Release of Melamine and Formaldehyde from Melamine-Formaldehyde Plastic Kitchenware
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals
2.3. Release Tests
2.4. Analytical Methods
2.4.1. Melamine
2.4.2. Formaldehyde
2.5. CP/MAS 13C-NMR
3. Results and Discussion
3.1. Stirring of Spoons During the Release Test
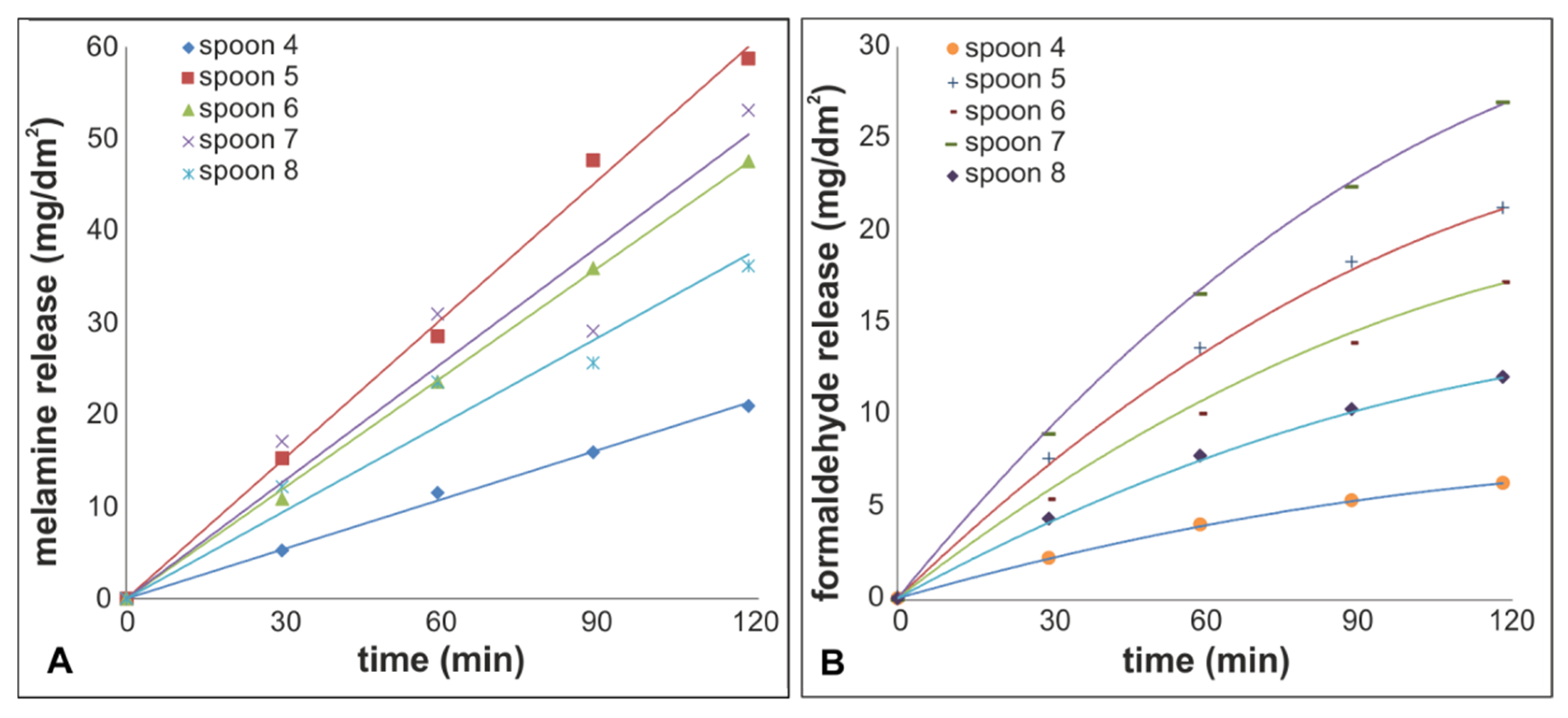
3.2. Release into Food Simulants and Food
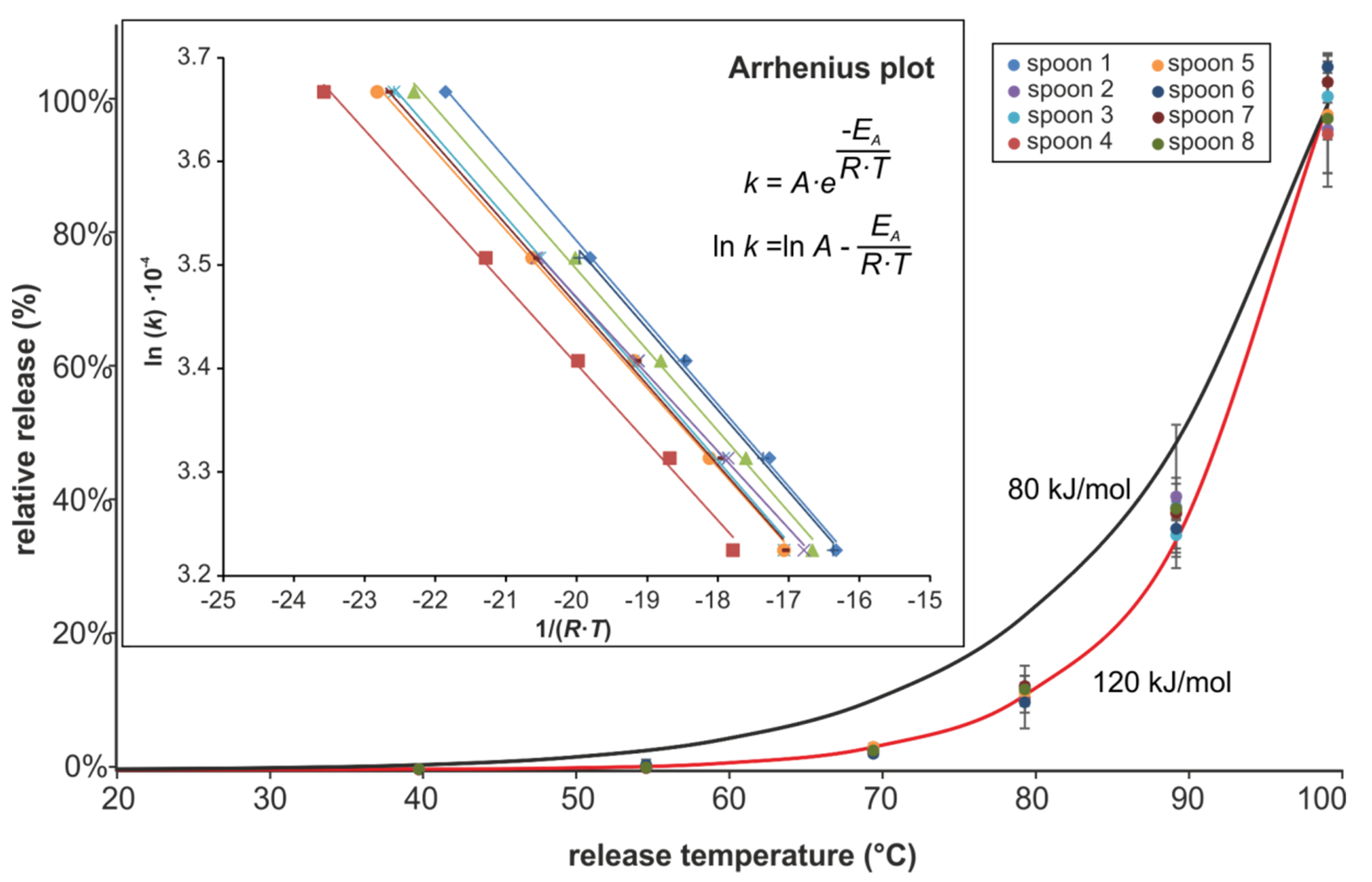
3.3. Temperature Dependence
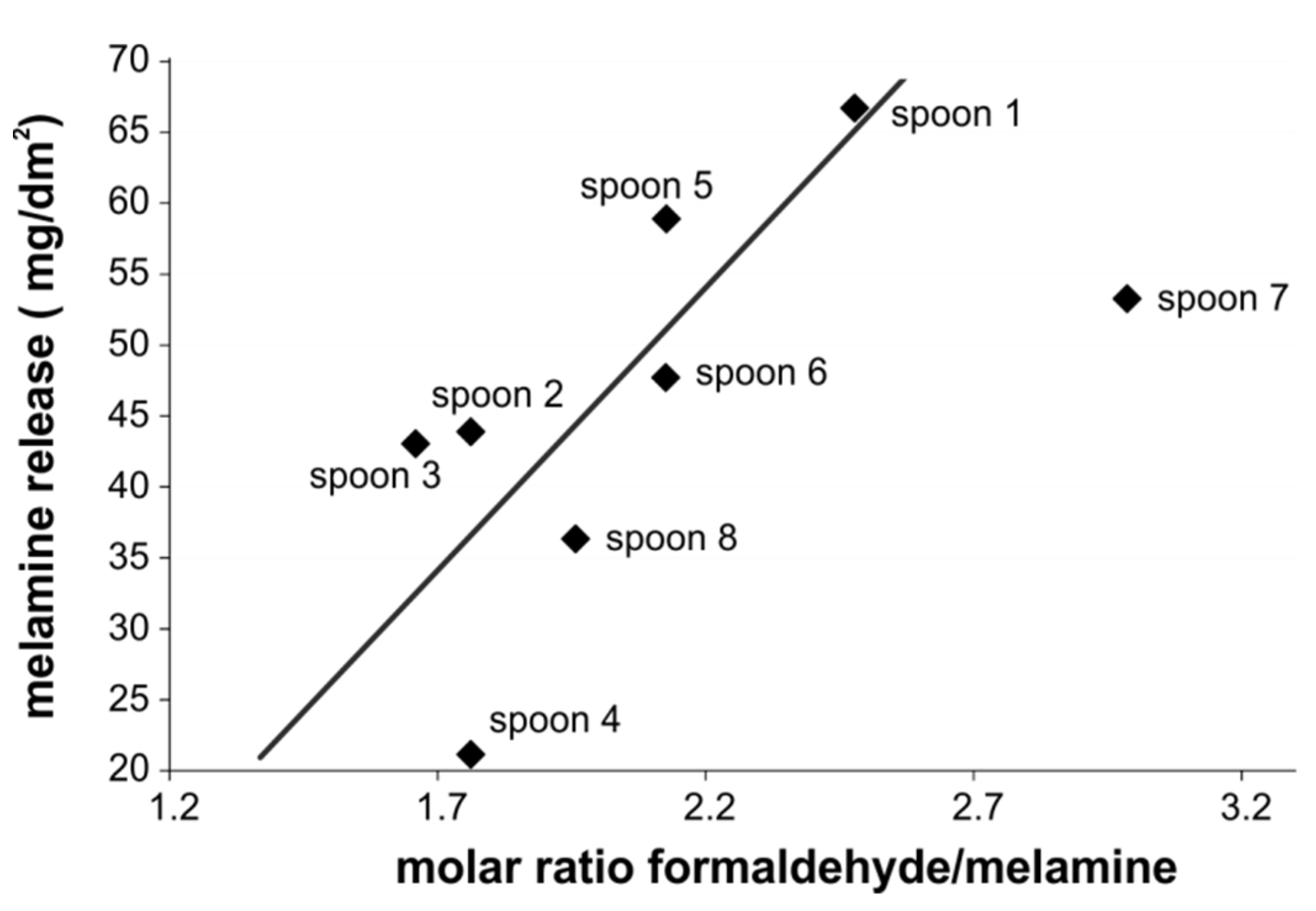
3.4. Ratio of Formaldehyde/Melamine
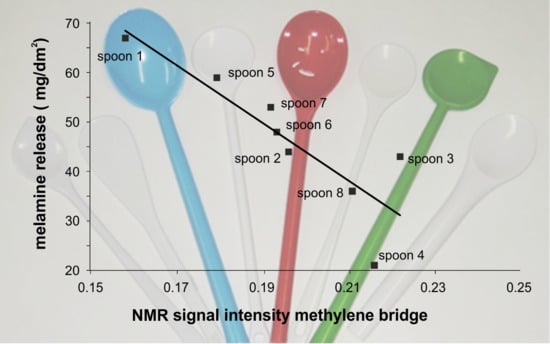
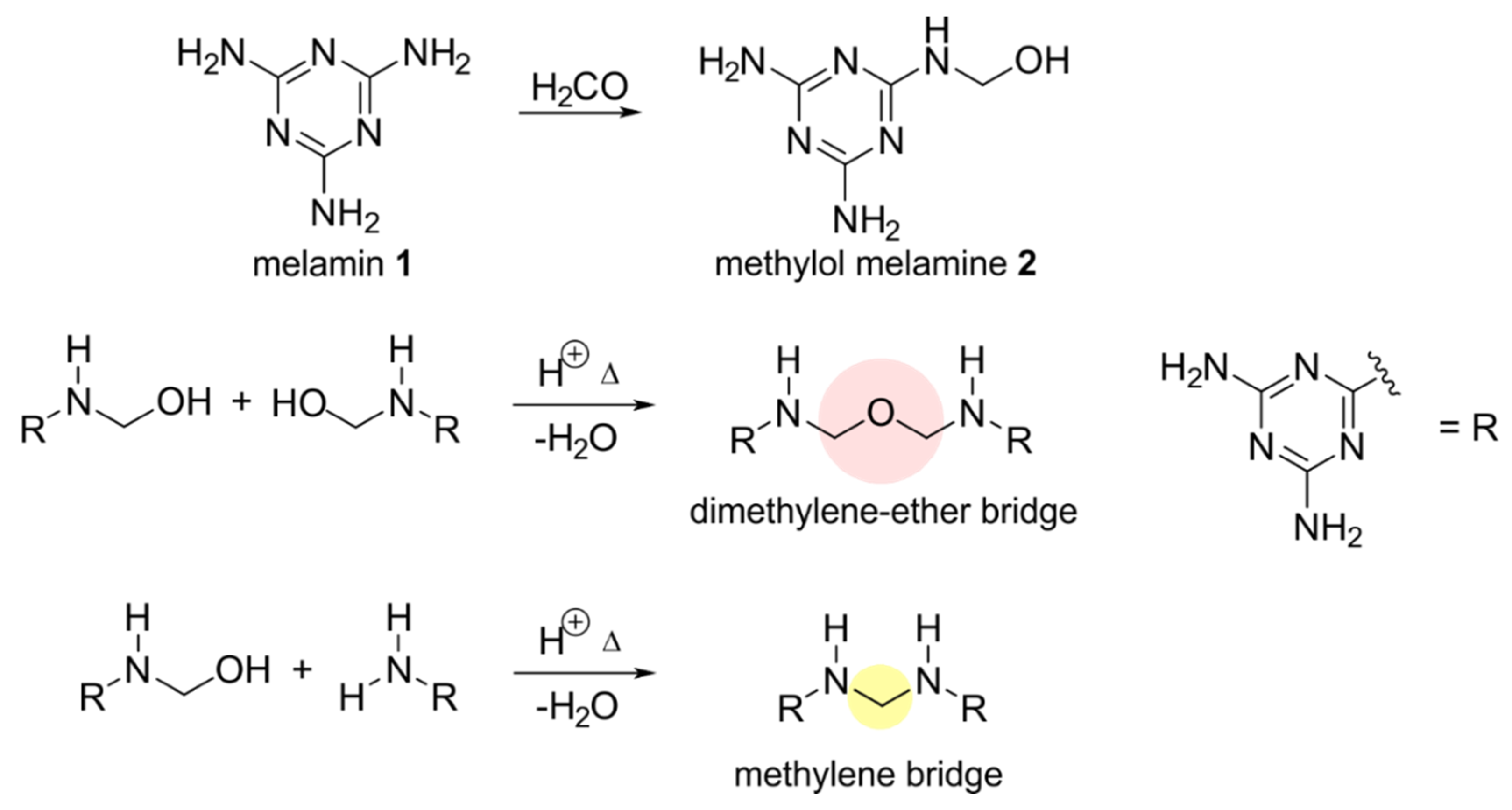
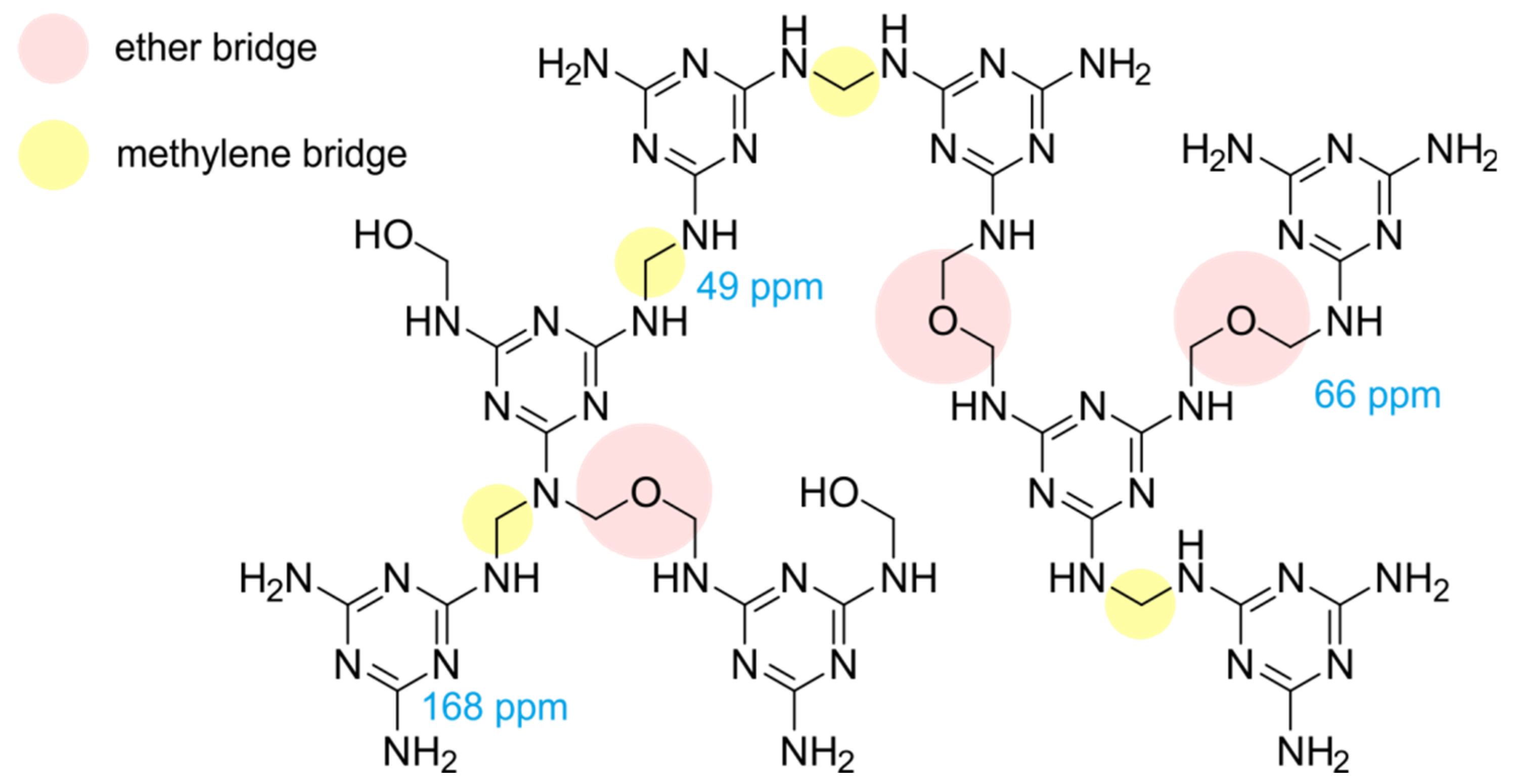
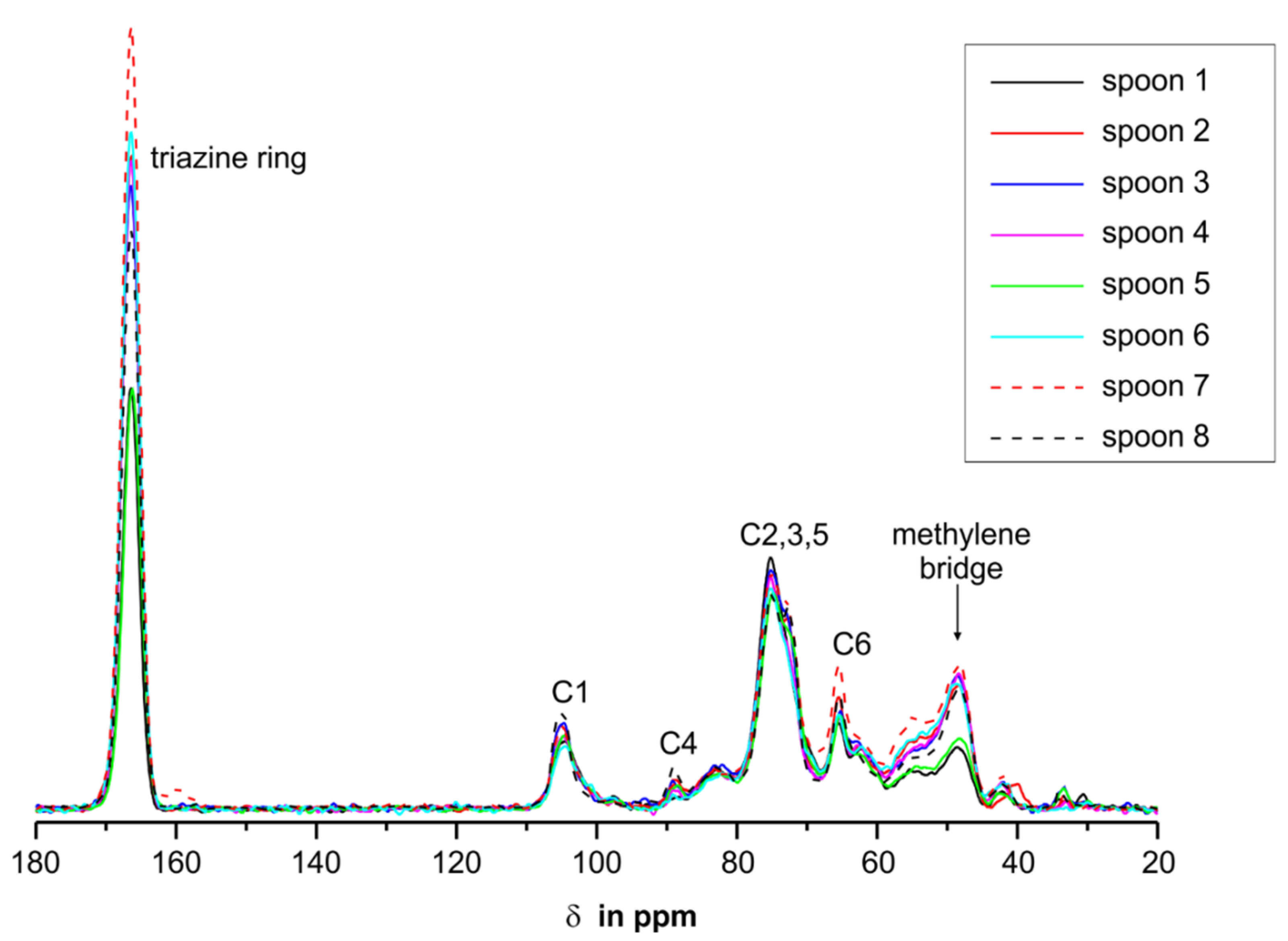
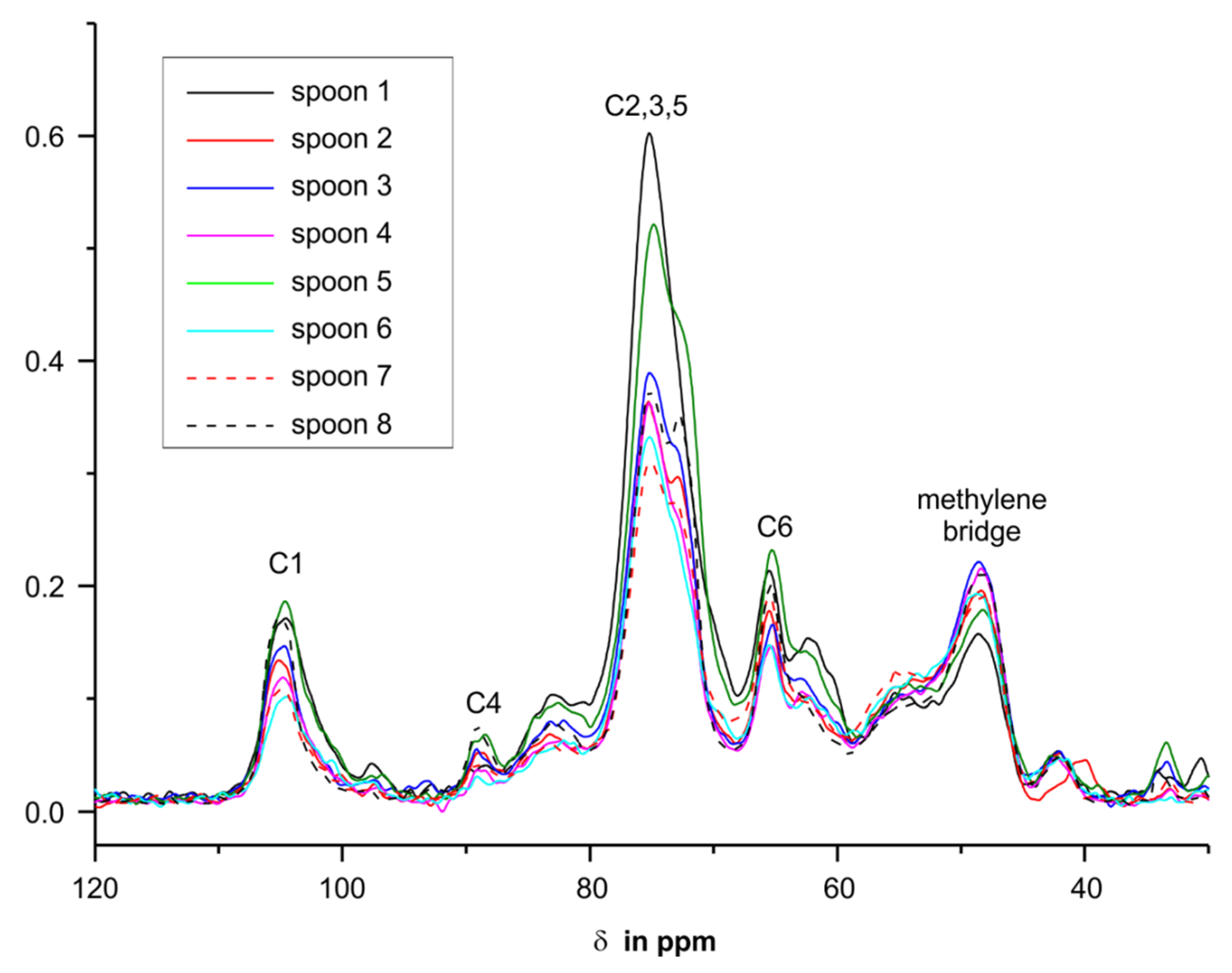
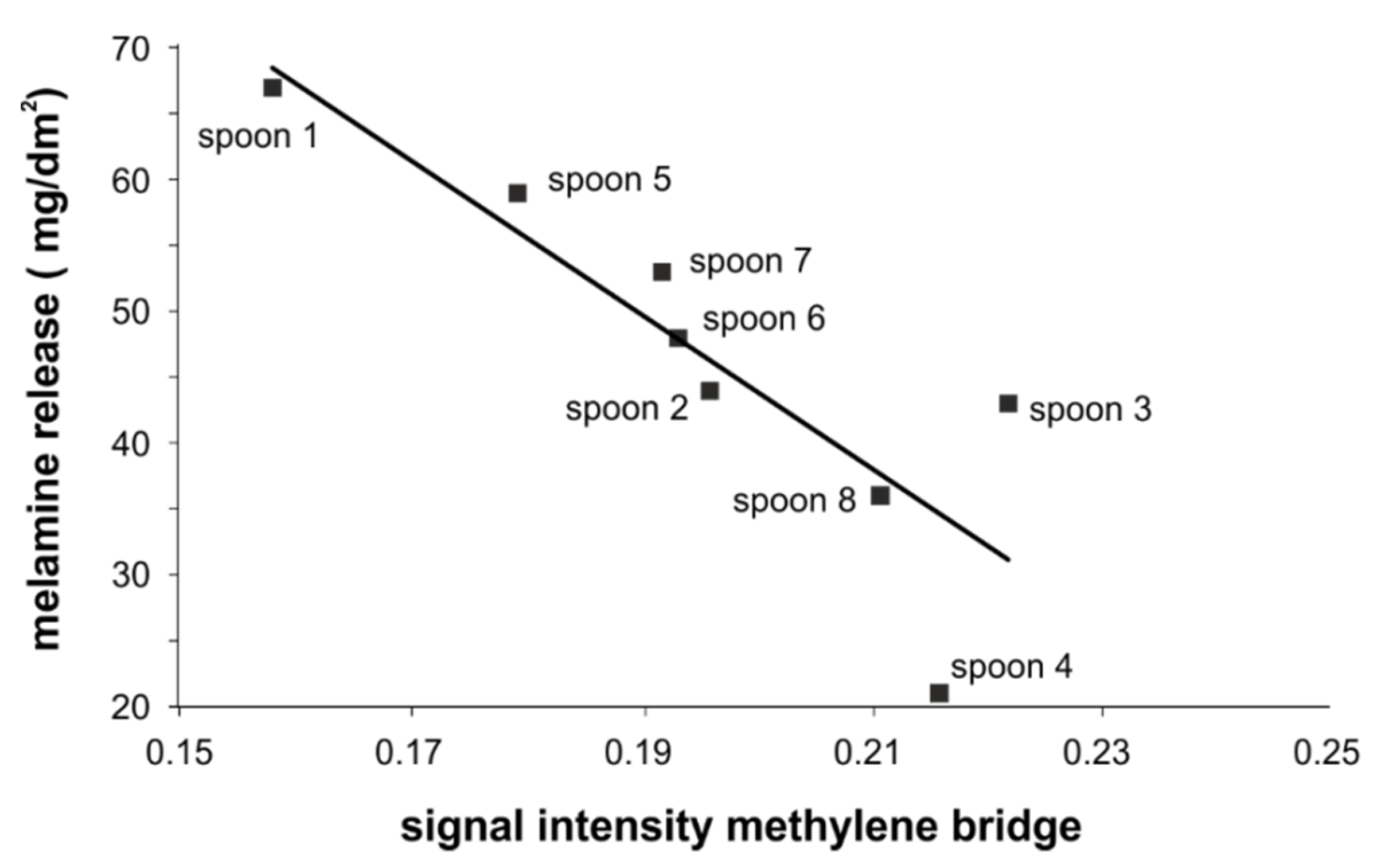
3.5. NMR Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merline, D.J.; Vukusic, S.; Abdala, A.A. Melamine formaldehyde: Curing studies and reaction mechanism. Polym. J. 2013, 45, 413–419. [Google Scholar] [CrossRef]
