Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review
Abstract
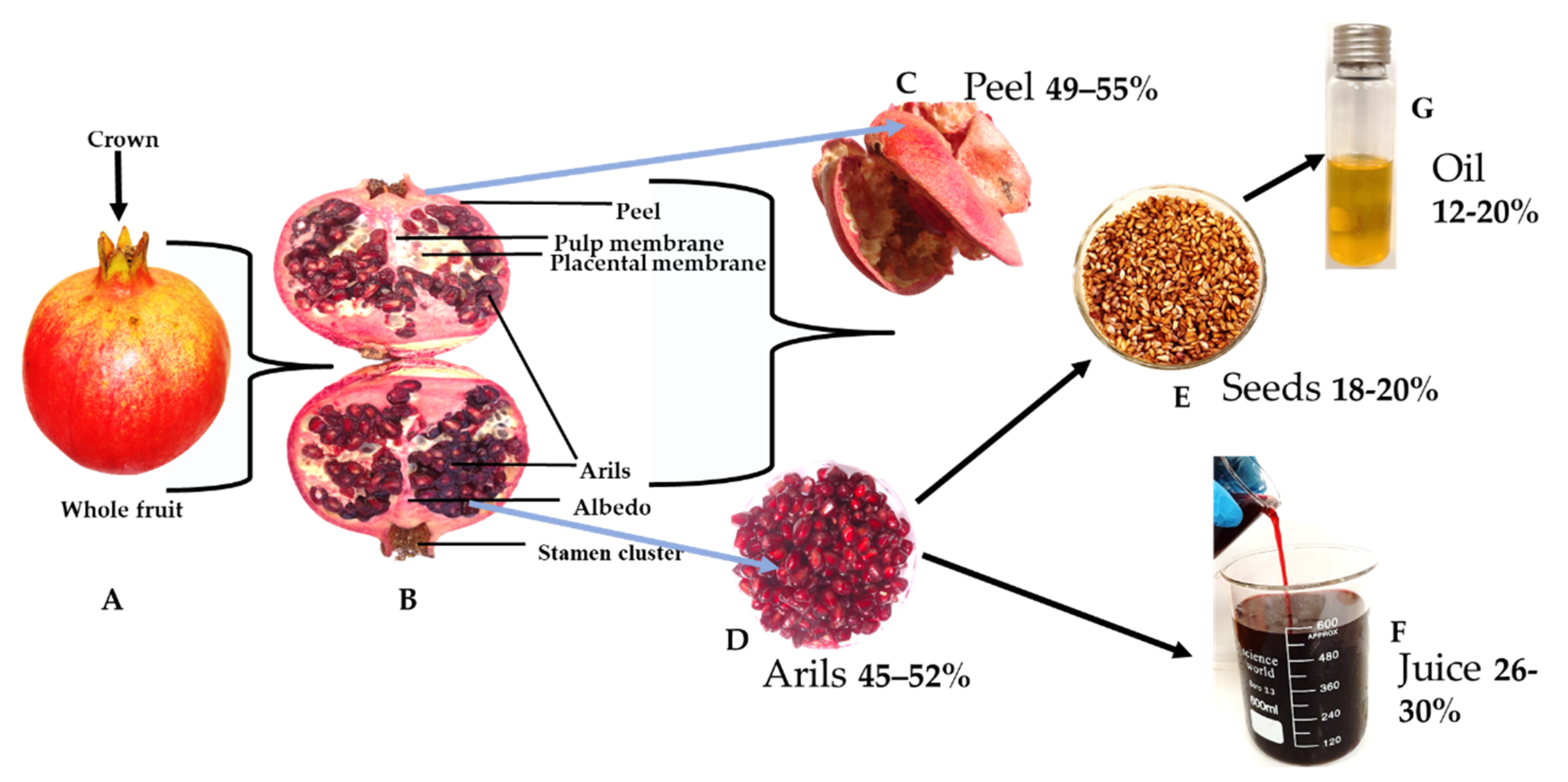
1. Introduction
2. Pharmacological, Phytochemical, and Nutritional Composition of Pomegranate Peel—An Overview
2.1. Phytochemistry and Nutritional Constituents of the Pomegranate Peel
2.2. Organic Acids and Total Sugars
2.3. Vitamin and Mineral Contents
3. Effects of Drying Methods
3.1. Sun Drying
3.2. Microwave Drying
3.3. Freeze-Drying
3.4. Vacuum Drying
3.5. Oven Drying
4. Effects of Extraction Methods
4.1. Solvent Extraction
4.2. Supercritical Fluid Extraction (SFE)
| Extraction Technique | Cultivar | Country | Phytochemical and Nutritional Properties | Key Findings | Reference |
|---|---|---|---|---|---|
| Solvent extraction 80% (v/v) methanol and distilled water (aqueous) | Ganesh, Molla de Elche, Arakta, Bhagwa, Wonderful, Herskawitz, Ganesh, and Ruby | South Africa | Total flavonoid content (TFC), gallotannin content (GTC), total anthocyanin content (TAC), catechin, epicatechin, ellagic acid, and gallic acid | High amounts of phenolic compounds were found in peel extracts, with the highest total phenolic content (TPC) of 295.5 mg/g dry extract found in Ganesh and the lowest in cultivar Molla de Elche at 179.3 mg/g dry extract. Catechin, epicatechin, ellagic acid, and gallic acid were found in all cultivars, with ellagic acid being the most abundant. None of the aqueous extracts exhibited good antibacterial activity at the highest screening concentration (>12.5 mg/mL). | [8] |
| Solvent extraction water, methanol, and ethanol | Helow | Sultanate of Oman | Total phenolic content (TPC) | Water extracted the highest amount of total phenolic content (TPC), followed by methanol and ethanol. Fresh peel extracts contained 5990, 4530, and 8460 mg gallic acid equivalent (GAE)/100 g dry peel solids (recorded as dry mass (DM)) for methanol, ethanol, and water extracts, respectively. | [85] |
| Solvent extraction Aqueous and methanolic extracts | Unknown | Egypt | Total phenolic content (TPC) and total flavonoid content (TFC) | Methanol extracts showed highest total phenolic content (TPC) and total flavonoid content (TFC) amounts, regardless of the mode of drying used—methanolic oven-dried peel extracts ranged from 16.00 to 17.78 mg gallic acid/g. The total phenolic content (TPC) of aqueous peel ranged from 14.23 to 16.34 mg gallic acid/g with the same drying technique. Total flavonoid content (TFC) values ranged from 6.99 to 7.98 and 6.54 to 6.86 mg rutin equivalent (RE)/g for methanolic and aqueous oven-dried extracts. | [18] |
| Solvent extraction Water, methanol (MeOH), ethanol, acetone, and ethyl acetate (EtOAc) extraction (15:1 (w/w) at 40 °C for 4 h) | Wonderful | USA | Extract yield, total phenolic content (TPC), proanthocyanidins, and total flavonoid content (TFC) | Highest extract yield in methanol (MeOH) at 46.51 ± 0.86, followed by water (H2O) at 43.19 ± 2.24. The lowest was ethyl acetate at 0.88 ± 0.08 g dried extract/100 g pomegranate marc peel(PMP). MeOH followed by H2O had the highest phytochemical yields, with total phenolic content (TPC) values of 5.90% and 8.26%, respectively. The concentrations of total phenolic content (TPC), pro-anthocyanidins, and (total flavonoid content (TFC) were highest using ethyl acetate at ≤20%, followed by methanol (MeOH) at 18%, distilled water (H2O) at 14%, and ethanol at 9%. | [109] |
| Solvent extraction 80% methanol (MeOH), distilled water (H2O), and diethyl ether extracts | Yemeni varieties | Saudi Arabia | Extract yield, total phenolic content (TPC), total flavonoid content (TFC), and ascorbic acid | 80% methanol (MeOH) extract had the highest yields for total phenolic content (TPC) and total flavonoid content (TFC) at 45.4 ± 5.3% and 274 ± 17 mg gallic acid equivalent (GAE)/g (27.4%) and 56.4 ± 2.7 mg for flavonoids rutin equivalent (RE)/g. Ascorbic acid (AA) was only present in small amounts (2 mg/g), and thus it was unlikely to substantially contribute to the antioxidant activity of methanol (MeOH). | [112] |
| Solvent extraction MeOH at 30 °C for 4 h | Ganesh | India | Extract yield, total phenolic content (TPC), gallic acid, and ellagic acid | Methanol (MeOH) extract had the highest yield at 10.38 ± 0.89% weight for weight (w/w). The total phenolic content of the extract [(+)-catechin equivalent] was found to be 42% weight for weight (w/w). The high performance liquid chromatography (HPLC) pattern of the methanol (MeOH) extract of peel showed the presence of gallic acid and ellagic acid as the major components. Methanol (MeOH) pomegranate peel extract was reported to enhance or maintain the activity of hepatic enzymes. | [114] |
| Solvent extraction Water, ethanol (50, 70 and 100% ethanol), and methanol (100%) | Ganesh | India | Extract yield, total phenolic content (TPC) | The highest yield was reported using 50% ethanol at 16.3 ± 1.99%, while aqueous extract had the highest total phenolic content (TPC) at 438.3 mg/g. Higher 2,2-diphenyl-1-picrylhydrazy (DPPH) and 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) inhibition was reported using methanol and 70% ethanol at 79.5 ± 6.5 and 94.6 ± 6.10, respectively. | [106] |
| Solvent extraction Aqueous, ethanol, chloroform, acetone, and petroleum ether | Unknown | India | Extract yield, tannins, saponins, flavonoids, quinones, cardiac glycosides, terpenoids, phenol, steroid, coumarins, and alkaloids | The optimum tannin yield was reported at 87.3 mg tannic acid equivalent (TAE)/g using ethanol extract. Tannins, saponins, flavonoids, quinones, cardiac glycosides, terpenoids, phenol, steroid, coumarins, and alkaloids. Highest antioxidant activity was reported at 94.5% using ethanol extract. | [124] |
| Soxhlet extraction (solvent extraction) Hexane, dichloromethane, ethyl acetate, and methanol | Unknown | India | Extract yield, 5-hydroxy- methylfurfural, furan-2,5- dicarbaldehyde | The highest exact yield was obtained at 37.85% using methanol and the lowest at 2.1% using hexane. Methanolic extract showed higher 5-hydroxymethylfurfural as a major compound at 60.11%, higher radical scavenging activity (RSA) of 75.36% at 100µg/mL, and a minimum value of 41.20% at 10µg/mL. Furan-2,5-dicarbaldehyde was reported with high radical scavenging activity (RSA) of 70.39% at 100µg/mL and 39.03% at 10µg/mL. | [113] |
| Solvent extraction Methanol, ethanol, acetone, chloroform, and ethyl acetate | Unknown | India | Extract yield, total phenolic content (TPC), methyl gallate, tocopherol, quercitin glucoside, polymeric flavonol, maclurin-3-c-(2-o-galloyl)-β-d-glucoside, epicatechin, and quercitin pentoside | Methanol had the highest yield at 23.56%, while the lowest yield was 2.2% using ethyl acetate. Similarly, the highest total phenolic content (TPC) value was found in methanol and the lowest in ethyl acetate peel extracts at 78.92 and 1.30 mg/gm gallic acid equivalent (GAE), respectively. | [115] |
| Solvent extraction ethyl acetate (EtOAc), methanol, and water | Ganesh | India | Moisture content (%), extract yield, total phenolic content (TPC), gallic acid | Methanolic peel extracts had the highest extract yield at 9.38% weight for weight (w/w), followed by water at 7.53% weight for weight (w/w) and ethyl acetate (EtOAc) at 1.04% weight for weight (w/w). Methanol (MeOH) extract had highest total phenolic content (TPC) at 44.0% weight for weight (w/w), while lowest was found in aqueous extract at 3.00% weight for weight (w/w). | [108] |
