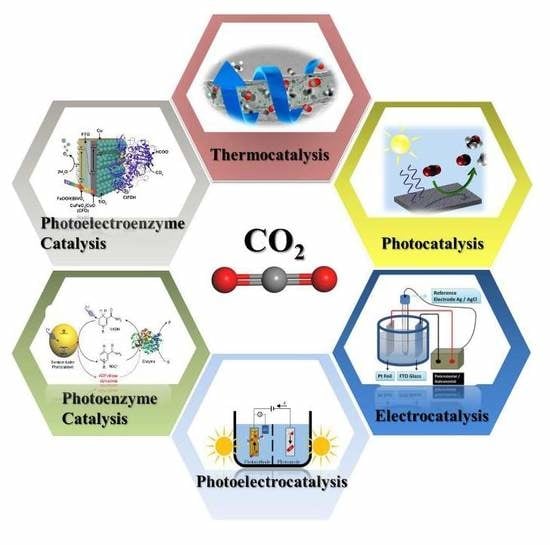
Research Progress in Conversion of CO2 to Valuable Fuels
Abstract
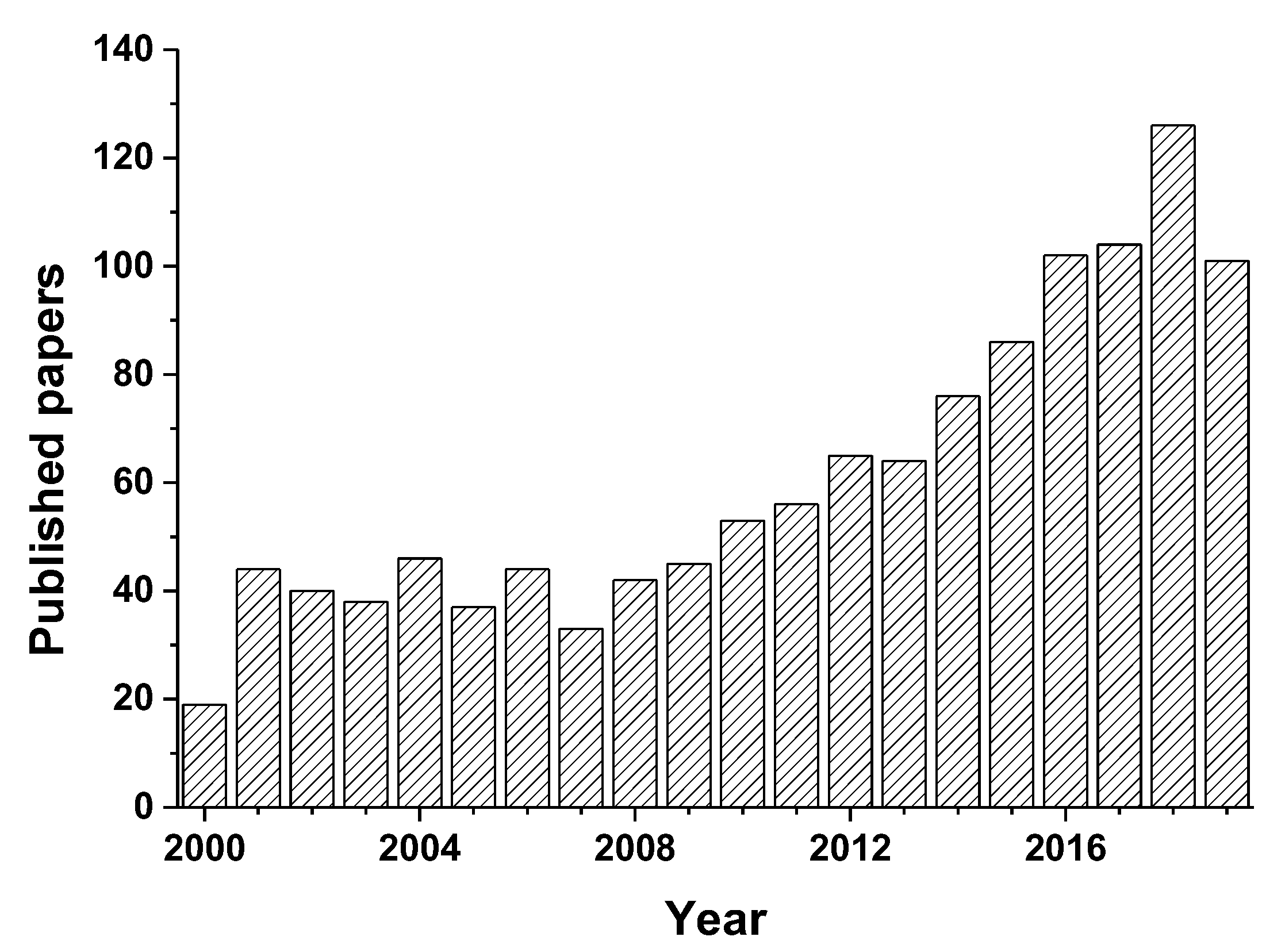
1. Introduction
2. Catalytic Reduction of CO2
2.1. Thermal Catalysis
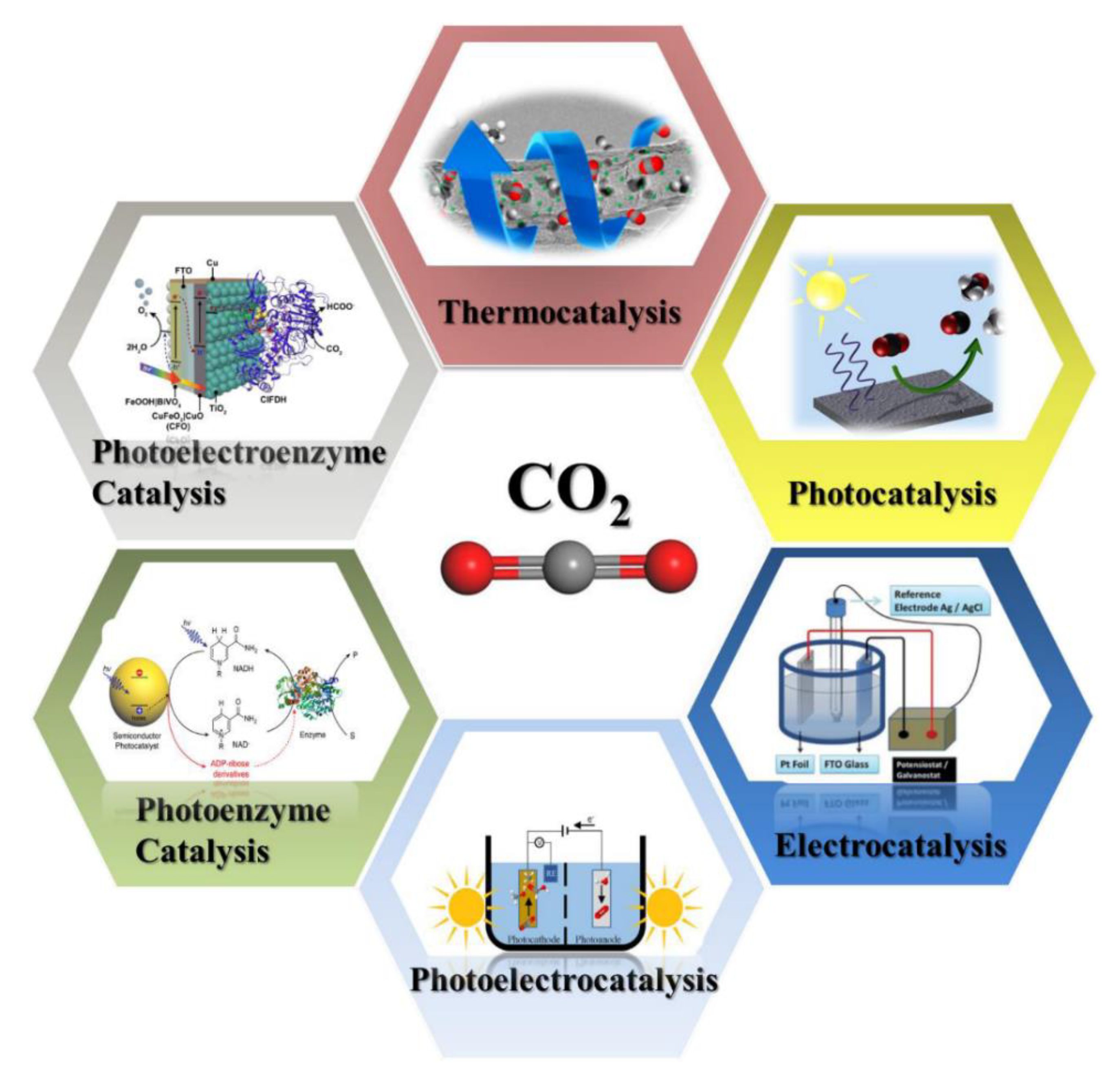
2.2. Photocatalysis
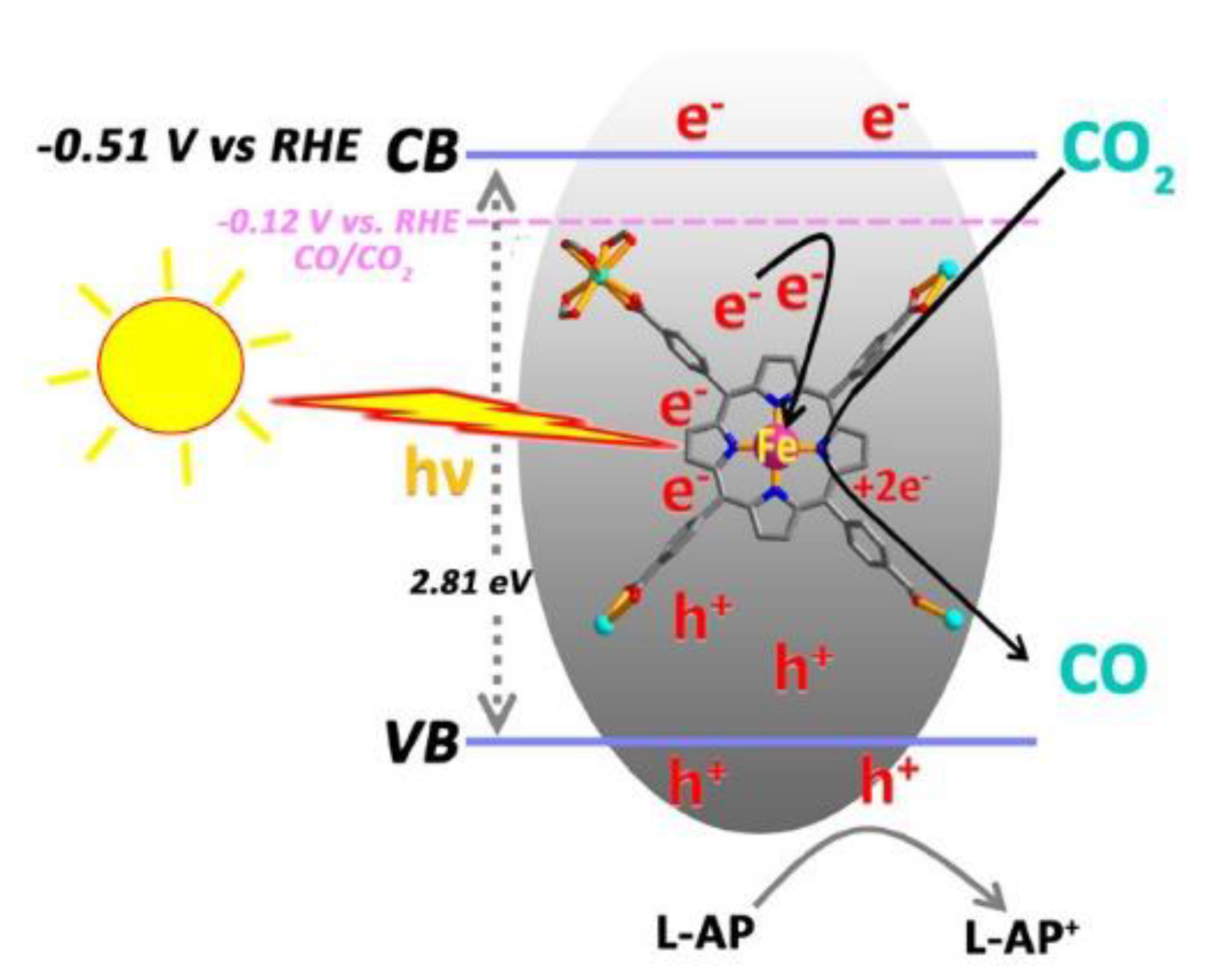
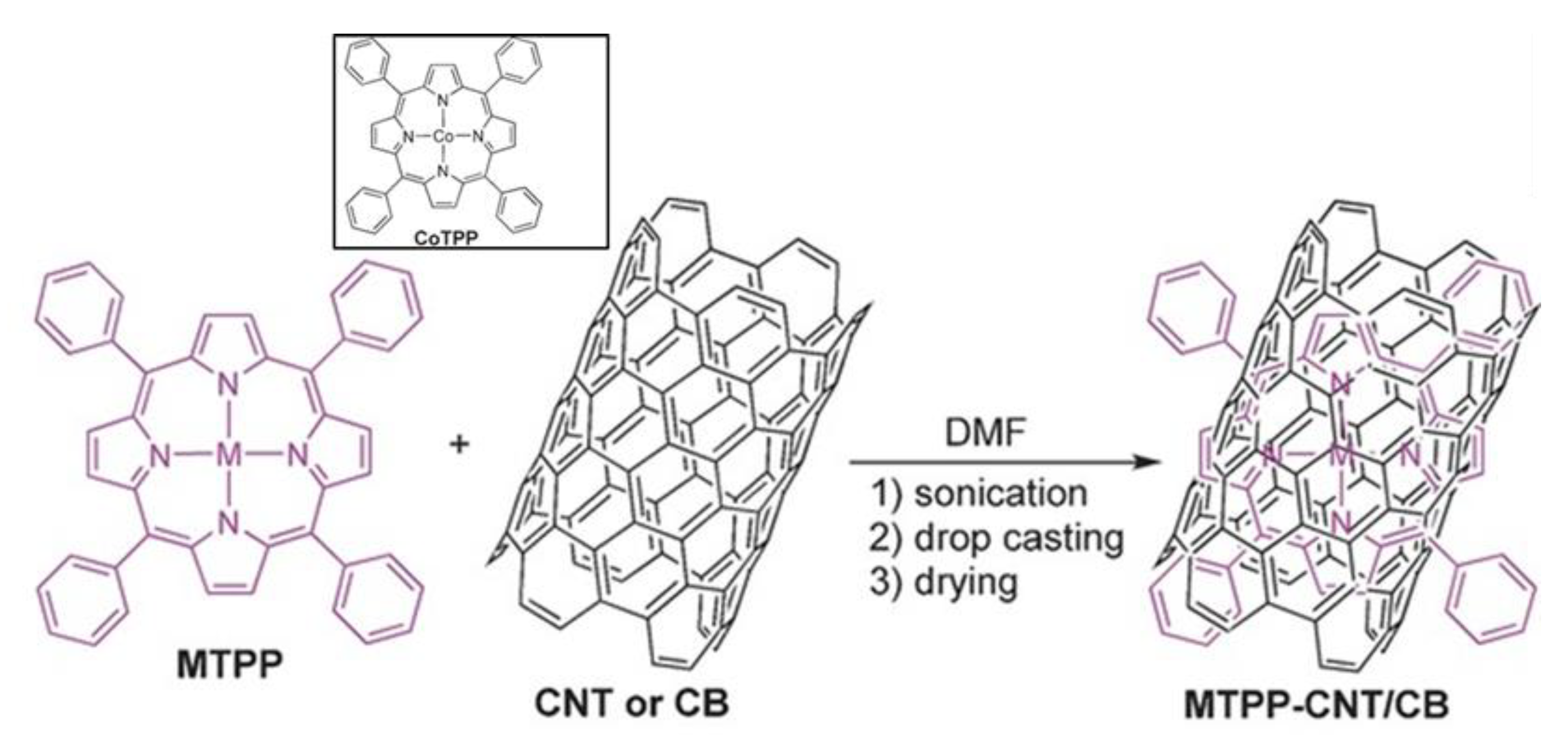
2.3. Electrocatalysis
2.4. Photoelectrocatalysis
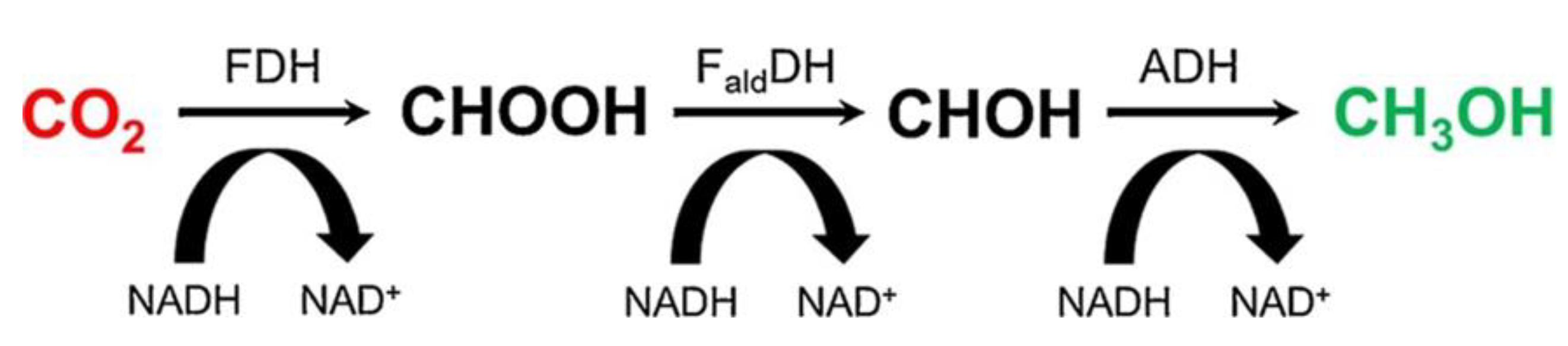
2.5. Enzyme
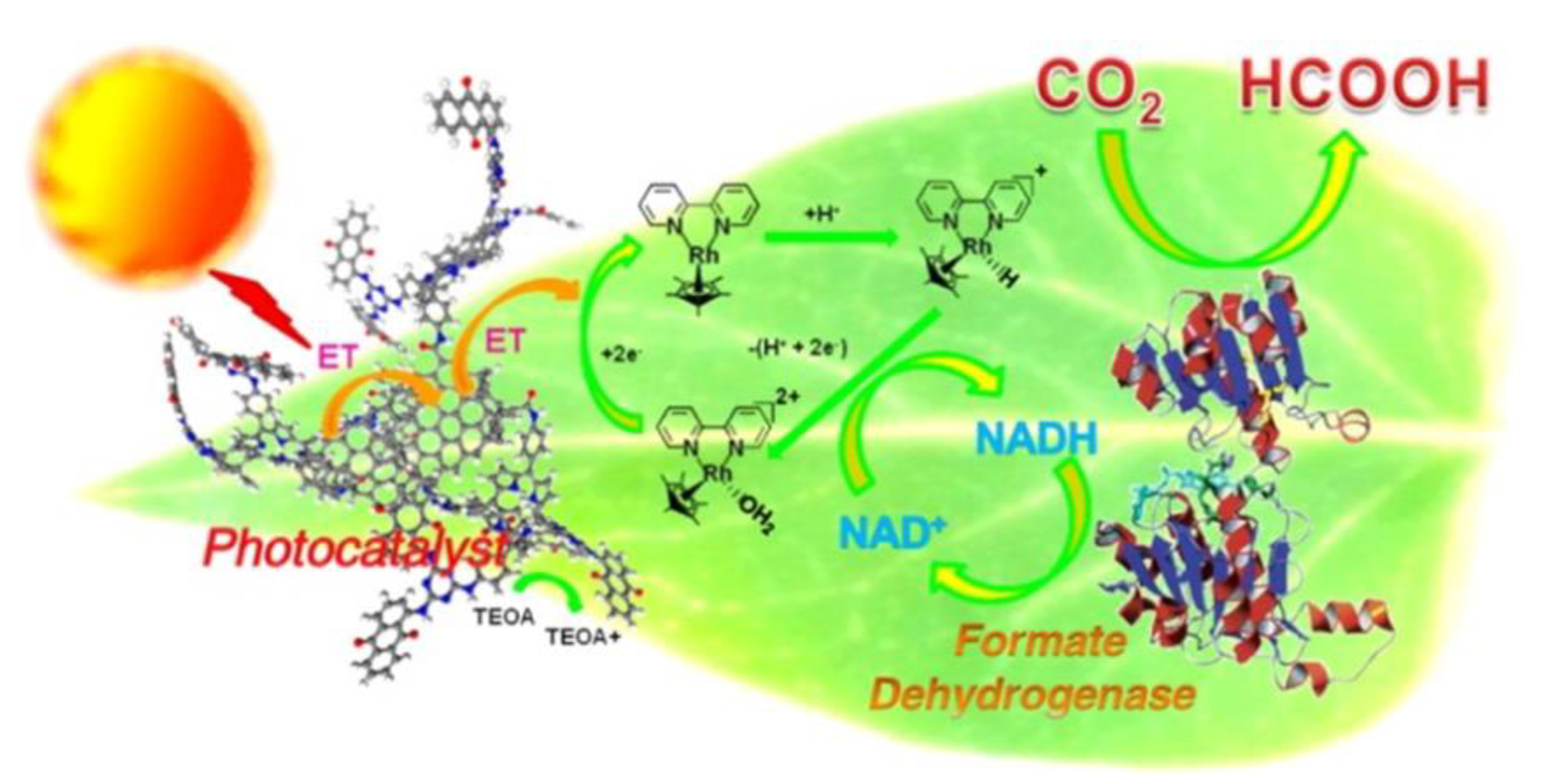
2.6. Enzyme Coupled to Photocatalysis
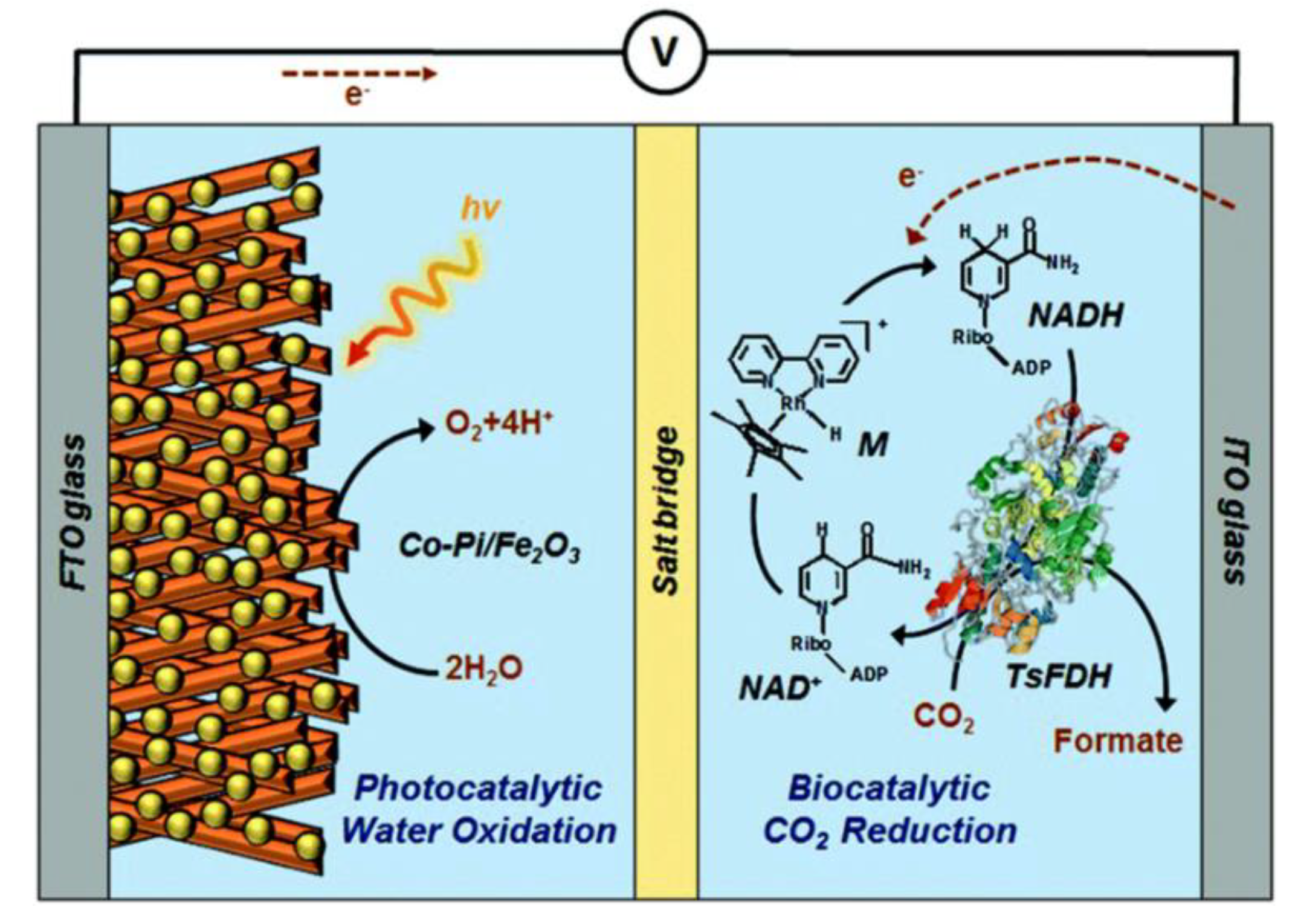
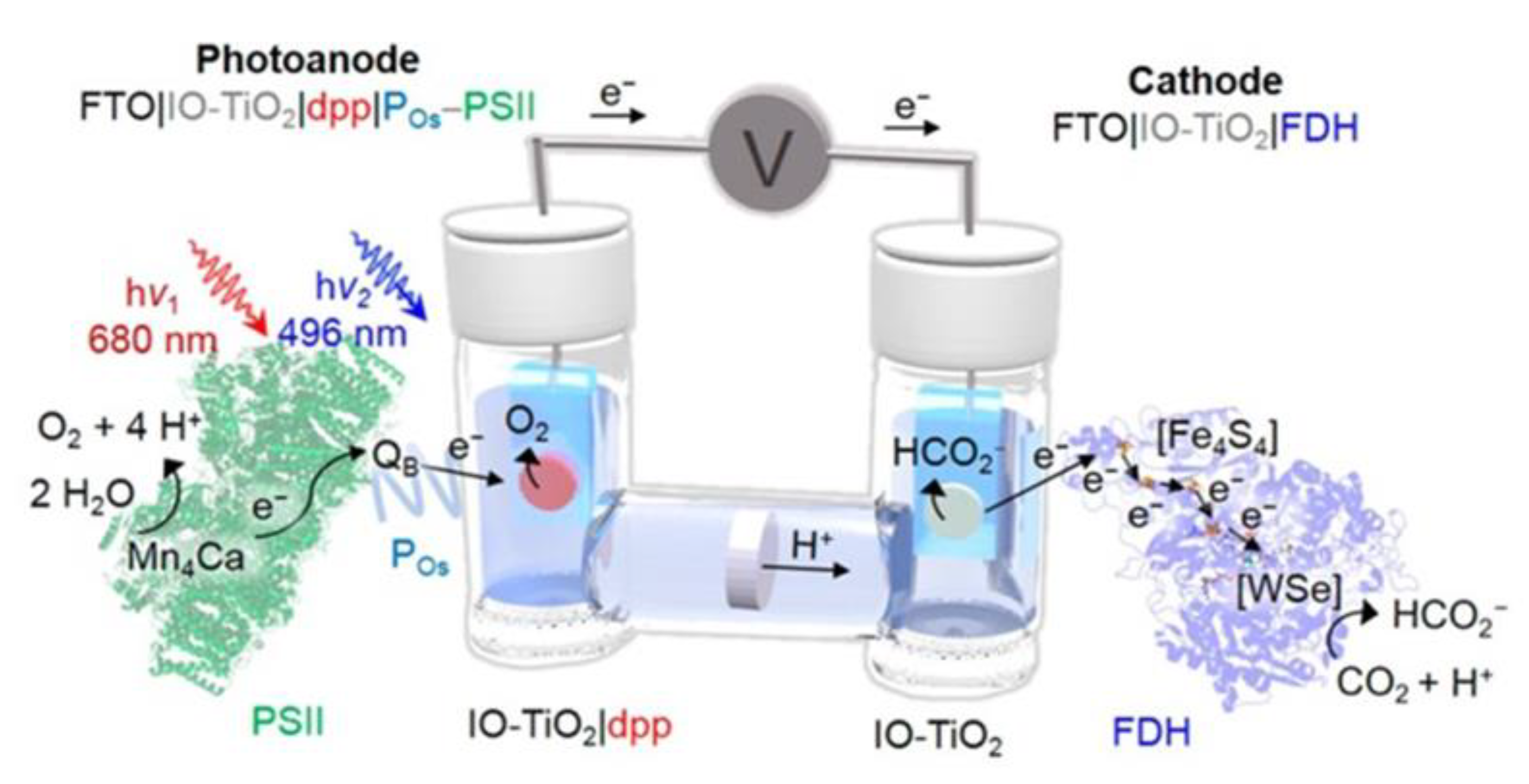
2.7. Enzyme Coupled to Photoelectrocatalysis
3. Conclusions and Outline
Author Contributions
Funding
Conflicts of Interest
References
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Medina-Elizade, M. Global temperature change. Proc. Natl. Acad. Sci. USA 2006, 103, 14288–14293. [Google Scholar] [CrossRef]
- Zachos, J.C.; Dickens, G.; Zeebe, R. An Early Cenozoic perspective on Greenhouse warming and carbon cycle dynamics. Nature 2008, 451, 279–283. [Google Scholar] [CrossRef]
- Hepp, S.; Jetter, M.; Portalupi, S.L.; Michler, P. Semiconductor Quantum Dots for Integrated Quantum Photonics. Adv. Quantum Technol. 2019, 2, 1900020. [Google Scholar] [CrossRef]
- Li, L.; Zhao, N.; Wei, W.; Sun, Y. A review of research progress on CO2 capture, storage, and utilization in Chinese Academy of Sciences. Fuel 2013, 108, 112–130. [Google Scholar] [CrossRef]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Tsang, S.C.E. Recent Advances in CO2 Capture and Utilization. ChemSusChem 2008, 1, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Treviño, M.A.; Kanani, N.; Jeong-Potter, C.W.; Farrauto, R.J. Bimetallic catalysts for CO2 capture and hydrogenation at simulated flue gas conditions. Chem. Eng. J. 2019, 375. [Google Scholar] [CrossRef]
- Kar, S.; Goeppert, A.; Prakash, G.K.S. Combined CO2 Capture and Hydrogenation to Methanol: Amine Immobilization Enables Easy Recycling of Active Elements. ChemSusChem 2019, 12, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Long, R.; Gao, C.; Xiong, Y. Recent progress on advanced design for photoelectrochemical reduction of CO2 to fuels. Sci. China Mater. 2018, 61, 771–805. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Studt, F.; Abild-Pedersen, F.; Schlögl, R.; Behrens, M. Hydrogenation of CO2 to methanol and CO on Cu/ZnO/Al2O3: Is there a common intermediate or not? J. Catal. 2015, 328, 43–48. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Xie, K.; Wang, G.; Bao, X. High-Temperature CO2 Electrolysis in Solid Oxide Electrolysis Cells: Developments, Challenges, and Prospects. Adv. Mater. 2019, 31, 1902033. [Google Scholar] [CrossRef] [PubMed]
- Currie, R.; Mottaghi-Tabar, S.; Zhuang, Y.; Simakov, D.S.A. Design of an Air-Cooled Sabatier Reactor for Thermocatalytic Hydrogenation of CO2: Experimental Proof-of-Concept and Model-Based Feasibility Analysis. Ind. Eng. Chem. Res. 2019, 58, 12964–12980. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Meng, X.; Li, P.; Ouyang, S.; Chang, K.; Liu, G.; Mei, Z.; Ye, J. Photoreduction of CO2 over the well-crystallized ordered mesoporous TiO2 with the confined space effect. Nano Energy 2014, 9, 50–60. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. TiO2–MnOx–Pt Hybrid Multiheterojunction Film Photocatalyst with Enhanced Photocatalytic CO2-Reduction Activity. ACS Appl. Mater. Interfaces 2019, 11, 5581–5589. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S.-Z. Cocatalysts in Semiconductor-based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal–Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–71. [Google Scholar] [CrossRef]
- Huang, J.; Buonsanti, R. Colloidal Nanocrystals as Heterogeneous Catalysts for Electrochemical CO2 Conversion. Chem. Mater. 2019, 31, 13–25. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; Shao, Q.; Yin, R.; Guo, J.; Li, Y.; Huang, X. Phase and structure modulating of bimetallic CuSn nanowires boosts electrocatalytic conversion of CO2. Nano Energy 2019, 59, 138–145. [Google Scholar] [CrossRef]
- Kim, C.; Cho, K.M.; Al-Saggaf, A.; Gereige, I.; Jung, H.-T. Z-scheme Photocatalytic CO2 Conversion on Three-Dimensional BiVO4/Carbon-Coated Cu2O Nanowire Arrays under Visible Light. ACS Catal. 2018, 8, 4170–4177. [Google Scholar] [CrossRef]
