Towards a Valorization of Corn Bioethanol Side Streams: Chemical Characterization of Post Fermentation Corn Oil and Thin Stillage
Abstract
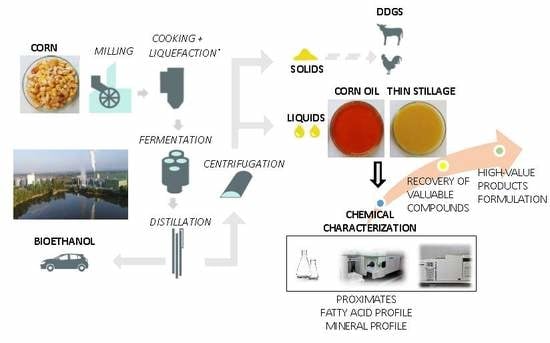
1. Introduction
2. Results and Discussion
2.1. Corn Oil
2.2. Thin Stillage
3. Materials and Methods
3.1. Collection of Side-Stream Samples
3.2. Treatment of Samples
3.3. Analytical Methods
3.3.1. Daily Routine Analyses
3.3.2. Proximate Composition
3.3.3. Minerals and Trace Elements
3.3.4. Total Lipids and Fatty Acids
3.4. Data Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. Consolidated version of the Treaty on the Functioning of the European Union. Official Journal of the European Union, 26 October 2012; C326/47. [Google Scholar]
- European Economic and Social Committee. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee, the Committee of the Regions and the European Investment Bank a Framework Strategy for a Resilient Energy Union with a Forward-Looking Climate Change Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52015DC0080 (accessed on 23 July 2020).
- European Economic and Social Committee. Communication from the Commission to the European Parliament and the Council Achieving the 10% Electricity Interconnection Target Making Europe’s Electricity Grid Fit for 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2015%3A82%3AFIN (accessed on 23 July 2020).
- European Commission. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. Official Journal of the European Union, 6 June 2009; L140/16. [Google Scholar]
- European Commission. Directive 2009/30/EC of The European Parliament and of the Council of 23 April 2009 amending Directive 98/70/EC as regards the specification of petrol, diesel and gas-oil and introducing a mechanism to monitor and reduce greenhouse gas emissions and amending Council Directive 1999/32/EC as regards the specification of fuel used by inland waterway vessels and repealing Directive 93/12/EEC, 2009. Official Journal of the European Union, 5 June 2009; L144/88. [Google Scholar]
- European Commission. Directive (EU) 2015/1513 of the European Parliament and of the Council of 9 September 2015 amending Directive 98/70/EC relating to the quality of petrol and diesel fuels and amending Directive 2009/28/EC on the promotion of the use of energy from renewable sources. Official Journal of the European Union, 15 September 2015; L239/1. [Google Scholar]
- European Commission. Renewable Energy Progress Report. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- ePURE, European Renewable Ethanol. Statistics and Infographics 2018. Share of European Renewable Ethanol Produced from Each Feedstock Type. Available online: https:/epure.org/media/1930/share-of-european-renewable-ethanol-produced-from-each-feedstock-type-2018.jpg (accessed on 30 July 2020).
- ePURE, European Renewable Ethanol. Statistics and Infographics 2018. European Renewable Ethanol Installed Production Capacity. Available online: https://epure.org/media/1925/european-renewable-ethanol-installed-production-capacity-2018.jpg (accessed on 30 July 2020).
- European Commission. A Sustainable Bioeconomy for Europe. Strengthening the Connection between Economy, Society and the Environment. Updated Bioeconomy Strategy 2018; Directorate-General for Research and Innovation: Brussels, Belgium, 2018. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Kim, Y.; Mosier, N.; Hendrickson, R.; Ezeji, T.; Blaschek, H.; Dien, B.; Dale, B.; Ladisch, M. Composition of corn dry-grind ethanol by-products: DDGS, wet cake, and thin stillage. Bioresour. Technol. 2008, 99, 5165–5176. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.R.; Rajendran, A.; Hu, B. New technologies in value addition to the thin stillage from corn-to-ethanol process. Rev. Environ. Sci. Biotechnol. 2017, 16, 175–206. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Production of ethanol and biomass from thin stillage by Neurospora intermedia: A pilot study for process diversification. Eng. Life Sci. 2015, 15, 751–759. [Google Scholar] [CrossRef]
