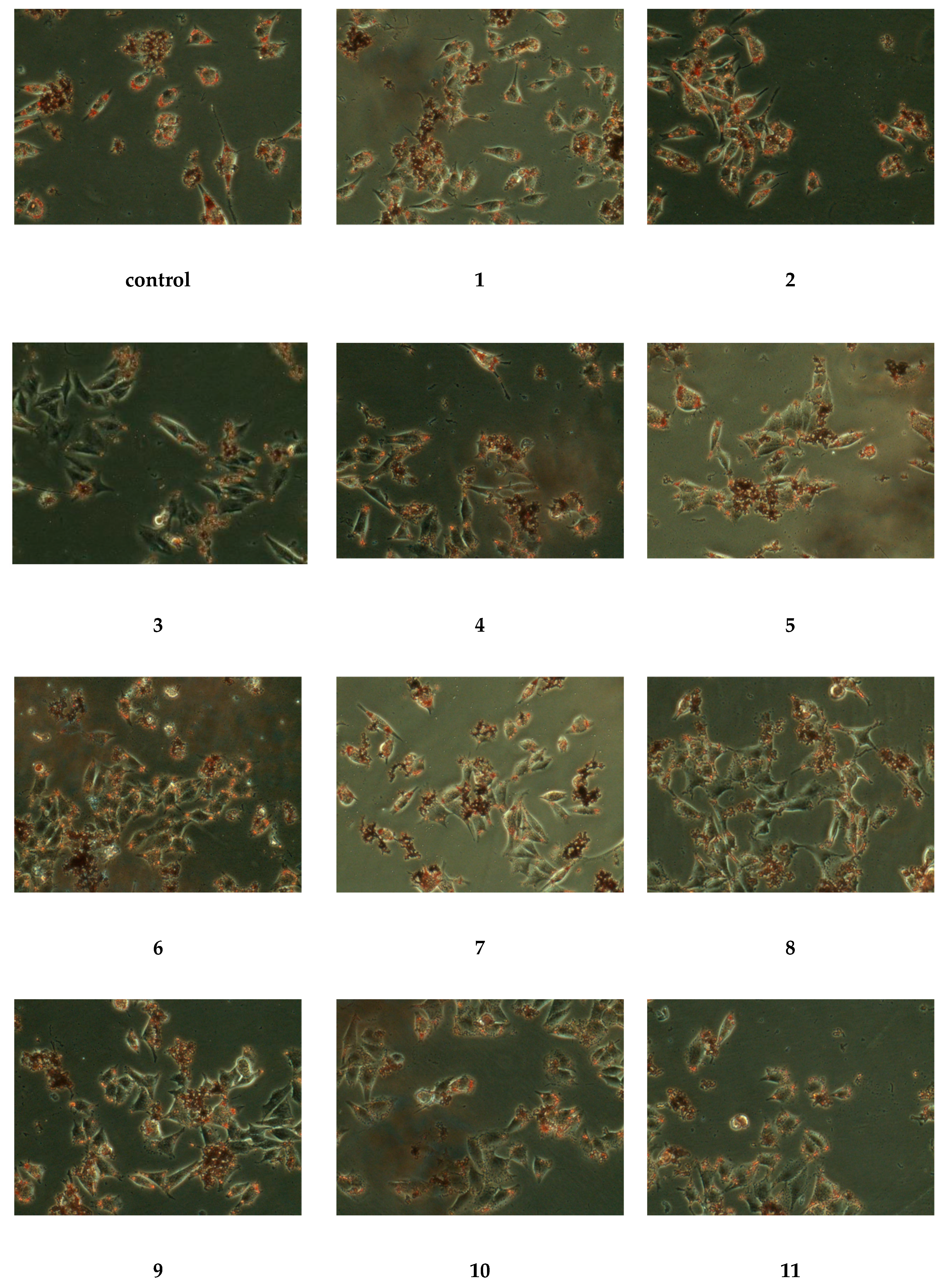
2.2.2. Hypolipidemia Activity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
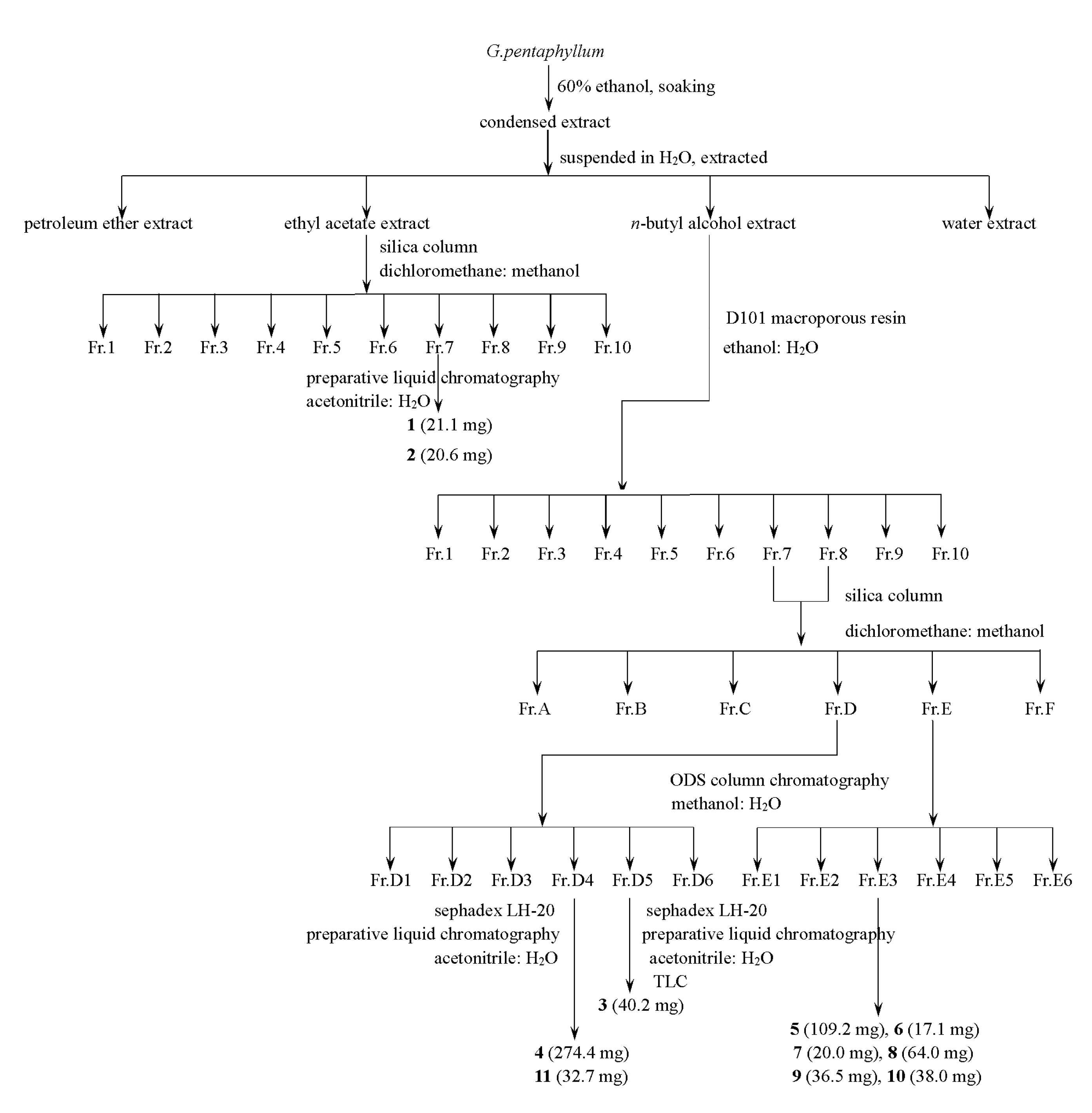
3.3. Extraction and Isolation
3.3.1. Yunnangypenoside A (1)
3.3.2. Yunnangypenoside B (2)
3.3.3. Yunnangypenoside C (3)
3.3.4. Yunnangypenoside D (4)
3.3.5. Yunnangypenoside E (5)
3.3.6. Yunnangypenoside F (6)
3.3.7. Yunnangypenoside G (7)
3.3.8. Yunnangypenoside H (8)
3.3.9. Yunnangypenoside I (9)
3.3.10. Yunnangypenoside J (10)
3.4. Acid Hydrolysis of Dammarane-Type Glycosides
3.5. Cytotoxic Bioactivity
3.5.1. Cell Culture and Reagents
3.5.2. Cell Viability Assays
3.5.3. Hypolipidemia Activity Assay
3.5.4. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gou, S.; Liu, B.; Han, X.; Wang, L.; Zhong, C.; Liang, S.; Liu, H.; Qiang, Y.; Zhang, Y.; Ni, J.M. Anti-atherosclerotic effect of Fermentum Rubrum and Gynostemma pentaphyllum mixture in high-fat emulsion- and vitamin D3-induced atherosclerotic rats. J. Chin. Med. Assoc. 2018, 81, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal medicine for cardiovascular diseases: Efficacy, mechanisms, and safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Y.; Ren, D.; Yang, X. Protective effect of saponins-enriched fraction of Gynostemma pentaphyllum against high choline-induced vascular endothelial dysfunction and hepatic damage in mice. Biol. Pharm. Bull. 2020, 43, 463–473. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Li, D.; Xie, T.; Li, Y.; Li, J.; Chen, X.; Wei, G. Integrating network pharmacology and component analysis study on anti-atherosclerotic mechanisms of total flavonoids of Engelhardia roxburghiana leaves in mice. Chem. Biodivers. 2020, 17, e1900629. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhu, J.; Zhang, H.; Fan, X. Investigating chemical features of Panax notoginseng based on integrating HPLC fingerprinting and determination of multiconstituents by single reference standard. J. Gins. Res. 2018, 42, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xie, W.; Luo, Y.; Lu, S.; Dai, Z.; Wang, R.; Sun, G.; Sun, X. Protective effects of total saponins of Aralia elata (Miq.) on endothelial cell injury induced by TNF-α via modulation of the PI3K/AKT and NF-κB signalling pathways. Int. J. Mol. Sci. 2018, 20, 36. [Google Scholar] [CrossRef]
- Shi, L.; Lu, F.; Zhao, H.; Zhao, Y. Two new triterpene saponins from Gynostemma pentaphyllum. J. Asian Nat. Prod. Res. 2012, 14, 856–861. [Google Scholar] [CrossRef]
- Wang, J.; Ha, T.K.Q.; Shi, Y.; Oh, W.; Yang, J. Hypoglycemic triterpenes from Gynostemma pentaphyllum. Phytochemistry 2018, 155, 171–181. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, M.; Cheng, X.; Han, Y.; Zhao, T.; Fan, M.; Zhu, L.; Yang, J. Dammarane-type saponins from Gynostemma pentaphyllum prevent hypoxia-induced neural injury through activation of ERK, Akt, and CREB pathways. J. Agric. Food Chem. 2020, 68, 193–205. [Google Scholar] [CrossRef]
