Effect of Drying Methods on Bioactive Compounds and Antioxidant Capacity in Grape Skin Residues from the New Hybrid Variety “BRS Magna”
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Composition
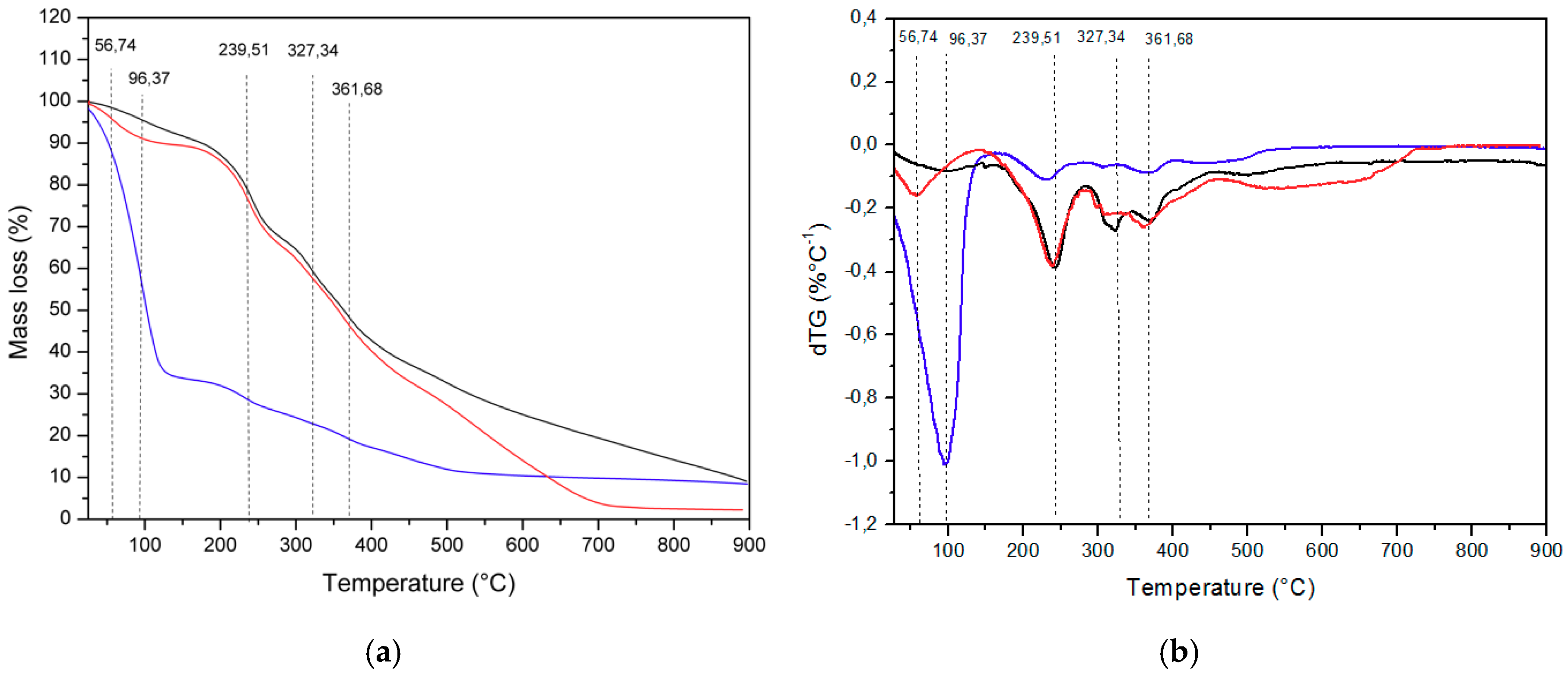
2.2. Thermogravimetric Analysis
2.3. Color Parameters
2.4. Identification and Quantification of Phenolic Compounds
2.5. Total Phenolic, Anthocyanin, and Flavonoid Contents and Antioxidant Activity
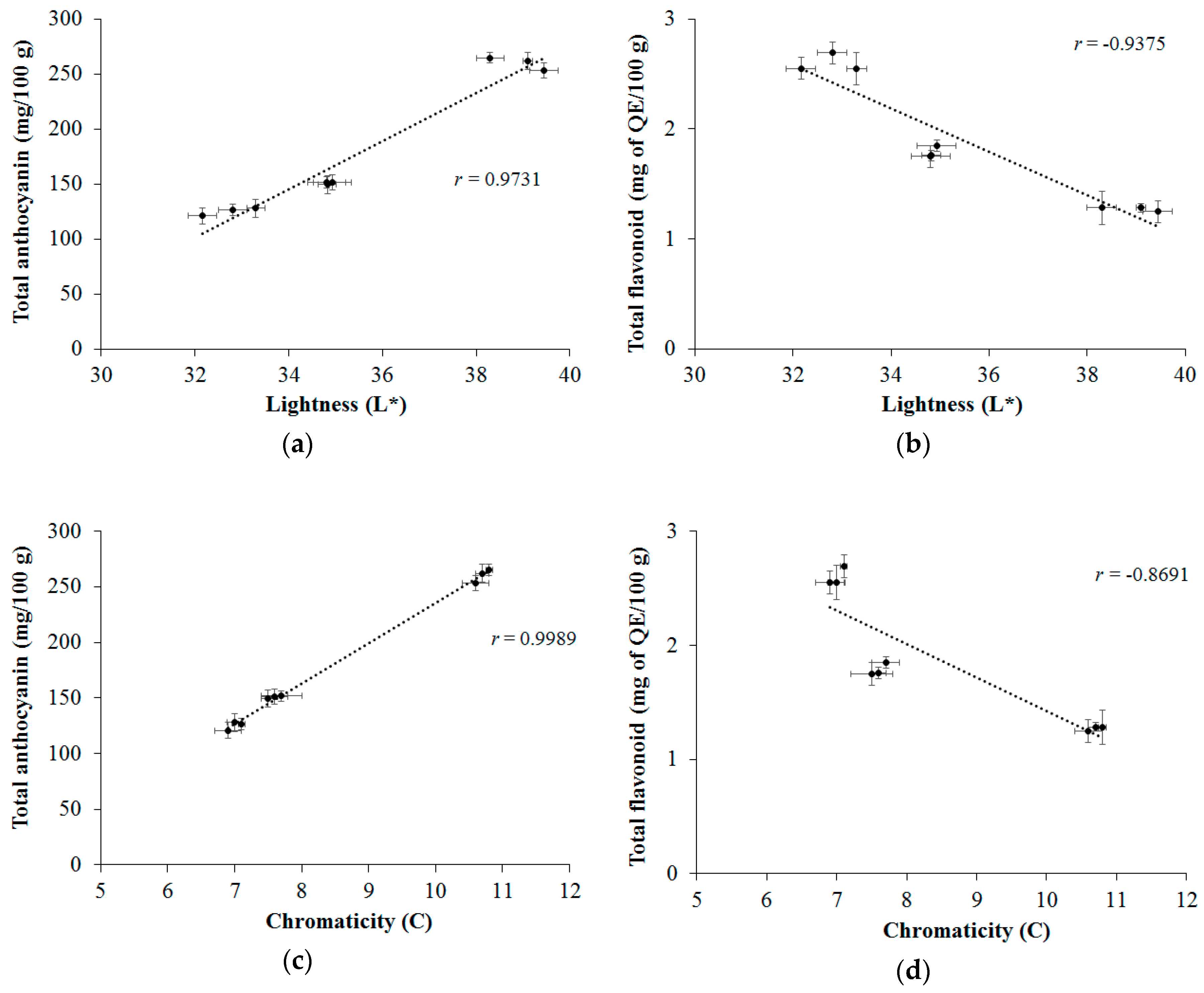
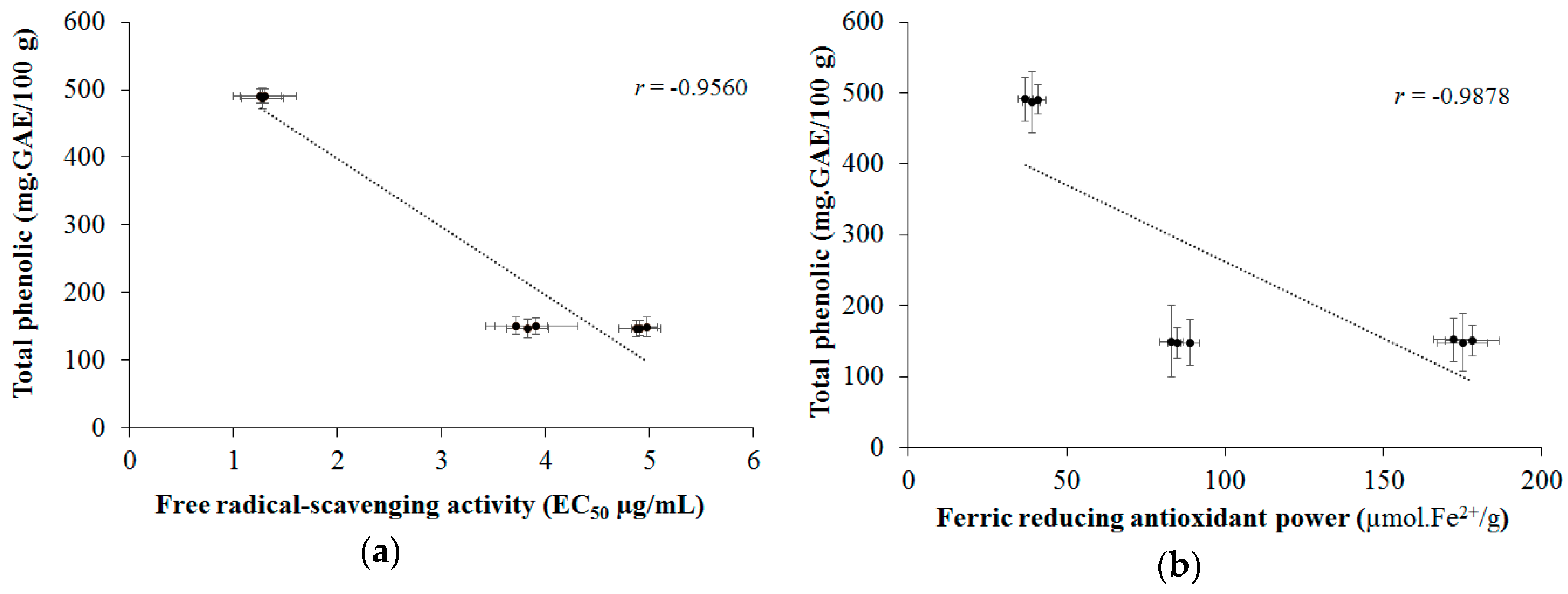
2.6. Correlation between Color Parameters and Antioxidant Activity with Phenolic Compounds
3. Materials and Methods
3.1. Materials
3.2. BRS Magna Grape Skin Residue and the Dehydration Process
3.3. Proximate Composition
3.4. Color Parameters
3.5. Individual Phenolic Compounds by HPLC-DAD-FD
3.6. Content of Phenolics, Anthocyanins and Flavonoids
3.7. Antioxidant Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Savini, F. The economy that runs on waste: Accumulation in the circular city. J. Environ. Policy Plan. 2019, 21, 675–691. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Krystosik, A.; Njoroge, G.; Odhiambo, L.; Forsyth, J.E.; Mutuku, F.; LaBeaud, A.D. Solid wastes provide breeding sites, burrows, and food for biological disease vectors, and urban zoonotic reservoirs: A call to action for solutions-based research. Front. Public Heal. 2020, 7, 405. [Google Scholar] [CrossRef]
- Amorim, F.L.; Silva, M.B.C.; Cirqueira, M.G.; Oliveira, R.S.; Machado, B.A.S.; Gomes, R.G.; Souza, C.O.; Druzian, J.I.; de Souza Ferreira, E.; Umsza-Guez, M.A. Grape peel (Syrah var.) jam as a polyphenol-enriched functional food ingredient. Food Sci. Nutr. 2019, 7, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, M.A.; Carmona, M.; Pardo, F.; Salinas, M.R.; Zalacain, A. Waste grape skins thermal dehydration: Potential release of colour, phenolic and aroma compounds into wine. CYTA J. Food. 2012, 10, 225–234. [Google Scholar] [CrossRef]
