C7-Prenylation of Tryptophan-Containing Cyclic Dipeptides by 7-Dimethylallyl Tryptophan Synthase Significantly Increases the Anticancer and Antimicrobial Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Prenylation of Tryptophan-Containing Cyclic Dipeptides by 7-DMATS
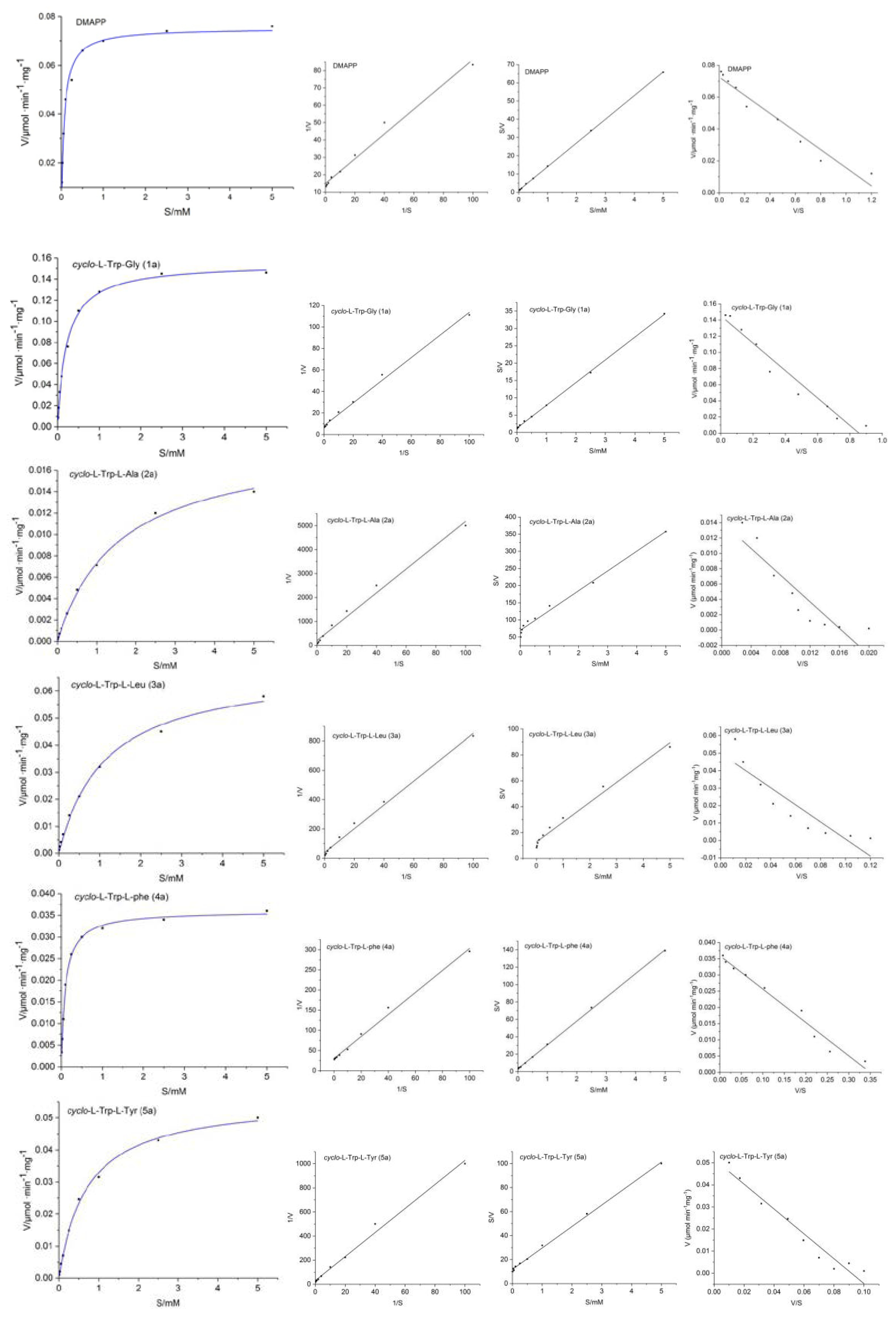
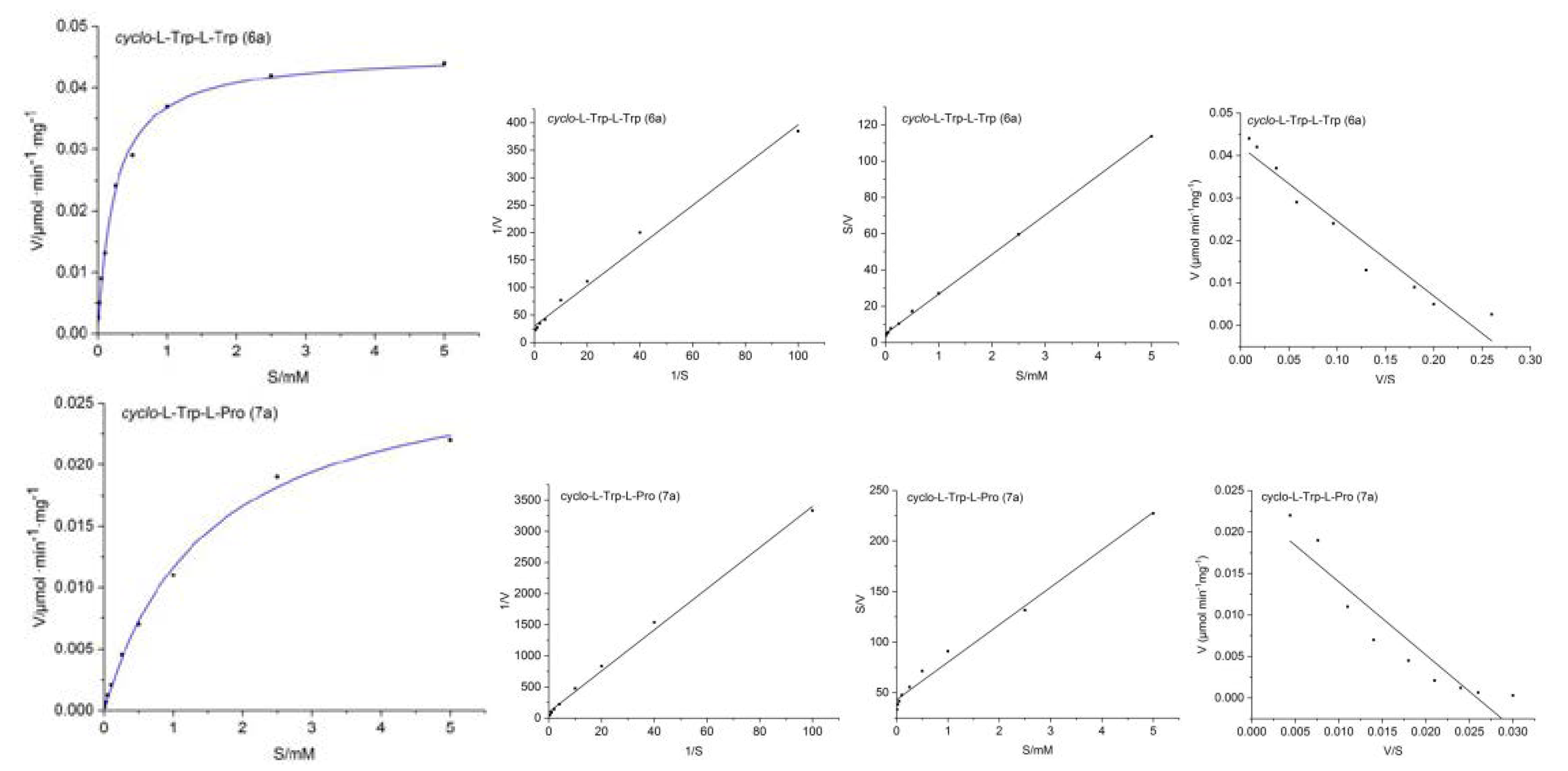
2.2. Kinetic Parameters of 7-DMATS
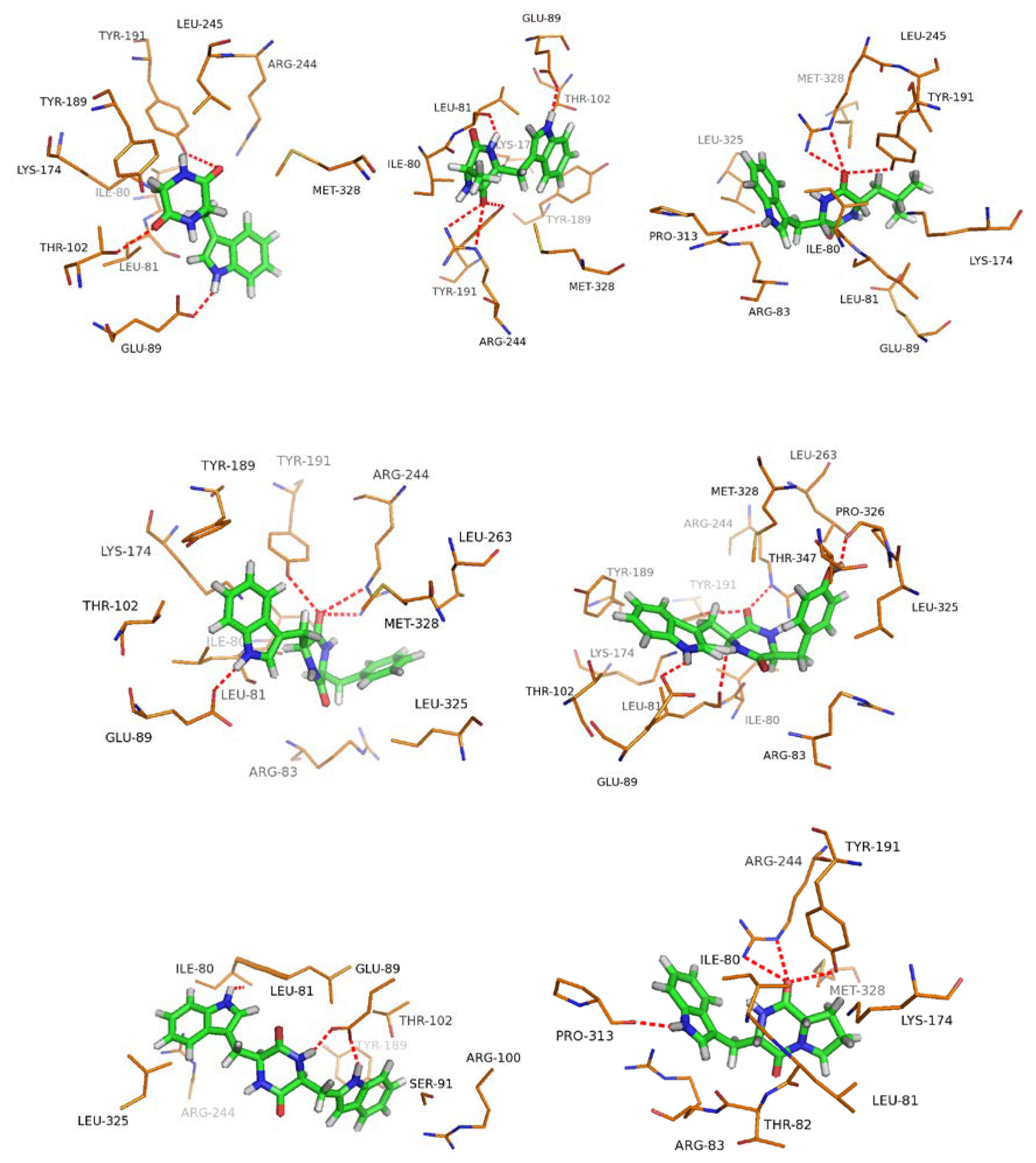
2.3. Docking with 1a–7a
2.4. Anticancer Activity
2.5. Antibacterial Activity
2.6. Antifungal Activity
2.7. Optimization of Enzyme-Catalyzed Reaction Conditions
3. Materials and Methods
3.1. General
3.2. Pathogen Microbial and Cell Lines
3.3. Overproduction and Purification of 7-DMATS as Well as Conditions for Enzymatic Reactions
3.4. HPLC Conditions for Analysis and Isolation of the Enzyme Products
3.5. Spectroscopic Analysis
3.6. Determination of the Kinetic Parameters
3.7. Molecular Docking
3.8. Anticancer Assay
3.9. Antibacterial Assay
3.10. Antifungal Assay
3.11. Optimize Enzymatic Reactions Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Tezgel, Ö.; Noinville, S.; Bennevault, V.; Illy, N.; Guégan, P. An alternative approach to create N-substituted cyclic dipeptides. Polym. Chem. 2019, 10, 776–785. [Google Scholar] [CrossRef]
- Haynes, S.W.; Gao, X.; Tang, Y.; Walsh, C.T. Complexity generation in fungal peptidyl alkaloid biosynthesis: A two-enzyme pathway to the hexacyclic MDR export pump inhibitor ardeemin. ACS Chem. Biol. 2013, 8, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010, 27, 57–78. [Google Scholar] [CrossRef]
- Li, S.M. Genome mining and biosynthesis of fumitremorgin-type alkaloids in ascomycetes. J. Antibio. 2010, 64, 45–49. [Google Scholar] [CrossRef][Green Version]
- Jain, H.D.; Zhang, C.; Zhou, S.; Zhou, H.; Ma, J.; Liu, X.; Liao, X.; Deveau, A.M.; Dieckhaus, C.M.; Johnson, M.A.; et al. Synthesis and structure-activity relationship studies on tryprostatin A, a potent inhibitor of breast cancer resistance protein. Bioorg. Med. Chem. 2008, 16, 4626–4651. [Google Scholar] [CrossRef][Green Version]
- Wollinsky, B.; Ludwig, L.; Hamacher, A.; Yu, X.; Kassack, M.U.; Li, S.M. Prenylation at the indole ring leads to a significant increase of cytotoxicity of tryptophan-containing cyclic dipeptides. Bioorg. Med. Chem. Lett. 2012, 22, 3866–3869. [Google Scholar] [CrossRef]
- Dalponte, L.; Parajuli, A.; Younger, E.; Mattila, A.; Jokela, J.; Wahlsten, M.; Leikoski, N.; Sivonen, K.; Jarmusch, S.A.; Houssen, W.E.; et al. N-Prenylation of Tryptophan by an Aromatic Prenyltransferase from the Cyanobactin Biosynthetic Pathway. Biochemistry 2018, 57, 6860–6867. [Google Scholar]
- Peng, J.X.; Gao, H.Q.; Li, J.; Ai, J.; Geng, M.Y.; Zhang, G.J.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Prenylated indole diketopiperazines from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2014, 79, 7895–7904. [Google Scholar] [CrossRef]
- Zou, X.W.; Li, Y.; Zhang, X.N.; Li, Q.; Liu, X.; Huang, Y.; Tang, T.; Zheng, S.J.; Wang, W.M.; Tang, J.T. A new prenylated indole diketopiperazine alkaloid from Eurotium cristatum. Molecules 2014, 19, 17839–17847. [Google Scholar] [CrossRef]