- Cui, W.; Jin, Y.; Liu, H.; Zu, M.; Zhai, X.; Yang, C.; Gu, Y.; Cheng, Y.; Piao, X. Dammarane-type saponins from Gynostemma pentaphyllum and their cytotoxicities. Nat. Prod. Res. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Chen, P.; Chang, C.; Huang, H.; Zhang, L.; Lian, C.; Lin, Y.; Nguyen, N.L.; Vo, T.H.; Cheng, Y.; Natschke, S.L.M.; et al. New dammarane-type saponins from Gynostemma pentaphyllum. Molecules 2019, 24, 1357. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Fanny, C.F.; Fu, G.; Pang, H.; Ye, W.; Nancy, Y. Dammarane saponins from Gynostemma pentaphyllum. Phytochemistry 2010, 10, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Kuang, Y.; Fan, D.; Jiang, T.; Chen, L.; Zhang, D.; Zhu, J.; Wang, Z.; Wang, D.; Li, C. Comparison of saponins from Gynostemma pentaphyllum leaves prepared by different processing methods. China J. Chin. Mater. Med. 2018, 43, 502–510. [Google Scholar]
- Ky, P.T.; Huong, P.T.; My, T.K.; Anh, P.T.; Kiem, P.V.; Minh, C.V.; Cuong, N.X.; Thao, N.P.; Nhiem, N.X.; Hyum, J.H.; et al. Dammarane-type saponins from Gynostemma pentaphyllum. Phytochemistry 2010, 71, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shen, Y.; Guo, X. Isolation, structures, and bioactivities of the polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino: A review. Biomed. Res. Int. 2018, 2018, 6285134. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Lin, M.; Wang, Y.; Chang, T.; Cui, W.; Piao, X. Novel dammarane-type saponins from Gynostemma pentaphyllum and their neuroprotective effect. Nat. Prod. Res. 2020, 34, 651–658. [Google Scholar] [CrossRef]
- Than, T.K.M.; Pham, T.K.; Pham, T.B.; Phuong, T.N.; Tien, D.N. New dammarane-type triterpenoid glycosides from Gynostemma burmanicum. Nat. Prod. Res. 2020, 34, 217–224. [Google Scholar] [CrossRef]
- Nakamura, S.; Sugimoto, S.; Matsuda, H.; Yoshikawa, M. Medicinal flowers. XVII. New dammarane-type triterpene glycosides from flower buds of american ginseng, Panax quinquefolium L. Chem. Pharm. Bull. 2007, 55, 1342–1348. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Sugimoto, S.; Nakamura, S.; Matsuda, H. Medicinal flowers. XI. Structures of new dammarane-type triterpene diglycosides with hydroperoxide group from flower buds of Panax ginseng. Chem. Pharm. Bull. 2007, 55, 571–576. [Google Scholar] [CrossRef]
- Yang, F.; Shi, H.; Zhang, X.; Yang, H.; Zhou, Q.; Yu, L. Two new saponins from tetraploid jiaogulan (Gynostemma pentaphyllum), and their anti-inflammatory and α-glucosidase inhibitory activities. Food Chem. 2013, 141, 3606–3613. [Google Scholar] [CrossRef]
- Min, C.Y.; Dong, S.S.; Jongki, H.; Sung, H.H.; Young, C.K.; Kang, R.L. Ginsenosides from the roots of Korean cultivated-wild ginseng. Nat. Prod. Sci. 2008, 14, 171–176. [Google Scholar]