- Falcão, J.S.; Sobral, T.S.; Cruz, L.F.S.; Philadelpho, B.O.; Santos, J.E.M.; Costa, J.A.V.; Druzian, J.I.; Ferreira, E.S. Protein-enriched umbu (Spondias tuberosa) jam prepared by supplementation with Spirulina sp. LEB-18. Brazilian J. Dev. 2020, 6, 22714–22729. [Google Scholar] [CrossRef]
- Makris, D.P.; Şahin, S. Polyphenolic antioxidants from agri-food waste biomass. Antioxidants 2019, 8, 624. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Plant polyphenol–based second-generation synbiotic agents: Emerging concepts, challenges, and opportunities. Nutrition 2020, 77, 110785. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, M.A.; Carmona, M.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. Pre-bottling use of dehydrated waste grape skins to improve colour, phenolic and aroma composition of red wines. Food Chem. 2013, 136, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Tavares, I.M.C.; Castilhos, M.B.M.; Mauro, M.A.; Ramos, A.M.; Souza, R.T.; Gómez-Alonso, S.; Gomes, E.; Da-Silva, R.; Hermosín-Gutiérrez, I.; Lago-Vanzela, E.S. BRS Violeta (BRS Rúbea × IAC 1398-21) grape juice powder produced by foam mat drying. Part I: Effect of drying temperature on phenolic compounds and antioxidant activity. Food Chem. 2019, 298, 124971. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Gomes, F.S.; Cabral, L.M.C.; Tonon, R.V. Effect of temperature on the degradation of bioactive compounds of Pinot Noir grape pomace during drying. Brazilian J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Chittrakorn, S.; Weerawatanakorn, M.; Dangpium, N. Effect of drying conditions on properties, pigments and antioxidant activity retentions of pretreated orange and purple-fleshed sweet potato flours. J. Food Sci. Technol. 