- Leung, J.J.; Warnan, J.; Ly, K.H.; Heidary, N.; Nam, D.H.; Kuehnel, M.F.; Reisner, E. Solar-driven reduction of aqueous CO2 with a cobalt bis(terpyridine)-based photocathode. Nat. Catal 2019, 2, 354–365. [Google Scholar] [CrossRef]
- Chen, J.; Yin, J.; Zheng, X.; Ait Ahsaine, H.; Zhou, Y.; Dong, C.; Mohammed, O.F.; Takanabe, K.; Bakr, O.M. Compositionally Screened Eutectic Catalytic Coatings on Halide Perovskite Photocathodes for Photoassisted Selective CO2 Reduction. ACS Energy Lett. 2019, 4, 1279–1286. [Google Scholar] [CrossRef]
- Kalamaras, E.; Belekoukia, M.; Tan, J.Z.Y.; Xuan, J.; Maroto-Valer, M.M.; Andresen, J.M. A microfluidic photoelectrochemical cell for solar-driven CO2 conversion into liquid fuels with CuO-based photocathodes. Faraday Discuss. 2019, 215, 329–344. [Google Scholar] [CrossRef]
- Sultana, S.; Sahoo, P.C.; Martha, S.; Parida, K. A review of harvesting clean fuels from enzymatic CO2 reduction. RSC Adv. 2016, 6, 44170–44194. [Google Scholar] [CrossRef]
- Rudroff, F.; Mihovilovic, M.D.; Groger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U.T. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 2018, 1, 12–22. [Google Scholar] [CrossRef]
- Tackett, B.M.; Gomez, E.; Chen, J.G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019, 2, 381–386. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Studt, F.; Sharafutdinov, I.; Abild-Pedersen, F.; Elkjær, C.F.; Hummelshøj, J.S.; Dahl, S.; Chorkendorff, I.; Nørskov, J.K. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 2014, 6, 320–324. [Google Scholar] [CrossRef]
- Martin, O.; Martín, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferré, D.; Pérez-Ramírez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef] [PubMed]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-based Metal–Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef] [PubMed]
- Roldán, L.; Marco, Y.; García-Bordejé, E. Origin of the Excellent Performance of Ru on Nitrogen-Doped Carbon Nanofibers for CO2 Hydrogenation to CH4. ChemSusChem 2017, 10, 1139–1144. [Google Scholar] [CrossRef]
- O’Byrne, J.P.; Owen, R.E.; Minett, D.R.; Pascu, S.I.; Plucinski, P.K.; Jones, M.D.; Mattia, D. High CO2 and CO conversion to hydrocarbons using bridged Fe nanoparticles on carbon nanotubes. Catal. Sci. Technol. 2013, 3, 1202–1207. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, W.; Chen, S.; Chu, W. Cerium Promoted Nano Nickel Catalysts Ni-Ce/CNTs and Ni-Ce/Al2O3 for CO2 Methanation. Integr. Ferroelectr. 2014, 151, 116–125. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Xiao, X.; Wang, W.; Wang, N.; Qian, W.; Chu, W. Regulation of Ni–CNT Interaction on Mn-Promoted Nickel Nanocatalysts Supported on Oxygenated CNTs for CO2 Selective Hydrogenation. ACS Appl. Mater. Interfaces 2018, 10, 41224–41236. [Google Scholar] [CrossRef]
- Wang, W.; Duong Viet, C.; Housseinou, B.; Baaziz, W.; Tuci, G.; Caporali, S.; Nguyen, D.-L.; Ersen, O.; Giambastiani, G.; Pham-Huu, C. Nickel Nanoparticles Decorated Nitrogen-Doped Carbon Nanotubes (Ni/N-CNT); A Robust Catalyst for the Efficient and Selective CO2 Methanation. ACS Appl. Energy Mater. 2018. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhu, L.; Li, Y.; Wang, K.; Qiu, K.; Tippayawong, N.; Aggarangsi, P.; Reubroycharoen, P.; Wang, S. Biomass derived N-doped biochar as efficient catalyst supports for CO2 methanation. J. CO2 Util. 2019, 34, 733–741. [Google Scholar] [CrossRef]
- Wang, W.; Duong-Viet, C.; Xu, Z.; Ba, H.; Tuci, G.; Giambastiani, G.; Liu, Y.; Truong-Huu, T.; Nhut, J.-M.; Pham-Huu, C. CO2 methanation under dynamic operational mode using nickel nanoparticles decorated carbon felt (Ni/OCF) combined with inductive heating. Catal. Today 2019. [Google Scholar] [CrossRef]
- Khalil, M.; Gunlazuardi, J.; Ivandini, T.A.; Umar, A. Photocatalytic conversion of CO2 using earth-abundant catalysts: A review on mechanism and catalytic performance. Renew. Sustain. Energy Rev. 2019, 113, 109246. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Ye, J. Electrostatic Self-Assembly of Nanosized Carbon Nitride Nanosheet onto a Zirconium Metal–Organic Framework for Enhanced Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2015, 25, 5360–5367. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal–Organic Framework (MOF) Compounds: Photocatalysts for Redox Reactions and Solar Fuel Production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar] [CrossRef] [PubMed]
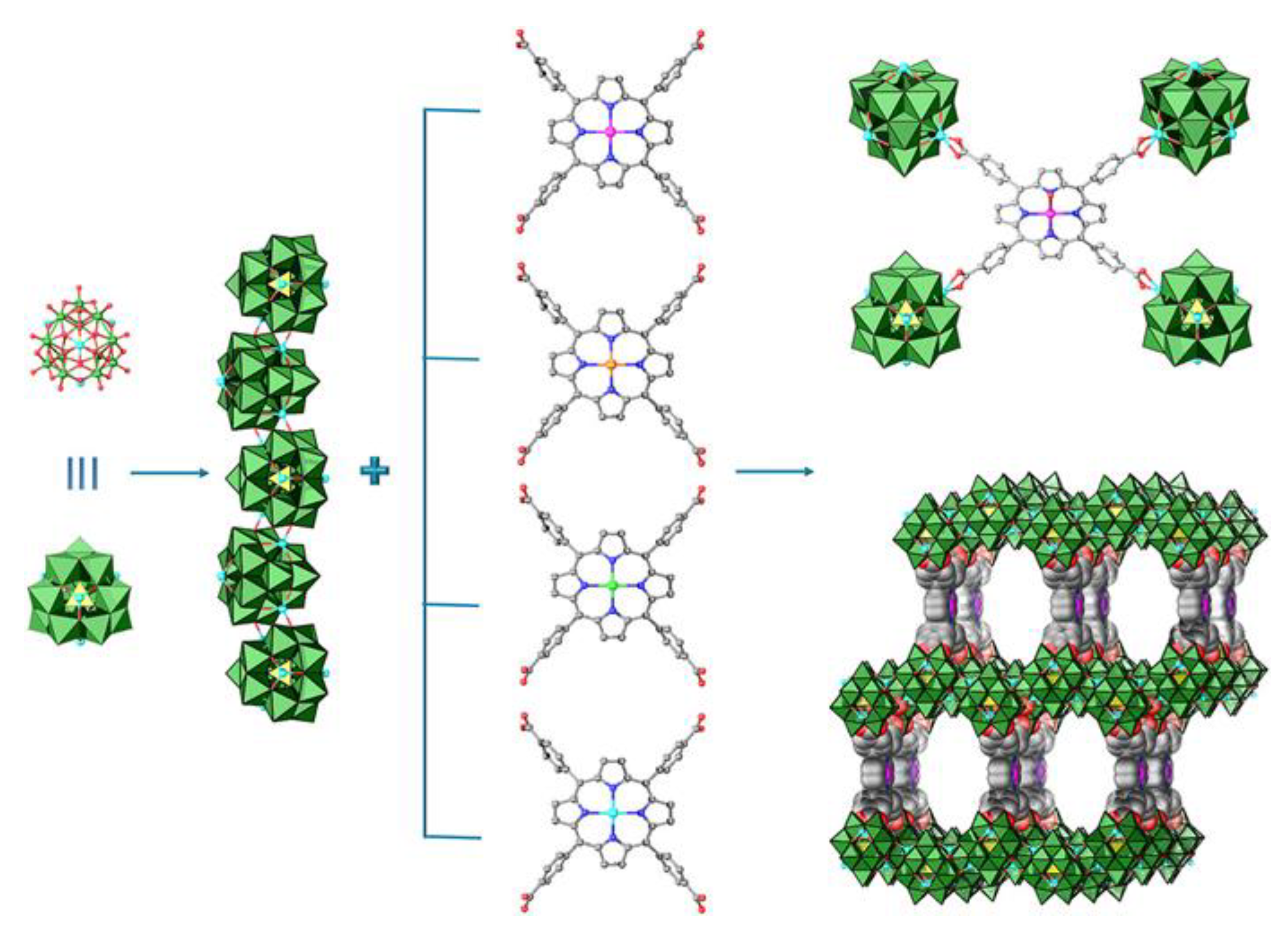