- Pietrzak, W.; Kawa-Rygielska, J. Backset valorization in dry-grind ethanol process by co-culture of edible filamentous fungi and fodder yeast. J. Clean. Prod. 2019, 220, 376–385. [Google Scholar] [CrossRef]
- Mitra, D.; van Leeuwen, J.; Lamsal, B. Heterotrophic/mixotrophic cultivation of oleaginous Chlorella vulgaris on industrial co-products. Algal Res. 2012, 1, 40–48. [Google Scholar] [CrossRef]
- Beigbeder, J.B.; Boboescu, J.Z.; Lavoie, J.M. Thin stillage treatment and co-production of bio-commodities through finely tuned Chlorella vulgaris cultivation. J. Clean. Prod. 2019, 216, 257–267. [Google Scholar] [CrossRef]
- Dowd, M.K.; Reilly, P.J.; Trahanovsky, W.S. Low molecular weight organic composition of ethanol stillage from corn. Cereal Chem. 1993, 70, 204–209. [Google Scholar]
- Moreau, R.A.; Hicks, K.B.; Johnston, D.B.; Laun, N.P. The Composition of Crude Corn Oil Recovered after Fermentation via Centrifugation from a Commercial Dry Grind Ethanol Process. J. Am. Oil Chem. Soc. 2010, 87, 895–902. [Google Scholar] [CrossRef]
- Moreau, R.A.; Liu, K.; Winkler-Moser, J.K.; Singh, V. Changes in lipid composition during dry grind ethanol processing of corn. J. Am. Oil Chem. Soc. 2011, 88, 435–442. [Google Scholar] [CrossRef]
- Ratanapariyanuch, K.; Shen, J.; Jia, Y.; Tyler, R.T.; Shim, Y.Y.; Reaney, M.J.T. Rapid NMR Method for the Quantification of Organic Compounds in Thin Stillage. J. Agric. Food Chem. 2011, 59, 10454–10460. [Google Scholar] [CrossRef] [PubMed]
- Winkler-Moser, J.K.; Breyer, L. Composition and oxidative stability of crude oil extracts of corn germ and distillers grains. Ind. Crops Prod. 2011, 33, 572–578. [Google Scholar] [CrossRef]
- Wood, C.; Rosentrater, K.A.; Muthukumarappan, K.; Gu, Z. Quantification of physical and chemical properties and identification of potentially valuable components from fuel ethanol process streams. Cereal Chem. 2013, 90, 70–79. [Google Scholar] [CrossRef]
- Banga, S.; Varshney, P. Effect of impurities on performance of biodiesel: A review. J. Sci. Ind. Res. 2010, 69, 575–579. [Google Scholar]
- Kai, T.; Kubo, A.; Nakazato, T.; Takanashi, H.; Uemura, Y. Biomass and renewables influence of the acid value on biodiesel fuel production using a two-step batch process with a homogeneous catalyst. Int. J. Biomass Renew. 2012, 1, 15–20. [Google Scholar]
- Gerpen, J.H.; He, B.B. Biodiesel and Renewable Diesel Production Methods. In Advances in Biorefineries: Biomass and Waste Supply Chain Exploitation; Waldron, K., Ed.; Woodhead Publishing, Elsevier: Cambridge, UK, 2014. [Google Scholar]
- Alves, B.; Carvalho, F.; Cruz, A.; Filho, H.; Dantas, K. Determination of Ca, Mg, Na, and K in Biodiesel of Oilseed from Northern Brazil. Rev. Virtual Quím. 2018, 10, 542–550. [Google Scholar] [CrossRef]
- CREA. Italian Food Composition Tables; Research Centre for Food and Nutrition: Rome, Italy; Available online: https://www.alimentinutrizione.it/tabelle-nutrizionali/009660 (accessed on 25 July 2020).
- Barrera-Arellano, D.; Badan-Ribeiro, A.P.; Serna-Saldivar, S.O. Chapter 21—Corn Oil: Composition, Processing, and Utilization. In Corn: Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Duxford, UK, 2019; pp. 593–613. ISBN 9780128118863. [Google Scholar]
- EN ISO 12937/2000 Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method.
- EN 12662/2014 Liquid Petroleum Products—Determination of total contamination in middle distillates, diesel fuels and fatty acid methyl esters.
- EN 14104/2003 Fat and oil derivatives. Fatty acid methyl esters (FAME). Determination of acid value.
- EN 14107/2003 Fat and oil derivatives. Fatty acid methyl esters (FAME). Determination of phosphorous content by inductively coupled plasma (ICP) emission spectrometry.
- Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1999. [Google Scholar]
- Bligh, E.G.; and Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, L.D.; Schmitz, A.A. The rapid preparation of fatty acid esters for gas chromatographic analysis. Anal. Chem. 1961, 33, 363–364. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Lot 1 | Lot 2 | Lot 3 | Lot 4 | Lot 5 | Lot 6 | Lot 7 | Lot 8 | Lot 9 | mean | sd | min | max | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water content (g/100 g) | 0.51 ± 0.07 | 0.41 ± 0.07 | 0.45 ± 0.08 | 0.44 ± 0.07 | 0.44 ± 0.08 | 0.34 ± 0.02 | 0.36 ± 0.03 | 0.46 ± 0.23 | 0.43 ± 0.09 | 0.43 | 0.05 | 0.34 | 0.51 |
| Sedimentation (vol %) | 5.88 ± 2.09 | 7.29 ± 3.96 | 13.88 ± 4.64 | 8.8 ± 0.82 | 7.14 ± 1.89 | 6.50 ± 2.51 | 9.20 ± 2.09 | 11.8 ± 3.24 | 13.0 ± 2.53 | 9.28 | 2.95 | 5.88 | 13.88 |
| Total contamination (g/100 g) | 0.01 ± 0.01 | 0.06 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.28 ± 0.03 | 0.19 ± 0.04 | 0.01 ± 0.01 | 0.06 | 0.10 | 0.01 | 0.28 |
| Acid value (mg KOH/g) | 21.02 ± 1.26 | 24.29 ±1.51 | 21.33 ± 1.91 | 19.72 ± 0.78 | 21.79 ± 1.72 | 20.90 ± 0.79 | 21.9 ± 1.95 | 22.86 ± 0.77 | 20.75 ± 1.21 | 21.62 | 1.33 | 19.72 | 24.29 |
| Minerals: | |||||||||||||
| P (mg/kg) | 7.5 ± 1.6 | 6.18 ± 1.08 | 18.81 ± 11.34 | 9.78 ± 5.77 | 34.45 ± 9.44 | 10.6 ± 2.2 | 9.75 ± 3.65 | 3.52 ± 1.28 | 10.87 ± 2.72 | 12.38 | 4.34 | 3.52 | 34.45 |
| K (mg/kg) | 3.27 ± 1.06 | 1.61 ± 0.40 | 11.83 ± 7.42 | 7.10 ± 2.94 | 6.33 ± 3.64 | <1 | 7.40 ± 3.58 | 1.66 ± 0.41 | 15.53 ± 6.97 | - | - | <1 | 15.53 |
| Na (mg/kg) | 0.57 ± 0.31 | 1.99 ± 1.20 | <1 | <1 | 2.57 ± 1.66 | 2.78 ± 1.66 | 2.94 ± 1.41 | 1.02 ± 0.42 | 4.74 ± 2.53 | - | - | 0.57 | 4.74 |
| Mg (mg/kg) | 0.84 ± 0.34 | <1 | <1 | <1 | 1.63 ± 0..47 | <1 | 1.36 ± 0.87 | <1 | 1.37 ±1.17 | - | - | 0.84 | 1.63 |
| Ca (mg/kg) | 0.05 ± 0.03 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | - | - | 0.05 | <1 |
| Lot 1 | Lot 2 | Lot 3 | Lot 4 | Lot 5 | Lot 6 | Lot 7 | Lot 8 | mean | sd | min | max | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of total fatty acids | ||||||||||||
| Lauric acid (C12:0) | 0.00 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 | 0.01 | 0.00 | 0.02 |
| Myristic acid (C14:0) | 0.00 ± 0.00 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.03 ± 0.01 | 0.08 ± 0.03 | 0.12 ± 0.03 | 0.12 ± 0.02 | 0.00 ± 0.00 | 0.07 | 0.04 | 0.03 | 0.12 |
| Pentadecylic acid (C15:0) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ±.0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.09 ± 0.04 | 0.01 | 0.03 | 0.00 | 0.09 |
| Palmitic acid (C16:0) | 12.11 ± 0.30 | 11.56 ± 0.34 | 15.11 ± 1.10 | 14.27 ± 2.13 | 15.38 ± 0.57 | 16.31 ± 0.48 | 17.33 ± 1.85 | 13.39 ± 1.03 | 14.43 | 2.00 | 11.56 | 17.33 |
