Synthesis of Novel Sulfamethaoxazole 4-Thiazolidinone Hybrids and Their Biological Evaluation
Abstract
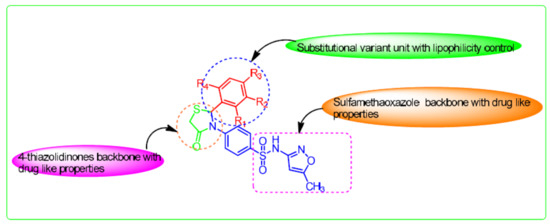
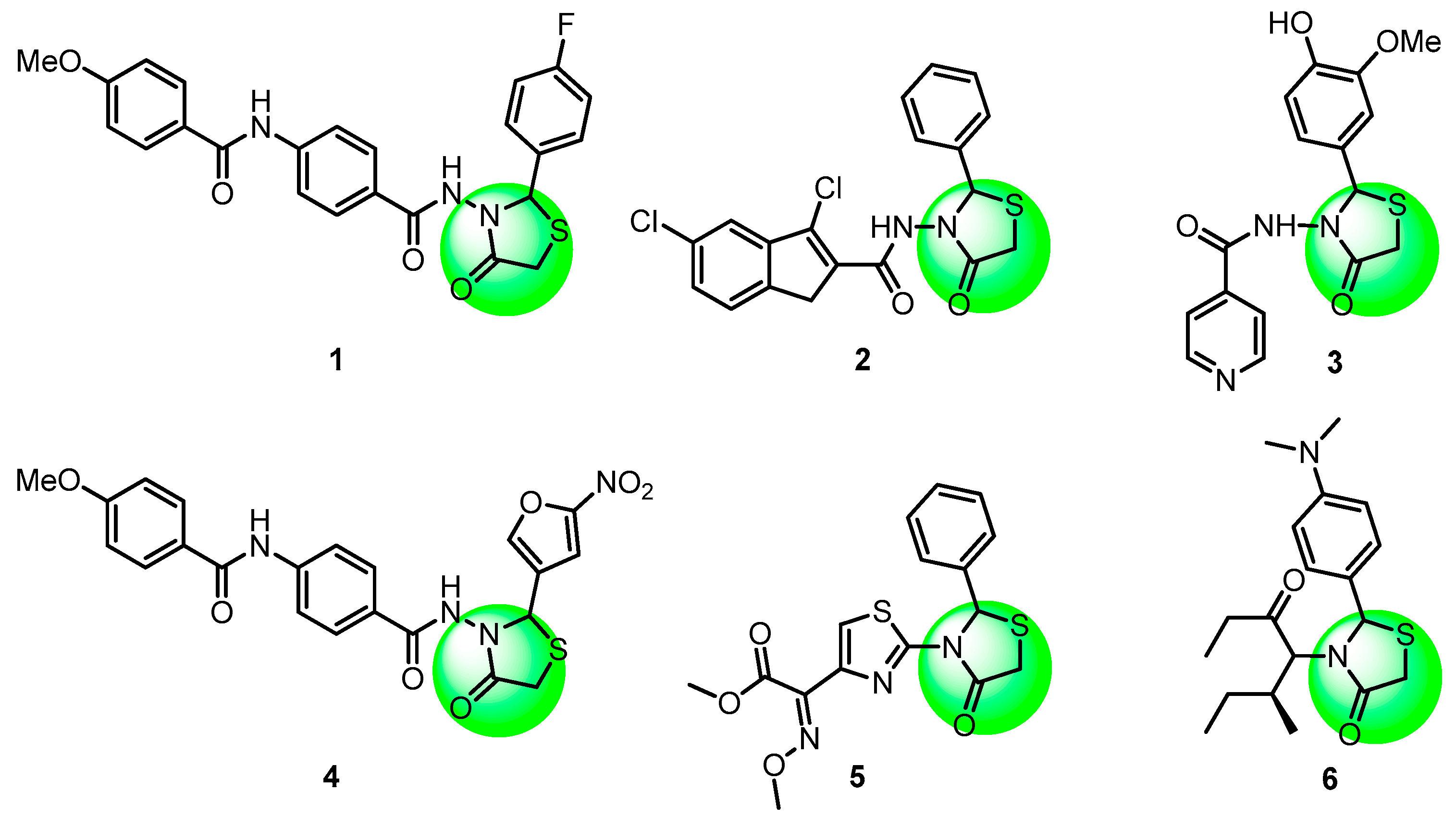
1. Introduction
2. Results and Discussion
2.1. Anti-Mycobacterial Activity
2.2. Structure Activity Relationship (SAR)
2.3. Cytotoxicity
2.4. Selectivity Index (SI)
2.5. Antibacterial Activity
2.6. ADME Properties
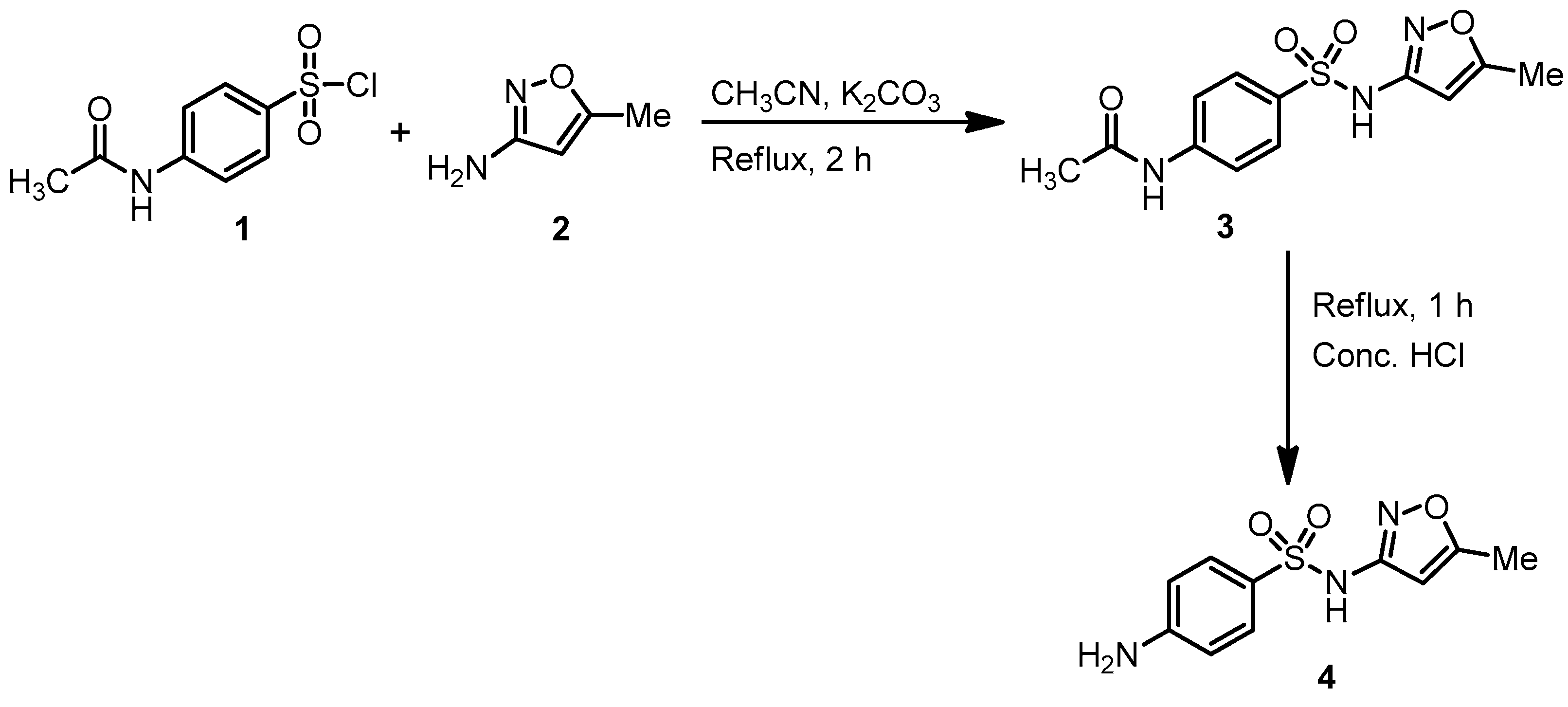
3. Materials and Methods
3.1. Materials
3.2. Typical Experimental Procedure for the Synthesis of 4-Amino-N-(5-methylisoxazol-3-yl)benzenesulfonamide (4)
3.3. Typical Experimental Procedure for the Synthesis of Sulfamethaoxazole Incorporated Substituted 4-Thiazolidinone Hybrid (7a)
3.3.1. N-(5-methylisoxazol-3-yl)-4-(4-oxo-2-phenylthiazolidin-3-yl)benzenesulfonamide (7a)
3.3.2. N-(5-methylisoxazol-3-yl)-4-(4-oxo-2-(p-tolyl)thiazolidin-3-yl)benzenesulfonamide (7b)