- Bauer, D.R.; Dickie, R.A.; Koenig, J.L. Magic-angle 13C-NMR of cured and degraded acrylic copolymer/melamine formaldehyde coatings. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 2009–2020. [Google Scholar] [CrossRef]
- Kamide, K.; Okajima, K.; Kowsake, K.; Matsui, T. CP/MASS 13C NMR Spectra of Cellulose Solids: An Explanation by the Intramolecular Hydrogen Bond Concept. Polym. J. 1985, 17, 701–706. [Google Scholar] [CrossRef]
- Ebdon, J.R.; Hunt, B.J.; O’Rourke, W.T.S.; Parkin, J. Characterisation of some melamine-formaldehyde condensates and some cured resins by 1H, 13C and 15N n.m.r. spectroscopy. Br. Polym. J. 1988, 20, 327–334. [Google Scholar] [CrossRef]
- Angelatos, A.S.; Burgar, M.I.; Dunlop, N.; Separovic, F. NMR structural elucidation of amino resins. J. Appl. Polym. Sci. 2004, 91, 3504–3512. [Google Scholar] [CrossRef]
- Mercer, A.T.; Pizzi, A. A 13C-NMR analysis method for MF and MUF resins strength and formaldehyde emission from wood particleboard. II. MF resins. J. Appl. Polym. Sci. 1996, 61, 1697–1702. [Google Scholar] [CrossRef]
