Base-Promoted Annulation of Amidoximes with Alkynes: Simple Access to 2,4-Disubstituted Imidazoles
Abstract
1. Introduction
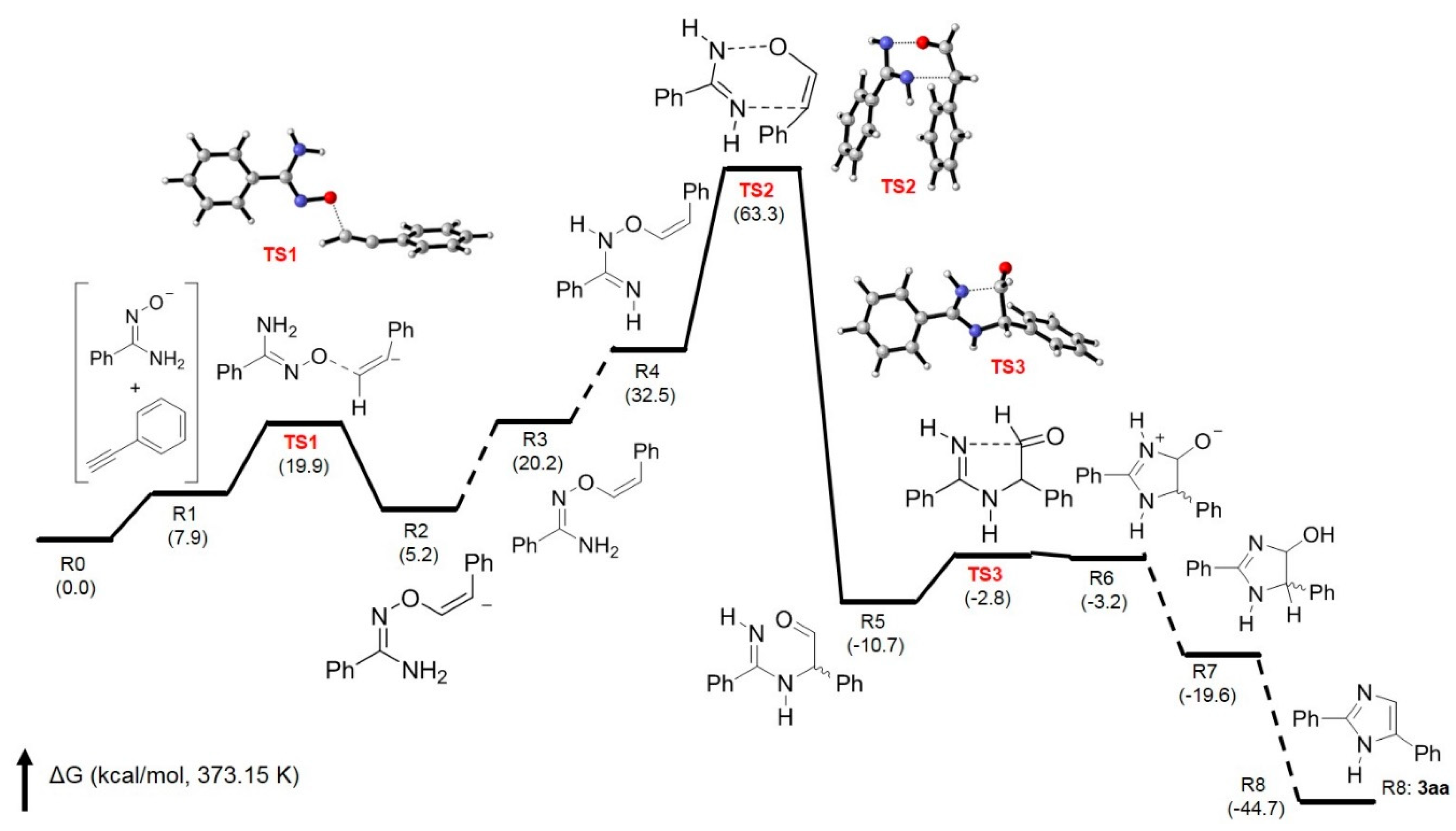
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Typical Experimental Procedure for the Synthesis of 2,4-Diphenyl-1H-imidazole (3aa)
3.3. Characterization Data of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Ward, K.E.; Hume, A.L. Olmesartan (Benicar) for hypertension. Am. Fam. Physician 2005, 72, 673–674. [Google Scholar]
- Ianiro, G.; Bibbo, S.; Montalto, M.; Ricci, R.; Gasbarrini, A.; Cammarota, G. Systematic review: Sprue-like enteropathy associated with olmesartan. Aliment. Pharmacol. Ther. 2014, 40, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.K.; McKenna, B.J. Olmesartan-associated enteropathy: A review of clinical and histologic findings. Arch. Pathol. Lab. Med. 2015, 139, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Redon, J.; Weber, M.A.; Reimitz, P.-E.; Wang, J.-G. Comparative effectiveness of an angiotensin receptor blocker, olmesartan medoxomil, in older hypertensive patients. J. Clin. Hypertens. 2018, 20, 356–365. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, M.; Caffe, S.E.; Michalak, R.A.; Reid, J.L. Losartan, an orally active angiotensin (AT1) receptor antagonist: A review of its efficacy and safety in essential hypertension. Pharmacol. Ther. 1997, 74, 181–194. [Google Scholar] [CrossRef]
- Conlin, P.R. Efficacy and safety of angiotensin receptor blockers: A review of Losartan in essential hypertension. Curr. Ther. Res. 2001, 62, 79–91. [Google Scholar] [CrossRef]
- Rubio-Guerra, A.F.; Garro-Almendaro, A.K.; Elizalde-Barrera, C.I.; Suarez-Cuenca, J.A.; Duran-Salgado, M.B. Effect of losartan combined with amlodipine or with a thiazide on uric acid levels in hypertensive patients. Ther. Adv. Cardiovasc. Dis. 2017, 11, 57–62. [Google Scholar] [CrossRef]
- Oxford, A.W.; Bell, J.A.; Kilpatrick, G.J.; Ireland, S.J.; Tyers, M.B. Ondansetron and related 5-HT, antagonists: Recent advances. Prog. Med. Chem. 1992, 29, 239–270. [Google Scholar]
- Del Favero, A.; Roila, F.; Tonato, M. Reducing chemotherapy-induced nausea and vomiting. Current perspectives and future possibilities. Drug Saf. 1993, 9, 410–428. [Google Scholar] [CrossRef]
- Christofaki, M.; Papaioannou, A. Ondansetron: A review of pharmacokinetics and clinical experience in postoperative nausea and vomiting. Expert. Opin. Drug Metab. Toxicol. 2014, 10, 437–444. [Google Scholar] [CrossRef]