3.3.3. 4-(2-(4-methoxyphenyl)-4-oxothiazolidin-3-yl)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (7c)
3.3.4. 4-(2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (7d)
3.3.5. 4-(2-(2-chlorophenyl)-4-oxothiazolidin-3-yl)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (7e)
3.3.6. 4-(2-(3-chlorophenyl)-4-oxothiazolidin-3-yl)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (7f)
3.3.7. 4-(2-(4-chlorophenyl)-4-oxothiazolidin-3-yl)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (7g)
3.3.8. 4-(2-(4-bromophenyl)-4-oxothiazolidin-3-yl)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (7h)
3.3.9. N-(5-methylisoxazol-3-yl)-4-(4-oxo-2-(4-(trifluoromethyl)phenyl)thiazolidin-3-yl)benzenesulfonamide (7i)
3.3.10. N-(5-methylisoxazol-3-yl)-4-(2-(4-nitrophenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide (7j)
3.3.11. 4-(2-(2,6-difluorophenyl)-4-oxothiazolidin-3-yl)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (7k)
3.3.12. 4-(2-(2,6-dichlorophenyl)-4-oxothiazolidin-3-yl)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (7l)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dye, C.; Williams, B.G. The Population Dynamics and Control of Tuberculosis. Science 2010, 328, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug. Discov. 2013, 5, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.I.; Gillespie, S.H.; Hoelscher, M.; Philips, P.P.; Cole, S.T.; Abubakar, I.; McHugh, T.D.; Schito, M.; Maeurer, M.; Nunn, A.J. New antituberculosis drugs, regimens, and adjunct therapies: Needs, advances, and future prospects. Lancet Infect. Dis. 2014, 4, 327–340. [Google Scholar] [CrossRef]
- Global Tuberculosis Control: WHO Report. 2019. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 25 July 2020).