- Samimi, R.; Xu, W.; Lui, E.M.K.; Charpentier, P.A. Isolation and immunosuppressive effects of 6’’-O-acetylginsenoside Rb-1 extracted from north american ginseng. Planta Med. 2014, 80, 509–516. [Google Scholar] [PubMed]
- Yoshikawa, M.; Murakami, T.; Ueno, T.; Yashiro, K.; Hirokawa, N.; Murakami, N.; Yamahara, J.; Matsuda, H.; Saijoh, R.; Tanaka, O. Bioactive saponins and glycosides. VIII. Notoginseng (1): New dammarane-type triterpene oligoglycosides, notoginsenosides-A, -B, -C, and -D, from the dried root of Panax notoginseng (Burk.) F.H. CHEN. Chem. Pharm. Bull. 1997, 45, 1309–1345. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Kasai, R.; Ohtani, K.; Ito, A.; Nguyen, T.N.; Yamasaki, K.; Tanaka, O. Saponins from vietnamese ginseng, Panax vietnamensis HA in Grushv. Collected in central Vietnam. II. Chem. Pharm. Bull. 1994, 42, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Chen, J.; Tu, S.; Gu, H.; Hu, W.; Qiu, M. Six new triterpenoid glycosides from Gynostemma pentaphyllum. Helv. Chim. Acta 2009, 92, 2737–2745. [Google Scholar] [CrossRef]
- Ten, L.N.; Chae, S.M.; Yoo, S.A. Biotransformation of ginsenoside Rb1 into F-2 and compound K by bacterium sphingomonas sp. BG 25. Chem. Nat. Compd. 2014, 49, 1168–1169. [Google Scholar] [CrossRef]
- Takemoto, T.; Arihara, S.; Yoshikawa, K.; Kusumoto, K.; Yano, I.; Hayashi, T. Studies on the constituents of cucurbitaceae plants. VI. on the saponin constituents of Luffa cylindrica roem. (1). Yakugaku Zasshi. 1984, 104, 246–255. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, G.; Li, F.; Wang, T.; Suo, T.; Wang, C.; Li, Z.; Zhu, Y. Chemical constituents from the roots of polygala arillata and their anti-inflammatory activities. J. Chem. 2019, 2019, 8079619. [Google Scholar] [CrossRef]
- Zhang, G.; Geng, H.; Zhao, C.; Li, F.; Li, Z.; Lun, B.; Wang, C.; Yu, H.; Bie, S.; Li, Z. Chemical constituents with inhibitory activity of NO production from a wild edible mushroom, Russula vinosa lindbl, may be its nutritional ingredients. Molecules 2019, 24, 1305. [Google Scholar] [CrossRef]
- Lun, B.; Shao, L.; Wang, Y.; Li, C.; Yu, H.; Wang, C.; Zhu, Y. Taxanes from Taxus wallichiana var. mairei cultivated in the southern area of the Yangtze River in china. Nat. Prod. Res. 2017, 31, 2341–2347. [Google Scholar] [CrossRef]
- Di, T.; Yang, S.; Du, F.; Zhao, L.; Xia, T.; Zhang, X. Cytotoxic and hypoglycemic activity of triterpenoid saponins from Camellia oleifera abel. Seed pomace. Molecules 2017, 22, 1562. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.; Zhang, D.; Lv, Y.; Wei, Y.; Wu, W.; Zhou, F.; Tang, M.; Miao, T.; Li, M.; et al. Inhibitory effect of blueberry polyphenolic compounds on oleic acid-induced hepatic steatosis in vitro. J. Agric. Food Chem. 2011, 59, 12254–12263. [Google Scholar] [CrossRef] [PubMed]
