Acorn Flour as a Source of Bioactive Compounds in Gluten-Free Bread
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Characteristics of GFB
2.2. Nutritional Composition of Bread
2.3. Bioactive Compounds and Antioxidant Activity
2.4. Sensory Evaluation
3. Materials and Methods
3.1. Raw Materials
3.2. Bread Making and Sampling
3.3. Physical Analysis of Bread
3.4. Nutritional Composition of Bread
3.5. Bioactive Compounds and Antioxidant Activity
3.5.1. Extract Preparation
3.5.2. Total Phenols Content (TPC) Determination
3.5.3. Ortho-Diphenols Content (ODC) Determination
3.5.4. Flavonoids Content (FlC) Determination
3.5.5. Determination of Antioxidant Capacity
3.5.6. Qualitative and Quantitative Analysis of Phenolic Compounds
3.6. Sensory Evaluation
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Milenkovic, D.; Rodriguez-Mateos, A.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; Morand, C. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Gujral, N.; Freeman, H.J.; Thomson, A.B.R. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Lionetti, E.; Gatti, S.; Pulvirenti, A.; Catassi, C. Celiac disease from a global perspective. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 365–379. [Google Scholar] [CrossRef]
- Fasano, A.; Catassi, C. Clinical practice. Celiac disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef]
- Comino, I.; Moreno, M.L.; Real, A.; Rodríguez-Herrera, A.; Barro, F.; Sousa, C. The gluten-free diet: Testing alternative cereals tolerated by celiac patients. Nutrients 2013, 5, 4250–4268. [Google Scholar] [CrossRef]
- Leonard, M.M.; Vasagar, B. US perspective on gluten-related diseases. Clin. Exp. Gastroenterol. 2014, 7, 25–37. [Google Scholar]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J. Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef]
- Brouns, F.J.P.H.; Van Buul, V.J.; Shewry, P.R. Does wheat make us fat and sick. J. Cereal Sci. 2013, 58, 209–215. [Google Scholar] [CrossRef]
- Gobbetti, M.; Pontonio, E.; Filannino, P.; Rizzello, C.G.; De Angelis, M.; Di Cagno, R. How to improve the gluten-free diet: The state of the art from a food science perspective. Food Res. Int. 2018, 110, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Available online: https://www.grandviewresearch.com/press-release/global-gluten-free-products-market (accessed on 23 January 2020).
- O’Shea, N.; Arendt, E.; Gallagher, E. State of the Art in Gluten-Free Research. J. Food Sci. 2014, 79, 1067–1075. [Google Scholar] [CrossRef]
- Capriles, V.D.; Dos Santos, F.G.; Arêas, J.A.G. Gluten-free breadmaking: Improving nutritional and bioactive compounds. J. Cereal Sci. 2016, 67, 83–91. [Google Scholar] [CrossRef]