- Lugosi, L. Theoretical and methodological aspects of BCG vaccine from the discovery of Calmette and Guerin to molecular biology. A review. Tuber. Lung Dis. 1992, 73, 252–261. [Google Scholar] [CrossRef]
- Udwadia, Z.F.; Amale, R.A.; Ajbani, K.K.; Rodrigues, C. Totally drug-resistant tuberculosis in India. Clin. Infect. Dis. 2012, 54, 579–581. [Google Scholar] [CrossRef]
- Butler, M.S.; Blaskovich, M.A.; Cooper, M.A. Antibiotics in the clinical pipeline in 2013. J. Antibiot (Tokyo) 2013, 66, 571–591. [Google Scholar] [CrossRef]
- Diacon, A.H.; Donald, P.R.; Pym, A.; Grobusch, M.; Patientia, R.F.; Mahanyele, R.; Bantubani, N.; Narasimooloo, R.; De Marez, T.; van Heeswijk, R.R. Andomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: Long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob. Agents Chemother. 2012, 56, 3271–3276. [Google Scholar] [CrossRef]
- Gouvea, F.A.; Vasconcellos, G.A.; Berwaldt, A.C.P.S.; Neto, G.; Fischer, R.P.; Sakata, W.P.; Almeida, W.P.; Cunico, W. 2-Aryl-3-(2-morpholinoethyl)thiazolidin-4-ones: Synthesis, anti-inflammatory in vivo, cytotoxicity in vitro and molecular docking studies. Eur. J. Med. Chem. 2016, 118, 259–265. [Google Scholar] [CrossRef]
- Monte, C.D.; Carradori, S.; Bizzarri, B.; Bolasco, A.; Caprara, F.; Mollica, A.; Rivanera, D.; Mari, E.; Zicari, A.; Akdemir, A. Anti-Candida activity and cytotoxicity of a large library of new N-substituted-1,3-thiazolidin-4-onederivatives. Eur. J. Med. Chem. 2016, 107, 82–96. [Google Scholar] [CrossRef]
- Rane, R.A.; Sahu, N.U.; Shah, C.P. Synthesis and antibiofilm activity of marine natural product-based 4-thiazolidinones derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 7131–7134. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Li, D.; Qua, G.; Wang, J.; Loiseau, P.M.; Fan, X. Ionic liquid mediated and promoted eco-friendly preparation of thiazolidinone and pyrimidine nucleoside-thiazolidinone hybrids and their antiparasitic activities. Bioorg. Med. Chem. Lett. 2009, 19, 6280–6283. [Google Scholar] [CrossRef] [PubMed]
- Onen-Bayram, F.E.; Buran, K.; Durmaz, I.; Berk, B.; Cetin-Atalay, R. 3-Propionyl-thiazolidine-4-carboxylic acid ethyl esters: A family of antiproliferative thiazolidines. Med. Chem. Commun. 2015, 6, 90–93. [Google Scholar] [CrossRef]
- Barrec, M.L.; Chimirri, A.; De Luca, L.; Monforte, A.M.; Monforte, P.; Rao, A.; Zappala, M.; Balzarini, J.; De Clercq, E.; Pannecouque, C.; et al. Discovery of 2,3-diaryl-1,3-thiazolidin-4-ones as potentanti-HIV-1 agents. Bioorg. Med. Chem. Lett. 2001, 11, 1793–1796. [Google Scholar] [CrossRef]
- Ottana, R.; Maccari, R.; Giglio, M.; Del Corso, A.; Cappiello, M.; Mura, U.; Cosconati, S.; Marinelli, L.; Novellino, E.; Sartini, S.; et al. Identification of 5-arylidene-4 thiazolidinone derivatives endowed with dual activity as aldose reductase inhibitors and antioxidant agents for the treatment of diabetic complications. Eur. J. Med. Chem. 2011, 46, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.M.; Vicini, P.; Geronikaki, A.; Incerti, M.; Cardile, V.; Crasci, L.; Messina, R.; Ronsisvalle, S. Heteroarylimino-4-thiazolidinones as inhibitors of cartilage degradation. Bioorg. Chem. 2011, 39, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, J.; Jette, J.; Kao, W.; Rogers, J.; Di, L.; Chi, J.; Perez, M.C.; Chen, G.C.; Shen, E.S. 5-Alkylated thiazolidinones as follicle-stimulating hormone (FSH) receptor agonists. Bioorg. Med. Chem. 2006, 14, 5729–5741. [Google Scholar] [CrossRef] [PubMed]