| Solvent extraction Ethanol, methanol, acetone, and combinations | Unknown | China | Extract yield, total phenolic content (TPC), flavonoids, pro-athocyanidins, and ascorbic acid | Peel combination solvent extract had higher ferric reducing antioxidant power (FRAP) value (approximately 4.5 mmol/L) than those obtained using individual solvents. Extract yield, total phenolic content (TPC), flavonoids, proanthocyanidins, and ascorbic acid of peel extract were reported at 31.5 ± 3.1%, 249.4 ± 17.2 mg/g, 59.1 ± 4.8 mg/g, 10.9 ± 0.5 mg/g, and 0.99 ± 0.02 mg/g, respectively. | [125] |
| Solvent extraction ethyl acetate (EtOAc), acetone, methanol (MeOH), and water | Unknown | India | Extract yield and Total phenolic content (TPC) | Methanol (MeOH) extract had highest total phenolic content (TPC) yields at 9.4% and 460 mg/g, respectively. | [116] |
| Solvent and ultrasound-assisted solvent extraction methods (acetone, methanol (MeOH), ethanol (EtOH), water (H2O), ethyl acetate) and supercritical fluid extraction (SFE) of (CO2) | Poost Syah | Iran | Phenolic compounds, punicalagin | Punicalagin content range was 0.32–0.84 g/100 g dryweight (DW) using supercritical fluid extraction (SFE). Acetone with sonication produced the highest phenolic compound contents (in either solvent or ultrasound-assisted solvent extraction methods, 40.0 and 35.0% for sonication and solvent extraction, respectively; lowest values were found in ethyl acetate extracts (0.2 and 0.2%)). The ethyl acetate extract and extract of modified supercritical fluid extraction carbon dioxide (SFE CO2) had similar but comparatively small extraction yields. | [126] |
| Supercritical fluid extraction (SFE) and hydrodistillation | Malas | Iran | Over 76 essential oils including oleic acid, palmitic acid, and (−)-borneol | Over 76 essential oils were identified using gas chromatography mass spectrometry (GCMS), with oleic acid, palmitic acid, and (−)-borneol being the major compounds in both extracts. The optimum extraction yields were 1.18% weight for weight (w/w) for supercritical fluid extraction (SFE) and 0.21% volume per weight (v/w) for hydrodistillation. | [118] |
| Supercritical carbon dioxide (SC-CO2) using a Box–Behnken design | Wonderful | Chile | Extract yield, total phenolic content (TPC), phenolic compounds (punicalagin (PU) and punicic acid (PA)) | Extract yields ranged from 0.2% to 8.5%, with the highest yields at 200 and 300 bar, 40–50 °C, and 20% cosolvent. Punicalagin (PU) contents of 0.4–9.5% with optimal extraction conditions of 400 bar, 43 °C, and 20% ethanol. Once the peel extract was extracted under optimal conditions, the extract had a punicalagin (PU) content of 9.7%, total phenolic content (TPC) of 10.01 mg gallic acid equivalent (GAE) per g, and an antioxidant activity of 99.4 (lg Trolox equivalent per g). The punicalagin EE ranged from 35.1% to 72.4%. | [119] |
| Enzyme-assisted supercritical fluid extraction (EASCFE) and enzyme-assisted solvent extraction (EASE) | Unknown | Pakistan | Extract yield, Total phenolic content (TPC), phenolic acids: vanillic acid, ferulic, syringic, sinapic acid | The highest extract yield of 65.89% was obtained using enzyme-assisted solvent extraction (EASE). Enzyme-assisted supercritical fluid extraction (EASCFE) had the highest total phenolic content (TPC) at 301.53 mg gallic acid equivalent (GAE)/g pretreated under optimised conditions involving cocktail enzyme concentration (3.8%) at 49 °C and pH of 6.7 for 85 min. Numerous phenolic compounds were present in high amounts, such as vanillic acid ranging at 65.87 and 108.36 µg/g in enzyme-assisted solvent extraction (EASE) and enzyme-assisted supercritical fluid extraction (EASCFE), respectively. The p-coumaric acid (0.12–14.87 µg/g), gallic acid (0.041–0.37 µg/g), caffeic acid (3.88–75.19 µg/g), ferulic acid (0.15–8.84µg/g), syringic acid (15.17–88.24 µg/g), and sinapic acid (2.13–3.58 µg/g) were assayed after enzyme-assisted solvent extraction (EASE) and enzyme-assisted supercritical fluid extraction (EASCFE). | [120] |
| Ultrasound-assisted extraction (UAE) Water | Sishe Kape-Ferdos | Iran | Moisture content, extract yield, and total phenolic content (TPC) | The moisture content was reported at 46.62 wet basis (%wb). The optimal conditions based on both individual and combinations of all process variables were ultrasonic amplitude (UA) of 60% and ultrasonic exposure time (UET) of 6.2 min. With these optimum conditions, the predicted maximum yield and total phenolic content (TPC) values were 13.1% and 42.2 mg gallic acid (GA)/g, respectively. | [127] |
| Ultrasound-assisted enzymatic extraction | Dalim | India | Total phenolic content (TPC) and total flavonoid content (TFC) | The optimum conditions for maximum extractability were an ultrasonication time of 41.45 min, enzyme concentration of 1.32 mL/100 mL, incubation time of 1.821 h, and incubation temperature of 44.85 °C. The total phenolic content (TPC), total flavonoid content (TFC), and radical scavenging activity (RSA) values in optimised conditions were 19.77 mg gallic acid equivalent (GAE)/g, 17.97 mg quercetin equivalent (QE)/g, and 74.213%, respectively. | [128] |
| Ultrasound-assisted extraction Ethanol | Unknown | Serbia | Total phenolic content (TPC), phenolic compounds: ellagic acid, gallic acid, punicalagin, and punicalin | Optimal extraction process conditions were as follows: extraction time of 25 min, ethanol concentration of 59%, solid-to-solvent ratio of 1:44, and extraction temperature of 80 °C. Total phenolic content (TPC) values in extracts obtained using ultrasound-assisted extraction (UAE) technique varied between 81.61 and 190.94 mg gallic acid equivalent (GAE)/g dry weight (DW). Ellagic acid, gallic acid, punicalin, and punicalagin content ranges were 4.05–12.54, 1.13–3.58, 28.38–65.67 and 7.04–35.05 mg/g dry weight (DW), respectively. | [129] |
| Ultrasound-assisted extraction Sunflower and soy oils | Unknown | Greece | Extract yield and carotenoids | The optimum extraction yield was about 0.3255 mg carotenoids/100 g of dry peels. The levels of extraction using the green solvents and ultrasound were about 85.7 and 93.8% of the total carotenoids present in the waste material, respectively. The highest concentrations obtained were 0.6134 and 0.6715 mg carotenoids/100 g of dry peels using sunflower oil and soy oils, respectively. | [130] |
| Pulsed ultrasound-assisted extraction (PUAE) | Malas | Iran | Extract yield, punicalagin, ellagic acid, gallic acid, and hydroxybenzoic acids | The application of 10 min extraction under the pulsed mode also resulted in a high yield (41.6%). Punicalagin contents (α and β) varied from 128.02 to 146.61 mg/g, with the highest content obtained at an intensity level of 105 W/cm2 and duty cycle of 50% for a short time (10 min). Ellagic acid content varied from 10.12 to 22.53 mg/g with different pulsed ultrasound-assisted extraction (PUAE) conditions. High performance liquid chromatography (HPLC) analysis revealed that punicalagin, ellagic acid, and gallic acid constitute almost 168.55 mg/g of the peel extract. | [131] |
| Ultrasound-assisted extraction | Unknown | India | Extract yield of pectin | Optimal conditions were solid liquid (SL) ratio of 1:18 g/mL, pH of 1.3, extraction time of 29 min, and extraction temperature of 62 °C. Under these conditions, the experimental yield of pectin was reported at 23.87%, close to the predicted value (23.92%). | [132] |
| Ultrasound-assisted polysaccharide extraction | Unknown | China | Polysaccharide yield | Optimum extraction parameters were as follows: ratio of water-to-raw material of 24 mL/g, ultrasonic power of 148 W, extraction time and temperature of 63 min and 55 °C, respectively. Under these conditions, the polysaccharide yield was 13.658 ± 0.133%, similar to the predicted value of 13.787%. | [133] |