- Scheepers, M.L.; Gelan, J.M.; Carleer, R.A.; Adriaensens, P.J.; Vanderzande, D.J.; Kip, B.J.; Brandts, P.M. Investigation of melamine-formaldehyde cure by Fourier transform Raman spectroscopy. Vib. Spectrosc. 1993, 6, 55–69. [Google Scholar] [CrossRef]
- Meier, R.J.; Tiller, A.; Vanhommerig, S.A.M. Molecular Modeling of Melamine-Formaldehyde Resins. 2. Vibrational Spectra of Methylolmelamines and Bridged Methylolmelamines. J. Phys. Chem. 1995, 99, 5457–5464. [Google Scholar] [CrossRef]
- Weiss, S.; Urdl, K.; Mayer, H.A.; Zikulnig-Rusch, E.M.; Kandelbauer, A. IR spectroscopy: Suitable method for determination of curing degree and crosslinking type in melamine–formaldehyde resins. J. Appl. Polym. Sci. 2019, 136, 47691. [Google Scholar] [CrossRef]
- Chien, C.-Y.; Wu, C.-F.; Liu, C.-C.; Chen, B.-H.; Huang, S.-P.; Chou, Y.-H.; Chang, A.-W.; Lee, H.-H.; Pan, C.-H.; Wu, W.-J.; et al. High melamine migration in daily-use melamine-made tableware. J. Hazard. Mater. 2011, 188, 350–356. [Google Scholar] [CrossRef]