- Mehlem, A.; Hagberg, C.E.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1-11 are available from the authors. |

| No. | 1 | 2 | 3 | 4 | 5 | 6 | 9 |
|---|---|---|---|---|---|---|---|
| 1 | 1.81 (m),1.07 (m) | 1.81 (m), 1.05 (m) | 2.50 (m),1.37 (m) | 1.61 (m), 0.83 (m) | 1.57 (m), 0.86 (m) | 1.62 (m), 0.84 (m) | 1.62 (m), 0.88 (m) |
| 2 | 1.79 (m), 1.67 (m) | 1.83 (m), 1.74 (m) | 4.11 (m) | 2.06 (m), 1.76 (m) | 2.23 (m), 2.12 (m) | 2.03 (m), 1.75 (m) | 1.95 (m), 1.39 (m) |
| 3 | 3.41 (m) | 3.43 (dd, 11.4, 4.4) | 3.43 (d, 9.4) | 3.25 (dd, 11.7, 4.6) | 3.33 (dd, 11.7, 4.5) | 3.22 (dd, 11.7, 4.6) | 3.35 (dd, 11.8, 4.5) |
| 4 | - | - | - | - | - | - | - |
| 5 | 0.83 (m) | 0.85 (m) | 0.98 (m) | 0.66 (m) | 0.73 (m) | 0.63 (m) | 0.72 (m) |
| 6 | 1.58 (m), 1.46 (m) | 1.61 (m), 1.50 (m) | 1.57 (m), 1.48 (m) | 1.49 (m), 1.36 (m) | 1.51 (m), 1.38 (m) | 1.66 (m), 1.47 (m) | 1.50 (m), 1.37 (m), |
| 7 | 1.48 (m), 1.23 (m) | 1.51 (m), 1.26 (m) | 1.49 (m), 1.24 (m) | 1.42 (m), 1.20 (m) | 1.48 (m), 1.23 (m) | 1.39 (m), 1.18 (m) | 1.46 (m), 1.20 (m) |
| 8 | - | - | - | - | - | - | - |
| 9 | 1.51 (m) | 1.54 (m) | 1.59 (m) | 1.43 (m) | 1.44 (m) | 1.42 (m) | 1.45 (m) |
| 10 | - | - | - | - | - | - | - |
| 11 | 2.70 (m), 1.30 (m) | 1.61 (m), 1.01 (m) | 1.58 (m), 1.02 (m) | 2.19 (m), 1.43 (m) | 2.04 (m), 1.45 (m) | 1.57 (m), 0.99 (m) | 1.57 (m), 1.01 (m) |
| 12 | 4.17 (m) | 4.12 (m) | 4.12 (m) | 4.17 (m) | 3.98 (m) | 4.18 (m) | 4.16 (m) |
| 13 | 2.05 (m) | 2.03 (m) | 2.00 (m) | 2.00 (m) | 2.00 (m) | 2.04 (m) | 1.99 (m) |
| 14 | - | - | - | - | - | - | - |
| 15 | 2.30 (m), 1.52 (m) | 2.28 (m), 1.55 (m) | 2.35 (m), 1.34 (m) | 1.57 (m), 1.01 (m) | 1.57 (m), 1.08 (m) | 2.20 (m), 1.42 (m) | 2.20 (m), 1.45 (m) |
| 16 | 2.22 (m), 2.12 (m) | 1.92 (m), 1.47 (m) | 1.99 (m), 1.41 (m) | 1.97 (m), 1.40 (m) | 1.86 (m), 1.55 (m) | 1.93 (m), 1.44 (m) | 1.62 (m), 1.59 (m) |
| 17 | 2.54 (m) | 2.57 (m) | 2.57 (m) | 2.56 (m) | 2.62 (m) | 2.53 (m) | 2.56 (m) |
| 18 | 0.97 (s) | 1.05 (s) | 0.96 (s) | 0.94 (s) | 0.98 (s) | 0.93 (s) | 0.93 (s) |
| 19 | 0.87 (s) | 0.93 (s) | 0.94 (s) | 0.78 (s) | 0.85 (s) | 0.76 (s) | 0.78 (s) |
| 20 | - | - | - | - | - | - | - |
| 21 | 1.62 (s) | 1.60 (s) | 1.64 (s) | 1.62 (s) | 1.66 (s) | 1.54 (s) | 1.60 (s) |
| 22 | 2.47 (m), 2.21 (m) | 3.08 (m), 2.69(m) | 2.40 (m), 1.89 (m) | 2.38 (m), 1.86 (m) | 2.35 (m), 2.03 (m) | 2.66 (m), 2.17 (m) | 2.37 (m), 1.85 (m) |
| 23 | 1.90 (m), 1.38 (m) | 6.23 (m), | 2.47 (m), 2.29 (m) | 2.50 (m), 2.28 (m) | 2.35 (m) | 3.45 (m), 3.17 (m) | 2.48 (m), 2.25 (m) |
| 24 | 4.69 (d, 8.1, 4.9) | 6.02 (d, 15.8) | 5.26 (t, 7.0) | 5.23 (t, 7.0) | 5.25 (m) | - | 5.21 (m) |
| 25 | - | - | - | - | - | - | - |
| 26 | 5.23 (s), 5.07 (m) | 1.62 (s) | 1.63 (s) | 1.61 (s), | 1.63 (s) | 6.34 (s), 5.74 (s) | 1.62 (s) |
| 27 | 1.96 (s) | 1.62 (s) | 1.66 (s) | 1.65 (s) | 1.67 (s) | 1.90 (s) | 1.63 (s) |