- Naqash, F.; Gani, A.; Gani, A.; Masoodi, F.A. Gluten-free baking: Combating the challenges—A review. Trends Food Sci. Technol. 2017, 66, 98–107. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J. Sci. Food Agric. 2015, 95, 653–661. [Google Scholar] [CrossRef]
- Gallagher, E.; Gormley, T.R.; Arendt, E.K. Recent advances in the formulation of gluten-free cereal-based products. Trends Food Sci. Technol. 2004, 15, 143–152. [Google Scholar] [CrossRef]
- Skendi, A.; Mouselemidou, P.; Papageorgiou, M.; Papastergiadis, E. Effect of acorn meal-water combinations on technological properties and fine structure of gluten-free bread. Food Chem. 2018, 253, 119–126. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Dziki, D.; Różyło, R.; Gawlik-Dziki, U.; Swieca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Betoret, E.; Rosell, C.M. Enrichment of bread with fruits and vegetables: Trends and strategies to increase functionality. Cereal Chem. 2019, 97, 9–19. [Google Scholar] [CrossRef]
- Ziobro, R.; Gumul, D.; Korus, J.; Korus, A. Starch bread with a share of non-wheat flours as a source of bioactive compounds in gluten-free diet. J. Food Nutr. Res. Slov. 2016, 55, 11–21. [Google Scholar]
- Torres, M.D.; Arufe, S.; Chenlo, F.; Moreira, R. Coeliacs cannot live by gluten-free bread alone—Every once in a while they need antioxidant. Int. J. Food Sci. Technol. 2017, 52, 81–90. [Google Scholar] [CrossRef]
- Polimac, M.; Komlenic, D.K.; Lukinac, J. Possibilities of using acorn flour in products based on flour. In Proceedings of the 8th International Congress “Flour-Bread”, Opatija, Croatia, 29–30 October 2015. [Google Scholar]
- Silva, S.; Costa, E.M.; Borges, A.; Carvalho, A.P.; Monteiro, M.J.; Pintado, M.M.E. Nutritional characterization of acorn flour (a traditional component of the Mediterranean gastronomical folklore). Food Meas. 2016, 10, 584–588. [Google Scholar] [CrossRef]
- Toumi, L.; Lumaret, R. Allozyme characterisation of four Mediterranean evergreen oak species. Biochem. Syst. Ecol. 2001, 29, 799–817. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. The influence of acorn flour on rheological properties of gluten-free dough and physical characteristics of the bread. Eur. Food Res. Technol. 2015, 240, 1135–1143. [Google Scholar] [CrossRef]
- Molavi, E.H.; Keramat, J.; Raisee, B. Evaluation of the Cake Quality Made from Acorn-Wheat Flour Blends as a Functional Food. Int. J. Food Sci. Technol. 2015, 5, 53–60. [Google Scholar]
- De Oliveira Marques, A.H. Introdução à História da Agricultura em Portugal; Edições Cosmos: Lisboa, Portugal, 1987. [Google Scholar]
- Lopez-Bote, C.J. Sustained utilization of the Iberian pig breed. Meat. Sci. 1998, 49, S17–S27. [Google Scholar] [CrossRef]
