Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
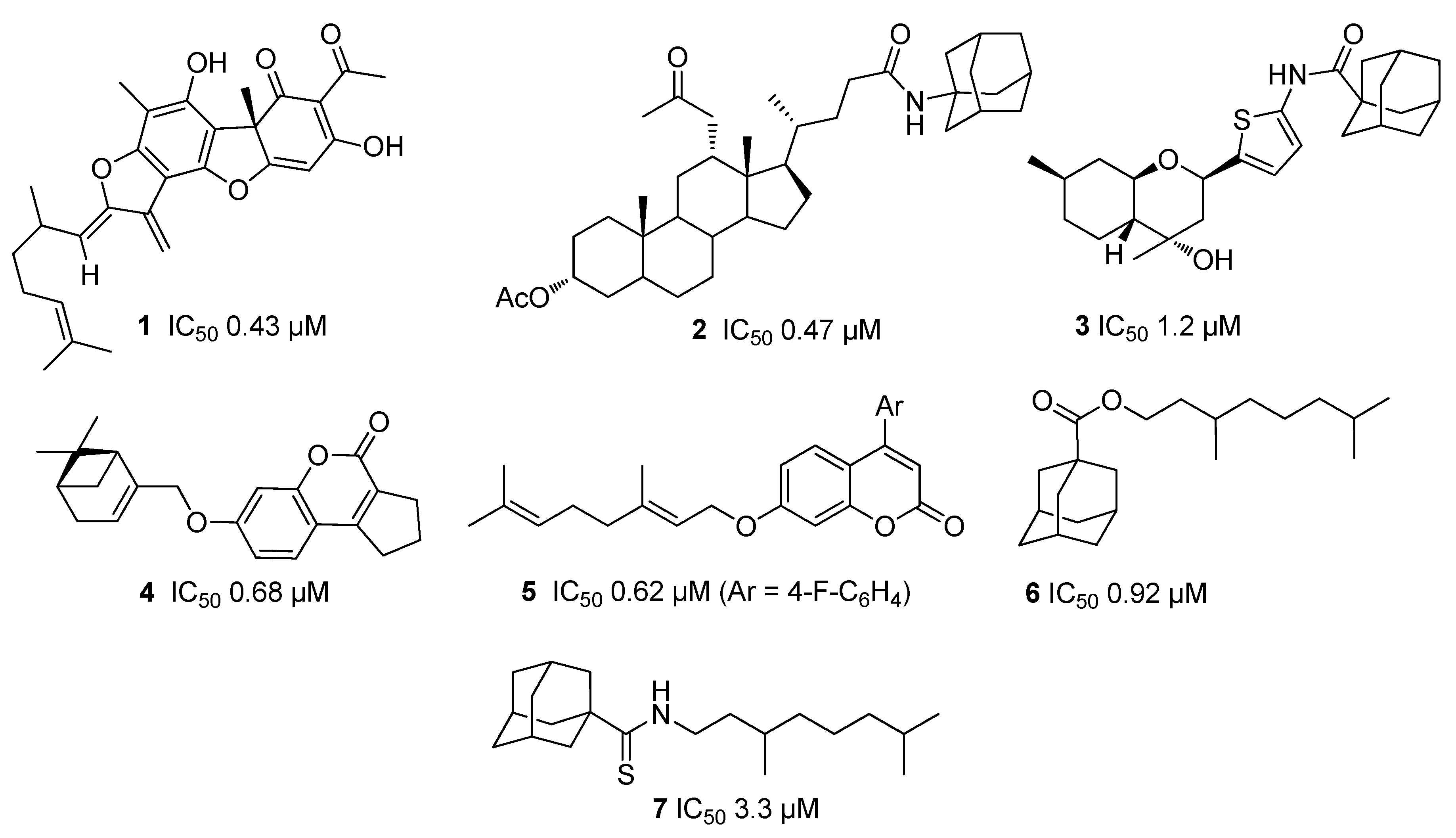
2.2.1. Structure–Activity Relationship Analysis
2.2.2. Cell Growth and Viability
Topotecan Cytotoxicity on HEK293FT Wild Type and TDP1−/− Cells
TDP1 Inhibitors’ Cytotoxicity on HEK293FT TDP1−/− Cells
Activity of 11h and 12k with Topotecan against Tumor Cells
2.2.3. Chemical Space
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure (GP)
3.1.2. Reaction of 2-Carene-Containing Mixture and 4-hydroxy-3-methoxybenzaldehyde 10a
3.1.3. Reaction of 2-Carene-Containing Mixture and Crotonaldehyde 10c
3.1.4. Reaction of 2-Carene-Containing Mixture and 2-Thiophenecarbaldehyde 10d
3.1.5. Reaction of 2-Carene-Containing Mixture and 3-methylthiophene-2-carbaldehyde 10e
3.1.6. Reaction of 2-Carene-Containing Mixture and 5-methylthiophene-2-carbaldehyde 10f
3.1.7. Reaction of 2-Carene-Containing Mixture and 4-bromothiophene-2-carbaldehyde 10g
3.1.8. Reaction of 2-Carene-Containing Mixture and 5-bromothiophene-2-carbaldehyde 10h
3.1.9. Reaction of 2-Carene-Containing Mixture and 5-nitrothiophene-2-carbaldehyde 10i
3.1.10. Reaction of 2-Carene-Containing Mixture and 5-nitrofuran-2-carbaldehyde 10j
3.1.11. Reaction of 2-Carene-Containing Mixture and thiophene-3-carbaldehyde 10k
3.1.12. Reaction of Limonene and 2-thiophenecarbaldehyde 10d
3.2. Real-Time Detection of TDP1 Activity
3.3. TDP1 Activity by Gel-Based Assay
3.4. Obtainment of TDP1 Knockout HEK293FT Clones
3.4.1. Plasmid Construction for Human TDP1 Gene Knockout
3.4.2. Knockout HEK293FT Clone Generation
3.4.3. Analysis of CRISPR/Cas9-Mediated Deletions in the TDP1 Gene
3.5. Cell Culture Assay
3.6. Calculation of Molecular Descriptors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, S.-Y.N.; Pommier, Y.; Marchand, C. Tyrosyl-DNA Phosphodiesterase 1 (Tdp1) inhibitors. Expert Opin. Ther. Patents 2011, 21, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Laev, S.S.; Salakhutdinov, N.F.; Lavrik, O.I. Tyrosyl-DNA phosphodiesterase inhibitors: Progress and potential. Bioorganic Med. Chem. 2016, 24, 5017–5027. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.; Dyrkheeva, N.; Lavrik, O.I. Dual DNA topoisomerase 1 and tyrosyl-DNA phosphodiesterase 1 inhibition for improved anticancer activity. Med. Res. Rev. 2019, 39, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Kawale, A.S.; Povirk, L.F. Tyrosyl-DNA phosphodiesterases: Rescuing the genome from the risks of relaxation. Nucleic Acids Res. 2018, 46, 520–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Interthal, H.; Pouliot, J.J.; Champoux, J.J. The tyrosyl-DNA phosphodiesterase Tdp1 is a member of the phospholipase D superfamily. Proc. Natl. Acad. Sci. USA 2001, 98, 12009–12014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Dexheimer, T.S.; Antony, S.; Marchand, C.; Pommier, Y. Tyrosyl-DNA phosphodiesterase as a target for anticancer therapy. Anti-Cancer Agents Med. Chem. 2008, 8, 381–389. [Google Scholar] [CrossRef]
- Beretta, G.L.; Cossa, G.; Gatti, L.; Zunino, F.; Perego, P. Tyrosyl-DNA Phosphodiesterase 1 Targeting for Modulation of Camptothecin-Based Treatment. Curr. Med. Chem. 2010, 17, 1500–1508. [Google Scholar] [CrossRef]
- Ledesma, F.C.; El Khamisy, S.F.; Zuma, M.C.; Osborn, K.; Caldecott, K.W. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 2009, 461, 674–678. [Google Scholar] [CrossRef]
- Filimonov, A.S.; Chepanova, A.A.; Luzina, O.A.; Zakharenko, A.L.; Zakharova, O.D.; Ilina, E.S.; Dyrkheeva, N.S.; Kupryushkin, M.S.; Kolotaev, A.V.; Khachatryan, D.S.; et al. New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors. Molecules 2019, 24, 3711. [Google Scholar] [CrossRef] [Green Version]
- Zakharenko, A.; Luzina, O.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.; Popova, N.; Patel, J.; Zakharova, O.; Chepanova, A.; Zafar, A.; et al. Novel tyrosyl-DNA phosphodiesterase 1 inhibitors enhance the therapeutic impact of topotecan on in vivo tumor models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Dyrkheeva, N.; Luzina, O.; Filimonov, A.; Zakharova, O.; Ilina, E.; Zakharenko, A.; Kuprushkin, M.; Nilov, D.; Gushchina, I.; Švedas, V.; et al. Inhibitory Effect of New Semisynthetic Usnic Acid Derivatives on Human Tyrosyl-DNA Phosphodiesterase 1. Planta Medica 2018, 85, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomatina, O.; Popadyuk, I.I.; Zakharenko, A.L.; Zakharova, O.D.; Fadeev, D.S.; Komarova, N.I.; Reynisson, J.; Arabshahi, H.J.; Chand, R.; Volcho, K.P.; et al. Novel Semisynthetic Derivatives of Bile Acids as Effective Tyrosyl-DNA Phosphodiesterase 1 Inhibitors. Molecules 2018, 23, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a renewable source of biologically active compounds. Pure Appl. Chem. 2017, 89, 1105–1117. [Google Scholar] [CrossRef]