- Dewinter, G.; Staelens, W.; Veef, E.; Teunkens, A.; Van de Velde, M.; Rex, S. Simplified algorithm for the prevention of postoperative nausea and vomiting: A before-and-after study. Br. J. Anaesth. 2018, 120, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Nikbin, N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011, 7, 442–495. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Sharma, A.; Singh, R. Imidazoles as promising scaffolds for antibacterial activity: A review. Mini Rev. Med. Chem. 2013, 13, 1812–1835. [Google Scholar]
- Zhang, L.; Peng, X.-M.; Damu, G.L.V.; Geng, R.-X.; Cheng-He Zhou, C.-H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Ali, I.; Lonea, M.N.; Aboul-Enein, H.Y. Imidazoles as potential anticancer agents. Med. Chem. Commun. 2017, 8, 1742–1773. [Google Scholar] [CrossRef] [PubMed]
- Bauman, J.E., Jr.; Wang, J.C. Imidazole complexes of Nickel (II), Copper (II), Zinc (II) and Silver (I). Inorg. Chem. 1963, 3, 368–373. [Google Scholar] [CrossRef]
- Gómez, E.; Huertos, W.A.; Pérez, J.; Riera, L.; Menéndez-Velázquez, A. Organometallic complexes with terminal imidazolato ligands and their use as metalloligands. Inorg. Chem. 2010, 49, 9527–9534. [Google Scholar] [CrossRef]
- Kamijo, S.; Yamamoto, Y. Recent progress in the catalytic synthesis of imidazoles. Chem. Asian J. 2007, 2, 568–578. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Rossi, R. Synthesis and biological activity of vicinal diaryl-substituted 1H-imidazoles. Tetrahedron 2007, 63, 4571–4624. [Google Scholar] [CrossRef]
- Daraji, D.G.; Prajapati, N.P.; Patel, H.D. Synthesis and applications of 2-substituted imidazole and its derivatives: A review. J. Heterocycl. Chem. 2019, 56, 2299–2317. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Hu, W.-P.; Yan, P.-C.; Senadi, G.C.; Wang, J.-J. Metal-free, acid-promoted synthesis of imidazole derivatives via a multicomponent reaction. Org. Lett. 2013, 15, 6116–6119. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Holownia, A.; Bennett, J.M.; Elkins, J.M.; St. Denis, J.D.; Adachi, S.; Yudin, A.K. Oxalyl boronates enable modular synthesis of bioactive imidazoles. Angew. Chem. Int. Ed. 2017, 56, 6264–6267. [Google Scholar] [CrossRef] [PubMed]
- Dubovtsev, A.Y.; Dar’in, D.V.; Krasavin, M.; Kukushkin, V.Y. Gold-catalyzed oxidation of internal alkynes into benzils and its application for one-pot synthesis of five-, six- and seven-membered azaheterocycles. Eur. J. Org. Chem. 2019, 1856–1864. [Google Scholar] [CrossRef]
- Naidoo, S.; Jeena, V. One-pot, two-step metal and acid-free synthesis of trisubstituted imidazole derivatives via oxidation of internal alkynes using an iodine/DMSO system. Eur. J. Org. Chem. 2019, 1107–1113. [Google Scholar] [CrossRef]