| Palmitoleic acid (C16:1 n-7) | 0.00 ± 0.00 | 0.08 ± 0.00 | 0.05 ± 0.02 | 0.05 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.06 | 0.02 | 0.05 | 0.08 |
| Margaric acid (C17:0) | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.05 | 0.01 | 0.04 | 0.05 |
| Stearic acid (C18:0) | 1.40 ± 0.08 | 1.47 ± 0.09 | 1.91 ± 0.34 | 1.84 ± 0.66 | 1.61 ± 0.05 | 1.17 ± 0.10 | 1.06 ± 0.71 | 1.41 ± 0.03 | 1.48 | 0.29 | 1.06 | 1.91 |
| Oleic acid (C18:1 n-9) | 26.44 ± 1.78 | 25.99 ± 1.80 | 23.44 ± 0.43 | 23.02 ± 0.78 | 23.13 ± 0.72 | 25.20 ± 1.32 | 25.37 ± 2.15 | 26.85 ± 1.07 | 24.93 | 1.53 | 23.02 | 26.85 |
| Linoleic acid (C18:2 n-6) | 57.99 ± 1.40 | 58.63 ± 1.41 | 57.30 ± 0.75 | 57.06 ± 2.51 | 57.70 ± 0.29 | 55.50 ± 0.81 | 54.12 ± 0.48 | 56.96 ± 0.27 | 56.91 | 1.45 | 54.12 | 58.63 |
| α-Linolenic acid (C18:3 n-3) | 1.78 ± 0.30 | 1.78 ± 0.31 | 1.78 ± 0.38 | 2.68 ± 1.63 | 1.98 ± 0.10 | 1.64 ± 0.09 | 1.70 ± 0.05 | 1.73 ± 0.07 | 1.88 | 0.34 | 1.64 | 2.68 |
| Arachidic acid (C20:0) | 0.18 ± 0.05 | 0.16 ± 0.07 | 0.07 ± 0.04 | 0.00 ± 0.00 | 0.11 ± 0.02 | 0.00 ± 0.00 | 0.34 ± 0.08 | 0.27 ± 0.10 | 0.19 | 0.10 | 0.07 | 0.34 |
| Gondoic acid (C20:1 n-9) | 0.29 ± 0.03 | 0.21 ± 0.06 | 0.27 ± 0.02 | 0.00 ± 0.00 | 0.18 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.24 | 0.05 | 0.18 | 0.29 |
| Eicosadienoic acid (C20:2 n-6) | - | - | - | - | - | - | - | - | - | - | - | - |
| Behenic acid (C22:0) | - | - | - | - | - | - | - | - | - | - | - | - |
| Total SFA | 13.73 ± 0.30 | 13.27 ± 0.36 | 17.18 ± 1.10 | 16.14 ± 1.64 | 17.13 ± 0.61 | 17.67 ± 0.49 | 18.81 ± 2.51 | 15.14 ± 1.06 | 16.13 | 1.95 | 13.27 | 18.81 |
| Total MUFA | 26.73 ± 1.70 | 26.26 ± 1.80 | 23.67 ± 0.53 | 23.04 ± 0.75 | 23.19 ± 0.69 | 25.20 ± 1.32 | 25.37 ± 2.15 | 26.85 ± 1.07 | 25.04 | 1.56 | 23.04 | 26.85 |
| Total PUFA | 59.77 ± 1.60 | 60.40 ± 1.66 | 59.08 ± 0.64 | 59.74 ± 0.88 | 59.67 ± 0.23 | 57.14 ± 0.90 | 55.82 ± 0.53 | 58.12 ± 1.08 | 58.72 | 1.57 | 55.82 | 60.40 |
| Total n-6 PUFA | 57.99 ± 0.50 | 58.63 ± 1.41 | 57.30 ± 0.75 | 57.06 ± 2.51 | 57.70 ± 0.29 | 55.50 ± 0.81 | 54.14 ± 0.48 | 56.96 ± 0.27 | 56.91 | 1.45 | 54.12 | 58.63 |
| Total n-3 PUFA | 1.78 ± 0.40 | 1.78 ± 0.31 | 1.78 ± 0.38 | 2.68 ± 1.63 | 1.98 ± 0.10 | 1.64 ± 0.09 | 1.70 ± 0.05 | 1.73 ± 0.07 | 1.88 | 0.34 | 1.64 | 2.68 |
| n-6/n-3 PUFA ratio | 32.58 ± 1.50 | 33.50 ± 4.79 | 33.17 ± 6.89 | 26.54 ± 13.00 | 29.26 ± 1.58 | 33.85 ± 1.39 | 31.81 ± 0.72 | 32.96 ± 1.02 | 31.71 | 2.53 | 26.54 | 33.85 |
| Lot 1 | Lot 2 | Lot 3 | Lot 4 | Lot 5 | Lot 6 | Lot 7 | Lot 8 | Lot 9 | mean | sd | min | max | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | - | 4.06 ± 0.11 | 4.48 ± 0.01 | 4.22 ± 0.02 | 4.61 ± 0.07 | 4.68 ± 0.04 | 4.48 ± 0.02 | 4.05 ± 0.01 | 4.49 ± 0.02 | 4.38 | 0.24 | 4.05 | 4.68 |
| g 100 g−1 wet mass | |||||||||||||