| Ultrasound-assisted extraction | Unknown | Greece | Extract yield | Direct ultrasound-assisted extraction of total phenolics gave a maximum yield of 13.85% (g gallic acid equivalent (GAE)/100 g of dry peels) with an extraction time of 10 min. The extraction yield increased with increasing extraction temperature from 25 to 35 °C, with amplitude levels up to around 40%, solvent/peel ratios of up to 33:1, and decreasing pulse duration/pulse interval ratios | [134] |
| Ultrasound-assisted extraction Ethanol–water mixture | Unknown | Iran | Extract yield, total phenolic content (TPC) | The extraction yield with water–ethanol as the solvent (45.4%) was four times better than the values for water, which were 11–14%.The extraction yield with water–ethanol as the solvent (45.4%) was also better than methanol (29–35.5%). Total phenolic content (TPC) varied from 5506.42 to 8923.24 mg gallic acid equivalent/100 g of dry weight. Optimal conditions were 70% ethanol–water mixture as the solvent, temperature of 60 °C, and extraction time of 30 min. | [135] |
| Ultrasonic-assisted extraction | Bhagwa | India | Extract yield, total phenolic content (TPC), total tannins (TE), phenolic compounds: gallic acid, ellagic acid, and punicalagin (α and β) | Optimum process conditions of 15.12 min (extraction time) and amplitude of 30% gave the highest yield, total phenolic content (TPC), and total tannins (TE) values of 42.45%, 354.67 mg gallic acid equivalent (GAE)/g, and 348.0 g TAE/g, respectively. High performance liquid chromatography (HPLC) analysis revealed gallic acid, ellagic acid, and punicalagin (α and β) as the major ellagitannin compounds at 0.96, 7.58, and 163.52 mg/g, respectively. | [136] |
| Ultrasound-assisted extractions in continuous (CUAE) and pulsed modes (PUAE) compared with convectional extraction (CE) | Wonderful | USA | Total phenolic yield (%) | High antioxidant yield (14.8%) was achieved at an intensity of 59.2 W/cm2 and treatment time of 60 min for ultrasound-assisted extractions in continuous (CUAE). 2,2-diphenyl-1-picrylhydrazy (DPPH) scavenging activities of 5.5 g/g at an extraction time of 60 min, temperature of 25 ± 2 °C, and water/peel ratio of 50:1 w/w elevated the antioxidant yield by 24% and decreased the extraction time by 90% compared to convectional extraction (CE). An intensity level of 59.2 W/cm2, pulse duration of 5, and interval of 5 s gave an antioxidant yield of 14.5%. Pulsed ultrasound-assisted extraction (PUAE) elevated the antioxidant yield by 22% and decreased the extraction time by 87% in comparison to convectional extraction (CE). It also conserved 50% more electrical energy than ultrasound-assisted extractions in continuous (CUAE). Pulsed ultrasound-assisted extraction (PUAE) was superior to ultrasound-assisted extractions in continuous (CUAE). | [137] |
| Microwave-assisted extraction (MAE) Used water | Unknown | China | Phenolic yield | The average experimental phenolic yield under the optimum conditions was found to be 210.36 ± 2.85 mg gallic acid equivalent (GAE)/g, similar to the predicted value of 214.46 mg GAE/g. It was found that the extract was an effective scavenger in quenching 2,2-diphenyl-1-picrylhydrazy (DPPH) radicals, with an inhibitory concentration (IC50) of 14.53 µg/mL. | [138] |
| Microwave-assisted extraction (MAE) Ethanol | Unknown | China | Flavonoids content and yield of flavonoids | A maximum extraction yield of 4.26% was achieved at an ethanol concentration of 60%, solvent-to-material ratio of 40:1, and microwave-assisted extraction (MAE) time of 3 min. The extract exhibited a strong 2,2-diphenyl-1-picrylhydrazy (DPPH) radical scavenging ability, with an inhibitory concentration (IC50) value of 0.187 mg/mL. | [139] |
| Microwave-assisted extraction, ultra-assisted extraction, conventional solid–liquid extraction (maceration and decoction) | Unknown | Mexico | Total phenolic content (TPC), phenolic compounds | The microwave extraction method obtained the highest total phenolic content (TPC) at 18.92 mg gallic acid equivalent (GAE)/g, and 14 compounds were identified using both extraction methods. Both extraction methods showed important differences. Cinnamic acid (m/z 146.9279) and granatin B (Galloyl-HHDP-DHHDP-hexoside) (m/z 950.7491) were only reported in decoction peel extracts. Secoisolariciresinol di-O-glucoside (m/z 540.8878) and pedunculagin I (m/z 782.8182) were reported in microwave-assisted extraction (MAE) peel extracts. | [140] |
| Pressurised liquid extraction Deionised water | Izmir 8, Izmir 10, Izmir 16, Izmir 23, Izmir 26, Izmir 1264, Izmir 1479, Izmir 1499, Izmir 1513 | Turkey | Extract yield, total phenolic content (TPC), total flavonoid content (TFC), condensed tannins (CT), hydrolysable tannins (HT), punicalagin (α and β), gallic acid and ellagic acid | Extract yields of 43.3, 46.5, and 16.7% reported for pressurised water extraction (PWE), methanol, and water extraction, respectively. Total phenolic content (TPC) values of 264.3, 258.2 and 80.5 mg tannic acid equivalent (TAE)/g reported using pressurised water (PWE), methanol, and water extraction, respectively. Total flavonoid content (TFC) values of 13.0, 18.1, and 6.2 mg catechin equivalent (CE)/g reported using pressurised water extraction (PWE), methanol, and water extraction, respectively. Hydrolysable tannins (HT) ranged from 262.70 to 82.6 mg tannic acid equivalent (TAE)/g (water extraction). Condensed tannins (CT) values of 9.5, 9.2, and 3.7 mg catechin equivalent (CE)/g dry weight (DW) using PWE, methanol, and water extraction, respectively. Punicalagin content (α and β), ellagic acid, and gallic acid values of 116.6, 1.25, and 1.43 mg/g, respectively. | [141] |
| Ultrasound-assisted pressurised liquid extraction (UAPLE) Water, ethanol + water 30, 50, and 70% v/v) | Wonderful | USA | Extract yield, α- and β-punicalagin, ellagic acid hexoside. and ellagic acid. Ellagic acid pentoside, ellagic acid deoxyhexoside, pedunculagin I, and unidentified compounds | Ultrasound extraction was reported to have a higher influence on the extraction yields when utilising large particles measuring 1.05 mm and intermediate ultrasound power of 480–640 W at the generator or 23.1–30.8 W at the tip of the probe. Using ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS), a total of 24 different compounds were detected. Using a larger peel particle size of 1.05 mm, water extraction, extraction temperature of 70 °C, ultrasound power of 480 W, and 3 cycles, am enhanced phenolic recovery yield of 61.72 ± 7.70 mg/g of phenolic compounds was achieved from the pomegranate peel. | [142] |
| High-pressure assisted extraction | Unknown | Portugal | Extract yield, individual phenolics, tannins, and anthocyanins | Increase of the extraction time and pressure and decrease of ethanol concentration, as well as enhanced extract yields (only until a pressure of 382 MPa and ethanol concentration of 36%). The optimum extraction conditions were comparable for all compounds, excluding anthocyanins, which varied between 356 and 600 MPa, with an extraction time of 30 min and an ethanol concentration of 56%. The optimum conditions were reported at an extraction time of 30 min, pressure of 492 MPa, and an extraction concentration of 37%. | [143] |
4.3. Ultrasound-Assisted Extraction (UAE)
4.4. Microwave-Assisted Extraction (MAE)
4.5. Pressurised Liquid Extraction (PLE)
5. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajaei, H.; Yazdanpanah, P. Buds and leaves in pomegranate (Punica granatum L.): Phenology in relation to structure and development. Flora Morphol. Distrib. Funct. Ecol. Plants 2015, 214, 61–69. [Google Scholar] [CrossRef]