2016, 53, 1811–1822. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ştefănescu, B.E.; Crişan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and nutraceutical potential of bioactive compounds extracted from fruit residues. Crit. Rev. Food Sci. Nutr 2015, 55, 319–337. [Google Scholar] [CrossRef]
- Grosso, G. Effects of polyphenol-rich foods on human health. Nutrients 2018, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Padwad, Y. Perspectives of the potential implications of polyphenols in influencing the interrelationship between oxi-inflammatory stress, cellular senescence and immunosenescence during aging. Trends Food Sci. Technol. 2020, 98, 41–52. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. Food Saf. 2017, 156, 186–195. [Google Scholar] [CrossRef]
- Belović, M.; Torbica, A.; Pajić-Lijaković, I.; Mastilović, J. Development of low calorie jams with increased content of natural dietary fibre made from tomato pomace. Food Chem. 2017, 237, 1226–1233. [Google Scholar] [CrossRef]
- Gowman, A.C.; Picard, M.C.; Rodriguez-Uribe, A.; Misra, M.; Khalil, H.; Thimmanagari, M.; Mohanty, A.K. Physicochemical analysis of apple and grape pomaces. BioResources 2019, 14, 3210–3230. [Google Scholar] [CrossRef]
- Botelho, T.; Costa, M.; Wilk, M.; Magdziarz, A. Evaluation of the combustion characteristics of raw and torrefied grape pomace in a thermogravimetric analyzer and in a drop tube furnace. Fuel 2018, 212, 95–100. [Google Scholar] [CrossRef]
- Natividade, M.M.P.; Corrêa, L.C.; Souza, S.V.C.; Pereira, G.E.; Lima, L.C.O. Simultaneous analysis of 25 phenolic compounds in grape juice for HPLC: Method validation and characterization of São Francisco Valley samples. Microchem. J. 2013, 110, 665–674. [Google Scholar] [CrossRef]