- Akcan, T.; Gökçe, R.; Asensio, M.; Estévez, M.; Morcuende, D. Acorn (Quercus spp.) as a novel source of oleic acid and tocopherols for livestock and humans: Discrimination of selected species from Mediterranean forest. J. Food Sci. Technol. 2017, 54, 3050–3057. [Google Scholar] [CrossRef]
- Tejerina, D.; García-Torres, S.; Vaca, M.; Vázquez, F.M.; Cava, R. Acorns (Quercus rotundifolia Lam.) and grass as natural sources of antioxidants and fatty acids in the “montanera” feeding of Iberian pig: Intra- and inter-annual variations. Food Chem. 2011, 124, 997–2004. [Google Scholar] [CrossRef]
- Vinha, A.F.; Barreira, J.C.M.; Costa, A.S.; Oliveira, M.B.P.P. A New Age for Quercus spp. Fruits: Review on Nutritional and Phytochemical Composition and Related Biological Activities of Acorns. Compr. Rev. Food Sci. Food Saf. 2016, 15, 947–981. [Google Scholar] [CrossRef]
- Korus, A.; Gumul, D.; Krystyjan, M.; Juszczak, L.; Korus, J. Evaluation of the quality, nutritional value and antioxidant activity of gluten-free biscuits made from corn-acorn flour or corn-hemp flour composites. Eur. Food Res. Technol. 2017, 243, 1–10. [Google Scholar] [CrossRef]
- Pasqualone, A.; Makhlouf, F.Z.; Barkat, M.; Difonzo, G.; Summo, C.; Squeo, G.; Caponio, F. Effect of acorn flour on the physico-chemical and sensory properties of biscuits. Heliyon 2019, 5, e02242. [Google Scholar] [CrossRef] [PubMed]
- Hrusková, M.; Svec, I.; Kadlcíková, I. Effect of chestnut and acorn flour on wheat/wheat-barley flour properties and bread quality. Int. J. Food Stud. 2019, 8, 41–57. [Google Scholar] [CrossRef]
- Švec, I.; Hrušková, M.; Kadlčíková, I. Features of flour composites based on the wheat or wheat-barley flour combined with acorn and chestnut. Croat. J. Food Sci. Technol. 2018, 10, 89–97. [Google Scholar] [CrossRef]
- Parsaei, M.; Goli, M.; Abbasi, H. Oak flour as a replacement of wheat and corn flour to improve biscuit antioxidant activity. Food Sci. Nutr. 2018, 6, 253–258. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Notarnicola, B.; Tassielli, G.; Renzulli, P.A.; Monforti, F. Energy flows and greenhouses gases of EU (European Union) national breads using an LCA (Life Cycle Assessment) approach. J. Clean. Prod. 2017, 140, 455–469. [Google Scholar] [CrossRef]
- Vinha, A.F.; Costa, A.S.G.; Barreira, J.C.M.; Pacheco, R.; Oliveira, M.B.P.P. Chemical and antioxidant profiles of acorn tissues from Quercus spp.: Potential as new industrial raw materials. Ind. Crop. Prod. 2016, 94, 143–151. [Google Scholar] [CrossRef]