- Chen, X.Q.; Si, L.L.; Liu, D.; Proksch, P.; Zhang, L.H.; Zhou, D.M.; Lin, W.H. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015, 93, 82–195. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanchis, P.; Savina, S.A.; Albericio, F.; Alvarez, M. Structure, bioactivity and synthesis of natural products with hexahydropyrrolo [2–b] indole. Chem-Eur. J. 2011, 17, 1388–1408. [Google Scholar] [CrossRef] [PubMed]
- Haarmann, T.; Rolke, Y.; Giesbert, S.; Tudzynski, P. Ergot: From Witchcraft to Biotechnology. Mol. Plant. Pathol. 2009, 10, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Shiomi, S.; Ishikawa, H. Bioinspired Indole Prenylation Reactions in Water. J. Nat. Prod. 2017, 80, 2371–2378. [Google Scholar] [CrossRef]
- Cui, C.B.; Kakeya, H.; Okada, G.; Onose, R.; Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus Fumigatus. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 1996, 49, 527–533. [Google Scholar] [CrossRef]
- Zhang, P.P.; Jia, C.X.; Deng, Y.L.; Chen, S.H.; Chen, B.; Yan, S.J.; Li, J.; Liu, L. Anti-inflammatory prenylbenzaldehyde derivatives isolated from Eurotium cristatum. Phytochemistry 2019, 158, 120–125. [Google Scholar] [CrossRef]
- Huisman, M.; Rahaman, M.; Asad, S.; Oehm, S.; Novin, S.; Rheingold, A.L.; Hossain, M.M. Total Synthesis of Tryprostatin B: Synthesis and Asymmetric Phase-Transfer-Catalyzed Reaction of Prenylated Gramine Salt. Org. Lett. 2019, 21, 134–137. [Google Scholar] [CrossRef]
- Schkeryantz, J.M.; Woo, J.C.G.; Siliphaivanh, P.; Depew, K.M.; Danishefsky, S.J. Total synthesis of gypsetin, deoxybrevianamide E, brevianamide E, and tryprostatin B: Novel constructions of 2,3-disubstituted indoles. J. Am. Chem. Soc. 1999, 121, 11964–11975. [Google Scholar] [CrossRef]
- Yamakawa, T.; Ideue, E.; Shimokawa, J.; Fukuyama, T. Total synthesis of tryprostatins A and B. Angew. Chem. Int. Ed. Engl. 2010, 49, 9262–9265. [Google Scholar] [CrossRef]
- Zhao, S.; Smith, K.S.; Deveau, A.M.; Dieckhaus, C.M.; Johnson, M.A.; Macdonald, T.L.; Cook, J.M. Biological activity of the tryprostatins and their diastereomers on human carcinoma cell lines. J. Med. Chem. 2002, 45, 1559–1562. [Google Scholar]
- Zhao, L.; May, J.P.; Huang, J.; Perrin, D.M. Stereoselective synthesis of brevianamide E. Org. Lett. 2012, 14, 90–93. [Google Scholar] [PubMed]
- De Bruijn, W.J.C.; Levisson, M.; Beekwilder, J.; van Berkel, W.J.H.; Vincken, J.P. Plant Aromatic Prenyltransferases: Tools for Microbial Cell Factories. Trends Biotechnol. 2020, 38, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Zhang, L.H.; Awakawa, T.; Hoshino, S.; Okada, M.; Morita, H.; Abe, I. Manipulation of prenylation reactions by structure-based engineering of bacterial indolactam prenyltransferases. Nat. Commun. 2016, 7, 10849. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, S.M. Prenyltransferases of the dimethylallyltryptophan synthase superfamily. In Natural Product Biosynthesis by Microorganisms and Plants; Part, B., Hopwood, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 516, pp. 259–278. [Google Scholar]