- Alencar, N.M.M.; Cazarin, C.B.B.; Corrêa, L.C.; Maróstica Junior, M.R.; Biasoto, A.C.T.; Behrens, J.H. Influence of maceration time on phenolic compounds and antioxidant activity of the Syrah must and wine. J. Food Biochem. 2018, 42, e12471. [Google Scholar] [CrossRef]
- Planinic, M.; Aliakbarian, B.; Perego, P.; Greganic, K.; Tomas, S.; Bucic-Kojic, A. Influence of temperature and drying time on extraction yield of phenolic compounds from grape pomace variety “portogizac”. Chem. Biochem. Eng. Q. 2015, 29, 343–350. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Alcaraz, L.; Pérez-Ferreras, S.; León-González, M.E.; Rosales-Conrado, N.; López, F.A. Extraction of polyphenols and synthesis of new activated carbon from spent coffee grounds. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vanzela, E.S.; Da-Silva, R.; Gomes, E.; García-Romero, E.; Hermosín-Gutiérrez, I. Phenolic composition of the edible parts (flesh and skin) of bordô grape (Vitis labrusca) using HPLC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2011, 59, 13136–13146. [Google Scholar] [CrossRef]
- Lima, M.D.S.; Silani, I.D.S.V.; Toaldo, I.M.; Corrêa, L.C.; Biasoto, A.C.T.; Pereira, G.E.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Food Chem. 2014, 161, 94–103. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Martins, I.M.; Roberto, B.S.; Blumberg, J.B.; Chen, C.Y.O.; Macedo, G.A. Enzymatic biotransformation of polyphenolics increases antioxidant activity of red and white grape pomace. Food Res. Int. 2016, 89, 533–539. [Google Scholar] [CrossRef]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lees, D.; Francis, F. Standardization of pigment analyses in cranberries. HortSci. 1972, 7, 83–84. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