- Maina, J.W.; Pozo-Gonzalo, C.; Kong, L.; Schütz, J.; Hill, M.; Dumée, L.F. Metal organic framework based catalysts for CO2 conversion. Mater. Horiz. 2017, 4, 345–361. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, J.Y.; Zhang, D.; Kang, Y.S. Selective construction of junctions on different facets of BiVO4 for enhancing photo-activity. New J. Chem. 2015, 39, 9918–9925. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Wang, Y.-F.; Li, Y.-Y.; Zhang, L.; Yao, H.-C.; Li, Z.-J. Enhanced photocatalytic CO2 reduction activity of Z-scheme CdS/BiVO4 nanocomposite with thinner BiVO4 nanosheets. J. CO2 Util. 2018, 28, 15–25. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Shi, R.; Waterhouse, G.I.N.; Tang, J.; Zhang, T. Two-dimensional photocatalyst design: A critical review of recent experimental and computational advances. Mater. Today 2020, 34, 78–91. [Google Scholar] [CrossRef]
- Mao, X.; Tang, C.; He, T.; Wijethunge, D.; Yan, C.; Zhu, Z.; Du, A. Computational screening of MN4 (M = Ti–Cu) based metal organic frameworks for CO2 reduction using the d-band centre as a descriptor. Nanoscale 2020, 12, 6188–6194. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, L.; Li, P.; Zhang, B.; Zhao, G.; He, T. A computational study on linear and bent adsorption of CO2 on different surfaces for its photoreduction. Catal. Today 2019, 335, 278–285. [Google Scholar] [CrossRef]
- Guharoy, U.; Le Saché, E.; Cai, Q.; Reina, T.R.; Gu, S. Understanding the role of Ni-Sn interaction to design highly effective CO2 conversion catalysts for dry reforming of methane. J. CO2 Util. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Guo, T.; Zhu, L.; Ling, C.; Xue, Q.; Xing, W. Charge-modulated CO2 capture of C3N nanosheet: Insights from DFT calculations. Chem. Eng. J. 2018, 338, 92–98. [Google Scholar] [CrossRef]
- Jiang, Y.-B.; Sun, Z. Self-assembled porphyrin and macrocycle derivatives: From synthesis to function. MRS Bull. 2019, 44, 167–171. [Google Scholar] [CrossRef]
- Wang, S.-S.; Huang, H.-H.; Liu, M.; Yao, S.; Guo, S.; Wang, J.-W.; Zhang, Z.-M.; Lu, T.-B. Encapsulation of Single Iron Sites in a Metal–Porphyrin Framework for High-Performance Photocatalytic CO2 Reduction. Inorg. Chem. 2020, 59, 6301–6307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wen, J.; Low, J.; Fang, Y.; Yu, J. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci. China Mater. 2014, 57, 70–100. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Xu, X.; Wang, J. Review on porous nanomaterials for adsorption and photocatalytic conversion of CO2. Chin. J. Catal. 2017, 38, 1956–1969. [Google Scholar] [CrossRef]
- Kočí, K.; Matějů, K.; Obalová, L.; Krejčíková, S.; Lacný, Z.; Plachá, D.; Čapek, L.; Hospodková, A.; Šolcová, O. Effect of silver doping on the TiO2 for photocatalytic reduction of CO2. Appl. Catal. B Environ. 2010, 96, 239–244. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, J.; Zhang, J.; Zhang, J.; Liu, G. Cubic anatase TiO2 nanocrystals with enhanced photocatalytic CO2 reduction activity. Chem. Commun. 2015, 51, 7950–7953. [Google Scholar] [CrossRef]
- Jang, J.-W.; Cho, S.; Magesh, G.; Jang, Y.J.; Kim, J.Y.; Kim, W.Y.; Seo, J.K.; Kim, S.; Lee, K.-H.; Lee, J.S. Aqueous-Solution Route to Zinc Telluride Films for Application to CO2 Reduction. Angew. Chem. Int. Ed. 2014, 53, 5852–5857. [Google Scholar] [CrossRef]
- Nesbitt, N.T.; Ma, M.; Trześniewski, B.J.; Jaszewski, S.; Tafti, F.; Burns, M.J.; Smith, W.A.; Naughton, M.J. Au Dendrite Electrocatalysts for CO2 Electrolysis. J. Phys. Chem. C 2018, 122, 10006–10016. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xu, J.; Wei, Z.; Ma, J.; Guo, S.; Wang, S.; Liu, H.; Dou, S. Metal-Free Carbon Materials for CO2 Electrochemical Reduction. Adv. Mater. 2017, 29, 1701784. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Chen, P.; Jaroniec, M.; Qiao, S.-Z. Molecular Scaffolding Strategy with Synergistic Active Centers To Facilitate Electrocatalytic CO2 Reduction to Hydrocarbon/Alcohol. J. Am. Chem. Soc. 2017, 139, 18093–18100. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Rosen, J.; Jiao, F. Nanostructured Metallic Electrocatalysts for Carbon Dioxide Reduction. ChemCatChem 2015, 7, 38–47. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.-J.; Gong, J. Nanostructured Materials for Heterogeneous Electrocatalytic CO2 Reduction and their Related Reaction Mechanisms. Angew. Chem. Int. Ed. 2017, 56, 11326–11353. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Quan, F.; Huang, J.; Jia, F.; Zhang, L. Selective electro-reduction of CO2 to formate on nanostructured Bi from reduction of BiOCl nanosheets. Electrochem. Commun. 2014, 46, 63–66. [Google Scholar] [CrossRef]