- Winkelblech, J.; Li, S.M. Biochemical investigations of two 6-DMATS enzymes from Streptomyces reveal new features of L -tryptophan prenyltransferases. ChemBioChem 2014, 15, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.E. Mechanistic studies on the indole prenyltransferases. Nat. Prod. Rep. 2015, 32, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gavia, D.J.; Tang, Y. Biosynthesis of fungal indole alkaloids. Nat. Prod. Rep. 2014, 31, 1474–1487. [Google Scholar] [CrossRef]
- Winkelblech, J.; Fan, A.L.; Li, S.M. Prenyltransferases as key enzymes in primary and secondary metabolism. Appl. Microbiol. Biotechnol. 2015, 99, 7379–7397. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ishikawa, F.; Nakamura, S.; Hayashi, Y.; Nakanishi, I.; Kakeya, H. A 7-dimethylallyl tryptophan synthase from a fungal Neosartorya sp.: Biochemical characterization and structural insight into the regioselective prenylation. Bioorg. Med. Chem. 2014, 22, 2517–2528. [Google Scholar] [CrossRef]
- Bonitz, T.; Alva, V.; Saleh, O.; Lupas, A.N.; Heide, L. Evolutionary relationships of microbial aromatic prenyltransferases. PLoS ONE 2011, 6, e27336. [Google Scholar] [CrossRef]
- Pockrandt, D.; Sack, C.; Kosiol, T.; Li, S.M. A promiscuous prenyltransferase from Aspergillus oryzae catalyses C-prenylations of hydroxynaphthalenes in the presence of different prenyl donors. Appl. Microbiol. Biotechnol. 2014, 98, 4987–4994. [Google Scholar] [CrossRef]
- Zheng, L.J.; Mai, P.; Fan, A.L.; Li, S.M. Switching a regular tryptophan C4-prenyltransferase to a reverse tryptophancontaining cyclic dipeptide C3-prenyltransferase by sequential site-directed mutagenesis. Org. Biomol. Chem. 2018, 16, 6688–6694. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Okada, M.; Nakashima, Y.; Tian, T.; Abe, I. A Tryptophan Prenyltransferase with Broad Substrate Tolerance from Bacillus subtilis subsp. Natto. Chembiochem. 2018, 19, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Tarcz, S.; Ludwig, L.; Li, S.M. AstPT catalyses both reverse N1- and regular C2-prenylation of a methylated bisindolyl benzoquinone. Chembiochem 2014, 15, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Nagia, M.; Gaid, M.; Biedermann, E.; Fiesel, T.; El-Awaad, I.; Hänsch, R.; Wittstock, U.; Beerhues, L. Sequential regiospecific gem-diprenylation of tetrahydroxyxanthone by prenyltransferases from Hypericum sp. New Phytol. 2019, 222, 318–334. [Google Scholar] [CrossRef]
- Bandari, C.; Scull, E.M.; Masterson, J.M.; Tran, R.H.Q.; Foster, S.B.; Nicholas, K.M.; Singh, S. Determination of alkyl-donor promiscuity of tyrosine-Oprenyltransferase SirD from Leptosphaeria maculans. ChemBioChem 2017, 23, 2323–2327. [Google Scholar] [CrossRef]
- Mai, P.; Coby, L.; Li, S.M. Different behaviors of cyclic dipeptide prenyltransferases toward the tripeptide derivative ardeemin fumiquinazoline and its enantiomer. Appl. Microbiol. Biotechnol. 2019, 103, 3773–3781. [Google Scholar] [CrossRef]
- Zhou, K.; Wunsch, C.; Dai, J.G.; Li, S.M. gem-Diprenylation of Acylphloroglucinols by a Fungal Prenyltransferase of the Dimethylallyltryptophan Synthase Superfamily. Org. Lett. 2017, 19, 388–391. [Google Scholar] [CrossRef]