| Dry matter | 7.69 ± 0.05 | 7.99 ± 0.05 | 7.86 ± 0.01 | 8.44 ± 0.01 | 6.81 ± 0.07 | 7.96 ± 0.02 | 8.74 ± 0.07 | 9.93 ± 0.08 | 8.57 ± 0.03 | 8.22 | 0.86 | 6.81 | 9.93 |
| Water content | 92.32 ± 0.02 | 92.01 ± 0.05 | 92.14 ± 0.01 | 91.56 ± 0.01 | 93.19 ± 0.07 | 92.04 ± 0.02 | 91.26 ± 0.07 | 90.07 ± 0.08 | 91.43 ± 0.03 | 91.78 | 0.86 | 90.07 | 93.19 |
| Total N | 0.22 ± 0.01 | 0.23 ± 0.03 | 0.24 ± 0.01 | 0.32 ± 0.02 | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.32 ± 0.00 | 0.26 ± 0.01 | 0.27 | 0.03 | 0.22 | 0.32 |
| Nonprotein N | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.13 ± 0.00 | 0.13 ± 0.01 | 0.10 ± 0.00 | 0.13 ± 0.00 | 0.12 ± 0.00 | 0.12 | 0.01 | 0.10 | 0.15 |
| True Protein | 0.68 ± 0.09 | 0.71 ± 0.16 | 0.78 ± 0.01 | 1.07 ± 0.13 | 0.87 ± 0.08 | 0.83 ± 0.08 | 1.03 ± 0.02 | 1.19 ± 0.02 | 0.91 ± 0.03 | 0.90 | 0.17 | 0.68 | 1.19 |
| Ash | 0.73 ± 0.05 | 0.69 ± 0.02 | 0.71 ± 0.01 | 0.75 ± 0.02 | 0.78 ± 0.01 | 0.81 ± 0.01 | 0.73 ± 0.03 | 0.77 ± 0.02 | 0.69 ± 0.02 | 0.74 | 0.04 | 0.69 | 0.81 |
| Crude fat | 2.33 ± 0.02 | 1.52 ± 0.06 | 1.83 ± 0.02 | 1.81 ± 0.01 | 1.46 ± 0.05 | 1.64 ± 0.02 | 1.67 ± 0.12 | - | - | 1.75 | 0.29 | 1.46 | 2.33 |
| Total dietary fiber | 0.65 ± 0.08 | 0.83 ± 0.10 | 0.95 ± 0.06 | 1.06 ± 0.01 | 1.21 ± 0.45 | 1.31 ± 0.54 | 2.14 ± 0.08 | 2.03 ± 0.06 | - | 1.27 | 0.54 | 0.65 | 2.14 |
| Other solubles ** | 3.29 ± 0.13 | 4.24 ± 0.29 | 3.57 ± 0.11 | 3.82 ± 0.03 | 2.51 ± 0.50 | 3.43 ± 0.60 | 3.14 ± 0.24 | - | - | 3.43 | 0.54 | 2.51 | 4.24 |
| g 100 g−1 dry mass | |||||||||||||
| Dry matter | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |||
| Total N | 2.87 ± 0.11 | 2.88 ± 0.34 | 3.10 ± 0.08 | 3.79 ± 0.21 | 3.96 ± 0.14 | 3.35 ± 0.07 | 3.03 ± 0.10 | 3.22 ± 0.03 | 3.07 ± 0.08 | 3.25 | 0.39 | 2.87 | 3.96 |
| Nonprotein N | 1.45 ± 0.10 | 1.47 ± 0.12 | 1.50 ± 0.09 | 1.77 ± 0.07 | 1.92 ± 0.05 | 1.68 ± 0.11 | 1.15 ± 0.05 | 1.30 ± 0.04 | 1.38 ± 0.02 | 1.51 | 0.24 | 1.15 | 1.92 |
| True Protein | 8.85 ± 1.22 | 8.83 ± 2.05 | 9.99 ± 0.19 | 12.67 ± 1.58 | 12.76 ± 1.12 | 10.46 ± 1.06 | 11.79 ± 0.28 | 12.03 ± 0.30 | 10.59 ± 0.40 | 10.88 | 1.51 | 8.83 | 12.76 |
| Ash | 9.54 ± 0.63 | 8.64 ± 0.26 | 9.04 ± 0.12 | 8.89 ± 0.20 | 11.40 ± 0.11 | 10.22 ± 0.08 | 8.39 ± 0.41 | 7.75 ± 0.26 | 8.05 ± 0.21 | 9.10 | 1.14 | 7.75 | 11.40 |
| Crude fat | 30.31 ± 0.14 | 19.03 ± 0.80 | 23.33 ± 0.29 | 21.40 ± 0.05 | 21.44 ± 0.61 | 20.57 ± 0.27 | 19.07 ± 2.01 | - | - | 22.18 | 3.88 | 19.03 | 30.31 |
| Total dietary fiber | 8.41 ± 0.97 | 10.43 ± 1.30 | 12.09 ± 1.50 | 12.56 ± 1.80 | 17.69 ± 6.72 | 16.40 ± 6.78 | 24.43 ± 2.00 | 20.44 ± 1.50 | - | 15.31 | 5.42 | 8.41 | 24.43 |
| Other solubles ** | 42.84 ± 1.67 | 53.07 ± 3.51 | 45.42 ± 1.44 | 45.20 ± 0.25 | 36.86 ± 7.00 | 43.05 ± 7.71 | 35.94 ± 2.56 | - | - | 43.20 | 5.77 | 35.94 | 53.07 |