- Liu, J.; Guo, C.; Vasileff, A.; Qiao, S. Nanostructured 2D Materials: Prospective Catalysts for Electrochemical CO2 Reduction. Small Methods 2017, 1, 1600006. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Senthil Kumar, S.; Anbu Kulandainathan, M. Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 2012, 25, 70–73. [Google Scholar] [CrossRef]
- Shen, J.-Q.; Liao, P.-Q.; Zhou, D.-D.; He, C.-T.; Wu, J.-X.; Zhang, W.-X.; Zhang, J.-P.; Chen, X.-M. Modular and Stepwise Synthesis of a Hybrid Metal–Organic Framework for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2017, 139, 1778–1781. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Rønne, M.H.; Pedersen, S.U.; Skrydstrup, T.; Daasbjerg, K. Enhanced Catalytic Activity of Cobalt Porphyrin in CO2 Electroreduction upon Immobilization on Carbon Materials. Angew. Chem. Int. Ed. 2017, 56, 6468–6472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-R.; Huang, Q.; He, C.-T.; Chen, Y.; Liu, J.; Shen, F.-C.; Lan, Y.-Q. Oriented electron transmission in polyoxometalate-metalloporphyrin organic framework for highly selective electroreduction of CO2. Nat. Commun. 2018, 9, 4466. [Google Scholar] [CrossRef]
- Davethu, P.A.; de Visser, S.P. CO2 Reduction on an Iron-Porphyrin Center: A Computational Study. J. Phys. Chem. A 2019, 123, 6527–6535. [Google Scholar] [CrossRef]
- Feng, S.; Zheng, W.; Zhu, J.; Li, Z.; Yang, B.; Wen, Z.; Lu, J.; Lei, L.; Wang, S.; Hou, Y. Porous metal-porphyrin triazine-based frameworks for efficient CO2 electroreduction. Appl. Catal. B Environ. 2020, 270, 118908. [Google Scholar] [CrossRef]
- Elgrishi, N.; Chambers, M.B.; Artero, V.; Fontecave, M. Terpyridine complexes of first row transition metals and electrochemical reduction of CO2 to CO. Phys. Chem. Chem. Phys. 2014, 16, 13635–13644. [Google Scholar] [CrossRef]
- Rao, G.K.; Pell, W.; Korobkov, I.; Richeson, D. Electrocatalytic reduction of CO2 using Mn complexes with unconventional coordination environments. Chem. Commun. 2016, 52, 8010–8013. [Google Scholar] [CrossRef]
- Liu, F.-W.; Bi, J.; Sun, Y.; Luo, S.; Kang, P. Cobalt Complex with Redox-Active Imino Bipyridyl Ligand for Electrocatalytic Reduction of Carbon Dioxide to Formate. ChemSusChem 2018, 11, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Myren, T.H.T.; Lilio, A.M.; Huntzinger, C.G.; Horstman, J.W.; Stinson, T.A.; Donadt, T.B.; Moore, C.; Lama, B.; Funke, H.H.; Luca, O.R. Manganese N-Heterocyclic Carbene Pincers for the Electrocatalytic Reduction of Carbon Dioxide. Organometallics 2019, 38, 1248–1253. [Google Scholar] [CrossRef]
- Wesselbaum, S.; vom Stein, T.; Klankermayer, J.; Leitner, W. Hydrogenation of Carbon Dioxide to Methanol by Using a Homogeneous Ruthenium–Phosphine Catalyst. Angew. Chem. Int. Ed. 2012, 51, 7499–7502. [Google Scholar] [CrossRef] [PubMed]
- Kothandaraman, J.; Goeppert, A.; Czaun, M.; Olah, G.A.; Prakash, G.K.S. Conversion of CO2 from Air into Methanol Using a Polyamine and a Homogeneous Ruthenium Catalyst. J. Am. Chem. Soc. 2016, 138, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Everett, M.; Wass, D.F. Highly productive CO2 hydrogenation to methanol—A tandem catalytic approach via amide intermediates. Chem. Commun. 2017, 53, 9502–9504. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sen, R.; Kothandaraman, J.; Goeppert, A.; Chowdhury, R.; Munoz, S.B.; Haiges, R.; Prakash, G.K.S. Mechanistic Insights into Ruthenium-Pincer-Catalyzed Amine-Assisted Homogeneous Hydrogenation of CO2 to Methanol. J. Am. Chem. Soc. 2019, 141, 3160–3170. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, T.; Tao, H.; Fan, Q.; Han, B. Fundamentals and Challenges of Electrochemical CO2 Reduction Using Two-Dimensional Materials. Chem 2017, 3, 560–587. [Google Scholar] [CrossRef]
- Sahara, G.; Kumagai, H.; Maeda, K.; Kaeffer, N.; Artero, V.; Higashi, M.; Abe, R.; Ishitani, O. Photoelectrochemical Reduction of CO2 Coupled to Water Oxidation Using a Photocathode with a Ru(II)–Re(I) Complex Photocatalyst and a CoOx/TaON Photoanode. J. Am. Chem. Soc. 2016, 138, 14152–14158. [Google Scholar] [CrossRef]
- Song, J.T.; Ryoo, H.; Cho, M.; Kim, J.; Kim, J.-G.; Chung, S.-Y.; Oh, J. Nanoporous Au Thin Films on Si Photoelectrodes for Selective and Efficient Photoelectrochemical CO2 Reduction. Adv. Energy Mater. 2017, 7, 1601103. [Google Scholar] [CrossRef]