- Grundmann, A.; Li, S.M. Overproduction, Purification and Characterization of FtmPT1, a Brevianamide F Prenyltransferase From Aspergillus Fumigatus. Microbiology 2005, 151, 2199–2207. [Google Scholar] [CrossRef]
- Mundt, K.; Li, S.M. CdpC2PT, a reverse prenyltransferase from Neosartorya fischeri with distinct substrate preference from known C2-prenyltransferases. Microbiology 2013, 159, 2169–2179. [Google Scholar] [CrossRef]
- Yin, W.B.; Xie, X.L.; Matuscheka, M.; Li, S.M. Reconstruction of pyrrolo [2–b] indoles carrying an α-configured reverse C3-dimethylallyl moiety by using recombinant enzymes. Org. Biomol. Chem. 2010, 8, 1133–1141. [Google Scholar] [CrossRef]
- Unsöld, I.A.; Li, S.M. Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 2005, 151, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, Y.; Xie, X.; Zheng, X.D.; Li, S.M. Biochemical characterization of indole prenyltransferases: Filling the lastgapofprenylation positions by a 5-dimethylallyltryptophan synthase from Aspergillus clavatus. J. Biol. Chem. 2012, 287, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Winkelblech, J.; Liebhold, M.; Gunera, J.; Xie, X.L.; Kolb, P.; Li, S.M. Tryptophan C5-, C6- and C7-prenylating enzymes displaying a preference for C-6 of the indole ring in the presence of unnatural dimethylallyl diphosphate analogues. Adv. Synth. Catal. 2015, 357, 975–986. [Google Scholar] [CrossRef]
- Wunsch, C.; Zou, H.X.; Linne, U.; Li, S.M. C7-prenylation of tryptophanyl and O-prenylation of tyrosyl residues in dipeptides by an Aspergillus terreus prenyltransferase. Appl. Microbiol. Biotechnol. 2015, 99, 1719–1730. [Google Scholar] [CrossRef]
- Zou, H.X.; Xie, X.L.; Linne, U.; Zheng, X.D.; Li, S.M. Simultaneous C7- and N1-prenylation of cyclo-L-Trp-L-Trp catalyzed by a prenyltransferase from Aspergillus oryzae. Org. Biomol. Chem. 2010, 8, 3037–3044. [Google Scholar] [CrossRef]
- Kremer, A.; Li, S.M. Potential of a 7-dimethylallyltryptophan synthase as a tool for production of prenylated indole derivatives. Appl. Microbiol. Biotechnol. 2008, 79, 951–961. [Google Scholar] [CrossRef]
- Yin, S.; Yu, X.; Wang, Q.; Liu, X.Q.; Li, S.M. Identification of a brevianamide F reverse prenyltransferase BrePT from Aspergillus versicolor with a broad substrate specificity towards tryptophancontaining cyclic dipeptides. Appl. Microbiol Biotechnol. 2013, 97, 1649–1660. [Google Scholar] [CrossRef]
- Schuller, J.M.; Zocher, G.; Liebhold, M.; Xie, X.; Stahl, M.; Li, S.M.; Stehle, T. Structure and catalytic mechanism of a cyclic dipeptide prenyltransferase with broad substrate promiscuity. J. Mol. Biol. 2012, 422, 87–99. [Google Scholar] [CrossRef]
- Yin, W.B.; Yu, X.; Xie, X.L.; Li, S.M. Preparation of pyrrolo [2–b] indoles carrying a β-configured reverse C3-dimethylallyl moiety by using a recombinant prenyltransferase CdpC3PT. Org. Biomol. Chem. 2010, 8, 2430–2438. [Google Scholar] [CrossRef]
- Steffan, N.; Grundmann, A.; Yin, W.B.; Kreme, A.; Li, S.M. Indole prenyltransferases from fungi: A new enzyme group with high potential for the production of prenylated indole derivatives. Curr. Med. Chem. 2009, 16, 218–231. [Google Scholar] [CrossRef]