- Fadavi, A.; Barzegar, M.; Azizi, M.H.; Bayat, M. Physicochemical composition of ten pomegranate cultivars (Punica granatum L.) grown in Iran. Food Sci. Technol. Int. 2005, 11, 113–119. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci. Hortic. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Effects of maturity status on biochemical content, polyphenol composition and antioxidant capacity of pomegranate fruit arils (cv. ‘Bhagwa’). S. Afr. J. Bot. 2013, 85, 23–31. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, horticulture, breeding. Hortic. Sci. 2009, 35, 127–191. [Google Scholar]
- Al-Said, F.A.; Opara, L.U.; Al-Yahyai, R.A. Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman. J. Food Eng. 2009, 90, 129–134. [Google Scholar] [CrossRef]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosine-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200–225. [Google Scholar] [CrossRef]
- Opara, L.U.; Al-Ani, M.R.; Al-Shuaibi, Y.S. Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food Bioprocess Technol. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L.; Theron, K.I. Chemical and phytochemical properties and antioxidant activities of three pomegranate cultivars grown in South Africa. Food Bioprocess Technol. 2012, 5, 425–444. [Google Scholar] [CrossRef]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green chemistry based benign routes for nanoparticle synthesis. J. Nanopart. 2014. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. Green nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Thilagavathi, T.; Renuka, R.; Priya, R.S. Bio-synthesis of silver nanoparticles using Punica granatum (pomegranate) peel extract: A novel approach toward waste utilization. Int. J. Adv. Sci. Eng. 2016, 3, 234–236. [Google Scholar]
- Shanmugavadivu, M.; Selvam, K.; Ranjithkumar, R. Synthesis of pomegranate peel extract mediated silver nanoparticles and its antibacterial activity. Am. J. Adv. Drug Deliv. 2014, 2, 174–182. [Google Scholar]
- Yang, H.; yu Ren, Y.; Wang, T.; Wang, C. Preparation and antibacterial activities of Ag/Ag+/Ag3+nanoparticle composites made by pomegranate (Punica granatum) rind extract. Results Phys. 2016, 6, 299–304. [Google Scholar] [CrossRef]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Goudarzi, M.; Salavati-Niasari, M. Using pomegranate peel powders as a new cap-ping agent for synthesis of CuO/ZnO/Al2O3 nanostructures; enhancement of visible light photocatalytic activity. Int. J. Hydrog. Energy 2018, 43, 14406–14416. [Google Scholar] [CrossRef]
- El-Said, M.M.; Haggag, H.F.; El-Din, H.M.F.; Gad, A.S.; Farahat, A.M. Antioxidant activities and physical properties of stirred yoghurt fortified with pomegranate peel extracts. Ann. Agric. Sci. 2014, 59, 207–212. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible coatings incorporating pomegranate peel extract and biocontrol yeast to reduce Penicillium digitatum postharvest decay of oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Characterization and antifungal activity of pomegranate peel extract and its use in polysaccharide-based edible coatings to extend the shelf-life of Capsicum (Capsicum annuum L.). Food Bioprocess Technol. 2018, 11, 1317–1327. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhang, Z.H.; Li, L.; Xiong, W.; Shi, J.Y.; Zhao, T.R.; Fan, J. Antioxidant effect of pomegranate rind powder extract, pomegranate juice, and pomegranate seed powder extract as antioxidants in raw ground pork meat. Food Sci. Biotechnol. 2013, 22, 1063–1069. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Aelma Husna, A.B.; Nurul Syahida, S.; Nor Khaizura, M.A.B.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [Google Scholar] [CrossRef]
- Emam-Djomeh, Z.; Moghaddam, A.; Ardakani, Y.S.A. Antimicrobial activity of pomegranate (Punica granatum L.) peel extract, physical, mechanical, barrier and antimicrobial properties of pomegranate peel extract-incorporated sodium caseinate film and application in packaging for ground beef. Packag. Technol. Sci. 2015, 28, 869–881. [Google Scholar] [CrossRef]
- He, L.; Lan, W.; Ahmed, S.; Qin, W.; Liu, Y. Electrospun polyvinyl alcohol film containing pomegranate peel extract and sodium dehydroacetate for use as food packaging. Food Packag. Shelf Life 2019. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Gani, A.; Punoo, H.A.; Masoodi, F.A. Use of pomegranate peel extract incorporated zein film with improved properties for prolonged shelf life of fresh Himalayan cheese (Kalari/kradi). Innov. Food Sci. Emerg. Technol. 2018, 48, 25–32. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhang, Z.H.; Li, L.; Yuan, M.L.; Fan, J.; Zhao, T.R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef]
- Yuan, G.L.H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Technol. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Thorat, P.; Manjunatha, M. Effect of vacuum packaging and pomegranate peel extract on quality aspects of ground goat meat and nuggets. J. Food Sci. Technol. 2014, 51, 2685–2691. [Google Scholar] [CrossRef]
- Firuzi, M.R.; Niakousari, M.; Eskandari, M.H.; Keramat, M.; Gahruie, H.H.; Khaneghah, A.M. Incorporation of pomegranate juice concentrate and pomegranate rind powder extract to improve the oxidative stability of frankfurter during refrigerated storage. LWT J. Food Sci. Technol. 2019, 102, 237–245. [Google Scholar] [CrossRef]
- Juneja, V.K.; Cadavez, V.; Gonzales-Barron, U.; Mukhopadhyay, S.; Friedman, M. Effect of pomegranate powder on the heat inactivation of Escherichia coli O104: H4 in ground chicken. Food Control 2016, 70, 26–34. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, Y.; Jia, S.; Hong, H.; Liu, Y.; Luo, Y. Effects of pomegranate peel extract on quality and microbiota composition of bighead carp (Aristichthys nobilis) fillets during chilled storage. Food Microbiol. 2019, 82, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.M.; Lazarides, H.N. Integrated processes can turn industrial food waste into valuable food by-products and/or ingredients: The cases of olive mill and pomegranate wastes. J. Food Eng. 2015, 167, 45–50. [Google Scholar] [CrossRef]
- Saadi, W.; Rodríguez-Sánchez, S.; Ruiz, B.; Souissi-Najar, S.; Ouederni, A.; Fuente, E. Pyrolysis technologies for pomegranate (Punica granatum L.) peel wastes. Prospects in the bioenergy sector. Renew. Energy 2019, 136, 373–382. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Nizamuddin, S.; Mubarak, N.M.; Shirin, K.; Aijaz, M.; Hussain, M.; Baloch, H.A. Characterization and process optimization of biochar produced using novel biomass, waste pomegranate peel: A response surface methodology approach. Waste Biomass Valoriz. 2019, 10, 521–532. [Google Scholar] [CrossRef]
- Ebrahimi, I.; Gashti, M.P. Extraction of polyphenolic dyes from henna, pomegranate rind, and Pterocarya flaxinifolia for nylon 6 dyeing. Color. Technol. 2016, 132, 162–176. [Google Scholar] [CrossRef]
- Ragheb, A.A.; Mosaad, M.M.; Tawfik, S.; Abd-El Thalouth, J.I. The impact of nanoparticles on developing the printing of natural fabrics with pomegranate peel. Egypt. J. Chem. 2019, 62, 1249–1261. [Google Scholar]
- Shams-Nateri, A.; Hajipour, A.; Dehnavi, E.; Ekrami, E. Colorimetric study on polyamides dyeing with weld and pomegranate peel natural dyes. Cloth. Text. Res. J. 2014, 32, 124–135. [Google Scholar] [CrossRef]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Pomegranate. In Integrated Processing Technologies for Food and Agricultural By-Products, 1st ed.; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 181–216. [Google Scholar]
- Pomegranate Association of South Africa (POMASA). Pomegranate Industry Overview. Available online: http://www.hortgro.co.za/portfolio/pomegranates (accessed on 12 March 2018).