- Cutshall, N.S.; O’Day, C.; Prezhdo, M. Rhodanine derivatives as inhibitors of JSP-1. Bioorg. Med. Chem. Lett. 2005, 15, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Anderson, C.; Binch, H.; Hadida, S.; Yoo, S.; Bergeron, D.; Decker, C.; terHaar, E.; Moore, J.; Garcia-Guzman, M.; et al. Identification of potent CNS-penetrant thiazolidinones as novel CGRP receptor antagonists. Bioorg. Med. Chem. Lett. 2014, 24, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, L.; Le, N.T.; Zhao, C.; Sidduri, A.; Lou, J.P.; Michoud, C.; Portland, L.; Jackson, N.; Liu, J.J.; et al. Synthesis and activity of quinolinyl-methylene-thiazolinones as potent and selective cyclin-dependent kinase 1 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 2134–2138. [Google Scholar] [CrossRef]
- Unsal-Tan, O.; Ozadali, K.; Piskin, K.; Balkan, A. Molecular modeling, synthesis and screening of some new 4-thiazolidinone derivatives with promising selective COX-2 inhibitory activity. Eur. J. Med. Chem. 2012, 57, 59–64. [Google Scholar] [CrossRef]
- Raza, S.; Srivastava, S.P.; Srivastava, D.S.; Srivastava, A.K.; Haq, W.; Katt, S.B. Thiazolidin-4-one and thiazinan-4-one derivatives analogous to rosiglitazone as potential antihyperglycemic and antidyslipidemic agents. Eur. J. Med. Chem. 2013, 63, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Kucukguzel, S.G.; Oruc, E.E.; Rollas, S.; Sahin, F.; Ozbek, A. Synthesis, characterization and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002, 37, 197–206. [Google Scholar] [CrossRef]
- Jaju, S.; Palkar, M.; Maddi, V.; Ronad, P.K.; Mamledesai, S.; Satyanarayana, D.; Ghatole, M. Synthesis and antimycobacterial activity of a novel series of isonicotinyl hydrazide derivatives. Arch. Pharm. Chem. Life Sci. 2009, 342, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Babaoglu, K.; Page, M.A.; Jones, V.C.; McNeil, M.R.; Dong, C.; Naismith, J.H.; Lee, R.E. Novel inhibitors of an emerging target in Mycobacterium tuberculosis; substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorg. Med. Chem. Lett. 2003, 13, 3227–3230. [Google Scholar] [CrossRef]
- Parekh, H.H.; Parikh, K.H.; Parikh, A.R. Synthesis of some 4-thiazolidinone derivatives as antitubercular ragents. J. Sci. Islm. Rep. Iran. 2004, 15, 143–148. [Google Scholar]
- Aridoss, G.; Amirthaganesan, S.; Kim, M.S.; Kim, J.T.; Jeong, Y.T. Synthesis, spectral and biological evaluation of some new thiazolidinones and thiazoles base dont-3-alkyl-r-2,c-6-diarylpiperidin-4-ones. Eur. J. Med. Chem. 2009, 44, 4199–4210. [Google Scholar] [CrossRef]
- Patel, A.B.; Chikhalia, K.H.; Kumari, P. Facile synthesis of benzonitrile/nicotinonitriles baseds-triazines as new potential antimycobacterial agents. Eur. J. Med. Chem. 2014, 79, 57–65. [Google Scholar] [CrossRef]
- Ramani, A.V.; Monika, A.; Indira, V.L.; Karyavardhi, G.; Venkatesh, J.; Jeankumar, V.U.; Manjashetty, T.H.; Yogeeswari, P.; Sriram, D. Synthesis of highly potent novel anti-tubercular isoniazid analogues with preliminary pharmacokinetic evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 2764–2767. [Google Scholar] [CrossRef]