- De la Hera, E.; Rosell, M.C.; Gomez, M. Effect of water content and flour particle size on gluten-free bread quality and digestibility. Food Chem. 2014, 151, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Beltrão-Martins, R.; Nunes, M.C.; Ferreira, L.M.; Peres, J.A.; Barros, A.I.R.N.A.; Raymundo, A. Impact of Acorn Flour on Gluten-Free Dough Rheology Properties. Foods 2020, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Turkut, G.M.; Cakmak, H.; Kumcuoglu, S.; Tavman, S. Effect of quinoa flour on gluten-free bread batter rheology and bread quality. J. Food Sci. 2016, 69, 174–181. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food Colour and Appearance; Springer: Boston, MA, USA, 1994; p. 610. [Google Scholar]
- Purlis, E. Browning development in bakery products–A review. J. Food Eng. 2009, 99, 239–249. [Google Scholar] [CrossRef]
- Rogowska, A.M. Synaesthesia and Individual Differences; Cambridge University Press: Cambridge, UK, 2015; p. 204. [Google Scholar]
- Hager, A.S.; Wolter, A.; Jacob, F.; Zannini, E.; Arendt, E.K. Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J. Cereal. Sci. 2012, 56, 239–247. [Google Scholar] [CrossRef]
- Encina-Zelada, C.R.; Cadavez, V.; Monteiro, F.; Teixeira, J.A.; Gonzales-Barron, U. Combined effect of xanthan gum and water content on physicochemical and textural properties of gluten-free batter and bread. Food Res. Int. 2018, 111, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Gellynck, X.; Kühne, B.; Van Bockstaele, F.; Van de Walle, D.; Dewettinck, K. Consumer perception of bread quality. Appetite 2009, 53, 16–23. [Google Scholar] [CrossRef]
- Angioloni, A.; Collar, C. Effects of pressure treatment of hydrated oat, finger millet and sorghum flours on the quality and nutritional properties of composite wheat breads. J. Cereal. Sci. 2012, 56, 713–719. [Google Scholar] [CrossRef]
- Maggio, A.; Orecchio, S. Fatty Acid Composition of Gluten-Free Food (Bakery Products) for Celiac People. Foods 2018, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Krumina-Zemture, G.; Beitane, I. Fatty Acid Composition in Buckwheat (Fagopyrum Esculentum, M.) Flours and Their Extruded Products. In Proceedings of the 8th International Scientific Conference Rural Development, Kaunas, Lithuania, 23–24 November 2017. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015, 13, 63. [Google Scholar]
- Bilgiçli, N.; İbanoğlu, S. Effect of pseudo cereal flours on some physical, chemical and sensory properties of bread. Int. J. Food Sci. Technol. 2015, 52, 7525–7529. [Google Scholar] [CrossRef]
- Torrinha, A.; Oliveira, M.; Marinho, S.; Paíga, P.; Delerue-Matos, C.; Morais, S. Mineral Content of Various Portuguese Breads: Characterization, Dietary Intake, and Discriminant Analysis. Molecules 2019, 24, 2787. [Google Scholar] [CrossRef]
- Chlopicka, J.; Pasko, P.; Gorinstein, S.; Jedryas, A.; Zagrodzki, P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT Food Sci. Technol. 2012, 46, 548–555. [Google Scholar] [CrossRef]