- Shema-Didi, L.; Kristal, B.; Sela, S.; Geron, R.; Ore, L. Does pomegranate intake attenuate cardiovascular risk factors in hemodialysis patients? Nutr. J. 2014, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tehranifar, A.; Selahvarzi, Y.; Kharrazi, M.; Bakhsh, V.J. High potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Ind. Crops Prod. 2011, 34, 1523–1527. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Khan, M.S.Y.; Vohora, S.B. Anti-bacterial, anti-fungal and anthelmintic investigations on Indian medicinal plants. Nutr. Rev. 1991, 62, 221–228. [Google Scholar]
- Lev, E.; Amar, Z. Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J. Ethnopharmacol. 2002, 82, 131–145. [Google Scholar] [CrossRef]
- Lad, V.; Frawley, D. The Yoga of Herbs: An Ayurvedic Guide to Herbal Medicine, 2nd ed.; Lotus Press: Twin Lakes, WI, USA, 1986; pp. 135–136. [Google Scholar]
- Ong, H.C.; Nordiana, M. Malay ethno-medico botany in Machang, Kelantan, Malaysia. Fitoterapia 1999, 70, 502–513. [Google Scholar] [CrossRef]
- Ganguly, S. Medicinal utility of pomegranate fruit in regular human diet: A brief review. Int. J. For. Hortic. 2017, 3, 17–18. [Google Scholar]
- Lasure, P.P.; Munot, N.M.; Lawande, S.S. Determination of antibacterial activity of Punica granatum fruit. Int. J. Pharm. Sci. Res. 2012, 3, 4421–4424. [Google Scholar]
- Egharevba, H.O.; Kunle, O.F.; Iliya, I.; Orji, P.N.; Abdullahi, M.S.; Okwute, S.K.; Okogun, J.I. Phytochemical analysis and antimicrobial activity of Punica granatum L. (fruit bark and leaves). N. Y. Sci. J. 2010, 3, 91–98. [Google Scholar]
- Reddy, M.; Gupta, S.; Jacob, M.; Khan, S.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef]
- Jafri, M.A.; Aslam, M.; Javed, K.; Singh, S. Effect of Punica granatum Linn. (flowers) on blood glucose level in normal and alloxan-induced diabetic rats. Res. J. Pharm. Technol. 2000, 70, 309–314. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef]
- Kim, N.D.; Mehta, R.; Yu, W.; Neeman, I.; Livney, T.; Amichay, A.; Poirier, D.; Nicholls, P.; Kirby, A.; Jiang, W.; et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granutum) for human breast cancer. Breast Cancer Res. Treat. 2002, 71, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.Z.Y.; Zhu, C.; Kim, M.S.; Yamahara, J.; Li, Y. Pomegranate flower ame-liorates fatty liver in an animal model of type 2 diabetes and obesity. J. Ethnopharmacol. 2009, 123, 280–287. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.G.; Liang, W.L.; Wanga, C.C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010, 118, 315–322. [Google Scholar] [CrossRef]
- Altunkaya, A.; Hedegaard, R.V.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Antioxidant capacity versus chemical safety of wheat bread enriched with pomegranate peel powder. Food Funct. 2013, 4, 722–727. [Google Scholar] [CrossRef] [PubMed]
- El-Hadary, A.E.; Ramadan, M.F. Phenolic profiles, antihyperglycemic, antihyperlipidemic, and antioxidant properties of pomegranate (Punica granatum) peel extract. J. Food Biochem. 2019, 43, 1–9. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hortic. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Arun, N.; Singh, D.P. Punica granatum: A review on pharmacological and therapeutic properties. Int. J. Pharm. Sci. Res. 2012, 3, 1240–1245. [Google Scholar]
- Morgunov, I.G.; Kamzolova, S.V.; Dedyukhina, E.G.; Chistyakova, T.I.; Lunina, J.N.; Mironov, A.A.; Stepanova, N.N.; Shemshura, O.N.; Vainshtein, M.B. Application of organic acids for plant protection against phytopathogens. Appl. Microbiol. Biotechnol. 2017, 101, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Dafny-Yalin, M.; Glazer, I.; Bar-Ilan, I.; Kerem, Z.; Holland, D.; Amir, R. Color, sugars and organic acids composition in aril juices and peel homogenates prepared from different pomegranate accessions. J. Agric. Food Chem. 2010, 58, 4342–4352. [Google Scholar] [CrossRef] [PubMed]
- Pande, G.; Akoh, C.C. Antioxidant capacity and lipid characterization of six Georgia-grown pomegranate cultivars. J. Agric. Food Chem. 2009, 57, 9427–9436. [Google Scholar] [CrossRef]
- Kareem, S.O.; Akpan, I.; Alebiowu, O.O. Production of citric acid by Aspergillus niger using pineapple waste. Malays. J. Microbiol. 2010, 6, 161–165. [Google Scholar]
- Harivaindaran, K.V.; Rebecca, O.P.S.; Chandran, S. Study of optimal temperature, pH and stability of dragon fruit (Hylocereus polyrhizus) peel use as potential natural colorant. Pak. J. Biol. Sci. 2008, 11, 2259–2263. [Google Scholar]
- Karthikeyan, A.; Sivakumar, N. Citric acid production by Koji fermentation using banana peel as a novel substrate. Bioresour. Technol. 2010, 101, 5552–5556. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.K.; Shanker, G.; Srivastava, A. Utilisation of fruits waste for citric acid production by solid state fermentation. Process Biochem. 2003, 38, 1725–1729. [Google Scholar] [CrossRef]
- Pinheiro, E.R.; Silva, I.M.D.A.; Gonzaga, L.V.; Amante, E.R.; Teo’filo, R.F.; Ferreira, M.M.C.; Amboni, R.D.M.C. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008, 99, 5561–5566. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged citric acid fermentation on orange peel autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef]
- Gil, M.I.; Garcia-Viguera, C.; Art, F.; Tomhs-Barberin, F.A. Changes in pomegranate juice pigmentation during ripening. J. Sci. Food Agric. 1995, 68, 77–81. [Google Scholar] [CrossRef]
- Poyrazoglu, E.; Goekmen, V.; Artik, N. Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J. Food Compos. Anal. 2002, 15, 567–575. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Composition of trace and major minerals in different parts of pomegranate (Punica granatum) fruit cultivars. Br. Food J. 2012, 114, 1518–1532. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants—A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Kushwaha, S.C.; Bera, M.B.; Kumar, P. Nutritional composition of detanninated and fresh pomegranate peel powder. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 7, 38–42. [Google Scholar] [CrossRef]
- Ismail, T.; Akhtar, S.; Riaz, M.; Ismail, A. Effect of pomegranate peel supplementation on nutritional, organoleptic and stability properties of cookies. Int. J. Food Sci. Nutr. 2014, 65, 661–666. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashin, N.; Janius, R.B.; Nawi, N.M.; Abdan, K. Modeling the thin-layer drying of fruits and vegetables: A review. Innov. Food Sci. Emerg. Technol. 2016, 15, 559–618. [Google Scholar] [CrossRef]
- Sablani, S.S. Drying of fruits and vegetables: Retention of nutritional/functional quality. Dry. Technol. 2006, 24, 123–135. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Mujumdar, A.S.; Wang, S. Trends in microwave related drying of fruits and vegetables. Trends Food Sci. Technol. 2006, 17, 524–534. [Google Scholar] [CrossRef]
- Sagar, V.R.; Kumar, S.P. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2010, 47, 15–26. [Google Scholar] [CrossRef]
- Al-Rawahi, A.S.; Rahman, M.S.; Guizani, N.; Essa, M.M. Chemical composition, water sorption isotherm, and phenolic contents in fresh and dried pomegranate peels. Dry. Technol. 2013, 31, 257–263. [Google Scholar] [CrossRef]
- Kumar, G.S.; Nagaraju, R.; Swarajyalakshmi, K.; Latha, P.; Balakrishna, M. Standardization of drying techniques for different fruit peel for making potpourris. Plant Arch. 2017, 17, 1587–1593. [Google Scholar]