- More, U.A.; Joshi, S.D.; Aminabhavi, T.M.; Gadad, A.K.; Nadagouda, M.N.; Kulkarni, V.H. Design, synthesis, molecular docking and 3D-QSAR studies of potent inhibitors of enoyl-acyl carrier protein reductase as potential antimycobacterial agents. Eur. J. Med. Chem. 2014, 71, 199–218. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Chen, J.; Wang, X.; He, M. Metal-free oxidative coupling of amines with sodium sulfinates: A mild access to sulfonamides. RSC Adv. 2014, 4, 64698–64701. [Google Scholar] [CrossRef]
- Pandya, R.; Murashima, T.; Tedeschi, L.; Barrett, A.G.M. Facile one-pot synthesis of aromatic and heteroaromatic sulfonamides. J. Org. Chem. 2003, 68, 8274–8276. [Google Scholar] [CrossRef] [PubMed]
- El-Mossalamy, E.H. Potentiometric and Spectroscopic Studies of Sulfonamide Azo-Dye Complexes with some Transition Metal Ions and Uranium Portu. Port. Electrochim. Acta 2009, 27, 143–152. [Google Scholar] [CrossRef]
- Singhal, A.; Parumala, S.K.R.; Sharma, A.; Peddinti, R.K. Hypervalent iodine mediated synthesis of di-and tri-substituted isoxazoles via [3+2] cycloaddition of nitrile oxides. Tetrahedron Lett. 2015, 57, 719–722. [Google Scholar] [CrossRef]
- Murugesan, V.; Makwana, N.; Suryawanshi, R.; Saxena, R.; Tripathi, R.; Paranjape, R.; Kulkarni, S.; Katti, S.B. Rational design and synthesis of novel thiazolidin-4-ones as non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorg. Med. Chem. 2014, 22, 3159–3170. [Google Scholar] [CrossRef]
- Bhat, M.A.; Al-Omar, M.A.; Naglah, A.M.; Khan, A.A. [Et3NH][HSO4]-mediated efficient synthesis of novel xanthene derivatives and their biological evaluation. J. Saudi Chem. Soc. 2020, 24, 425–433. [Google Scholar] [CrossRef]
- Bhat, M.A. Synthesis and anti-mycobacterial activity of new 4-thiazolidinone and 1,3,4-oxadiazole derivatives of isoniazid. Acta Pol. Pharm. 2014, 71, 763–770. [Google Scholar]
- Boyle, J.; Otty, S.; Sarojini, V. A Safer and Convenient Synthesis of Sulfathiazole for Undergraduate Organic and Medicinal Chemistry Classes. J. Chem. Educ. 2012, 89, 141–143. [Google Scholar] [CrossRef]
- Iona, E.; Pardini, M.; Gagliardi, M.C.; Colone, M.; Stringaro, A.R.; Teloni, R.; Brunori, L.; Nisini, R.; Fattorini, L.; Giannoni, F. Infection of human THP-1 cells with dormant Mycobacterium tuberculosis. Microbes Infect. 2012, 14, 959–967. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.A.; Beelen, R.H.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Hartkoorn, R.C.; Chandler, B.; Owen, A.; Ward, S.A.; Squire, S.B.; Back, D.J.; Khoo, S.H. Differential drug susceptibility of intracellular and extracellular tuberculosis, and the impact of P-glycoprotein. Tuberculosis 2007, 87, 248–255. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, L.; Dominy, B.W.; Freeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Molinspiration Chemoinformatics Brastislava, Slovak Republic. 2014. Available online: http://www.molinspiration.com/cgi-bin/properties (accessed on 25 July 2020).
- Zhao, Y.H.; Abraham, M.H.; Lee, J.; Hersey, A.; Luscombe, N.C.; Beck, G.; Sherborne, B.; Cooper, I. Rate-Limited Steps of Human Oral Absorption and QSAR Studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Drug-Likeness and Molecular Property Prediction. Available online: http://www.molsoft.com/mprop/ (accessed on 25 July 2020).