- Manava, H.M.; Gurbuzb, O.; Cumbulc, D.; Tokatc, I.E.; Korkmazd, E.; Dagdelene, A.F. Investigation of overall and melamine migration from melamine bowls. Acta Aliment. 2019, 48, 187–195. [Google Scholar] [CrossRef]
- Ishiwata, H.; Inoue, T.; Tanimura, A. Migration of melamine and formaldehyde from tableware made of melamine resin. Food Addit. Contam. 1986, 3, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Hizo, C.B.; Ong, A.M.; Alba, O.M.; Ishiwata, H. Release of Formaldehyde and Melamine from Melamine Tableware Manufactured in the Philippines. J. Food Prot. 1992, 55, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, J.R. Melamine and the Global Implications of Food Contamination. N. Engl. J. Med. 2008, 359, 2745–2748. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xiao, J.; Yang, D.-J.; Wang, Z.-T.; Jiang, D.-G.; Fang, C.-R.; Yang, J. Study on Migration of Melamine from Food Packaging Materials on Markets. Biomed. Environ. Sci. 2009, 22, 104–108. [Google Scholar] [CrossRef]
- Tyan, Y.-C.; Yang, M.-H.; Jong, S.-B.; Wang, C.-K.; Shiea, J. Melamine contamination. Anal. Bioanal. Chem. 2009, 395, 729–735. [Google Scholar] [CrossRef]