- DuChene, J.S.; Tagliabue, G.; Welch, A.J.; Cheng, W.-H.; Atwater, H.A. Hot Hole Collection and Photoelectrochemical CO2 Reduction with Plasmonic Au/p-GaN Photocathodes. Nano Lett. 2018, 18, 2545–2550. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Hu, Y.; Deng, J.; Chen, J.; Zha, C.; Yang, H.; Han, N.; Gong, Q.; Li, L.; Wang, T.; et al. Controlled chemical etching leads to efficient silicon–bismuth interface for photoelectrochemical CO2 reduction to formate. Mater. Today Chem. 2019, 11, 80–85. [Google Scholar] [CrossRef]
- Castro, S.; Albo, J.; Irabien, A. Continuous conversion of CO2 to alcohols in a TiO2 photoanode-driven photoelectrochemical system. J. Chem. Technol. Biotechnol. 2020, 95, 1876–1882. [Google Scholar] [CrossRef]
- Gurudayal; Beeman, J.W.; Bullock, J.; Wang, H.; Eichhorn, J.; Towle, C.; Javey, A.; Toma, F.M.; Mathews, N.; Ager, J.W. Si photocathode with Ag-supported dendritic Cu catalyst for CO2 reduction. Energy Environ. Sci. 2019, 12, 1068–1077. [Google Scholar] [CrossRef]
- Choi, S.K.; Kang, U.; Lee, S.; Ham, D.J.; Ji, S.M.; Park, H. Sn-Coupled p-Si Nanowire Arrays for Solar Formate Production from CO2. Adv. Energy Mater. 2014, 4, 1301614. [Google Scholar] [CrossRef]
- Rajeshwar, K.; de Tacconi, N.R.; Ghadimkhani, G.; Chanmanee, W.; Janáky, C. Tailoring Copper Oxide Semiconductor Nanorod Arrays for Photoelectrochemical Reduction of Carbon Dioxide to Methanol. ChemPhysChem 2013, 14, 2251–2259. [Google Scholar] [CrossRef]
- Shan, B.; Vanka, S.; Li, T.-T.; Troian-Gautier, L.; Brennaman, M.K.; Mi, Z.; Meyer, T.J. Binary molecular-semiconductor p–n junctions for photoelectrocatalytic CO2 reduction. Nat. Energy 2019, 4, 290–299. [Google Scholar] [CrossRef]
- Rao, K.R.; Pishgar, S.; Strain, J.; Kumar, B.; Atla, V.; Kumari, S.; Spurgeon, J.M. Photoelectrochemical reduction of CO2 to HCOOH on silicon photocathodes with reduced SnO2 porous nanowire catalysts. J. Mater. Chem. A 2018, 6, 1736–1742. [Google Scholar] [CrossRef]
- Huang, X.; Shen, Q.; Liu, J.; Yang, N.; Zhao, G. A CO2 adsorption-enhanced semiconductor/metal-complex hybrid photoelectrocatalytic interface for efficient formate production. Energy Environ. Sci. 2016, 9, 3161–3171. [Google Scholar] [CrossRef]
- Liu, J.; Guo, C.; Hu, X.; Zhao, G. Bio-proton coupled semiconductor/metal-complex hybrid photoelectrocatalytic interface for efficient CO2 reduction. Green Chem. 2019, 21, 339–348. [Google Scholar] [CrossRef]
- Li, P.; Jing, H.; Xu, J.; Wu, C.; Peng, H.; Lu, J.; Lu, F. High-efficiency synergistic conversion of CO2 to methanol using Fe2O3 nanotubes modified with double-layer Cu2O spheres. Nanoscale 2014, 6, 11380–11386. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fugate, E.A.; Mueanngern, Y.; Baker, L.R. Photoelectrochemical CO2 Reduction to Acetate on Iron–Copper Oxide Catalysts. ACS Catal. 2017, 7, 177–180. [Google Scholar] [CrossRef]
- Ardao, I.; Hwang, E.T.; Zeng, A.-P. In Vitro Multienzymatic Reaction Systems for Biosynthesis. In Fundamentals and Application of New Bioproduction Systems; Zeng, A.-P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 153–184. [Google Scholar] [CrossRef]
- Kuwabata, S.; Tsuda, R.; Nishida, K.; Yoneyama, H. Electrochemical Conversion of Carbon Dioxide to Methanol with Use of Enzymes as Biocatalysts. Chem. Lett. 1993, 22, 1631–1634. [Google Scholar] [CrossRef]
- Kuwabata, S.; Tsuda, R.; Yoneyama, H. Electrochemical conversion of carbon dioxide to methanol with the assistance of formate dehydrogenase and methanol dehydrogenase as biocatalysts. J. Am. Chem. Soc. 1994, 116, 5437–5443. [Google Scholar] [CrossRef]
- Obert, R.; Dave, B.C. Enzymatic Conversion of Carbon Dioxide to Methanol: Enhanced Methanol Production in Silica Sol−Gel Matrices. J. Am. Chem. Soc. 1999, 121, 12192–12193. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, R.; Sivakumar, D.; Kondaveeti, S.; Kim, T.; Li, J.; Sung, B.H.; Cho, B.-K.; Kim, D.R.; Kim, S.C.; et al. Insights into Cell-Free Conversion of CO2 to Chemicals by a Multienzyme Cascade Reaction. ACS Catal. 2018, 8, 11085–11093. [Google Scholar] [CrossRef]
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbek, H.; DuBois, D.L.; Dupuis, M.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.A.; et al. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef]