- Fan, A.L.; Li, S.M. Prenylation of tyrosine and derivatives by a tryptophan C7-prenyltransferase. Tetrahedron Lett. 2014, 55, 5199–5202. [Google Scholar] [CrossRef]
- Mai, P.; Zocher, G.; Stehle, T.; Li, S.M. Structure-based protein engineering enables prenyl donor switching of a fungal aromatic prenyltransferase. Org. Biomol. Chem. 2018, 16, 7461–7469. [Google Scholar] [CrossRef] [PubMed]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.H.; Chong, L.L.; Lee, M.; Lee, T.S.; Duan, Y.; Wang, W.; et al. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Sugiyama, A.; Purqon, A.; Nagao, H.; Nishikawa, K. Binding free energy calculation and structural analysis for antigen-antibody complex. AIP Conf. Proc. 2006, 832, 566–569. [Google Scholar]
- Wang, R.S.; Chen, R.D.; Li, J.H.; Liu, X.; Xie, K.B.; Chen, D.W.; Yin, Y.Z.; Tao, X.Y.; Xie, D.; Zou, J.H.; et al. Molecular characterization and phylogenetic analysis of two novel regio-specific flavonoid prenyltransferases from Morus alba and Cudrania tricuspidata. J. Biol. Chem. 2014, 289, 35815–35825. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Jiang, W.B.; Xia, Y.Y.; Wang, X.M.; Shen, G.A.; Pang, Y.Z. Genistein-specific G6DT gene for the inducible production of wighteone in Lotus Japonicus. Plant Cell Physiol. 2018, 59, 128–141. [Google Scholar] [CrossRef]
- Yoneyama, K.; Akashi, T.; Aoki, T. Molecular characterization of soybean pterocarpan 2-dimethylallyltransferase in glyceollin biosynthesis: Local gene and whole-genome duplications of prenyltransferase genes led to the structural diversity of soybean prenylated isoflavonoids. Plant Cell Physiol. 2016, 57, 2497–2509. [Google Scholar] [CrossRef]
- Shen, G.A.; Huhman, D.; Lei, Z.T.; Snyder, J.; Sumner, L.W.; Dixon, R.A. Characterization of an Isoflavonoid-Specific Prenyltransferase from Lupinus albus. Plant Physiol. 2012, 159, 70–80. [Google Scholar] [CrossRef]
- He, J.B.; Dong, Z.Y.; Hu, Z.M.; Kuang, Y.; Fan, J.R.; Qiao, X.; Ye, M. Regio-specific prenylation of pterocarpans by a membrane-bound prenyltransferase from Psoralea corylifolia. Org. Biomol. Chem. 2018, 16, 6760–6766. [Google Scholar] [CrossRef]
- Woodside, A.B.; Huang, Z.; Poulter, C.D. Trisammonium geranyl diphosphate. Org. Synth. 1988, 66, 211–215. [Google Scholar]
- Jeedigunta, S.; Krenisky, J.M.; Kerr, R.G. Diketopiperazines as advanced intermediates in the biosynthesis of ecteinascidins. Tetrahedron 2000, 56, 3303–3307. [Google Scholar] [CrossRef]
- Yu, X.; Zocher, G.; Xie, X.; Liebhold, M.; Schütz, S.; Stehle, T.; Li, S.M. Catalytic mechanism of stereospecific formation of cis-configured prenylated pyrroloindoline diketopiperazines by indole prenyltransferases. Chem. Biol. 2013, 20, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.; Westrich, L.; Li, S.M. A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: Overproduction, purification and biochemical characterization. Microbiology 2007, 153, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Raasakka, A.; Myllykoski, M.; Laulumaa, S.; Lehtimäki, M.; Härtlein, M.; Moulin, M.; Kursula, I.; Kursula, P. Determinants of ligand binding and catalytic activity in the myelin enzyme 2′, 3′-cyclic nucleotide 3′-phosphodiesterase. Sci. Rep. UK 2015, 5, 16520. [Google Scholar] [CrossRef] [PubMed]