- John, K.M.M.; Bhagwat, A.A.; Luthria, D.L. Swarm mortility inhibitory and antioxidant activities of pomegranate peel processed under three drying conditions. Food Chem. 2017, 235, 145–153. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Hernández, F.; Melgarejo, P.; Lech, K.; Carbonell-Barrachina, Á.A. Chemical composition, antioxidant capacity, and sensory quality of pomegranate (Punica granatum L.) arils and rind as affected by drying method. Food Bioprocess Technol. 2013, 6, 1644–1654. [Google Scholar] [CrossRef]
- Turrini, F.; Zunin, P.; Catena, S.; Villa, C.; Alfei, S.; Boggia, R. Traditional or hydro-diffusion and gravity microwave coupled with ultrasound as green technologies for the valorization of pomegranate external peels. Food Bioprod. Process. 2019, 117, 30–37. [Google Scholar] [CrossRef]
- Akpinar, E.K. Drying of mint leaves in a solar dryer and under open sun: Modelling, performance analyses. Energy Convers. Manag. 2010, 51, 2407–2418. [Google Scholar] [CrossRef]
- Doymaz, I.; Ismail, O. Drying characteristics of sweet cherry. Food Bioprod. Process. 2011, 89, 31–38. [Google Scholar] [CrossRef]
- Purohit, P.; Kumar, A.; Kandpal, T.C. Solar drying vs. open sun drying: A framework for financial evaluation. Sol. Energy 2006, 80, 1568–1579. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Effects of drying conditions on physicochemical and antioxidant properties of banana (Musa cavendish) peels. Dry. Technol. 2017, 35, 1141–1151. [Google Scholar] [CrossRef]
- Anuar, M.S.; Tahir, S.M.; Najeeb, M.I.; Ahmad, S. Banana (Musa acuminata) peel drying and powder characteristics obtained through shade and microwave drying processes. Adv. Mater. Process. Technol. 2019, 5, 181–190. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: Changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. J. Food Meas. Charact. 2020, 14, 862–875. [Google Scholar] [CrossRef]
- Talens, C.; Castro-Giraldez, M.; Fito, P.J. Effect of microwave power coupled with hot air drying on sorption isotherms and microstructure of orange peel. J. Food Eng. 2018, 11, 723–734. [Google Scholar] [CrossRef]
- Tekgül, Y.; Baysal, T. Comparative evaluation of quality properties and volatile profiles of lemon peels subjected to different drying techniques. J. Food Process Eng. 2018, 41, 1–9. [Google Scholar] [CrossRef]
- Manzoor, S.; Yusof, Y.A.; Ling, C.N.; Tawakkal, I.S.M.A.; Fikry, M.; Sin, C.L. Thin-layer drying characteristics of papaya (Carica papaya) peel using convection oven and microwave drying. Pertanika J. Sci. Technol. 2019, 27, 1207–1226. [Google Scholar]
- Chew, Y.M.; King, V.A.E. Microwave drying of pitaya (Hylocereus) peel and the effects compared with hot-air and freeze-drying. Trans. ASABE 2019, 62, 919–928. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.Z.; Chen, R.R.; Bao, J.Y.; Yang, G.M. Comparison of volatiles of banana powder dehydrated by vacuum belt drying, freeze-drying and air-drying. Food Chem. 2007, 104, 1516–1521. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Effects of four different drying methods on the carotenoid composition and antioxidant capacity of dried gac peel. J. Sci. Food Agric. 2017, 97, 1656–1662. [Google Scholar] [CrossRef]
- Rafiq, S.; Singh, B.; Gat, Y. Effect of different drying techniques on chemical composition, color and antioxidant properties of kinnow (Citrus reticulata) peel. J. Food Sci. Technol. 2019, 56, 2458–2466. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef]
- Hadrich, F.; Cherif, S.; Gargouri, Y.T.; Adel, S. Antioxidant and lipase inhibitory activities and essential oil composition of pomegranate peel extracts. J. Oleo Sci. 2014, 63, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Jha, A.; Hettiarachchy, N. Antioxidant and antibacterial potential of pomegranate peel extracts. J. Food Sci. Technol. 2014, 51, 4132–4137. [Google Scholar] [CrossRef]
- Qu, W.; Pan, Z.; Zhang, R.; Ma, H.; Chen, X.; Zhu, B.; Wang, Z.; Atungulu, G.G. Integrated extraction and anaerobic digestion process for recovery of nutraceuticals and biogas from pomegranate marc. Trans. ASABE 2009, 52, 1997–2006. [Google Scholar] [CrossRef]
- Singh, R.P.; Murthy, K.N.C.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Z.; Ma, H.; Atungulu, G.G. Extract of phenolics from pomegranate peels. Open Food Sci. J. 2011, 5, 17–25. [Google Scholar] [CrossRef]
- Rashad, M.M.; Mahmoud, A.E.; Ali, M.M.; Nooman, M.U.; Al-Kashef, A.S. Antioxidant and anticancer agents produced from pineapple waste by solid state fermentation. Int. J. Toxicol. Pharmacol. Res. 2015, 7, 287–296. [Google Scholar]
- Gnanasaraswathi, M.; Lakshmipraba, S.; Jesudoss, R.R.P.; Abhinayashree, M.; Beevi, F.M.; Lakshmipriya, A.V.; Kamatchi, S. Potent anti-oxidant behaviour of citrus fruit peels and their bactericidal activity against multi drug resistant organism Pseudomonas aeruginosa. J. Chem. Pharm. Sci. 2014, 2, 139–144. [Google Scholar]
- Shiban, M.S.; Al-Otaibi, M.M.; Al-Zoreky, N.S. Antioxidant activity of pomegranate (Punica granatum L.) fruit peels. Food Nutr. Sci. 2012, 3, 991–996. [Google Scholar]
- Kaur, R.; Kaushal, S.; Sharma, P. Antimicrobial and antioxidant potential of pomegranate (Punica granatum L.) peel. Int. J. Chem. Stud. 2018, 6, 3441–3449. [Google Scholar]
- Murthy, K.C.; Jayaprakasha, G.; Singh, R. Antioxidant activity of pomegranate peel extracts in vivo models. J. Agric. Food Chem. 2002, 50, 4791–4795. [Google Scholar] [CrossRef]
- Padmaja, A.; Prasad, N.B.L. Pomegranate (Punica granatum L.) peel extract as a source of natural antioxidant. J. Food Sci. Eng. 2011, 1, 171–182. [Google Scholar]
- Negi, P.; Jayaprakasha, J. Antioxidant and antibacterial activities of Punica granatum peel extracts. J. Food Sci. 2003, 68, 1473–1477. [Google Scholar] [CrossRef]
- Ghasemian, A.; Mehrabian, S.; Majd, A. Peel extracts of two Iranian cultivars of pomegranate (Punica granatum) have antioxidant and antimutagenic activities. Pak. J. Biol. Sci. 2006, 9, 1402–1405. [Google Scholar]
- Janarthanam, B.; Sumathi, E. Phytochemical composition, tannin content, DPPH assay and antibacterial activity of peel extracts of Punica granatum L. World J. Pharm. Res. 2015, 4, 1895–1908. [Google Scholar]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Yasoubi, P.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Total phenolic contents and antioxidant activity of pomegranate (Punica granatum L.) peel extracts. J. Agric. Sci. Technol. 2007, 9, 35–42. [Google Scholar]
- Ara, K.M.; Raofie, F. Application of response surface methodology for the optimization of supercritical fluid extraction of essential oil from pomegranate (Punica granatum L.) peel. J. Food Sci. Technol. 2016, 53, 3113–3121. [Google Scholar] [CrossRef]
- Bustamante, A.; Hinojosa, A.; Robert, P.; Escalona, V. Extraction and microencapsulation of bioactive compounds from pomegranate (Punica granatum var. Wonderful) residues. Int. J. Food Sci. Technol. 2017, 52, 1452–1462. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Nag, S.; Sit, N. Optimization of ultrasound assisted enzymatic extraction of polyphenols from pomegranate peels based on phytochemical content and antioxidant property. J. Food Meas. Charact. 2018, 12, 1734–1743. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Kazemi, M.; Karim, R.; Mirhosseini, H.; Hamid, A.A. Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: Punicalagin and hydroxybenzoic acids. Food Chem. 2016, 206, 156–166. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Surya, S.M.; Naganyashree, S.; Shivamathi, C.S. Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int. J. Biol. Macromol. 2015, 72, 1323–1328. [Google Scholar] [CrossRef]