- Schlager, S.; Dibenedetto, A.; Aresta, M.; Apaydin, D.H.; Dumitru, L.M.; Neugebauer, H.; Sariciftci, N.S. Biocatalytic and Bioelectrocatalytic Approaches for the Reduction of Carbon Dioxide using Enzymes. Energy Technol. 2017, 5, 812–821. [Google Scholar] [CrossRef]
- Fu, Q.; Xiao, S.; Li, Z.; Li, Y.; Kobayashi, H.; Li, J.; Yang, Y.; Liao, Q.; Zhu, X.; He, X.; et al. Hybrid solar-to-methane conversion system with a Faradaic efficiency of up to 96%. Nano Energy 2018, 53, 232–239. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Pastore, C. Biotechnology to develop innovative syntheses using CO2. Environ. Chem. Lett. 2005, 3, 113–117. [Google Scholar] [CrossRef][Green Version]
- Lee, S.Y.; Lim, S.Y.; Seo, D.; Lee, J.-Y.; Chung, T.D. Light-Driven Highly Selective Conversion of CO2 to Formate by Electrosynthesized Enzyme/Cofactor Thin Film Electrode. Adv. Energy Mater. 2016, 6, 1502207. [Google Scholar] [CrossRef]
- Kuk, S.K.; Ham, Y.; Gopinath, K.; Boonmongkolras, P.; Lee, Y.; Lee, Y.W.; Kondaveeti, S.; Ahn, C.; Shin, B.; Lee, J.-K.; et al. Continuous 3D Titanium Nitride Nanoshell Structure for Solar-Driven Unbiased Biocatalytic CO2 Reduction. Adv. Energy Mater. 2019, 9, 1900029. [Google Scholar] [CrossRef]
- El-Khouly, M.E.; El-Mohsnawy, E.; Fukuzumi, S. Solar energy conversion: From natural to artificial photosynthesis. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 36–83. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, J.; Chen, Y.; Huo, Q.; Li, W.; Wu, Y.; Sun, Y.; Zhang, Y.; Wang, X.; Jiang, Z. Unraveling and Manipulating of NADH Oxidation by Photogenerated Holes. ACS Catal. 2020, 10, 4967–4972. [Google Scholar] [CrossRef]
- Yadav, R.K.; Baeg, J.-O.; Oh, G.H.; Park, N.-J.; Kong, K.-J.; Kim, J.; Hwang, D.W.; Biswas, S.K. A Photocatalyst–Enzyme Coupled Artificial Photosynthesis System for Solar Energy in Production of Formic Acid from CO2. J. Am. Chem. Soc. 2012, 134, 11455–11461. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, R.K.; Ram, K.; Aguiar, A.; Koh, J.; Sobral, A.J.F.N. Graphene oxide modified cobalt metallated porphyrin photocatalyst for conversion of formic acid from carbon dioxide. J. CO2 Util. 2018, 27, 107–114. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, J.; Sun, Y.; Wu, Y.; Zhang, Y.; Cai, Z.; Chen, Y.; You, C.; Han, P.; Jiang, Z. Artificial Thylakoid for the Coordinated Photoenzymatic Reduction of Carbon Dioxide. ACS Catal. 2019, 9, 3913–3925. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Porous metal–organic frameworks as platforms for functional applications. Chem. Commun. 2011, 47, 3351–3370. [Google Scholar] [CrossRef]
- Nadar, S.S.; Vaidya, L.; Rathod, V.K. Enzyme embedded metal organic framework (enzyme–MOF): De novo approaches for immobilization. Int. J. Biol. Macromol. 2020, 149, 861–876. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, S.; Kang, H.; He, R.; Ning, Y.; Yu, Y.; Wang, M.; Chen, B. Construction of multi-enzyme cascade biomimetic carbon sequestration system based on photocatalytic coenzyme NADH regeneration. Renew. Energy 2020, 156, 107–116. [Google Scholar] [CrossRef]
- Yadav, R.K.; Oh, G.H.; Park, N.-J.; Kumar, A.; Kong, K.-J.; Baeg, J.-O. Highly Selective Solar-Driven Methanol from CO2 by a Photocatalyst/Biocatalyst Integrated System. J. Am. Chem. Soc. 2014, 136, 16728–16731. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cazelles, R.; Chen, Z.P.; Zhou, H.; Galarneau, A.; Antonietti, M. The bioinspired construction of an ordered carbon nitride array for photocatalytic mediated enzymatic reduction. Phys. Chem. Chem. Phys. 2014, 16, 14699–14705. [Google Scholar] [CrossRef] [PubMed]
- Amao, Y.; Kataoka, R. Methanol production from CO2 with the hybrid system of biocatalyst and organo-photocatalyst. Catal. Today 2018, 307, 243–247. [Google Scholar] [CrossRef]
- Yadav, D.; Yadav, R.K.; Kumar, A.; Park, N.-J.; Kim, J.Y.; Baeg, J.-O. Fullerene polymer film as a highly efficient photocatalyst for selective solar fuel production from CO2. J. Appl. Polym. Sci. 2020, 137, 48536. [Google Scholar] [CrossRef]
- Gu, F.; Wang, Y.; Meng, Z.; Liu, W.; Qiu, L. A coupled photocatalytic/enzymatic system for sustainable conversion of CO2 to formate. Catal. Commun. 2020, 136, 105903. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Y.; Zong, Y.; Li, J.; Yang, N.; Zhang, M.; Guo, Z.; Song, H. Construction of Functionally Compartmental Inorganic Photocatalyst–Enzyme System via Imitating Chloroplast for Efficient Photoreduction of CO2 to Formic Acid. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Ma, K.; Yehezkeli, O.; Park, E.; Cha, J.N. Enzyme Mediated Increase in Methanol Production from Photoelectrochemical Cells and CO2. ACS Catal. 2016, 6, 6982–6986. [Google Scholar] [CrossRef]