| 28 | 1.25 (s) | 1.26 (s) | 1.29 (s) | 1.32 (s) | 1.32 (s) | 1.31 (s) | 1.34 (s) |
| 29 | 1.03 (s) | 0.95 (s) | 1.09 (s) | 1.10 (s) | 1.11 (s) | 1.09 (s) | 1.00 (s) |
| 30 | 0.93 (s) | 0.96 (s) | 0.95 (s) | 1.00 (s) | 1.09 (s) | 0.96 (s) | 1.00 (s) |
| 20-O-Glc | |||||||
| 1’ | 5.12 (d, 7.8) | 5.18 (d, 7.8) | 5.16 (d, 7.8) | 5.16 (d, 7.7) | 5.27 (d, 7.9) | 5.10 (br, s) | 5.16 (d, 7.8) |
| 2’ | 4.28 (m) | 4.25 (m) | 4.24 (m) | 4.28 (m) | 4.10 (m) | 4.31 (m) | 4.27 (m) |
| 3’ | 3.88 (m) | 4.29 (m) | 4.40 (m) | 4.36 (m) | 4.28 (m) | 4.29 (m) | 4.00 (m) |
| 4’ | 4.15 (m) | 4.10 (m) | 4.31 (m) | 4.34 (m) | 4.17 (m) | 4.32 (m) | 4.28 (m) |
| 5’ | 4.35 (m) | 3.94 (m) | 3.94 (m) | 4.28 (m) | 4.28 (m) | 4.37 (m) | 4.00 (m) |
| 6’ | 4.43 (m) | 4.51 (m) | 4.45 (m) | 4.48 (m) | 4.29 (m) | 4.72 (m) | 4.30 (m) |
| 2’-O-Glc | |||||||
| 1’’ | 5.80 (d, 7.8) | 5.67 (d, 7.8) | 5.65 (d, 7.8) | 5.73 (d, 7.7) | 5.29 (d, 7.9) | 5.82 (d, 7.8) | 5.73 (d, 7.8) |
| 2’’ | 4.14 (m) | 4.13 (m) | 4.15 (m) | 4.14 (m) | 4.14 (m) | 4.13 (m) | 4.05 (m) |
| 3’’ | 3.82 (m) | 4.30 (m) | 3.85 (m) | 4.28 (m) | 3.94 (m) | 4.29 (m) | 4.37 (m) |
| 4’’ | 4.31 (m) | 4.29 (m) | 4.22 (m) | 4.17 (m) | 4.35 (m) | 4.62 (m) | 4.27 (m) |
| 5’’ | 4.30 (m) | 3.94 (m) | 4.31 (m) | 4.29 (m) | 3.85 (m) | 4.29 (m) | 3.85 (m) |
| 6’’ | 4.28 (m) | 4.30 (m) | 4.32 (m) | 4.31 (m) | 4.38 (m) | 4.49 (m) | 4.55 (m) |
| 3-O-Glc | |||||||
| 1’’’ | - | - | - | 4.93 (d, 7.6) | 4.95 (d, 7.3) | 4.90 (d, 7.7) | 4.96 (d, 7.8) |
| 2’’’ | - | - | - | 4.25 (m) | 4.26 (m) | 4.24 (m) | 4.14 (m) |
| 3’’’ | - | - | - | 4.30 (m) | 3.85 (m) | 4.32 (m) | 4.28 (m) |
| 4’’’ | - | - | - | 4.18 (m) | 4.35 (m) | 3.91 (m) | 4.27 (m) |
| 5’’’ | - | - | - | 3.86 (m) | 4.28 (m) | 4.29 (m) | 3.92 (m) |
| 6’’’ | - | - | - | 4.49 (m) | 4.50 (m) | 4.49 (m) | 4.45 (m) |
| 2’’’-O-Glc | |||||||
| 1’’’’ | - | - | - | 5.41 (d, 7.6) | 5.40 (d, 7.5) | 5.40 (d, 7.7) | - |
| 2’’’’ | - | - | - | 4.15 (m) | 4.14 (m) | 4.13 (m) | - |
| 3’’’’ | - | - | - | 4.28 (m) | 3.85 (m) | 3.91 (m) | - |
| 4’’’’ | - | - | - | 4.18 (m) | 4.17 (m) | 4.12 (m) | - |
| 5’’’’ | - | - | - | 3.93 (m) | 4.08 (m) | 3.91 (m) | - |
| 6’’’’ | - | - | - | 4.39 (m) | 5.01 (m) | 4.26 (m) | - |
| 6’’’’-OCOCH3 | - | - | - | - | - | - | - |
| CH3COO | - | - | - | - | 2.06 (s) | - | - |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 9 |
|---|---|---|---|---|---|---|---|
| 1 | 39.9 | 39.7 | 48.5 | 39.3 | 39.5 | 39.3 | 39.3 |
| 2 | 28.4 | 28.5 | 69.1 | 26.9 | 27.2 | 26.8 | 27.4 |
| 3 | 78.3 | 78.3 | 83.9 | 89.1 | 89.3 | 89.1 | 89.0 |
| 4 | 39.7 | 39.9 | 40.2 | 40.0 | 40.1 | 40.0 | 40.0 |
| 5 | 56.5 | 56.6 | 56.7 | 56.6 | 56.7 | 56.5 | 56.6 |
| 6 | 19.1 | 19.1 | 19.1 | 18.8 | 18.8 | 18.8 | 18.8 |
| 7 | 35.4 | 35.5 | 35.3 | 35.4 | 35.4 | 35.3 | 35.3 |
| 8 | 40.3 | 40.4 | 40.4 | 40.3 | 40.3 | 40.3 | 40.3 |