Sample Availability: Samples of the material are available from the authors. |
| Parameters (% Dry Weight) | Method Applied ‡ | |||
|---|---|---|---|---|
| Fresh Peel | Oven-Drying | Freeze-Drying | p-Value | |
| Protein | 7.0 ± 0.2 | 7.0 ± 0.8 | 7.2 ± 1.2 | 0.098 |
| Ash | 6.6 ± 0.2 | 6.4 ± 0.5 | 5.1 ± 0.6 | 0.058 |
| Total lipids | 2.9 ± 0.4 | 2.9 ± 0.1 | 3.3 ± 0.2 | 0.054 |
| Total Carbohydrate † | 83.5 ± 0.8 | 83.8 ± 0.6 | 84.5 ± 0.9 | 0.063 |
| Crude fiber | 35.4 ± 2.0 | 38.0 ± 3.6 | 41.6 ± 1.8 | 0.082 |
| Parameters (% Dry Weight) | Method Applied ‡ | |||
|---|---|---|---|---|
| Fresh Peel | Oven-Drying | Freeze-Drying | p-Value | |
| L* | 32.8 ± 0.6 c | 34.9 ± 0.1 b | 39.3 ± 0.2 a | <0.001 |
| C* | 7.0 ± 0.5 c | 7.6 ± 0.1 b | 10.7 ± 0.1 a | <0.001 |
| H° | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.420 |
| Phenolic Compounds (mg/kg) | Method Applied ‡ | |||
|---|---|---|---|---|
| Fresh Peel | Oven-Drying | Freeze-Drying | p-Value | |
| Anthocyanins | ||||
| Cyanidin-3,5-di-O-glucoside | 27.9 ± 4.1 a | 11.8 ± 0.1 b | 34.0 ± 3.7 a | <0.001 |
| Malvidin-3,5-di-O-glucoside | 536.9 ± 11.3 b | 253.2 ± 5.5 c | 848.3 ± 6.7 a | <0.001 |
| Pelargonidin-3-O-glucoside | 23.5 ± 1.7 a | 14.0 ± 0.6 b | 13.1 ± 1.0 b | <0.001 |
| Delfinidin-3-O-glucoside | 76.6 ± 2.9 b | 37.1 ± 0.6 c | 115.9 ± 4.0 a | <0.001 |
| Cyanidin-3-O-glucoside | 35.7 ± 5.8 b | 13.1 ± 0.1 c | 59.3 ± 6.6 a | <0.001 |
| Malvidin-3-O-glucoside | 35.7 ± 4.1 a | 16.2 ± 1.2 b | 13.5 ± 1.6 b | <0.001 |
| Peonidina-3-O-glucoside | 2.6 ± 0.1 a | 0.0 ± 0.0 c | 1.5 ± 0.2 b | <0.001 |
| Petunidin-3-O-glucoside | 45.3 ± 1.2 a | 28.4 ± 1.2 b | 25.5 ± 2.3 b | <0.001 |
| Flavonols and stilbene | ||||
| Kaempferol-3-O-glucoside | 13.1 ± 0.1 a | 7.9 ± 0.1 b | 4.1 ± 0.2 c | <0.001 |