| Lot 1 | Lot 2 | Lot 3 | Lot 4 | Lot 5 | Lot 6 | Lot 7 | Lot 8 | Lot 9 | mean | sd | min | max | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| content per 100 g wet weight | |||||||||||||
| K (mg) | 151.69 ± 2.51 | 171.55 ± 2.05 | 172.78 ± 0.57 | 198.70 ± 3.83 | 189.29± 5.15 | 177.84 ± 7.1 | 196.48 ± 8.15 | 179.68 ± 1.29 | 172.20 ± 5.50 | 178.91 | 14.52 | 151.69 | 198.70 |
| P (mg) | 108.78 ± 1.51 | 111.38 ± 4.1 | 110.27 ± 1.87 | 135.10 ± 2.64 | 126.25 ± 2.0 | 125.23 ± 1.9 | 129.17 ± 5.84 | 126.99 ± 8.64 | 116.71 ± 4.52 | 121.10 | 9.50 | 108.78 | 135.10 |
| Na (mg) | 47.10 ± 0.52 | 43.10 ± 0.82 | 39.71 ±1.53 | 36.52 ± 0.43 | 60.49 ± 1.1 | 66.70 ± 1.66 | 73.66 ± 3.58 | 69.74 ± 4.73 | 47.22 ± 1.59 | 53.81 | 13.966 | 36.52 | 73.66 |
| Mg (mg) | 41.02 ± 0.25 | 44.55 ± 0.52 | 41.07 ± 3.79 | 51.42 ± 1.02 | 54.04 ± 0.96 | 51.63 ± 1.47 | 52.30 ±2.61 | 48.34 ± 3.66 | 50.43 ± 1.47 | 48.31 | 4.92 | 41.02 | 54.04 |
| Ca (mg) | 4.72 ± 0.03 | 5.19 ± 0.26 | 4.82 ± 0.46 | 5.71 ± 0.08 | 6.08 ± 0.11 | 5.53 ± 0.09 | 6.50 ± 0.46 | 7.08 ± 0.55 | 6.04 ± 0.22 | 5.74 | 0.78 | 4.72 | 7.08 |
| Fe (mg) | 0.66 ± 0.02 | 0.71 ± 0.02 | 0.63 ± 0.01 | 0.82 ± 0.02 | 0.81 ± 0.02 | 0.90 ± 0.03 | 0.85 ± 0.06 | 0.87 ± 0.07 | 0.58 ± 0.02 | 0.76 | 0.12 | 0.58 | 0.90 |
| Zn (mg) | 0.59 ± 0.02 | 0.77 ± 0.03 | 0.62 ± 0.02 | 0.75 ± 0.02 | 0.71 ± 0.01 | 0.66 ± 0.02 | 0.75 ± 0.04 | 0.77 ± 0.05 | 0.66 ± 0.03 | 0.70 | 0.07 | 0.59 | 0.77 |
| Mn (mg) | 0.22 ± 0.01 | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.24 ± 0.01 | 0.23 ± 0.02 | 0.20 ± 0.01 | 0.22 | 0.02 | 0.19 | 0.24 |
| Cu (mg) | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.03 | 0.01 | 0.02 | 0.04 |
| content per 100 g dry weight | |||||||||||||
| K (g) | 1.97 ± 0.03 | 2.15 ± 0.03 | 2.20 ± 0.09 | 2.35 ± 0.05 | 2.78 ± 0.08 | 2.23± 0.09 | 2.25 ± 0.09 | 1.81 ± 0.13 | 2.01 ± 0.06 | 2.19 | 0.27 | 1.81 | 2.78 |
| P (g) | 1.41 ± 0.02 | 1.39 ± 0.05 | 1.40 ± 0.02 | 1.60 ± 0.03 | 1.85 ± 0.03 | 1.57 ± 0.02 | 1.48 ± 0.07 | 1.28 ± 0.08 | 1.36 ± 0.05 | 1.48 | 0.17 | 1.28 | 1.85 |
| Na (g) | 0.61 ± 0.01 | 0.54 ± 0.01 | 0.51 ± 0.02 | 0.43 ± 0.01 | 0.89± 0.02 | 0.84 ± 0.02 | 0.84 ± 0.04 | 0.70 ± 0.05 | 0.55± 0.02 | 0.66 | 0.17 | 0.43 | 0.89 |
| Mg (g) | 0.53 ± 0.00 | 0.56 ± 0.01 | 0.52 ± 0.05 | 0.61 ± 0.01 | 0.79 ± 0.01 | 0.65± 0.02 | 0.60 ± 0.03 | 0.49 ± 0.04 | 0.59 ± 0.02 | 0.59 | 0.09 | 0.49 | 0.79 |
| Ca (mg) | 61.32 ± 0.40 | 64.95 ± 3.21 | 61.32 ± 5.81 | 67.64 ± 0.94 | 89.21 ± 1.54 | 69.46 ± 1.16 | 74.40 ± 5.24 | 71.31 ± 5.50 | 70.50 ± 2.57 | 70.01 | 8.46 | 61.32 | 89.21 |
| Fe (mg) | 8.63 ± 0.21 | 8.84 ± 0.22 | 7.96 ± 0.15 | 9.75 ± 0.24 | 11.92 ± 0.28 | 11.26 ± 0.37 | 9.67 ± 0.68 | 8.74 ± 0.71 | 6.76 ± 0.24 | 9.28 | 1.59 | 6.76 | 11.92 |