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Bouz, G.; Juhas, M.; Otero, L.P.; Red, C.P.; Jandourek, O.; Konecna, K.; Paterova, P.; Kubicek, V.; Janousek, J.; Dolezal, M.; et al. Substituted N-(Pyrazin-2-yl)benzenesulfonamides; Synthesis, Anti-Infective Evaluation, Cytotoxicity, and In Silico Studies. Molecules 2020, 25, 138. [Google Scholar] [CrossRef] [PubMed]
- Tauro, M.; Laghezza, A.; Loiodice, F.; Piemontese, L.; CaraDonna, A.; Capelli, D.; Montanari, R.; Pochetti, G.; Di Pizio, A.; Agamennone, M. Catechol-based matrix metalloproteinase inhibitors with additional anti oxidative activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 25–37. [Google Scholar] [CrossRef]
- Ogryzek, M.; Chylewska, A.; Turecka, K.; Lesiak, D.; Krolicka, A.; Banasiuk, R.; Nidzworski, D.; Makowski, M. Coordination chemistry of pyrazine derivatives analogues of PZA: Design, synthesis, characterization and biological activity. RSC Adv. 2016, 6, 52009–52025. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Perlepe, P.; Aubrey, M.L.; Woodruff, D.N.; Reyes-Lillo, S.E.; Reinholdt, A.; Voigt, L.; Li, Z.; Borup, K.; Rouzieres, M. Formation of the layered conductive magnet CrCl2(pyrazine) (2) through redox-active coordination chemistry. Nat. Chem. 2018, 10, 1056–1061. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Entry | Structures | M. Bovis BCG | MTBH37Ra | ||
|---|---|---|---|---|---|
| IC50 | IC90 | IC50 | IC90 | ||
| 7a | 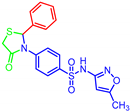 | >30 | >30 | >30 | >30 |
| 7b | 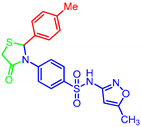 | >30 | >30 | >30 | >30 |
| 7c |  | >30 | >30 | >30 | >30 |
| 7d | 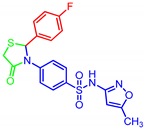 | 0.03 | 0.22 | 0.54 | 0.70 |
| 7e | 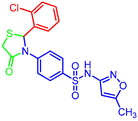 | 2.86 | >30 | 1.26 | >30 |
| 7f | 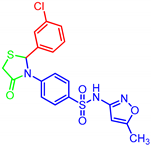 | 16.75 | >30 | 1.68 | >30 |
| 7g | 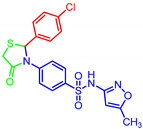 | 0.02 | 0.13 | 0.12 | 5.31 |
| 7h |  | >30 | >30 | >30 | >30 |
| 7i | 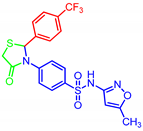 | 0.28 | 0.17 | 0.16 | 1.08 |
| 7j |  | >30 | >30 | >30 | >30 |
| 7k | 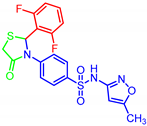 | 0.026 | 0.15 | 0.13 | 0.71 |
| 7l | 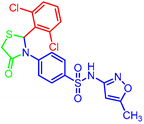 | 0.016 | 0.058 | 0.07 | 0.43 |
| bRP | - | 0.0043 ± 0.00028 | 0.0173 ± 0.039 | 0.0019 ± 0.00022 | 0.020 ± 0.0021 |
| Entry | MCF-7 (Breast) Cell Line | HCT 116 (Colorectal) Cell Line | A549 (Lung) Cell Line | |||
|---|---|---|---|---|---|---|
| GI50 (µg/mL) | GI90 (µg/mL) | GI50 (µg/mL) | GI90 (µg/mL) | GI50 (µg/mL) | GI90 (µg/mL) | |
| 7d | >100 | >100 | >100 | >100 | >100 | >100 |
| 7g | >100 | >100 | >100 | >100 | >100 | >100 |
| 7i | >100 | >100 | >100 | >100 | >100 | >100 |
| 7k | >100 | >100 | >100 | >100 | >100 | >100 |
| 7l | >100 | >100 | >100 | >100 | >100 | >100 |
| Paclitaxel | 0.0048 | 0.075 | 0.1279 | 5.715 | 0.0035 | 0.0706 |
| Rifampicin | >100 | >100 | >100 | >100 | >100 | >100 |