- Bradley, E.L.; Castle, L.; Day, J.S.; Ebner, I.; Ehlert, K.; Helling, R.; Koster, S.; Leak, J.; Pfaff, K. Comparison of the migration of melamine from melamine–formaldehyde plastics (‘melaware’) into various food simulants and foods themselves. Food Addit. Contam. Part A Chem. 2010, 27, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.L.; Castle, L.; Day, J.S.; Leak, J. Migration of melamine from can coatings cross-linked with melamine-based resins, into food simulants and foods. Food Addit. Contam. Part A Chem. 2011, 28, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Chik, Z.; Haron, D.E.M.; Ahmad, E.D.; Taha, H.; Mustafa, A.M. Analysis of melamine migration from melamine food contact articles. Food Addit. Contam. Part. A Chem. 2011, 28, 967–973. [Google Scholar] [CrossRef]
- Garcia Ibarra, V.; Rodriguez Bernaldo de Quiros, A.; Sendon, R. Study of melamine and formaldehyde migration from melamine tableware. Eur. Food Res. Technol. 2016, 242, 1187–1199. [Google Scholar] [CrossRef]
- Tittlemier, S.A. Methods for the analysis of melamine and related compounds in foods: A review. Food Addit. Contam. Part A Chem. 2010, 27, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Bueno, M.J.; Cimmino, S.; Silvestre, C.; Tadeo, J.L.; Garcia-Valcarcel, A.I.; Fernandez-Alba, A.R.; Hernando, M.D. Characterization of non-intentionally added substances (NIAS) and zinc oxide nanoparticle release from evaluation of new antimicrobial food contact materials by both LC-QTOF-MS, GC-QTOF-MS and ICP-MS. Anal. Methods 2016, 8, 7209–7216. [Google Scholar] [CrossRef]
- Lu, Y.; Xia, Y.; Liu, G.; Pan, M.; Li, M.; Lee, N.A.; Wang, S. A Review of Methods for Detecting Melamine in Food Samples. Crit. Rev. Anal. Chem. 2017, 47, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on Melamine in Food and Feed. EFSA J. 2010, 8, 1573. [CrossRef]
- Commission Regulation (EU) No 1282/2011 of 28 November 2011 amending and correcting Commission Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food Text with EEA relevance. Off. J. Eur. Union 2011, 54, 22–29.