| Rutin | 18.3 ± 0.1 a | 13.1 ± 0.1 b | 5.6 ± 0.5 c | <0.001 |
| Isorhamnetin-3-O-glucoside | 15.7 ± 1.7 a | 10.0 ± 0.6 b | 7.6 ± 0.8 b | <0.001 |
| Myricetin | 0.0 ± 0.0 c | 11.8 ± 0.1a | 4.1 ± 0.2 b | <0.001 |
| Trans-resveratrol | 7.8 ± 0.1 a | 5.2 ± 0.1 b | 1.3 ± 0.1 c | <0.001 |
| Quercetin-3-β-d-glucoside | 99.3 ± 7.0 b | 138.4 ± 3.2 a | 17.2 ± 2.1 c | <0.001 |
| Phenolic acid | ||||
| Gallic acid | 30.5 ± 2.3 a | 22.7 ± 1.5 b | 15.3 ± 2.7 c | <0.001 |
| Caftaric acid | 32.2 ± 1.2 b | 86.0 ± 0.6 a | 24.6 ± 2.6 c | <0.001 |
| Caffeic acid | 1.6 ± 0.1 b | 2.4 ± 0.1 a | 1.6 ± 0.2 b | <0.001 |
| Ferulic acid | 18.3 ± 0.1 a | 10.5 ± 0.1 b | 5.9 ± 0.2 c | <0.001 |
| Chlorogenic acid | 20.1 ± 1.2 a | 20.0 ± 0.6 a | 7.8 ± 0.8 b | <0.001 |
| p-Coumaric acid | 33.1 ± 4.6 a | 30.6 ± 1.5 a | 15.5 ± 1.4 b | <0.001 |
| Flavanols | ||||
| (–)-Epicatechin | 17.4 ± 1.2 b | 22.7 ± 1.5 a | 4.3 ± 0.4 c | <0.001 |
| (–)-Epicatechin gallate | 68.8 ± 4.6 a | 48.5 ± 1.7 b | 35.8 ± 3.1 c | <0.001 |
| (–)-Epigalatocatechin gallate | 68.8 ± 6.4 a | 44.5 ± 0.9 b | 22.7 ± 0.5 c | <0.001 |
| (+)-Catechin | 30.5 ± 2.3 a | 26.6 ± 0.6 ab | 24.6 ± 2.7 b | 0.034 |
| Procyanidins | ||||
| Procyanidin A2 | 20.9 ± 0.1 a | 15.7 ± 0.1 b | 5.9 ± 0.2 c | <0.001 |
| Procyanidin B1 | 36.6 ± 5.2 b | 62.9 ± 2.6 a | 13.8 ± 2.0 c | <0.001 |
| Procyanidin B2 | 20.0 ± 2.3 b | 77.7 ± 4.9 a | 20.0 ± 2.6 b | <0.001 |
| Total compounds | 1337.2 ± 71.1 a | 1031.0 ± 29.2 b | 1351.5 ± 49.2 a | <0.001 |
| Parameters (% Dry Weight) | Method Applied ‡ | |||
|---|---|---|---|---|
| Fresh Peel | Oven-Drying | Freeze-Drying | p-Value | |
| Total phenolic (mg.GAE/100 g) | 489.5 ± 1.8 a | 149.9 ± 1.4 b | 148.3 ± 0.9 b | <0.001 |
| Total anthocyanin (mg.CE/100 g) | 124.9 ± 2.7 c | 150.7 ± 0.9 b | 260.1 ± 4.6 a | <0.001 |