- Sakač, M.; Torbica, A.; Sedej, I.; Hadnađev, M. Influence of breadmaking on antioxidant capacity of gluten free breads based on rice and buckwheat flours. Food Res. Int. 2011, 44, 2806–2813. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; López-Bote, C.; de la Hoz, L.; Ordóñez, J.A.; Tomás-Barberán, F.A. Phenolic compounds and fatty acids from acorns (Quercus spp.), the main dietary constituent of free-ranged Iberian pigs. J. Agric. Food Chem. 2003, 51, 6248–6255. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Beitâne, I.; Krûmiòa-Zemture, G.; Krûma, Z.; Cinkmanis, I. Phenolics content in buckwheat flour. Proc. Latv. Acad. Sci. 2018, 72, 75–79. [Google Scholar] [CrossRef]
- Fernandes, I.C.X. Desenvolvimento de Pães Sem Glúten Enriquecidos com Tetraselmis Chuii. Ph.D. Thesis, Dissertação de Mestrado em Engenharia Alimentar. Instituto Superior de Agronomia da Universidade de Lisboa, Lisboa, Portugal, 2019. [Google Scholar]
- Approved Methods of Analysis—AACC Method 10-05.01. Guidelines for Measurement of Volume by Rapeseed Displacement; Cereals & Grains Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Graça, C.; Fradinho, P.; Raymundo, A.; Sousa, I. Impact of Chlorella vulgaris on the rheology of wheat flour dough and bread texture. LWT Food Sci. Technol. 2018, 89, 466–474. [Google Scholar] [CrossRef]
- ISO. International Standard ISO 20483:2006. In Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- NP 4168. Cereais e derivados: Determinação do teor de matéria gorda total; Instituto Português da Qualidade: Lisboa, Portugal, 1991. [Google Scholar]
- ISO. International Standard ISO 5509:2000. In Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids; ISO 5509: Geneva, Switzerland, 2000. [Google Scholar]
- Gouvinhas, I.; Pinto, R.; Santos, R.; Saavedra, M.J.; Barros, A.I. Enhanced phytochemical composition and biological activities of grape (Vitis vinifera L.) Stems growing in low altitude regions. Sci. Hortic. 2020, 265, 109248. [Google Scholar] [CrossRef]
- Machado, N.F.L.; Domínguez-Perles, R. Addressing Facts and Gaps in the Phenolics Chemistry of Winery By-Products. Molecules 2017, 22, 286. [Google Scholar] [CrossRef]
- Teixeira-Guedes, C.; Oppolzer, D.; Barros, A.I.R.N.A.; Pereira-Wilson, C. Impact of cooking method on phenolic composition and antioxidant potential of four varieties of Phaseolus vulgaris L. and Glycine max L. LWT Food Sci. Technol. 2019, 103, 238–246. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Teixeira, A.I.; Rosa, E.; Barros, A.I.R.N.A. Assessment of (poly)phenols in grape (Vitis vinifera L.) stems by using food/pharma industry compatible solvents and Response Surface Methodology. Food Chem. 2014, 164, 339–346. [Google Scholar] [CrossRef]