- Li, J.; Neuville, L. Copper-catalyzed oxidative deamination of terminal alkynes by amidines: Synthesis of 1,2,4-trisubstituted imidazoles. Org. Lett. 2013, 15, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Englert, U.; Bolm, C. Base-mediated syntheses of di- and trisubstituted imidazoles from amidine hydrochlorides and bromoacetylenes. Chem. Eur. J. 2015, 21, 13221–13224. [Google Scholar] [CrossRef]
- Su, J.; Chen, Q.; Lu, L.; Ma, Y.; Auyoung, G.H.L.; Hua, R. Base-promoted nucleophilic fluoroarenes substitution of C-F bonds. Tetrahedron 2018, 74, 303–307. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Lu, L.; Mehmood, H.; Khan, D.M.; Hua, R. Quinazolinone synthesis through base-promoted SNAr reaction of ortho-fluorobenzamides with amides followed by cyclization. ACS Omega 2019, 4, 8207–8213. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Mehmood, H.; Lv, J.; Hua, R. Base-promoted SNAr reactions of fluoro- and chloroarenes as a route to N-aryl indoles and carbazoles. Molecules 2019, 24, 1145. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Hua, R. Base-promoted chemodivergent formation of 1,4-benzoxazepin-5(4H)-ones and 1,3-benzoxazin-4(4H)-ones switched by solvents. Molecules 2019, 24, 3773. [Google Scholar] [CrossRef]
- Nizami, T.A.; Hua, R. Cycloaddition of 1,3-butadiynes: Efficient synthesis of carbo- and heterocycles. Molecules 2014, 19, 13788–13802. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Nizami, T.A. Synthesis of heterocycles by using propargyl compounds as versatile synthons. Mini-Rev. Org. Chem. 2018, 15, 198–207. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. C–H activation and alkyne annulation via automatic or intrinsic directing groups: Towards high step economy. Chem. Rec. 2018, 18, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.M.; Hua, R. Isoquinolone synthesis via Zn (OTf)2-catalyzed aerobic cyclocondensation of 2-(1-alkynyl) benzaldehydes with arylamines. Catalysts 2020, 10, 683. [Google Scholar] [CrossRef]
- One similar structural substrate reaction between ethyl amino-oximinoacetate (in 1, R = COOEt) and methyl propiolate (an active alkyne) in the presence of Et3N under reflux xylene giving 2-carbethoxy-4-carbomethoxyimidazole in 70% yield was reported, see: Branco, P.S.; Prabhakar, S.; Lobo, A.M.; Wiliams, D.J. Reactions of hydroxylamines with ethyl cyanoformate. Preparation of aminonitrones and their synthetic applications. Tetrahedron 1992, 48, 6335–6360. Very recently, a microwave-assisted synthesis of imidazoles via nucleophilic catalysis has been reported by the addition of amidoximes to activated alkynes followed by a thermally induced rearrangement of the in situ generated O-vinylamidoxime intermediates, see: Shabalin, D.A.; Dunsford, J.J.; Ngwerume, S.; Saunders, A.R.; Gill, D.M.; Camp, J.E. Synthesis of 2,4-disubstituted imidazoles via nucleophilic catalysis. Synlett 2020, 31, 797–800.