- King, P.W. Designing interfaces of hydrogenase–nanomaterial hybrids for efficient solar conversion. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1827, 949–957. [Google Scholar] [CrossRef]
- Sakimoto, K.K.; Kornienko, N.; Cestellos-Blanco, S.; Lim, J.; Liu, C.; Yang, P. Physical Biology of the Materials–Microorganism Interface. J. Am. Chem. Soc. 2018, 140, 1978–1985. [Google Scholar] [CrossRef]
- Nam, D.H.; Kuk, S.K.; Choe, H.; Lee, S.; Ko, J.W.; Son, E.J.; Choi, E.-G.; Kim, Y.H.; Park, C.B. Enzymatic photosynthesis of formate from carbon dioxide coupled with highly efficient photoelectrochemical regeneration of nicotinamide cofactors. Green Chem. 2016, 18, 5989–5993. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.J.; Svoboda, V.; Lau, C.; Martin, G.; Minteer, S.D. Enzyme catalysed biofuel cells. Energy Environ. Sci. 2008, 1, 320–337. [Google Scholar] [CrossRef]
- Choi, E.-G.; Yeon, Y.J.; Min, K.; Kim, Y.H. Communication—CO2 Reduction to Formate: An Electro-Enzymatic Approach Using a Formate Dehydrogenase from Rhodobacter capsulatus. J. Electrochem. Soc. 2018, 165, H446–H448. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Perspectives on poly(dopamine). Chem. Sci. 2013, 4, 3796–3802. [Google Scholar] [CrossRef]
- Shi, J.; Yang, C.; Zhang, S.; Wang, X.; Jiang, Z.; Zhang, W.; Song, X.; Ai, Q.; Tian, C. Polydopamine Microcapsules with Different Wall Structures Prepared by a Template-Mediated Method for Enzyme Immobilization. ACS Appl. Mater. Interfaces 2013, 5, 9991–9997. [Google Scholar] [CrossRef]
- Kuk, S.K.; Singh, R.K.; Nam, D.H.; Singh, R.; Lee, J.-K.; Park, C.B. Photoelectrochemical Reduction of Carbon Dioxide to Methanol through a Highly Efficient Enzyme Cascade. Angew. Chem. Int. Ed. 2017, 56, 3827–3832. [Google Scholar] [CrossRef]
- Zhang, L.; Vilà, N.; Kohring, G.-W.; Walcarius, A.; Etienne, M. Covalent Immobilization of (2,2′-Bipyridyl) (Pentamethylcyclopentadienyl)-Rhodium Complex on a Porous Carbon Electrode for Efficient Electrocatalytic NADH Regeneration. ACS Catal. 2017, 7, 4386–4394. [Google Scholar] [CrossRef]
- Srikanth, S.; Alvarez-Gallego, Y.; Vanbroekhoven, K.; Pant, D. Enzymatic Electrosynthesis of Formic Acid through Carbon Dioxide Reduction in a Bioelectrochemical System: Effect of Immobilization and Carbonic Anhydrase Addition. ChemPhysChem 2017, 18, 3174–3181. [Google Scholar] [CrossRef]
- Sokol, K.P.; Robinson, W.E.; Oliveira, A.R.; Warnan, J.; Nowaczyk, M.M.; Ruff, A.; Pereira, I.A.C.; Reisner, E. Photoreduction of CO2 with a Formate Dehydrogenase Driven by Photosystem II Using a Semi-artificial Z-Scheme Architecture. J. Am. Chem. Soc. 2018, 140, 16418–16422. [Google Scholar] [CrossRef]
- Amao, Y.; Fujimura, M.; Miyazaki, M.; Tadokoro, A.; Nakamura, M.; Shuto, N. A visible-light driven electrochemical biofuel cell with the function of CO2 conversion to formic acid: Coupled thylakoid from microalgae and biocatalyst immobilized electrodes. New J. Chem. 2018, 42, 9269–9280. [Google Scholar] [CrossRef]
- Srikanth, S.; Maesen, M.; Dominguez-Benetton, X.; Vanbroekhoven, K.; Pant, D. Enzymatic electrosynthesis of formate through CO2 sequestration/reduction in a bioelectrochemical system (BES). Bioresour. Technol. 2014, 165, 350–354. [Google Scholar] [CrossRef]
- Ali, I.; Gill, A.; Omanovic, S. Direct electrochemical regeneration of the enzymatic cofactor 1,4-NADH employing nano-patterned glassy carbon/Pt and glassy carbon/Ni electrodes. Chem. Eng. J. 2012, 188, 173–180. [Google Scholar] [CrossRef]
- Xiu, Y.; Zhang, X.; Feng, Y.; Wei, R.; Wang, S.; Xia, Y.; Cao, M.; Wang, S. Peptide-mediated porphyrin based hierarchical complexes for light-to-chemical conversion. Nanoscale 2020, 12, 15201–15208. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Zhang, X.; Yu, D.; Jiang, X.; Wang, Z.; Cao, M.; Xia, Y.; Liu, H. Short peptide-regulated aggregation of porphyrins for photoelectric conversion. Sustain. Energy Fuels 2019, 3, 529–538. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Patil, A.J.; Sun, S.; Tian, L.; Zhang, D.; Cao, M.; Mann, S. Design and construction of artificial photoresponsive protocells capable of converting day light to chemical energy. J. Mater. Chem. A 2017, 5, 24612–24616. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Amao, Y.; Shuto, N. Formate dehydrogenase–viologen-immobilized electrode for CO2 conversion, for development of an artificial photosynthesis system. Res. Chem. Intermed. 2014, 40, 3267–3276. [Google Scholar] [CrossRef]