- Zhu, C.P.; Zhai, X.C.; Li, L.Q.; Wu, X.X.; Li, B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215. [Google Scholar] [CrossRef]
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012, 98, 16–23. [Google Scholar] [CrossRef]
- Foujdar, R.; Bera, M.B.; Chopra, H.K. Optimization of process variables of probe ultrasonic-assisted extraction of phenolic compounds from the peel of Punica granatum var. Bhagwa and it’s chemical and bioactivity characterization. J. Food Process. Preserv. 2020, 44, 1–16. [Google Scholar]
- Pan, Z.; Qu, W.; Mab, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2012, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, B.; Li, L.; Zhu, X. Microwave-assisted extraction and antioxidant activity of total phenolic compounds from pomegranate peel. J. Med. Plants Res. 2011, 5, 1004–1011. [Google Scholar]
- Huang, J.; He, W.; Yan, C.; Du, X.; Shi, X. Microwave assisted extraction of flavonoids from pomegranate peel and its antioxidant activity. Bio Web Conf. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Castro-López, A.; Ventura-Sobrevilla, J.M.; González-Hernández, M.D.; Rojas, R.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Martínez-Ávila, G.C.G. Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem. 2017, 237, 1139–1148. [Google Scholar] [CrossRef]
- Ҫam, M.; Hıştıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar]
- Sumere, B.R.; de Souza, M.C.; dos Santos, M.P.; Bezerra, R.M.N.; da Cunha, D.T.; Martinez, J.; Rostagno, M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Araujo, P.; Duarte, M.F.; Freitas, V.; Pintado, M.; Saraiva, J.A. Experimental design, modeling, and optimization of high-pressure-assisted extraction of bioactive compounds from pomegranate peel. Food Bioprocess Technol. 2017, 10, 886–900. [Google Scholar] [CrossRef]
- Panichayupakarananta, P.; Issuriya, A.; Sirikatitham, A.; Wang, W. Antioxidant assay-guided purification and LC determination of ellagic acid in pomegranate peel. J. Chromatogr. Sci. 2010, 48, 456–459. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.P.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Kelly, N.P.; Kelly, A.L.; O’Mahony, J.A. Strategies for enrichment and purification of polyphenols from fruit-based materials. Trends Food Sci. Technol. 2019, 83, 248–258. [Google Scholar] [CrossRef]
- Boluda-Aguilar, M.; García-Vidal, L.; González-Castañeda, F.D.; López-Gómez, A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour. Technol. 2010, 101, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Kim, J.H.; Wi, S.G.; Kim, K.H.; Bae, H.J. Bioethanol production from mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl. Energy 2013, 102, 204–210. [Google Scholar] [CrossRef]
- Deylami, M.Z.; Rahman, R.A.; Tan, C.P.; Bakar, J.; Olusegun, L. Effect of blanching on enzyme activity, color changes, anthocyanin stability and extractability of mangosteen pericarp: A kinetic study. J. Food Eng. 2016, 178, 12–19. [Google Scholar] [CrossRef]
- Duarte, Y.; Chaux, A.; Lopez, N.; Largo, E.; Ramírez, C.; Nunez, H.; Simpson, R.; Vega, O. Effect of blanching and hot air-drying conditions on the physicochemical and technological properties of yellow passion fruit (Passiflora edulis var. Flavicarpa) by-products. J. Food Process Eng. 2016, 21, 59–67. [Google Scholar] [CrossRef]
- Nurhuda, H.H.; Maskat, M.Y.; Mamot, S.; Afiq, J.; Aminah, A. Effect of blanching on enzyme and antioxidant activities of rambutan (Nephelium lappaceum) peel. Int. Food Res. J. 2013, 20, 1725–1730. [Google Scholar]
- Peerajit, P.; Chiewchan, N.; Devahastin, S. Effects of pretreatment methods on health-related functional properties of high dietary fibre powder from lime residues. Food Chem. 2012, 132, 1891–1898. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Walia, N.; Chand, C.T.; Ramalingam, C. Microwave blanching: An emerging trend in food engineering and its effects on Capsicum annuum L. J. Food Process Eng. 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Mokapane, F.M.; Fawole, O.A.; Opara, U.L. Strategies to preserve quality and extend shelf life of dried fruits and vegetables: A review. Acta Hortic. 2018, 1201, 99–106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Medicinal Uses | Plant Part | Administration | Reference |
|---|---|---|---|
| Vermifuge and anthelmintic | Root and bark | Oral | [46] |
| Diarrhoea | Peel | Oral | [47] |
| Blood tonic | Juice | Oral | [48] |
| Treat vaginal white discharges | Peel | Oral | [49] |
| Weight loss | Juice | Oral | |
| Stomach disorders | Peel, bark, and leaves | Oral | [50] |
| To slow the development of cataracts and anaemia | Seed | Ophthalmic | |
| Fatigue and hear loss | Oral | ||
| Cure sore throat, dental plaque, dysentery, cholera | Juice | Oral | |
| Cure haemorrhoid flare ups | Topical | ||
| Stop nosebleeds | Oral and or nasal | ||
| Against diarrhoea, dysentery, and intestinal parasites | Peel and bark | Oral | [51] |
| Slow the development of cataracts | Juice | Ophthalmic | |
| Tonic for the heart and throat | Seeds and juice | Oral | |
| Nose bleeds | Flower juice, peel, and bark | Oral and or nasal | |
| Gum disease | Oral | ||
| Treat haemorrhoids | Topical | ||
| Wound healing | Leaves and bark | Topical | [52] |
| Antiparasitic | Oral | ||
| Beneficial in fevers and chronic debility due to malaria | Root | Oral | [53] |
| Antidiabetic properties | Flowers | Oral | [54] |
| Pharmacological Activity * | Plant Part | Test Method | Details | Reference |
|---|---|---|---|---|
| Antibacterial (Escherichia coli and Klebsiella pneumonia) and Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) | Peel extracts | Microdilution antibacterial assay | Methanolic peel extracts showed strong broad-spectrum activity against Gram-positive and Gram-negative bacteria, with the minimum inhibitory concentrations (MIC) ranging from 0.2 to 0.78 mg/mL. | [8] |
| Antityrosinase properties | Peel extracts | Spectrophotometric method | Active peel extracts against monophenolase and diphenolase had IC50 values of 3.66 and 15.88 µg/mL, respectively. | [8] |
| Anti-inflammatory | Peel extracts | Column chromatography combined with in vitro bioassay-guided fractionation | Pomegranate fractions showed potential nitric oxide (NO) inhibition in lipopolysaccharide (LPS) induced RAW 264.7 macrophage cells and also significantly reduced carrageenan-induced mice paw oedema for 1, 3, 4 and 5 h. | [60] |
| Antimicrobial (S. aureus, P. aeruginosa and E. coli) | Aril and peel extracts | Agar diffusion | All of the fruit fraction extracts exhibited higher antimicrobial activity on S. aureus than P. aeruginosa, while E. coli was resistant. | [9] |
| Antifungal (Penicillium italicum, Rhizopus stolonifera, and Botrytis cinerea) | Leaf, peel, and seed extracts | Agar diffusion | The highest inhibitory spore germination (ISG) value was reported at 30.92 ± 2.64% followed by 30.29 ± 2.58% for Botrytis cinerea and Rhizopus stolonifer, respectively. Penicillium italicum showed the lowest inhibition of spore germination at 17.86 ± 2.09%. | [44] |
| Antioxidant properties | Peel powder | 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) | Antioxidant levels ranged from 1.8 to 6.8 µmol Trolox equivalent antioxidant capacity (TEAC) per gram bread for fresh bread. Addition of peel powder up to 2.5% w/w to wheat bread significantly increased its oxidative stability, with no effect on innocuousness as assayed with the brine–shrimp larvae assay. | [61] |