| 9 | 50.6 | 50.6 | 50.5 | 50.4 | 50.3 | 50.5 | 50.4 |
| 10 | 37.7 | 37.7 | 38.9 | 37.3 | 37.3 | 37.3 | 37.3 |
| 11 | 30.3 | 31.1 | 31.2 | 30.9 | 31.5 | 31.2 | 31.2 |
| 12 | 71.0 | 71.2 | 71.0 | 71.1 | 71.2 | 71.1 | 71.1 |
| 13 | 49.5 | 49.7 | 49.6 | 49.5 | 49.6 | 49.4 | 49.5 |
| 14 | 51.9 | 52.0 | 51.9 | 52.0 | 52.0 | 52.0 | 52.0 |
| 15 | 30.8 | 31.0 | 31.1 | 31.3 | 31.3 | 30.7 | 30.9 |
| 16 | 27.0 | 27.1 | 27.2 | 27.4 | 27.1 | 27.5 | 26.9 |
| 17 | 52.9 | 52.9 | 53.1 | 53.0 | 53.6 | 53.3 | 53.0 |
| 18 | 16.3 | 16.3 | 16.3 | 16.2 | 16.1 | 16.3 | 16.2 |
| 19 | 16.6 | 16.7 | 17.9 | 16.6 | 16.6 | 16.6 | 16.6 |
| 20 | 84.5 | 84.1 | 84.4 | 84.4 | 84.1 | 84.2 | 84.4 |
| 21 | 22.3 | 22.9 | 22.2 | 22.4 | 22.9 | 22.0 | 22.3 |
| 22 | 33.2 | 40.2 | 36.6 | 36.6 | 36.3 | 29.9 | 36.6 |
| 23 | 27.2 | 126.9 | 23.8 | 23.8 | 24.3 | 33.3 | 23.8 |
| 24 | 90.3 | 138.4 | 126.3 | 126.3 | 126.2 | 202.6 | 126.3 |
| 25 | 146.4 | 81.8 | 131.1 | 131.2 | 131.2 | 144.9 | 131.2 |
| 26 | 113.6 | 25.8 | 26.1 | 26.1 | 26.1 | 125.2 | 26.1 |
| 27 | 18.1 | 25.5 | 18.1 | 18.2 | 18.2 | 18.2 | 18.1 |
| 28 | 29.0 | 29.0 | 29.6 | 28.5 | 28.5 | 28.5 | 28.6 |
| 29 | 16.6 | 16.7 | 17.7 | 17.0 | 17.0 | 17.0 | 17.2 |
| 30 | 17.8 | 17.7 | 17.8 | 17.8 | 17.5 | 17.8 | 17.8 |
| 20-O-Glc | |||||||
| 1’ | 97.0 | 97.1 | 97.1 | 97.2 | 97.1 | 97.0 | 97.2 |
| 2’ | 80.4 | 81.7 | 81.9 | 81.2 | 84.9 | 79.8 | 81.1 |
| 3’ | 78.5 | 79.0 | 79.2 | 79.3 | 78.2 | 78.7 | 78.6 |
| 4’ | 71.7 | 71.8 | 72.1 | 72.0 | 71.3 | 71.9 | 71.8 |
| 5’ | 79.5 | 78.6 | 78.7 | 78.7 | 78.2 | 79.7 | 78.7 |
| 6’ | 63.0 | 63.2 | 62.9 | 63.1 | 62.6 | 62.9 | 62.9 |
| 2’-O-Glc | |||||||
| 1’’ | 105.1 | 105.9 | 105.8 | 105.5 | 107.1 | 104.8 | 105.4 |
| 2’’ | 76.8 | 77.0 | 77.0 | 76.9 | 77.2 | 77.4 | 76.1 |
| 3’’ | 78.4 | 78.8 | 78.5 | 78.7 | 78.4 | 78.7 | 79.4 |
| 4’’ | 71.9 | 72.0 | 71.8 | 71.8 | 72.0 | 72.0 | 72.1 |
| 5’’ | 78.7 | 78.6 | 78.7 | 78.7 | 78.6 | 78.6 | 78.5 |
| 6’’ | 62.9 | 63.0 | 63.2 | 63.0 | 63.1 | 63.0 | 63.3 |
| 3-O-Glc | |||||||
| 1’’’ | - | - | - | 105.2 | 105.4 | 105.1 | 107.1 |
| 2’’’ | - | - | - | 83.6 | 83.7 | 83.6 | 76.9 |
| 3’’’ | - | - | - | 78.6 | 78.7 | 78.6 | 79.1 |
| 4’’’ | - | - | - | 71.8 | 71.9 | 71.7 | 71.8 |
| 5’’’ | - | - | - | 78.3 | 78.3 | 78.6 | 78.7 |
| 6’’’ | - | - | - | 63.1 | 63.2 | 63.1 | 63.0 |
| 2’’’-O-Glc | |||||||
| 1’’’’ | - | - | - | 106.3 | 106.3 | 106.3 | - |
| 2’’’’ | - | - | - | 77.4 | 77.5 | 76.7 | - |
| 3’’’’ | - | - | - | 78.6 | 78.7 | 78.3 | - |
| 4’’’’ | - | - | - | 71.8 | 71.4 | 71.7 | - |
| 5’’’’ | - | - | - | 78.3 | 75.9 | 78.3 | - |
| 6’’’’ | - | - | - | 62.8 | 64.8 | 63.1 | - |
| 6’’’’-OCOCH3 | - | - | - | - | 171.3 | - | - |
| CH3COO | - | - | - | - | 21.2 | - | - |
| Position | 13C | 1H | Position | 13C | 1H |
|---|---|---|---|---|---|
| 1 | 39.7 | 1.51 (m), 0.80 (m) | 3-O-Glc | ||