- The structure of 3aa was further confirmed by its x-ray diffraction studies (CCDC2012403), and the x-ray structural details are reported in Supplementary Materials.
- Tabolin, A.A.; Ioffe, S.L. Rearrangement of N-oxyenamines and related reactions. Chem. Rev. 2014, 114, 5426–5476. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Bokach, N.A.; Demakova, M.Y.; Kukushkin, V.Y. Metal-involving synthesis and reactions of oximes. Chem. Rev. 2017, 117, 13039–13122. [Google Scholar] [CrossRef]
- Sridhar Madabhushi, S.; Vangipuram, V.S.; Mallu, K.K.R.; Chinthala, N.; Beeram, C.R. Europium (III) triflate-catalyzed trofimov synthesis of polyfunctionalized pyrroles. Adv. Synth. Catal. 2012, 354, 1413–1416. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
Sample Availability: Samples of the products are not available from the authors. |





| Entry | 2a (equiv) | Base(equiv) | Solvent | Yield(%)b |
|---|---|---|---|---|
| 1 | 2.0 | Na2CO3(4) | DMSO | 10 |
| 2 | 2.0 | K2CO3(4) | DMSO | 34 |
| 3 | 2.0 | KOH(4) | DMSO | 53 |
| 4 | 2.0 | KOtBu(4) | DMSO | 41 |
| 5 | 2.0 | Cs2CO3(4) | DMSO | 75 |
| 6 | 2.0 | Cs2CO3(4) | THF | 10 |
| 7 | 2.0 | Cs2CO3(4) | Dioxane | 14 |
| 8 | 2.0 | Cs2CO3(4) | DMF | 21 |
| 9 | 1.0 | Cs2CO3(4) | DMSO | 59 |
| 10 | 1.5 | Cs2CO3(4) | DMSO | 68 |
| 11 12 13 14 | 2.0 2.0 2.0 2.0 | Cs2CO3(2.5) Cs2CO3(1.0) Cs2CO3(0.5) -- | DMSO DMSO DMSO DMSO | 73 33 19 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmood, H.; Iqbal, M.A.; Lu, L.; Hua, R. Base-Promoted Annulation of Amidoximes with Alkynes: Simple Access to 2,4-Disubstituted Imidazoles. Molecules 2020, 25, 3621. https://doi.org/10.3390/molecules25163621
Mehmood H, Iqbal MA, Lu L, Hua R. Base-Promoted Annulation of Amidoximes with Alkynes: Simple Access to 2,4-Disubstituted Imidazoles. Molecules. 2020; 25(16):3621. https://doi.org/10.3390/molecules25163621
Chicago/Turabian StyleMehmood, Hina, Muhammad Asif Iqbal, Le Lu, and Ruimao Hua. 2020. "Base-Promoted Annulation of Amidoximes with Alkynes: Simple Access to 2,4-Disubstituted Imidazoles" Molecules 25, no. 16: 3621. https://doi.org/10.3390/molecules25163621
APA StyleMehmood, H., Iqbal, M. A., Lu, L., & Hua, R. (2020). Base-Promoted Annulation of Amidoximes with Alkynes: Simple Access to 2,4-Disubstituted Imidazoles. Molecules, 25(16), 3621. https://doi.org/10.3390/molecules25163621