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 54, 1–89.
- Fillable Articles Made from Melamine Formaldehyde Resin, Such as Coffee-to-Go Cups Sold as ‘Bambooware’, May Leak Harmful Substances Into Hot Foods; BfR Opinion No. 046/2019; German Federal Institute for Risk Assessment: Berlin, Germany, 2019.
- Commission Regulation (EU) No 284/2011 of 22 March 2011 laying down specific conditions and detailed procedures for the import of polyamide and melamine plastic kitchenware originating in or consigned from the People’s Republic of China and Hong Kong Special Administrative Region, China. Off. J. Eur. Union 2011, 54, 25–29.
- Beldi, G.; Jakubowska, N.; Robouch, P.; Hoekstra, E. Testing Conditions for Kitchenware Articles in Contact with Foodstuffs—Part 1: Plastics; Technical Report JRC116750; European Union: Brussels, Belgium, 2019. [Google Scholar]
- Mannoni, V.; Padula, G.; Panico, O.; Maggio, A.; Arena, C.; Milana, M.-R. Migration of formaldehyde and melamine from melaware and other amino resin tableware in real life service. Food Addit. Contam. Part A Chem. 2017, 34, 113–125. [Google Scholar] [CrossRef]
- Castle, L. 1—Chemical migration into food: An overview. In Chemical Migration and Food Contact Materials; Barnes, K.A., Sinclair, C.R., Watson, D.H., Eds.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2007; pp. 1–13. [Google Scholar]
- No, B.Y.; Kim, M.G. Syntheses and properties of low-level melamine-modified urea–melamine–formaldehyde resins. J. Appl. Polym. Sci. 2004, 93, 2559–2569. [Google Scholar] [CrossRef]
- Li, T.; Cao, M.; Zhang, B.; Yang, L.; Du, G. Effects of Molar Ratio and pH on the Condensed Structures of Melamine-Formaldehyde Polymers. Materials 2018, 11, 2571. [Google Scholar] [CrossRef]
- Chiavarini, M.; Del Fanti, N.; Bigatto, R. Compositive characterization of melamine-formaldehyde condensates by NMR spectroscopy. Die Angew. Makromol. Chem. 1976, 56, 15–25. [Google Scholar] [CrossRef]
- Danish Standards Foundation. Materials and Articles in Contact with Foodstuffs—Plastics Substances Subject to Limitation—Part 23: Determination of Formaldehyde and Hexamethylenetetramine in Food Simulants; CEN/TS 13130-23:2005; Danish Standards Foundation: Copenhagen, Denmark, 2005. [Google Scholar]
- Sander, S. Optimierung der Acetylacetonmethode in Anwendung auf Migrate von Lebensmittelbedarfsgegenständen. Bericht zur externen Praxisphase; Beuth University of Applied Sciences: Berlin, Germany, 2011. [Google Scholar]
- Beldi, G.; Jakubowska, N.; Derda, M.; Simoneau, C. Report of the Interlaboratory Comparison Organised by the European Reference Laboratory for Food Contact Material—ILC01 2011-Formaldehyde in Food Contact Migration Solution; European Union: Brussels, Belgium, 2012. [Google Scholar]
- Comite Europeen de Normalization. Materials and Articles in Contact with Foodstuffs—Plastics Substances Subject to Limitation—Part 1: Guide to Test Methods for the Specific Migration of Substances from Plastics to Foods and Food Simulants and the Determination of Substances in Plastics and the Selection of Conditions of Exposure to Food Simulants; DIN EN 13130-1:2004; Comite Europeen de Normalization: Brussels, Belgium, 2004. [Google Scholar]
- Release of Melamine and Formaldehyde from Dishes and Kitchen Utensils; BfR Opinion No 012/2011; German Federal Institute for Risk Assessment: Berlin, Germany, 2011.