| Anticardiovascular properties | Juice | In vivo experiment, 101 haemodialysis (HD) patients were randomised to receive 100 cc of pomegranate juice containing about 0.7 mM polyphenols three times a week for one year | Consumption of juice yielded significant time response improvements in systolic blood pressure, pulse pressure, triglycerides, and high density lipoprotein (HDL) level. These beneficial outcomes were more pronounced among patients with hypertension, high levels of triglycerides and low levels of HDL. | [43] |
| Antihyperglycaemic, antihyperlipidaemic, and antioxidant properties | Peel extracts | In vivo experiment, 56 Wister albino rats were distributed into 8 groups and compared with standard drugs (glibenclamide and atorvastatin). For antioxidant activity: DPPH (2,2-diphenylpicrylhydrazyl) and ABTS (2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) | Peel extracts showed antihyperglycaemic and antihyperlipidaemic activities from a powerful reactive oxygen scavenger through its antioxidant compounds. In addition, the peel extracts enhanced liver and kidney functions when compared to standard drugs in diabetic and hyperlipidaemic rats. | [62] |
| Plant Part | Constituents |
|---|---|
| Roots and bark | Ellagitannins, including punicalagin and punicalin, and several piperidine alkaloids. |
| Leaf | Tannins (which include punicafolin and punicalin), flavone glycosides, luteolin and apigenin, and brevifolin carboxylic acid. |
| Pericarp (Peel) | Ellagitannins (punicalin and punicalagin), gallic acid and other fatty acids, catechin and epicatechin, quercetin, rutin and other flavonols, flavones, flavonones, procanthocyanidins, and anthocyanidins. |
| Flower | Gallic acid, triterpenoids, and ursolic acids, including maslinic and asiatic acids. |
| Seed oil | Punicic acid, hydroxybenzoic acids (gallic and ellagic), other fatty acids, sterols (daucosterol, campesterol, stigmasterol, beta-sitosterol), and γ-tocopherol. |
| Juice | Procanthocyanidins and anthocyanins, ellagitannins (punicalin and punicalagin), glucose, ascorbic acid, ellagic acid, gallic acid, caffeic acid, catechin and epicatechin, quercetin, rutin, amino acids (methionine, proline, and valine), and many minerals especially irons. |
| Drying Technique | Cultivar | Country | Phytochemical and Nutritional Properties | Key Findings | Reference |
|---|---|---|---|---|---|
| Sun (4 days) and oven drying (overnight at 100 °C) | Baghva, Ruby, Indian white, and Egypt | Sultanate of Oman | Moisture loss (%), pH, and vitamin C | Sun-dried fruit peel had highest water loss, pH, and vitamin C values, with ranges of 64.49–82.52%, 3.5–4.5, and 100–75 mg/100 g dry weight (DW), respectively. | [9] |
| Freeze-drying (20 °C for 96 h), air drying (48, 30, and 24 h), vacuum drying (60 kPa vacuum at 40, 60, or 90 °C for 24, 13, and 4.5 h), and sun drying for 11 h | Helow | Sultanate of Oman | Moisture loss (%) and total phenolics | Freeze-dried peels showed phenolic contents comparable to those of fresh peel (i.e., 4900 and 4010 mg gallic acid equivalent (GAE)/100 g dry peel solids). Vacuum drying ranged from 1200 to 5330 mg gallic acid equivalent (GAE)/100 g dry peel solids extracted in ethanol at 40 °C and in water at 90 °C, respectively. The highest sun-dried peel value of 4080 mg gallic acid equivalent (GAE)/100 g dry peel solids was achieved in water extract. | [85] |
| Microwave drying (up to 9 min) air drying (5 days), sun drying (5 days) silica gel drying (up to 7 days) and hot air oven drying (40 °C for 30 h) | Unknown | India | Moisture loss (%) | Highest drying weight for pomegranate peel using microwave dryer at 34.67 g. Lowest moisture loss for pomegranate peel reported using a microwave oven dryer at 65.33%. | [86] |
| Oven (40 °C for 48 h) and solar drying (50 °C for 2 h) | Unknown | Egypt | Total phenolic content (TPC) and total flavonoid content (TFC) | Total phenolic content (TPC) highest in oven-dried peel with 17.78 mg gallic acid/g in methanol extract. Total flavonoid content (TFC) values statistically similar for both oven- and sun-dried whole peel at 7.98 and 7.96 mg rutin equivalent (RE)/g, respectively. | [18] |
| Freeze (−88.7 °C for 16 h) and oven drying (40, 50, and 60 °C for 22, 17, and 12 h, respectively) | Wonderful | South Africa | Punicalin, rutin, p-coumaric, +catechin,-epicatechin, hesperidin, vitamin C | Higher punicalin and p-coumaric values at 60 °C. Freeze-dried (FD) peel had higher rutin, +catechin, -epicatechin, and hesperidin values at 4666.03 mg/kg dry mass (DM), 674.51, 70.56, and 16.45 mg/kg DM, respectively. Vitamin C was reported at ˂30 µg ascorbic acid equivalent (AAE)/g dry mass (DM) using an oven drier at 40 °C. | [34] |
| Freeze-drying at −80 °C, ambient temperature of ~25 °C, and oven drying at 50 °C | Unknown | USA | Total phenolic content (TPC), α-punicalin, β-punicalin, ellagic acid, α- and β-punicalagin | Higher total phenolic content (TPC), α- punicalin, and β-punicalin values in freeze-dried peel samples of 96 mg/g gallic acid equivalent, 0.8 mg/g, and 1.6 mg/g, respectively. Higher punicalagin (~38.6–50.3 mg/g) and ellagic acid (~2.8–3.2 mg) levels in the peel fractions. | [87] |
| Freeze-drying (FD) for 48 h; convective drying (CD) at 50, 60, or 70 °C; vacuum–microwave drying (VMD) at 3 different power levels of 240, 360, or 480 W; combined drying (CPD-VMFD) and predrying (CPD) at 60 °C for 90 or 150 min, VMFD with microwave wattage of 360 W. | Mollar de Elche | Spain | Punicalagin isomers (α-PC and β-PC), ellagic acid (EA), and total phenolic content (TPC) | The α-punicalagin and β-punicalagin isomers were highest in fresh peel at 139 and 143 mg/g DW, followed by freeze-dried peel at 113.4 and 98.7 mg/g dry weight (DW), respectively. High ellagic acid (EA) reported in fresh peel at 2.49 mg/g, followed by convective drying (60 °C) and freeze-drying, which were statistically similar at 1.95 mg/g at 1.71 mg/g, respectively. Fresh samples had higher total phenolic content (TPC) values at 125 mg/g gallic acid equivalent, followed by freeze-dried peel at 118 mg/g gallic acid equivalent. | [88] |
| Single-mode microwave oven dryer, microwave hydro-diffusion (MH), and gravity oven dryer (MHG): both at temperatures ˂ 100 °C for ˂5 min, and traditional oven dryer at 40 °C for 48 h | Acco and Wonderful | Italy | Total phenolic content (TPC), free ellagic acid (EA), and ellagitannins (ETs) | Microwave hydro-diffusion gravity oven dryer was reported with high amounts of total phenolic content (TPC) at 190–230 mg gallic acid equivalent (GAE)/100 mL, ellagitannins at 106–110 µg/mL, and radical scavenging activity (RSA) at 480–560 mg ascorbic acid equivalent antioxidant capacity (AEAC)/100 mL. | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules 2020, 25, 4690. https://doi.org/10.3390/molecules25204690
Magangana TP, Makunga NP, Fawole OA, Opara UL. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules. 2020; 25(20):4690. https://doi.org/10.3390/molecules25204690
Chicago/Turabian StyleMagangana, Tandokazi Pamela, Nokwanda Pearl Makunga, Olaniyi Amos Fawole, and Umezuruike Linus Opara. 2020. "Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review" Molecules 25, no. 20: 4690. https://doi.org/10.3390/molecules25204690
APA StyleMagangana, T. P., Makunga, N. P., Fawole, O. A., & Opara, U. L. (2020). Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules, 25(20), 4690. https://doi.org/10.3390/molecules25204690