- Miyatani, R.; Amao, Y. Photochemical synthesis of formic acid from CO2 with formate dehydrogenase and water-soluble zinc porphyrin. J. Mol. Catal. B Enzym. 2004, 27, 121–125. [Google Scholar] [CrossRef]
- Jayathilake, B.S.; Bhattacharya, S.; Vaidehi, N.; Narayanan, S.R. Efficient and Selective Electrochemically Driven Enzyme-Catalyzed Reduction of Carbon Dioxide to Formate using Formate Dehydrogenase and an Artificial Cofactor. Acc. Chem. Res. 2019, 52, 676–685. [Google Scholar] [CrossRef]
- Ishibashi, T.; Higashi, M.; Ikeda, S.; Amao, Y. Photoelectrochemical CO2 Reduction to Formate with the Sacrificial Reagent Free System of Semiconductor Photocatalysts and Formate Dehydrogenase. ChemCatChem 2019, 11, 6227–6235. [Google Scholar] [CrossRef]

| Photocathode a | Condition b | Efficiency c | Ref. |
|---|---|---|---|
| p+-n-n+-Si/TiO2 + Cu/Ag | 100 mW cm−2, 0.1 M CsHCO3 | C2H4, 10–25%, −8 mA cm−2 at −0.4 V vs. reversible hydrogen electrode (RHE) for 20 days | [94] |
| p-Si NWs + Sn | 100 mW cm−2, 0.1 M KHCO3 | HCOOH, 88%, 18.9 μmol h−1 cm−2, −0.875 V vs. RHE for 3 h | [95] |
| CuO + Cu2O | 70 mW cm−2, 0.1 M NaHCO3 | CH3OH, 95%, 85 mM at −0.2 V vs. standard hydrogen electrode (SHE) after 1.5 h | [96] |
| Si/GaN-NPhN4-Ru(CP RuCt | 100 mW cm−2, 0.05 M NaHCO3 | HCOOH, 35–64%, −1.1 mA cm−2 at −0.25 V vs. RHE for 20 h | [97] |
| p-n+-Si + SnO2 NW | 100 mW cm−2, 0.1 M KHCO3 | HCOOH, 59.2%, −18 mA cm−2 at −0.4 V vs. RHE for 3 h | [98] |
| Co3O4/CA + Ru(bpy)2dppz | 9 mW cm−2, 0.1 M NaHCO3 | HCOOH, 86%, 110 μmol h−1 cm−2 at −0.60 V vs. normal hydrogen electrode (NHE) for 8 h | [99] |
| FTO/TiO2/Cu2O + Ru-BNAH | 100 mW cm−2, 0.1 M KCl | HCOOH, NA, 409.5 umol at −0.9 V vs. NHE after 8 h | [100] |
| p-Si + Bi | 50 mW cm−2, 0.5 M KHCO3 | HCOOH, 70–95%, ~−4 mA cm−2 at −0.32 V vs. RHE for 7 h | [92] |
| Fe2O3 NTs + Cu2O | 100 mW cm−2, 0.1 M KHCO3 | CH3OH, 93%, 6 h, 4.94 mmol L−1 cm−2 at −1.3Vvs. SCE for 6 h | [101] |
| FTO/CuFeO2 + CuO | 100 mW cm−2, 0.1 M NaHCO3 | CH3COOH, 80%, 142 μM at −0.4 V vs. Ag/AgCl after 2 h | [102] |
| Photocatalyst | Enzyme | Cofactors | Efficiency a | Ref. |
|---|---|---|---|---|
| CCG-IP | FateDH, FaldDH, ADH | NADH + [Cp*Rh(bpy)H2O]2+Rh + TEOA | CH3OH, 11.21 μM after 1 h | [122] |
| CNA | FateDH, FaldDH, YADH | NADH + [Cp*Rh(bpy)H2O]2+Rh + TEOA | CH3OH, 0.21 mM min−1 | [123] |
| H2TPPS | FDH, AldDH, ADH | MV2+ | CH3OH, 6.8 μM after 100 min | [124] |
| C60 polymer film | FDH | NADH + TEOA | HCOOH, 239.46 μM after 2 h | [125] |
| TiO2 | FDH | NADH | HCOOH, 1.634 mM after 4.5 h | [126] |
| C3N4(TPE-C3N4) | MAF-7@FDH | NADH + Rh + TEOA | HCOOH, 16.75 mM after 9 h | [127] |
| CCGCMAQSP | FateDH, FaldDH, ADH | NADH + [Cp*Rh(bpy)H2O]2+ Rh + TEOA | CH3OH, 110.55 μM after 2 h | [116] |
| CdS QDs+PTi | ClFDH | NADH + Rh | HCOOH, 1500 μM h−1 | [118] |
| Photoanode | Photocathode | Efficiency a | Ref. |
|---|---|---|---|
| Co-Pi/Fe2O3 | ITO/FDH | HCOOH, 6.4 μM h−1 | [131] |
| CoPi/BiVO4 | EC-PDA | HCOOH, FE: 99.18% | [112] |
| Co-Pi/αoFe2O3 | BiFeO3-CcFDH/PcFaldDH/YADH | CH3OH, 220 μM h−1 | [137] |
| FTO/IO-TiO2/dPP/POs-PSII | FTO/IO-TiO2/FDH | HCOOH, 0.185 μM cm–2 | [140] |
| FTO/FeOOH/BiVO4 | FTO/3D TiN-ClFDH | HCOOH, 0.78 μM h−1, FE: 77.3% | [113] |
| TK/TiO2 | FDH-CH3V(CH2)9COOH | HCOOH, 30.0 nM after 3 h | [141] |
| Plain graphite rod | Pt-FDH | HCOOH, 15.49 μM mg Enzyme−1 min−1 | [142] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Xiu, Y.; Liu, F.; Liang, Y.; Wang, S. Research Progress in Conversion of CO2 to Valuable Fuels. Molecules 2020, 25, 3653. https://doi.org/10.3390/molecules25163653
Xu L, Xiu Y, Liu F, Liang Y, Wang S. Research Progress in Conversion of CO2 to Valuable Fuels. Molecules. 2020; 25(16):3653. https://doi.org/10.3390/molecules25163653
Chicago/Turabian StyleXu, Luyi, Yang Xiu, Fangyuan Liu, Yuwei Liang, and Shengjie Wang. 2020. "Research Progress in Conversion of CO2 to Valuable Fuels" Molecules 25, no. 16: 3653. https://doi.org/10.3390/molecules25163653
APA StyleXu, L., Xiu, Y., Liu, F., Liang, Y., & Wang, S. (2020). Research Progress in Conversion of CO2 to Valuable Fuels. Molecules, 25(16), 3653. https://doi.org/10.3390/molecules25163653