- Coullerez, G.; Léonard, D.; Lundmark, S.; Mathieu, H.J. XPS and ToF-SIMS study of freeze-dried and thermally cured melamine–formaldehyde resins of different molar ratios. Surf. Interface Anal. 2000, 29, 431–443. [Google Scholar] [CrossRef]


| 3% Acetic Acid | Apple Juice | Plum Puree | Sauerkraut Juice | Strained Tomatoes | |||||
|---|---|---|---|---|---|---|---|---|---|
| pH | 2.7 | 3.5 | 3.4 | 3.5 | 4.2 | ||||
| spoon 1 | |||||||||
| 1. release | 20 | 30 | 147% | 24 | 119% | 45 | 221% | 12 | 60% |
| 2. release | 58 | 40 | 69% | 16 | 28% | 75 | 130% | 33 | 57% |
| 3. release | 67 | 42 | 64% | 16 | 25% | 72 | 108% | 48 | 73% |
| spoon 2 | |||||||||
| 1. release | 12 | 25 | 206% | 10 | 79% | 36 | 288% | 8 | 65% |
| 2. release | 39 | 32 | 82% | 16 | 41% | 50 | 129% | 26 | 68% |
| 3. release | 44 | 23 | 53% | 16 | 36% | 49 | 112% | 32 | 74% |
| spoon 3 | |||||||||
| 1. release | 17 | 17 | 100% | 13 | 78% | 46 | 275% | 13 | 77% |
| 2. release | 46 | 30 | 66% | 20 | 44% | 65 | 140% | 32 | 68% |
| 3. release | 43 | 34 | 79% | 19 | 45% | 54 | 127% | 41 | 97% |
| 3% Acetic Acid | Apple Juice | Plum Puree | Sauerkraut Juice | Strained Tomatoes | |||||
|---|---|---|---|---|---|---|---|---|---|
| pH | 2.7 | 3.5 | 3.4 | 3.5 | 4.2 | ||||
| spoon 1 | |||||||||
| 1. release | 24 | 18 | 75% | 5 | 20% | 26 | 109% | 2 | 10% |
| 2. release | 24 | 15 | 62% | 3 | 10% | 26 | 110% | 3 | 14% |
| 3. release | 28 | 16 | 57% | 2 | 7% | 25 | 88% | 7 | 23% |
| spoon 2 | |||||||||
| 1. release | 9 | 9 | 101% | 2 | 20% | 12 | 141% | 2 | 20% |
| 2. release | 12 | 8 | 65% | 2 | 14% | 12 | 95% | 2 | 15% |
| 3. release | 13 | 6 | 49% | 2 | 12% | 12 | 93% | 4 | 27% |
| spoon 3 | |||||||||
| 1. release | 8 | 7 | 79% | 2 | 22% | 14 | 174% | 2 | 27% |
| 2. release | 12 | 8 | 61% | 2 | 15% | 15 | 118% | 3 | 27% |
| 3. release | 12 | 8 | 67% | 2 | 14% | 13 | 108% | 3 | 28% |
| 3% Acetic Acid | 3% Acetic Acid | Deionized Water | ||||
|---|---|---|---|---|---|---|
| Test Conditions | Hot Plate | Oven a | Hot Plate | |||
| Melamine | Formaldehyde | Melamine | Formaldehyde | Melamine | Formaldehyde | |
| spoon 1 | 67 | 28 | 32 (47%) | 21 (75%) | 1.5 (2%) | 6.9 (25%) |
| spoon 2 | 44 | 13 | 28 (65%) | 12 (92%) | 0.4 (1%) | 2.6 (20%) |
| spoon 3 | 43 | 12 | 26 (61%) | 11 (90%) | 0.8 (2%) | 1.8 (15%) |
| spoon 4 | 21 | 6.3 | 16 (76%) | 6.8 (108%) | 0.3 (2%) | 1.2 (20%) |
| spoon 5 | 59 | 21 | 51 (86%) | 26 (121%) | 1.3 (2%) | 5.4 (26%) |
| spoon 6 | 48 | 17 | 24 (50%) | 11 (64%) | 0.5 (1%) | 2.0 (12%) |
| spoon 7 | 53 | 27 | 32 (61%) | 18 (68%) | 0.4 (1%) | 4.3 (16%) |
| spoon 8 | 36 | 12 | 38 (106%) | 15 (124%) | 1.4 (4%) | 2.6 (21%) |
| Test Conditions | 3% Acetic Acid | 3% Acetic Acid | Deionized Water | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hot Plate | Oven a | Hot Plate | |||||||
| Release Test Step | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| spoon 1 | 6.9 | 2.4 | 2.5 | 13 | 3.8 | 2.8 | 42 | 34 | 27 |
| spoon 2 | 4.1 | 1.9 | 1.8 | 6.0 | 2.2 | 1.8 | 90 | 53 | 38 |
| spoon 3 | 2.9 | 1.6 | 1.7 | 9.2 | 2.4 | 1.7 | 60 | 27 | 13 |
| spoon 4 | 7.8 | 2.3 | 1.8 | 6.7 | 2.2 | 1.8 | 52 | 40 | 21 |
| spoon 5 | 6.3 | 2.6 | 2.1 | 8.8 | 2.7 | 2.1 | 86 | 48 | 25 |
| spoon 6 | 6.1 | 2.5 | 2.1 | 7.7 | 2.4 | 1.9 | 83 | 57 | 25 |
| spoon 7 | 4.3 | 3.4 | 3.0 | 10 | 3.0 | 2.4 | 140 | 102 | 66 |
| spoon 8 | 3.5 | 2.0 | 2.0 | 4.0 | 1.8 | 1.6 | 27 | 19 | 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebner, I.; Haberer, S.; Sander, S.; Kappenstein, O.; Luch, A.; Bruhn, T. Release of Melamine and Formaldehyde from Melamine-Formaldehyde Plastic Kitchenware. Molecules 2020, 25, 3629. https://doi.org/10.3390/molecules25163629
Ebner I, Haberer S, Sander S, Kappenstein O, Luch A, Bruhn T. Release of Melamine and Formaldehyde from Melamine-Formaldehyde Plastic Kitchenware. Molecules. 2020; 25(16):3629. https://doi.org/10.3390/molecules25163629
Chicago/Turabian StyleEbner, Ingo, Steffi Haberer, Stefan Sander, Oliver Kappenstein, Andreas Luch, and Torsten Bruhn. 2020. "Release of Melamine and Formaldehyde from Melamine-Formaldehyde Plastic Kitchenware" Molecules 25, no. 16: 3629. https://doi.org/10.3390/molecules25163629
APA StyleEbner, I., Haberer, S., Sander, S., Kappenstein, O., Luch, A., & Bruhn, T. (2020). Release of Melamine and Formaldehyde from Melamine-Formaldehyde Plastic Kitchenware. Molecules, 25(16), 3629. https://doi.org/10.3390/molecules25163629