- Eisenthal, R.; Danson, M.J.; Hough, D.W. Catalytic efficiency and Kcat/Km: A useful comparator? Trends Biotechnol. 2007, 25, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Metzger, U.; Schall, C.; Zocher, G.; Unsold, I.; Stec, E.; Li, S.M.; Heide, L.; Stehle, T.; Demain, A.L. The structure of dimethylallyl tryptophan synthase reveals a common architecture of aromatic prenyltransferases in fungi and bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 14309. [Google Scholar] [CrossRef]
- Ren, W.; Truong, T.M.; Ai, H.W. Study of the binding energies between unnatural amino acids and engineered orthogonal tyrosyltrna synthetases. Sci. Rep. UK 2015, 5, 12632. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.Y.; Yao, X.J.; Li, D.; Xu, L.; Li, Y.Y.; Tian, S.; Hou, T.J. Comprehensive evaluation of ten docking programs on a diverse set of protein-ligand complexes: The prediction accuracy of sampling power and scoring power. Phys. Chem. Chem. Phys. 2016, 18, 12964–12975. [Google Scholar] [CrossRef]
- Liu, J.Y.; Pang, Y.; Chen, J.; Huang, P.; Huang, W.; Zhu, X.Y.; Yan, D.Y. Hyperbranched polydiselenide as a selfassembling broad spectrum anticancer agent. Biomaterials 2012, 33, 7765–7774. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards (NCCLS). Performance Standards for Antimicrobial Susceptibility Testing; Document M100–S12; NCCLS: Wayne, PA, USA, 2002. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Brothdilution Antifungal Susceptibility Testing of Filamentous Fungi; As the Documentis M38-A2; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Brothdilution Antifungal Susceptibility Testing of Yeasts; As the Document is M27-S4; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
Sample Availability: Samples of the all compounds are available from the authors. |
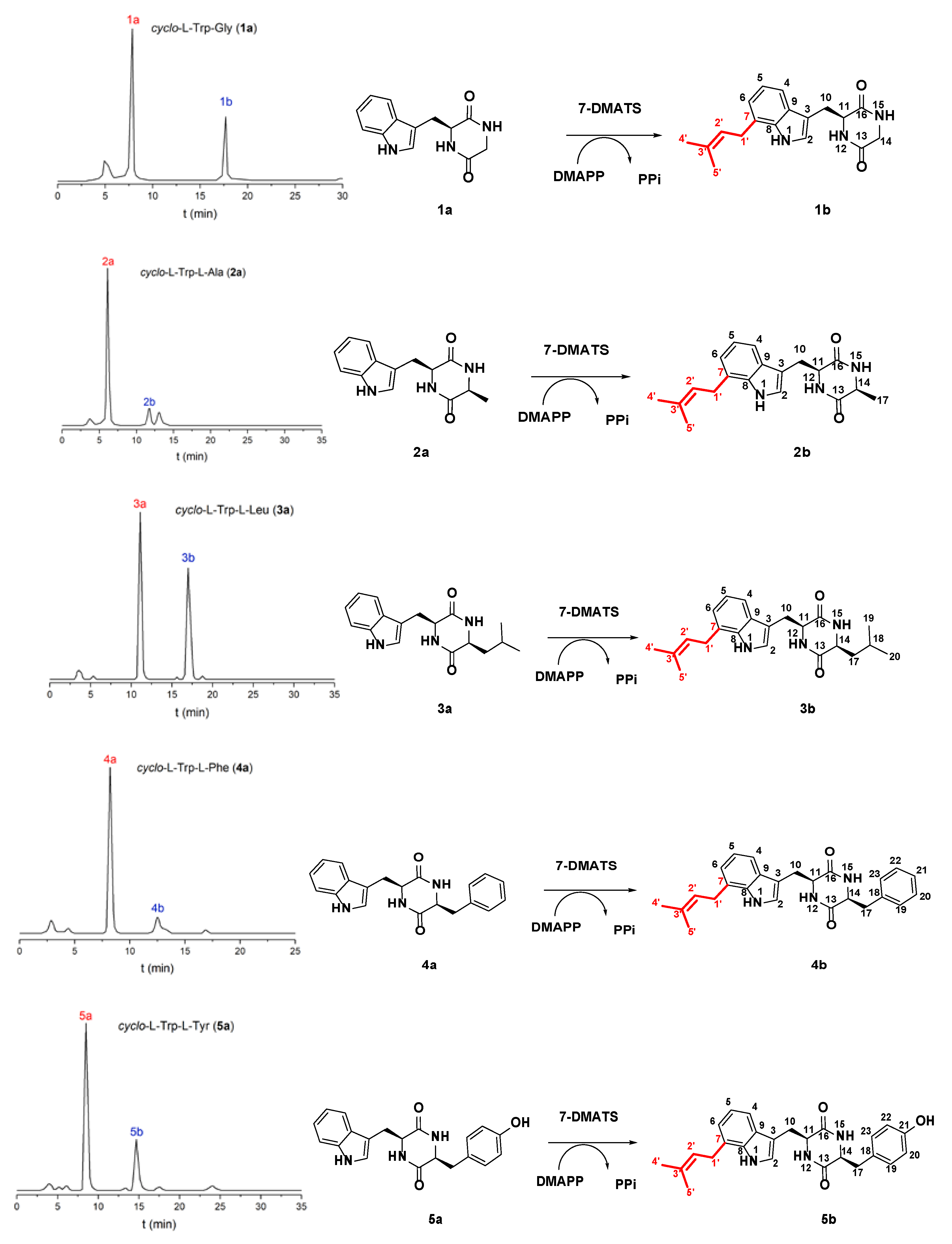
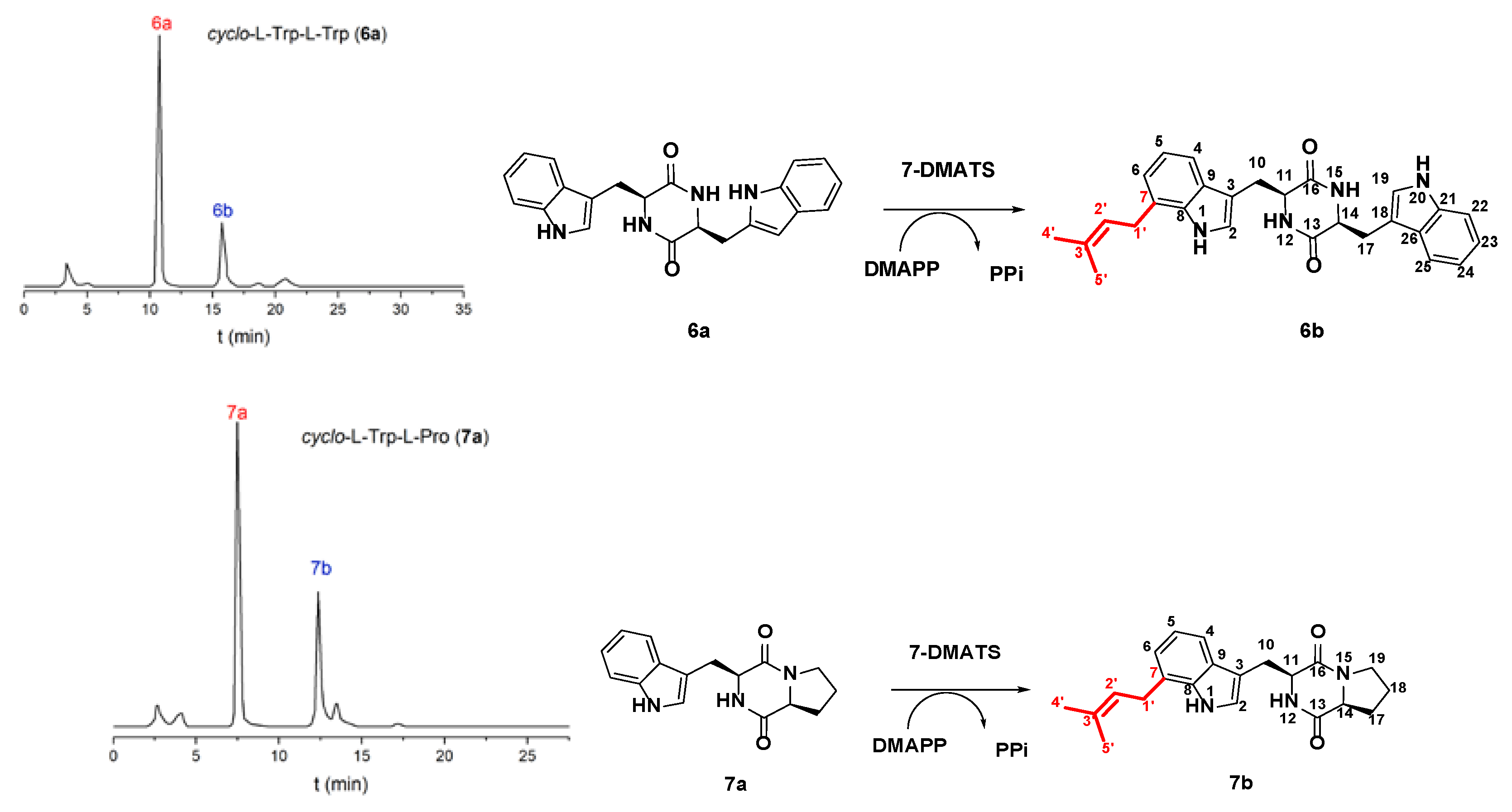


| Substrate | Product | The Final Conversion Rate (%) |
|---|---|---|
| cyclo-l-Trp-Gly (1a) | cyclo-l-7-dimethylallyl-Trp-Gly (1b) | 33.6 |
| cyclo-l-Trp-l-Ala (2a) | cyclo-l-7-dimethylallyl-Trp-l-Ala (2b) | 20.2 |
| cyclo-l-Trp-l-Leu (3a) | cyclo-l-7-dimethylallyl-Trp-l-Leu (3b) | 30.2 |
| cyclo-l-Trp-l-Phe (4a) | cyclo-l-7-dimethylallyl-Trp-l-Phe (4b) | 11.8 |
| cyclo-l-Trp-l-Tyr (5a) | cyclo-l-7-dimethylallyl-Trp-l-Tyr (5b) | 28.1 |
| cyclo-l-Trp-l-Trp (6a) | cyclo-l-7-dimethylallyl-Trp-l-Trp (6b) | 28.5 |
| cyclo-l-Trp-l-Pro (7a) | cyclo-l-7-dimethylallyl-Trp-l-Pro (7b) | 25.4 |
| Substrate | KM (μM) | Vmax (M s−1) | kcat (s−1) | kcat/KM (s−1M−1) | kcat/KM (%) |
|---|---|---|---|---|---|
| DMAPP | 79.6 | 1.2 × 10−9 | 0.0693 | 870.6 | 113.1 |
| cyclo-l-Trp-Gly (1a) | 169.7 | 2.42 × 10−9 | 0.1307 | 770.1 | 100 |
| cyclo-l-Trp-l-Ala (2a) | 867.8 | 2.35 × 10−10 | 0.0127 | 14.6 | 1.90 |
| cyclo-l-Trp-l-Leu (3a) | 823.2 | 1.09 × 10−9 | 0.0586 | 71.2 | 9.25 |