| Zn (mg) | 7.72 ± 0.21 | 9.61 ± 0.34 | 7.95 ± 0.22 | 8.83 ± 0.19 | 10.41 ± 0.16 | 8.27 ± 0.30 | 8.62 ± 0.42 | 7.77 ± 0.49 | 7.69 ± 0.30 | 8.54 | 0.94 | 7.69 | 10.41 |
| Mn (mg) | 2.80 ± 0.04 | 2.56 ± 0.09 | 2.48 ± 0.11 | 2.61 ± 0.06 | 3.41 ± 0.07 | 2.80 ± 0.05 | 2.71 ± 0.13 | 2.36 ± 0.18 | 2.36 ± 0.08 | 2.68 | 0.32 | 2.36 | 3.41 |
| Cu (mg) | 0.26 ± 0.01 | 0.37 ± 0.02 | 0.35 ± 0.01 | 0.37 ± 0.01 | 0.36 ± 0.02 | 0.36 ± 0.01 | 0.29 ± 0.02 | 0.32 ± 0.02 | 0.51 ± 0.02 | 0.36 | 0.07 | 0.26 | 0.51 |
| Lot 1 | Lot 2 | Lot 3 | Lot 4 | Lot 5 | Lot 6 | Lot 7 | mean | sd | min | max | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % of total fatty acids | |||||||||||
| Lauric acid (C12:0) | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.00 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.03 | 0.00 ± 0.00 | 0.02 | 0.02 | 0.00 | 0.06 |
| Myristic acid (C14:0) | 0.08 ± 0.02 | 0.06 ± 0.02 | 0.09 ± 0.00 | 0.06 ± 0.02 | 0.12 ± 0.02 | 0.15 ± 0.04 | 0.12 ± 0.02 | 0.10 | 0.03 | 0.06 | 0.15 |
| Pentadecylic acid (C15:0) | 0.00 ± 0.00 | 0.03 ± 0.01 | 0.02 ±. 000 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 | 0.01 | 0.00 | 0.03 |
| Palmitic acid (C16:0) | 23.29 ± 0.30 | 23.00 ± 0.37 | 22.12 ± 0.70 | 24.10 ± 0.47 | 23.28 ± 0.38 | 22.17 ± 2.39 | 21.40 ± 0.66 | 22.76 | 0.91 | 21.40 | 24.10 |
| Palmitoleic acid (C16:1 n-7) | 0.00 ± 0.00 | 0.09 ± 0.03 | 0.06 ± 0.01 | 0.05 ± 0.00 | 0.07 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.04 | 0.04 | 0.00 | 0.09 |
| Margaric acid (C17:0) | 0.00 ± 0.00 | 0.07 ± 0.00 | 0.10 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 | 0.05 | 0.00 | 0.10 |
| Stearic acid (C18:0) | 3.31 ± 0.07 | 3.16 ± 0.08 | 3.32 ± 0.03 | 3.48 ± 0.41 | 2.49 ± 0.06 | 1.37 ± 0.19 | 1.77 ± 0.14 | 2.70 | 0.85 | 1.37 | 3.48 |
| Oleic acid (C18:1 n-9) | 21.08 ± 0.50 | 21.35 ± 0.54 | 21.20 ± 0.67 | 20.36 ± 1.10 | 21.13 ± 0.17 | 24.79 ± 1.42 | 23.73 ± 0.47 | 21.95 | 1.64 | 20.36 | 24.79 |
| Linoleic acid (C18:2 n-6) | 49.14 ± 0.30 | 49.79 ± 0.32 | 50.76 ± 0.17 | 49.48 ± 1.07 | 50.55 ± 0.29 | 49.35 ± 1.32 | 50.95 ± 0.05 | 50.00 | 0.74 | 49.14 | 50.95 |
| α-Linolenic acid (C18:3 n-3) | 1.75 ± 0.05 | 1.82 ± 0.05 | 1.63 ± 0.08 | 1.92 ± 0.15 | 1.65 ± 0.04 | 1.40 ± 0.11 | 1.75 ± 0.05 | 1.70 | 0.17 | 1.40 | 1.92 |
| Arachidic acid (C20:0) | 0.16 ± 0.02 | 0.32 ± 0.02 | 0.34 ± 0.02 | 0.25 ± 0.07 | 0.30 ± 0.03 | 0.26 ± 0.04 | 0.29 ± 0.05 | 0.27 | 0.06 | 0.16 | 0.34 |
| Gondoic acid (C20:1 n-9) | 0.39 ± 0.04 | 0.18 ± 0.11 | 0.28 ± 0.07 | 0.00 ± 0.00 | 0.26 ± 0.03 | 0.41 ± 0.04 | 0.00 ± 0.00 | 0.25 | 0.15 | 0.00 | 0.41 |
| Eicosadienoic acid (C20:2 n-6) | 0.00 ± 0.00 | 0.07 ± 0.01 | 0.09 ± 0.03 | 0.29 ± 0.08 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.08 | 0.11 | 0.00 | 0.29 |