| Entry | MCF-7 | HCT 116 | A549 | |||
|---|---|---|---|---|---|---|
| MTB H37Ra | M. Bovis BCG | MTB H37Ra | M. Bovis BCG | MTB H37Ra | M. Bovis BCG | |
| 7d | 139.1 | 476.1 | 139.1 | 476.1 | 139.1 | 476.1 |
| 7g | 94.5 | 832.9 | 94.5 | 832.9 | 94.5 | 833.9 |
| 7i | 19.1 | 587.9 | 19.1 | 587.9 | 19.1 | 587.9 |
| 7k | 143.1 | 667.1 | 143.1 | 667.1 | 143.1 | 667.1 |
| 7l | 237.8 | 12.1 | 237.8 | 12.1 | 237.8 | 12.1 |
| Rifampicin | >5000 | 1754.4 | >5000 | 1754.4 | >5000 | 1754.4 |
| Entry | P. fluorescens | E. coli | B. subtillus | S. aureus |
|---|---|---|---|---|
| 7d | >100 | >100 | >100 | >100 |
| 7g | >100 | >100 | >100 | >100 |
| 7i | >100 | >100 | >100 | >100 |
| 7k | >100 | >100 | >100 | >100 |
| 7l | >100 | >100 | >100 | >100 |
| Ampicillin | 4.36 | 1.46 | 10.32 | 1 |
| Kanamycin | 0.49 | 1.62 | 1.35 | >30 |
| Com. | % ABS | TPSA (A2) | n-ROTB | MV | MW | miLog P | n-ON | n-OHNH | Lipinski Violation | Drug Likeness Model Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Rule | - | - | - | - | <500 | ≤5 | <10 | <5 | ≤1 | |
| 7a | 77.08 | 92.51 | 5 | 337.35 | 415.50 | 2.91 | 7 | 1 | 0 | −0.10 |
| 7b | 77.08 | 92.51 | 5 | 353.91 | 429.52 | 3.36 | 7 | 1 | 0 | −0.27 |
| 7c | 73.89 | 101.74 | 6 | 362.90 | 445.52 | 2.97 | 8 | 1 | 0 | 0.01 |
| 7d | 77.08 | 92.51 | 5 | 342.28 | 433.19 | 3.08 | 7 | 1 | 0 | 0.21 |
| 7e | 77.08 | 92.51 | 5 | 350.89 | 449.94 | 3.54 | 7 | 1 | 0 | 0.01 |
| 7f | 77.08 | 92.51 | 5 | 350.89 | 449.94 | 3.57 | 7 | 1 | 0 | 0.10 |
| 7g | 77.08 | 92.51 | 5 | 350.89 | 449.94 | 3.59 | 7 | 1 | 0 | 0.36 |
| 7h | 77.08 | 92.51 | 5 | 353.24 | 494.39 | 3.72 | 7 | 1 | 0 | −0.02 |
| 7i | 77.08 | 92.51 | 6 | 368.65 | 483.49 | 3.81 | 7 | 1 | 0 | −0.17 |
| 7j | 61.31 | 138.23 | 6 | 360.69 | 460.49 | 2.87 | 10 | 1 | 0 | −0.12 |
| 7k | 77.08 | 92.51 | 5 | 347.21 | 451.48 | 3.14 | 7 | 1 | 0 | −0.20 |
| 7l | 77.08 | 92.51 | 5 | 364.42 | 484.39 | 4.17 | 7 | 1 | 0 | −0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, M.A.; Al-Omar, M.A.; Naglah, A.M.; Khan, A.A. Synthesis of Novel Sulfamethaoxazole 4-Thiazolidinone Hybrids and Their Biological Evaluation. Molecules 2020, 25, 3570. https://doi.org/10.3390/molecules25163570
Bhat MA, Al-Omar MA, Naglah AM, Khan AA. Synthesis of Novel Sulfamethaoxazole 4-Thiazolidinone Hybrids and Their Biological Evaluation. Molecules. 2020; 25(16):3570. https://doi.org/10.3390/molecules25163570
Chicago/Turabian StyleBhat, Mashooq A., Mohamed A. Al-Omar, Ahmed M. Naglah, and Azmat Ali Khan. 2020. "Synthesis of Novel Sulfamethaoxazole 4-Thiazolidinone Hybrids and Their Biological Evaluation" Molecules 25, no. 16: 3570. https://doi.org/10.3390/molecules25163570
APA StyleBhat, M. A., Al-Omar, M. A., Naglah, A. M., & Khan, A. A. (2020). Synthesis of Novel Sulfamethaoxazole 4-Thiazolidinone Hybrids and Their Biological Evaluation. Molecules, 25(16), 3570. https://doi.org/10.3390/molecules25163570