| cyclo-l-Trp-l-Phe (4a) | 102.9 | 5.98 × 10−10 | 0.0323 | 314.0 | 40.77 |
| cyclo-l-Trp-l-Tyr (5a) | 562.4 | 8.58 × 10−10 | 0.0464 | 82.4 | 10.70 |
| cyclo-l-Trp-l-Trp (6a) | 225.8 | 7.65 × 10−10 | 0.0413 | 182.9 | 23.75 |
| cyclo-l-Trp-l-Pro (7a) | 880.1 | 3.80 × 10−10 | 0.0205 | 23.3 | 3.01 |
| Compound | Binding Free Energy ΔGbind(kcal·mol−1) | Hydrogen Bonding |
|---|---|---|
| 1a | −6.31 | TYR191, THR102, GLU89 |
| 2a | −4.78 | ARG244, TYR191, LEU81, GLU89 |
| 3a | −5.11 | PRO313, TYR191, MET328 |
| 4a | −6.05 | TYR191, GLU89, ARG244, MET328 |
| 5a | −5.54 | PRO326, THR343, TYR191, LEU81, ILE80 |
| 6a | −5.94 | ILE80, GLU89 |
| 7a | −4.96 | PRO313, ILE80, ARG244, TYR191, |
| IC50 (μM) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HeLa | HepG2 | A549 | MCF-7 | ||||||||||||
| Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | ||||||||
| 1a | >200 | 1b | 95.3 | 1a | >200 | 1b | 87.7 | 1a | >200 | 1b | 72.4 | 1a | 100 | 1b | 33.7 |
| 2a | >200 | 2b | 93.1 | 2a | >200 | 2b | >200 | 2a | >200 | 2b | >200 | 2a | >200 | 2b | 45.6 |
| 3a | >200 | 3b | 85.2 | 3a | >200 | 3b | >200 | 3a | >200 | 3b | >200 | 3a | >200 | 3b | 41.4 |
| 4a | 100 | 4b | 75.8 | 4a | >200 | 4b | 80.3 | 4a | 100 | 4b | 61.5 | 4a | 100 | 4b | 32.3 |
| 5a | >200 | 5b | 94.8 | 5a | >200 | 5b | 97.5 | 5a | >200 | 5b | 98.0 | 5a | >200 | 5b | 42.8 |
| 6a | >200 | 6b | 91.4 | 6a | >200 | 6b | 92.8 | 6a | >200 | 6b | 99.3 | 6a | >200 | 6b | 39.9 |
| 7a | >200 | 7b | 92.2 | 7a | >200 | 7b | 82.3 | 7a | >200 | 7b | 97.1 | 7a | >200 | 7b | 82.5 |
| MIC (μg·mL−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillus subtilis | Staphylococcus aureus | Staphylococcus epidermis | Staphylococcus simulans | ||||||||||||
| Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | ||||||||
| 1a | 32 | 1b | 16 | 1a | 32 | 1b | 8 | 1a | 128 | 1b | 32 | 1a | 32 | 1b | 4 |
| 2a | 16 | 2b | 8 | 2a | 8 | 2b | 8 | 2a | 32 | 2b | 16 | 2a | 32 | 2b | 4 |
| 3a | 256 | 3b | 64 | 3a | 128 | 3b | 64 | 3a | – | 3b | – | 3a | 128 | 3b | 32 |
| 4a | 4 | 4b | 0.5 | 4a | 8 | 4b | 2 | 4a | 2 | 4b | 1 | 4a | 16 | 4b | 2 |
| 5a | 32 | 5b | 16 | 5a | 64 | 5b | 16 | 5a | 8 | 5b | 2 | 5a | 32 | 5b | 4 |
| 6a | 32 | 6b | 16 | 6a | 16 | 6b | 1 | 6a | – | 6b | 256 | 6a | 128 | 6b | 16 |
| 7a | 16 | 7b | 4 | 7a | 16 | 7b | 2 | 7a | – | 7b | 256 | 7a | 64 | 7b | 8 |
| ampicillin 64 | 128 | 64 | – | ||||||||||||
| ciprofloxacin 2 | 2 | 2 | 4 | ||||||||||||
| MIC (μg·mL−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | Klebsiella pneumoniae | Proteus mirabilis | Pseudomonas aeruginosa | ||||||||||||
| Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | ||||||||
| 1a | 16 | 1b | 2 | 1a | 16 | 1b | 1 | 1a | 32 | 1b | 2 | 1a | 128 | 1b | 16 |
| 2a | 4 | 2b | 0.5 | 2a | 16 | 2b | 2 | 2a | 16 | 2b | 0.5 | 2a | 32 | 2b | 4 |
| 3a | 512 | 3b | 64 | 3a | – | 3b | 128 | 3a | – | 3b | 256 | 3a | – | 3b | 128 |
| 4a | 8 | 4b | 1 | 4a | 16 | 4b | 4 | 4a | 16 | 4b | 4 | 4a | 16 | 4b | 2 |
| 5a | 32 | 5b | 2 | 5a | 64 | 5b | 4 | 5a | 64 | 5b | 4 | 5a | 16 | 5b | 1 |
| 6a | 256 | 6b | 16 | 6a | – | 6b | – | 6a | – | 6b | – | 6a | 256 | 6b | 128 |