- Bolanos De La Torre, A.A.S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- ISO. International Standard ISO 11136:2014. In Sensory Analysis—Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area; ISO 11136: Geneva, Switzerland, 2014. [Google Scholar]
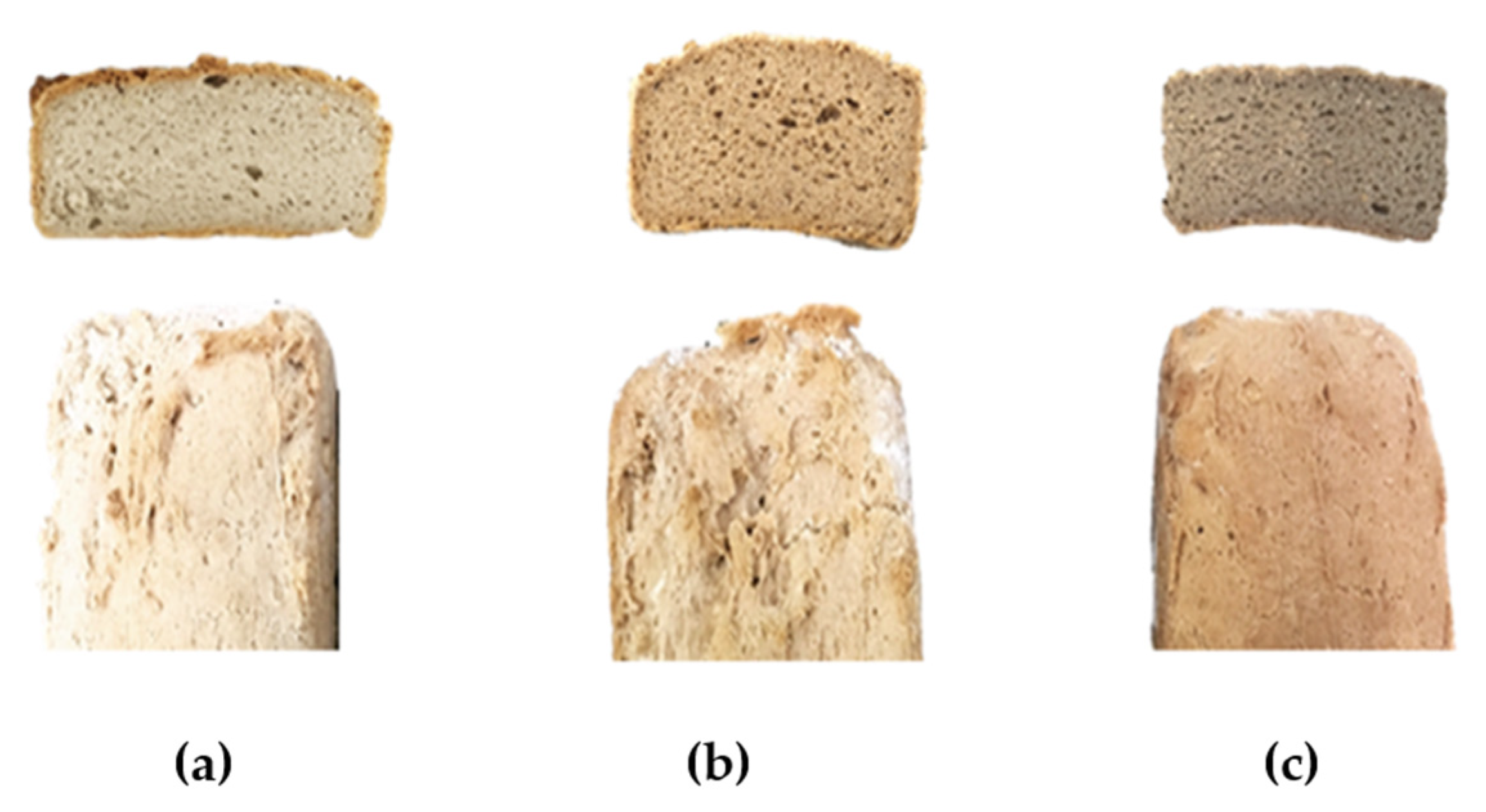

| Control | A23% | A35% | |
|---|---|---|---|
| Volume (cm3) | 920 b ± 92 | 1255 a ± 136 | 965.56 b ± 64 |
| Bake Loss (%) | 12.72 a ± 0.80 | 13.087 a ± 0.20 | 13.849 a ± 0.28 |
| Crumb Colour | |||
| L* | 70.66 a ± 1.54 | 59.76 b ± 0.57 | 51.19 c ± 1.85 |
| a* | 2.45 c ± 0.13 | 7.27 b ± 0.20 | 8.88 a ± 1.40 |
| b* | 11.64 b ± 0.13 | 16.08 a ± 0.20 | 17.12 a ± 1.40 |
| Crust Colour | |||
| L* | 62.23 a ± 7.09 | 47.96 b ± 3.27 | 50.22 b ± 2.17 |
| a* | 8.37 a ± 1.52 | 9.79 a ± 3.34 | 9.59 a ± 1.25 |
| b* | 19.96 a ± 3.00 | 19.49 a ± 1.81 | 18.38 a ± 1.85 |
| aw (water activity) | 0.965 a ± 0.008 | 0.949 b ± 0.005 | 0.955 b ± 0.005 |
| Firmness (N) | 20.83 b ± 2.65 | 28.90 a ± 3.78 | 22.74 b ± 3.60 |
| Cohesiveness | 0.76 a ± 0.02 | 0.74 a ± 0.05 | 0.64 b ± 0.05 |
| Control | A23% | A35% | |
|---|---|---|---|
| Protein (g/100 g) | 10.44 a ± 3.40 | 9.91 a ± 0.01 | 8.52 a ± 0.21 |
| Ash (g/100 g) | 2.44 a ± 0.01 | 2.26 b ± 0.05 | 2.34 a,b ± 0.03 |
| Moisture (g/100 g) | 42.27 a ± 0.40 | 41.10 a ± 1.81 | 41.18 a ± 0.51 |
| Carbohydrates (g/100 g) | 39.75 a ± 1.39 | 40.72 a ± 1.99 | 40.37 a ± 0.80 |
| Total Lipids (g/100 g) | 6.01 b ± 0.03 | 7.76 a ± 0.50 | 8.58 a ± 0.09 |
| FA (g/100 g of Fat) | Control | A23% | A35% |
|---|---|---|---|
| Palmitic | 7.979 b ± 0.13 | 8.882 a ± 0.25 | 9.574 a ±0.08 |
| Palmitoleic | 0.433 a ± 0.03 | 0.221 b ± 0.02 | 0.264 b ±0.01 |
| Stearic | 3.229 a ± 0.04 | 3.277 a ± 0.12 | 3.305 a ±0.02 |
| Oleic (ω9) | 34,222 c ± 0.16 | 4.543 b ± 0.91 | 46.824 a ±0.29 |
| Linoleic (ω6) | 50.726 a ± 0.68 | 41.608 b ± 0.83 | 37.605 c ±0.95 |
| α-Linolenic (ω3) | 0.326 a ± 0.04 | 0.302 a ± 0.01 | 0.343 a ±0.07 |
| Eicosonoic | 0.427 a ± 0.01 | 0.369 a ± 0.01 | 0.366 a ±0.00 |
| Eicosenoic | 0.592 a ± 0.04 | 0.441 b ± 0.02 | 0.423 b ±0.01 |
| Eicosapentaenoic | 0.882 a ± 0.01 | 0.609 a,b ± 0.02 | 0.527 b ±0.01 |
| Lignoceric | 0.390 a ± 0.01 | 0.000 b ± 0.00 | 0.000 b ±0.00 |
| Saturated | 12.121 c ± 0.11 | 12.622 b ± 0.38 | 13.348 a ±0.06 |
| Monounsaturated | 35.529 c ±0.17 | 44.538 b ± 0.94 | 47.879 a ±0.30 |
| Polyunsaturated | 52.350 a ±0.74 | 42.840 b ± 0.87 | 38.773 c ±1.00 |
| Minerals (mg/100 g DW) | Control | A23% | A35% |
|---|---|---|---|
| Na | 643.21 a ± 10 | 655.00 a ± 7 | 642.90 a ± 5 |
| K | 484.42 c ± 11 | 543.53 b ± 3 | 572.04 a ± 6 |