| 2 | 27.2 | 2.24 (m), 1.87 (m) | 1’ | 105.4 | 4.96 (d, 7.6) |
| 3 | 89.4 | 3.33 (dd,11.7, 4.6) | 2’ | 77.4 | 4.15 (m) |
| 4 | 40.1 | - | 3’ | 78.6 | 4.02 (m) |
| 5 | 56.8 | 0.74 (m) | 4’ | 72.0 | 4.36 (m) |
| 6 | 18.8 | 1.52 (m), 1.39(m) | 5’ | 78.3 | 4.28 (m) |
| 7 | 36.0 | 1.53 (m), 1.25 (m) | 6’ | 63.2 | 4.56 (m) |
| 8 | 41.0 | - | 20-O-Glc | ||
| 9 | 51.3 | 1.33 (m) | 1’’ | 97.5 | 5.13 (d, 7.6) |
| 10 | 37.3 | - | 2’’ | 83.7 | 4.23 (m) |
| 11 | 22.3 | 1.33 (m), 1.24 (m) | 3’’ | 83.7 | 4.34 (m) |
| 12 | 25.6 | 2.23 (m), 2.04 (m) | 4’’ | 72.0 | 4.36 (m) |
| 13 | 43.2 | 1.84 (m) | 5’’ | 78.4 | 3.95 (m) |
| 14 | 51.1 | - | 6’’ | 63.1 | 4.51 (m) |
| 15 | 31.8 | 1.67 (m),1.15 (m) | 2’’-O-Glc | ||
| 16 | 28.2 | 2.07 (m),1.44 (m) | 1’’’ | 106.3 | 5.38 (d, 7.6) |
| 17 | 48.2 | 2.34 (m) | 2’’’ | 77.7 | 4.16 (m) |
| 18 | 16.2 | 1.01 (s) | 3’’’ | 78.2 | 4.29 (m) |
| 19 | 16.8 | 0.83 (s) | 4’’’ | 71.9 | 4.19 (m) |
| 20 | 83.5 | - | 5’’’ | 78.7 | 4.29 (m) |
| 21 | 21.8 | 1.53 (s) | 6’’’ | 63.1 | 4.34 (m) |
| 22 | 39.8 | 2.01 (m), 1.86 (m) | 3’’-O-Glc | ||
| 23 | 23.6 | 2.60 (m), 2.35 (m) | 1’’’’ | 106.4 | 5.36 (d, 7.4) |
| 24 | 126.7 | 5.36 (s) | 2’’’’ | 77.9 | 3.84 (m) |
| 25 | 130.9 | - | 3’’’’ | 79.0 | 4.03 (m) |
| 26 | 26.2 | 1.71 (s) | 4’’’’ | 72.0 | 4.19 (m) |
| 27 | 18.4 | 1.71 (s) | 5’’’’ | 78.7 | 4.35 (m) |
| 28 | 28.4 | 1.31 (s) | 6’’’’ | 63.1 | 4.34 (m) |
| 29 | 17.0 | 1.13 (s) | - | - | - |
| 30 | 17.0 | 1.04 (s) | - | - | - |
| Position | 13C | 1H | Position | 13C | 1H |
|---|---|---|---|---|---|
| 1 | 39.7 | 1.57 (m), 0.89 (m) | 3-O-Glc | ||
| 2 | 27.4 | 1.98 (m), 1.41 (m) | 1’ | 105.7 | 4.96 (d, 7.7) |
| 3 | 89.1 | 3.33 (dd, 11.7, 4.4) | 2’ | 77.0 | 4.13 (m) |
| 4 | 40.0 | - | 3’ | 78.6 | 3.84 (m) |
| 5 | 56.9 | 0.68 (m) | 4’ | 72.5 | 3.95 (m) |
| 6 | 18.9 | 1.48 (m), 1.39 (m) | 5’ | 78.6 | 3.94 (m) |
| 7 | 35.4 | 1.43 (m), 1.20 (m) | 6’ | 63.1 | 4.50 (m) |
| 8 | 40.3 | - | 20-O-Glc | ||
| 9 | 50.4 | 1.41 (m) | 1’’ | 97.2 | 5.17 (d, 7.7) |
| 10 | 37.3 | - | 2’’ | 81.7 | 4.24 (m) |
| 11 | 31.4 | 1.58 (m), 1.02 (m) | 3’’ | 79.2 | 4.36 (m) |
| 12 | 71.2 | 4.10 (m) | 4’’ | 71.8 | 4.29 (m) |
| 13 | 49.5 | 1.99 (m) | 5’’ | 78.8 | 4.28 (m) |
| 14 | 52.1 | - | 6’’ | 62.8 | 4.45 (m) |
| 15 | 31.0 | 2.13 (m), 1.42 (m) | 2’’-O-Glc | ||
| 16 | 27.2 | 2.16 (m), 1.78 (m) | 1’’’ | 105.8 | 5.64 (d, 7.7) |
| 17 | 53.1 | 2.58 (m) | 2’’’ | 78.3 | 4.28 (m) |
| 18 | 17.3 | 1.18 (s) | 3’’’ | 78.5 | 4.16 (m) |
| 19 | 16.7 | 0.79 (s) | 4’’’ | 71.9 | 4.17 (m) |
| 20 | 84.4 | - | 5’’’ | 80.2 | 4.29 (m) |
| 21 | 22.5 | 1.61 (s) | 6’’’ | 63.2 | 4.38 (m) |
| 22 | 36.6 | 2.36 (m), 1.87 (m) | 2’’’-O-Rha | ||
| 23 | 23.9 | 2.45 (m), 2.27 (m) | 1’’’’ | 102.1 | 6.56 (s) |
| 24 | 126.4 | 5.23 (t) | 2’’’’ | 72.9 | 4.70 (m) |
| 25 | 131.2 | - | 3’’’’ | 72.8 | 4.88 (m) |
| 26 | 26.1 | 1.61 (s) | 4’’’’ | 74.5 | 4.35 (m) |
| 27 | 18.2 | 1.64 (s) | 5’’’’ | 70.0 | 4.79 (m) |