| 7a | 512 | 7b | 256 | 7a | 256 | 7b | 16 | 7a | – | 7b | – | 7a | 128 | 7b | 32 |
| ampicillin 128 | – | 64 | 256 | ||||||||||||
| ciprofloxacin 1 | 2 | 2 | 2 | ||||||||||||
| MIC (μg·mL−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspergillus flavus | Candida albicans | Cryptococcus gastricus | Trichophyton rubrum | ||||||||||||
| Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | ||||||||
| 1a | 32 | 1b | 4 | 1a | 16 | 1b | 4 | 1a | 64 | 1b | 8 | 1a | 16 | 1b | 8 |
| 2a | 32 | 2b | 8 | 2a | 8 | 2b | 1 | 2a | 8 | 2b | 4 | 2a | 16 | 2b | 2 |
| 3a | 16 | 3b | 2 | 3a | 16 | 3b | 8 | 3a | 64 | 3b | 16 | 3a | – | 3b | 256 |
| 4a | 32 | 4b | 4 | 4a | 16 | 4b | 4 | 4a | 8 | 4b | 1 | 4a | 4 | 4b | 0.5 |
| 5a | 256 | 5b | 64 | 5a | 256 | 5b | 128 | 5a | – | 5b | – | 5a | 512 | 5b | 64 |
| 6a | 64 | 6b | 8 | 6a | 128 | 6b | 32 | 6a | 64 | 6b | 4 | 6a | 64 | 6b | 16 |
| 7a | 32 | 7b | 16 | 7a | 64 | 7b | 4 | 7a | 32 | 7b | 1 | 7a | 64 | 7b | 8 |
| Amphotericin B 512 | 16 | 8 | 8 | ||||||||||||
| MIC (μg·mL−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fusarium oxysporum | Rhizoctonia solani | Penicillium expansum | Alternaria brassicae | ||||||||||||
| Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | Substrate | Prenylated | ||||||||
| 1a | 32 | 1b | 16 | 1a | 8 | 1b | 2 | 1a | 16 | 1b | 2 | 1a | 16 | 1b | 4 |
| 2a | 32 | 2b | 16 | 2a | 4 | 2b | 0.5 | 2a | 4 | 2b | 0.5 | 2a | 8 | 2b | 1 |
| 3a | 8 | 3b | 4 | 3a | 32 | 3b | 8 | 3a | 32 | 3b | 2 | 3a | 64 | 3b | 32 |
| 4a | 2 | 4b | 0.5 | 4a | 2 | 4b | 1 | 4a | 4 | 4b | 1 | 4a | 8 | 4b | 2 |
| 5a | 64 | 5b | 16 | 5a | 64 | 5b | 32 | 5a | 32 | 5b | 16 | 5a | 512 | 5b | 64 |
| 6a | 64 | 6b | 32 | 6a | 16 | 6b | 4 | 6a | 16 | 6b | 8 | 6a | – | 6b | – |
| 7a | 16 | 7b | 2 | 7a | 16 | 7b | 4 | 7a | 8 | 7b | 2 | 7a | 128 | 7b | 32 |
| Bavistin 8 | 16 | 32 | 32 | ||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Zhang, H.; Wu, W.; Li, H.; An, Z.; Zhou, F. C7-Prenylation of Tryptophan-Containing Cyclic Dipeptides by 7-Dimethylallyl Tryptophan Synthase Significantly Increases the Anticancer and Antimicrobial Activities. Molecules 2020, 25, 3676. https://doi.org/10.3390/molecules25163676
Liu R, Zhang H, Wu W, Li H, An Z, Zhou F. C7-Prenylation of Tryptophan-Containing Cyclic Dipeptides by 7-Dimethylallyl Tryptophan Synthase Significantly Increases the Anticancer and Antimicrobial Activities. Molecules. 2020; 25(16):3676. https://doi.org/10.3390/molecules25163676
Chicago/Turabian StyleLiu, Rui, Hongchi Zhang, Weiqiang Wu, Hui Li, Zhipeng An, and Feng Zhou. 2020. "C7-Prenylation of Tryptophan-Containing Cyclic Dipeptides by 7-Dimethylallyl Tryptophan Synthase Significantly Increases the Anticancer and Antimicrobial Activities" Molecules 25, no. 16: 3676. https://doi.org/10.3390/molecules25163676
APA StyleLiu, R., Zhang, H., Wu, W., Li, H., An, Z., & Zhou, F. (2020). C7-Prenylation of Tryptophan-Containing Cyclic Dipeptides by 7-Dimethylallyl Tryptophan Synthase Significantly Increases the Anticancer and Antimicrobial Activities. Molecules, 25(16), 3676. https://doi.org/10.3390/molecules25163676