| Ca | 9.33 a ± 1.84 | 16.77 b ± 1.87 | 17.59 b ± 1.23 |
| Mg | 104.02 a ± 1.48 | 72.61 b ± 2.61 | 56.97 c ± 0.74 |
| P | 252.71 a ± 3 | 186.56 b ± 2 | 151.70 c ± 2 |
| S | 101.58 a ± 2.9 | 77.05 b ± 1.8 | 64.50 c ± 2.6 |
| Zn | 1.82 a ± 0.21 | 1.37 a ± 0.28 | 1.60 a ± 0.30 |
| Mn | 0.60 a ± 0.61 | 2.0 b ± 0.29 | 2.67 c ± 0.07 |
| Control | A23% | A35% | ||
|---|---|---|---|---|
| Phenolic Composition | TPC (mg GA/g) | 0.395 c ± 0.020 | 0.613 b ± 0.20 | 0.848 a ± 0.10 |
| ODC (mg GA/g) | 0.99 c ± 0.160 | 5.08 b ± 0.43 | 7.49 a ± 0.58 | |
| FlC (mg CE/ g) | 4.41 b ± 0.65 | 5.39 a,b ± 0.96 | 6.30 a ± 0.78 | |
| Antioxidant Activity | ABTS (mmol trolox/g) | 0.014 b ± 0.003 | 0.066 a ± 0.012 | 0.073 a ± 0.011 |
| DPPH (mmol trolox/g) | 0.006 b ± 0.001 | 0.037 a ± 0.006 | 0.043 a ± 0.015 | |
| FRAP (mmol trolox/g) | 0.007 c ± 0.001 | 0.041 b ± 0.005 | 0.064 a ± 0.010 |
| Identification | RT (min) | HPLC-DAD λ max (nm) | Control | A23% | A35% |
|---|---|---|---|---|---|
| Gallic acid | 10.90 | 230; 270 | 0.48 c ±0.02 | 3.93 b ± 0.02 | 5.29 a ± 0.16 |
| Catechin | 18.35 | 230; 280 | ND | 1.27 b ± 0.02 | 2.07 a ± 0.04 |
| Syringic acid | 24.16 | 232; 275 | 0.77 c ± 0.03 | 7.16 b ± 0.25 | 10.13 a ± 0.28 |
| Benzoic acid unidentified | 24.33 | 233; 277 | ND | 0.94 b ± 0.07 | 1.37 a ± 0.03 |
| Ellagic acid | 24.57 | 250; 370 | ND | 1.0 b ± 0.05 | 1.59 a ± 0.04 |
| Rutin | 24.86 | 233; 254; 355 | ND | 2.09 a ± 0.19 | 3.24 a ± 0.05 |
| Flavanol unidentified | 25.08 | 233; 278 | ND | 16.47 b ± 1.70 | 27.78 a ± 0.29 |
| Flavanol unidentified | 25.49 | 233; 280 | ND | 13.69 b ± 0.91 | 19.97 a ± 0.47 |
| Luteolin | 30.54 | 235; 265; 350 | 0.24 ± 0.04 | ND | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrão Martins, R.; Gouvinhas, I.; Nunes, M.C.; Alcides Peres, J.; Raymundo, A.; Barros, A.I.R.N.A. Acorn Flour as a Source of Bioactive Compounds in Gluten-Free Bread. Molecules 2020, 25, 3568. https://doi.org/10.3390/molecules25163568
Beltrão Martins R, Gouvinhas I, Nunes MC, Alcides Peres J, Raymundo A, Barros AIRNA. Acorn Flour as a Source of Bioactive Compounds in Gluten-Free Bread. Molecules. 2020; 25(16):3568. https://doi.org/10.3390/molecules25163568
Chicago/Turabian StyleBeltrão Martins, Rita, Irene Gouvinhas, Maria Cristiana Nunes, José Alcides Peres, Anabela Raymundo, and Ana I.R.N.A. Barros. 2020. "Acorn Flour as a Source of Bioactive Compounds in Gluten-Free Bread" Molecules 25, no. 16: 3568. https://doi.org/10.3390/molecules25163568
APA StyleBeltrão Martins, R., Gouvinhas, I., Nunes, M. C., Alcides Peres, J., Raymundo, A., & Barros, A. I. R. N. A. (2020). Acorn Flour as a Source of Bioactive Compounds in Gluten-Free Bread. Molecules, 25(16), 3568. https://doi.org/10.3390/molecules25163568