| 28 | 16.2 | 0.93 (s) | 6’’’’ | 19.1 | 1.71 (d, 6.2) |
| 29 | 28.4 | 1.28 (s) | |||
| 30 | 17.8 | 1.00 (s) |
| Position | 13C | 1H | Position | 13C | 1H |
|---|---|---|---|---|---|
| 1 | 47.8 | 2.45 (m), 1.14 (m) | 3-O-Glc | ||
| 2 | 67.2 | 4.01 (m) | 1’ | 104.8 | 4.95 (d, 7.8) |
| 3 | 95.7 | 3.21 (m) | 2’ | 77.1 | 4.49 (m) |
| 4 | 41.3 | - | 3’ | 78.5 | 4.14 (m) |
| 5 | 56.4 | 0.80 (m) | 4’ | 71.7 | 4.29 (m) |
| 6 | 18.8 | 1.51 (m), 1.38 (m) | 5’ | 78.5 | 4.14 (m) |
| 7 | 35.3 | 1.46 (m), 1.22 (m) | 6’ | 63.1 | 4.44 (m) |
| 8 | 40.3 | - | 20-O-Glc | ||
| 9 | 50.4 | 1.54 (m) | 1’’ | 97.2 | 5.18 (d, 7.8) |
| 10 | 38.2 | - | 2’’ | 81.9 | 4.23 (m) |
| 11 | 31.2 | 1.56 (m), 1.02 (m) | 3’’ | 79.2 | 4.15 (m) |
| 12 | 71.0 | 4.15 (m) | 4’’ | 71.5 | 4.15 (m) |
| 13 | 49.5 | 2.00 (m) | 5’’ | 78.6 | 4.14 (m) |
| 14 | 51.9 | - | 6’’ | 62.8 | 4.29 (m) |
| 15 | 31.0 | 2.31 (m), 1.56 (m) | 2’’-O-Glc | ||
| 16 | 27.2 | 1.98 (m), 1.42 (m) | 1’’’ | 105.8 | 5.65 (d, 7.8) |
| 17 | 53.1 | 2.59 (m) | 2’’’ | 82.6 | 4.30 (1m) |
| 18 | 16.2 | 0.95 (s) | 3’’’ | 78.9 | 3.97 (m) |
| 19 | 17.7 | 0.88 (s) | 4’’’ | 71.9 | 4.29 (m) |
| 20 | 84.4 | - | 5’’’ | 78.5 | 4.14 (m) |
| 21 | 22.3 | 1.62 (s) | 6’’’ | 62.7 | 4.29 (m) |
| 22 | 36.5 | 2.38 (m), 1.89 (m) | 2’’’-O-Glc | ||
| 23 | 23.8 | 2.47 (m), 2.27 (m) | 1’’’’ | 105.9 | 5.52 (d, 7.8) |
| 24 | 126.3 | 5.24 (t, 7.0) | 2’’’’ | 77.1 | 4.49 (m) |
| 25 | 131.2 | - | 3’’’’ | 78.7 | 3.85 (m) |
| 26 | 26.1 | 1.62 (s) | 4’’’’ | 71.3 | 4.52 (m) |
| 27 | 18.2 | 1.65 (s) | 5’’’’ | 78.7 | 4.30 (m) |
| 28 | 28.6 | 1.34 (s) | 6’’’’ | 63.3 | 4.52 (m) |
| 29 | 18.0 | 1.20 (s) | |||
| 30 | 17.7 | 0.99 (s) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, M.; Zhang, J.; Wang, L.; Li, F.; Li, Z.; Xiang, W.; Bie, S.; Wang, C.; Li, Z. Ten New Dammarane-Type Saponins with Hypolipidemia Activity from a Functional Herbal Tea—Gynostemma pentaphyllum. Molecules 2020, 25, 3737. https://doi.org/10.3390/molecules25163737
Yin M, Zhang J, Wang L, Li F, Li Z, Xiang W, Bie S, Wang C, Li Z. Ten New Dammarane-Type Saponins with Hypolipidemia Activity from a Functional Herbal Tea—Gynostemma pentaphyllum. Molecules. 2020; 25(16):3737. https://doi.org/10.3390/molecules25163737
Chicago/Turabian StyleYin, Maojing, Jingjing Zhang, Lizhi Wang, Fangyi Li, Zhenfa Li, Wei Xiang, Songtao Bie, Chunhua Wang, and Zheng Li. 2020. "Ten New Dammarane-Type Saponins with Hypolipidemia Activity from a Functional Herbal Tea—Gynostemma pentaphyllum" Molecules 25, no. 16: 3737. https://doi.org/10.3390/molecules25163737
APA StyleYin, M., Zhang, J., Wang, L., Li, F., Li, Z., Xiang, W., Bie, S., Wang, C., & Li, Z. (2020). Ten New Dammarane-Type Saponins with Hypolipidemia Activity from a Functional Herbal Tea—Gynostemma pentaphyllum. Molecules, 25(16), 3737. https://doi.org/10.3390/molecules25163737