| Behenic acid (C22:0) | 0.00 ± 0.00 | 0.13 ± 0.01 | 0.13 ± 0.00 | 0.00 ± 0.00 | 0.14 ± 0.02 | 0.06 ± 0.04 | 0.00 ± 0.00 | 0.09 | 0.06 | 0.00 | 0.14 |
| Total SFA | 26.84 ± 0.50 | 26.69 ± 0.52 | 26.03 ± 0.70 | 27.90 ± 0.90 | 26.35 ± 0.45 | 24.06 ± 2.66 | 23.57 ± 0.46 | 25.92 | 1.56 | 23.57 | 27.90 |
| Total MUFA | 21.47 ± 0.40 | 21.63 ± 0.43 | 21.53 ± 0.60 | 20.41 ± 1.10 | 21.46 ± 0.17 | 25.19 ± 1.38 | 23.73 ± 0.47 | 22.20 | 1.65 | 20.41 | 25.19 |
| Total PUFA | 50.89 ± 0.30 | 51.69 ± 0.28 | 52.48 ± 0.26 | 51.69 ± 1.11 | 52.20 ± 0.32 | 50.75 ± 1.43 | 52.70 ± 0.02 | 51.77 | 0.75 | 50.75 | 52.70 |
| Total n-6 PUFA | 49.14 ± 0.30 | 49.87 ± 0.32 | 50.85 ± 0.18 | 49.77 ± 1.11 | 50.55 ± 0.29 | 49.35 ± 1.32 | 50.95 ± 0.05 | 50.07 | 0.72 | 49.14 | 50.95 |
| Total n-3 PUFA | 1.75 ± 0.05 | 1.82 ± 0.05 | 1.63 ± 0.08 | 1.92 ± 0.15 | 1.65 ± 0.04 | 1.40 ± 0.11 | 1.75 ± 0.05 | 1.70 | 0.17 | 1.40 | 1.92 |
| n-6/n-3 PUFA ratio | 28.08 ± 0.70 | 27.43 ± 0.88 | 31.29 ± 1.42 | 26.01 ± 2.26 | 30.61 ± 0.69 | 35.35 ± 1.84 | 29.07 ± 0.82 | 29.69 | 3.08 | 26.01 | 35.35 |
| PUFA/SFA ratio | 1.90 ± 0.05 | 1.94 ± 0.05 | 2.02 ± 0.06 | 1.85 ± 0.09 | 1.98 ± 0.05 | 2.13 ± 0.31 | 2.24 ± 0.04 | 2.01 | 0.14 | 1.85 | 2.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lena, G.; Ondrejíčková, P.; Pulgar, J.S.d.; Cyprichová, V.; Ježovič, T.; Lucarini, M.; Lombardi Boccia, G.; Ferrari Nicoli, S.; Gabrielli, P.; Aguzzi, A.; et al. Towards a Valorization of Corn Bioethanol Side Streams: Chemical Characterization of Post Fermentation Corn Oil and Thin Stillage. Molecules 2020, 25, 3549. https://doi.org/10.3390/molecules25153549
Di Lena G, Ondrejíčková P, Pulgar JSd, Cyprichová V, Ježovič T, Lucarini M, Lombardi Boccia G, Ferrari Nicoli S, Gabrielli P, Aguzzi A, et al. Towards a Valorization of Corn Bioethanol Side Streams: Chemical Characterization of Post Fermentation Corn Oil and Thin Stillage. Molecules. 2020; 25(15):3549. https://doi.org/10.3390/molecules25153549
Chicago/Turabian StyleDi Lena, Gabriella, Petra Ondrejíčková, Josè Sanchez del Pulgar, Veronika Cyprichová, Tomáš Ježovič, Massimo Lucarini, Ginevra Lombardi Boccia, Stefano Ferrari Nicoli, Paolo Gabrielli, Altero Aguzzi, and et al. 2020. "Towards a Valorization of Corn Bioethanol Side Streams: Chemical Characterization of Post Fermentation Corn Oil and Thin Stillage" Molecules 25, no. 15: 3549. https://doi.org/10.3390/molecules25153549
APA StyleDi Lena, G., Ondrejíčková, P., Pulgar, J. S. d., Cyprichová, V., Ježovič, T., Lucarini, M., Lombardi Boccia, G., Ferrari Nicoli, S., Gabrielli, P., Aguzzi, A., Casini, I., & Caproni, R. (2020). Towards a Valorization of Corn Bioethanol Side Streams: Chemical Characterization of Post Fermentation Corn Oil and Thin Stillage. Molecules, 25(15), 3549. https://doi.org/10.3390/molecules25153549