| Total flavonoids (mg.QE/100 g) | 2.6 ± 0.1 a | 1.8 ± 0.1 b | 1.3 ± 0.1 c | <0.001 |
| DPPH (EC50 in μg/mL) | 1.3 ± 0.2 c | 3.8 ± 0.1 b | 5.0 ± 0.1 a | <0.001 |
| FRAP (μmol.Fe2+/g) | 184.1 ± 8.9 a | 163.5 ± 10.9 a | 82.9 ± 9.3 b | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.V.d.; Machado, B.A.S.; Oliveira, W.P.d.; Silva, C.F.G.d.; Quadros, C.P.d.; Druzian, J.I.; Ferreira, E.d.S.; Umsza-Guez, M.A. Effect of Drying Methods on Bioactive Compounds and Antioxidant Capacity in Grape Skin Residues from the New Hybrid Variety “BRS Magna”. Molecules 2020, 25, 3701. https://doi.org/10.3390/molecules25163701
Silva GVd, Machado BAS, Oliveira WPd, Silva CFGd, Quadros CPd, Druzian JI, Ferreira EdS, Umsza-Guez MA. Effect of Drying Methods on Bioactive Compounds and Antioxidant Capacity in Grape Skin Residues from the New Hybrid Variety “BRS Magna”. Molecules. 2020; 25(16):3701. https://doi.org/10.3390/molecules25163701
Chicago/Turabian StyleSilva, Gabriela Viana da, Bruna Aparecida Souza Machado, Walkia Polliana de Oliveira, Camilla Fernanda Godinho da Silva, Cedenir Pereira de Quadros, Janice Izabel Druzian, Ederlan de Souza Ferreira, and Marcelo Andrés Umsza-Guez. 2020. "Effect of Drying Methods on Bioactive Compounds and Antioxidant Capacity in Grape Skin Residues from the New Hybrid Variety “BRS Magna”" Molecules 25, no. 16: 3701. https://doi.org/10.3390/molecules25163701
APA StyleSilva, G. V. d., Machado, B. A. S., Oliveira, W. P. d., Silva, C. F. G. d., Quadros, C. P. d., Druzian, J. I., Ferreira, E. d. S., & Umsza-Guez, M. A. (2020). Effect of Drying Methods on Bioactive Compounds and Antioxidant Capacity in Grape Skin Residues from the New Hybrid Variety “BRS Magna”. Molecules, 25(16), 3701. https://doi.org/10.3390/molecules25163701