- Patrusheva, O.; Volcho, K.; Salakhutdinov, N. Approaches to the synthesis of oxygen-containing heterocyclic compounds based on monoterpenoids. Russ. Chem. Rev. 2018, 87, 771–796. [Google Scholar] [CrossRef]
- Khomenko, T.; Zakharenko, A.; Odarchenko, T.; Arabshahi, H.J.; Sannikova, V.; Zakharova, O.; Korchagina, D.; Reynisson, J.; Volcho, K.; Salakhutdinov, N. New inhibitors of tyrosyl-DNA phosphodiesterase I (Tdp 1). Bioorg. Med. Chem. 2016, 24, 5573–5581. [Google Scholar] [CrossRef]
- Khomenko, T.M.; Zakharenko, A.L.; Chepanova, A.A.; Ilina, E.S.; Zakharova, O.D.; I. Kaledin, V.; Nikolin, V.P.; A. Popova, N.; Korchagina, D.V.; Reynisson, J.; et al. Promising New Inhibitors of Tyrosyl-DNA Phosphodiesterase I (Tdp 1) Combining 4-Arylcoumarin and Monoterpenoid Moieties as Components of Complex Antitumor Therapy. Int. J. Mol. Sci. 2019, 21, 126. [Google Scholar] [CrossRef] [Green Version]
- Mozhaitsev, E.S.; Zakharenko, A.L.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Vasil’eva, I.A.; Chepanova, A.A.; Black, E.; Patel, J.; Chand, R.; et al. Novel Inhibitors of DNA Repair Enzyme TDP1 Combining Monoterpenoid and Adamantane Fragments. Anti-Cancer Agents Med. Chem. 2019, 19, 463–472. [Google Scholar] [CrossRef]
- Mozhaitsev, E.; Suslov, E.V.; Demidova, Y.; Korchagina, D.; Volcho, K.P.; Zakharenko, A.; Vasil’eva, I.; Kupryushkin, M.; Chepanova, A.; Ayine-Tora, D.M.; et al. The Development of Tyrosyl-DNA Phosphodyesterase 1 (TDP1) Inhibitors Based on the Amines Combining Aromatic/Heteroaromatic and Monoterpenoid Moieties. Lett. Drug Des. Discov. 2019, 16, 597–605. [Google Scholar] [CrossRef]
- Chepanova, A.A.; Mozhaitsev, E.S.; Munkuev, A.A.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Zakharenko, A.L.; Patel, J.; Ayine-Tora, D.M.; Reynisson, J.; et al. The Development of Tyrosyl-DNA Phosphodiesterase 1 Inhibitors. Combination of Monoterpene and Adamantine Moieties via Amide or Thioamide Bridges. Appl. Sci. 2019, 9, 2767. [Google Scholar] [CrossRef] [Green Version]
- Ponomarev, K.Y.; Suslov, E.V.; Zakharenko, A.L.; Zakharova, O.D.; Rogachev, A.D.; Korchagina, D.V.; Zafar, A.; Reynisson, J.; Nefedov, A.A.; Volcho, K.P.; et al. Aminoadamantanes containing monoterpene-derived fragments as potent tyrosyl-DNA phosphodiesterase 1 inhibitors. Bioorganic Chem. 2018, 76, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Li-Zhulanov, N.S.; Zakharenko, A.L.; Chepanova, A.A.; Patel, J.; Zafar, A.; Volcho, K.P.; Salakhutdinov, N.F.; Reynisson, J.; Leung, I.K.H.; Lavrik, O.I. A Novel Class of Tyrosyl-DNA Phosphodiesterase 1 Inhibitors That Contains the Octahydro-2H-chromen-4-ol Scaffold. Molecules 2018, 23, 2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Il’ina, I.V.; Volcho, K.P.; Korchagina, D.V.; Salnikov, G.E.; Genaev, A.M.; Karpova, E.V.; Salakhutdinov, N.F. Unusual reactions of (+)-2- and (+)-3-carene with aldehydes on K10 clay. Helv. Chim. Acta 2010, 93, 2135–2150. [Google Scholar] [CrossRef]
- Pavlova, A.; Il’Ina, I.; Morozova, E.; Korchagina, D.; Kurbakova, S.; Sorokina, I.; Tolstikova, T.; Volcho, K.P.; Salakhutdinov, N. Potent Neuroprotective Activity of Monoterpene Derived 4-[(3aR,7aS)- 1,3,3a,4,5,7a-Hexahydro-3,3,6-trimethylisobenzofuran-1-yl]-2-methoxyphenol in MPTP Mice Model. Lett. Drug Des. Discov. 2013, 11, 611–617. [Google Scholar] [CrossRef]
- Acharya, S.P.; Brown, H.C. Hydroboration of Terpenes. III. Isomerization of (+)-3-Carene to (+)-2-Carene. Hydroboration of (+)-2-Carene ([UNK]4-Carene). Nuclear Magnetic Resonance Spectra with Absolute Configurational and Conformational Assignments for the 2-Caranols and 2-Caranones. J. Am. Chem. Soc. 1967, 89, 1925–1932. [Google Scholar] [CrossRef]
- Meyer, U.; Hoelderich, W. Application of basic zeolites in the decomposition reaction of 2-methyl-3-butyn-2-ol and the isomerization of 3-carene. J. Mol. Catal. A: Chem. 1999, 142, 213–222. [Google Scholar] [CrossRef]
- Julianto, T.S.; Jumina; Sastrohamidjojo, H.; Mustofa. Solvent-free isomerization of 3-carene to 2-carene using Na/o-chlorotoluene catalyst in trans-isolimonene production. Orient. J. Chem. 2017, 33, 3107–3111. [Google Scholar] [CrossRef]
- Eswaramoorthy, M.; Krishnasamy, V. Influence of coke on the aromatization of 3-carene in the vapour phase over zeolites. Indian J. Chem. 2001, 40, 264–269. [Google Scholar]
- Krishnasamy, V.; Yeddanapalli, L.M. Vapour phase catalytic transformations of terpene hydrocarbons in the C10H16 series. III. Dehydrogenation of Δ3-carene over modified chromia and chromia–alumina catalysts. Can. J. Chem. 1976, 54, 3458–3463. [Google Scholar] [CrossRef]
- Sidorenko, A.; Aho, A.; Ganbaatar, J.; Batsuren, D.; Utenkova, D.; Sen’Kov, G.; Wärnå, J.; Murzin, D.; Agabekov, V. Catalytic isomerization of α-pinene and 3-carene in the presence of modified layered aluminosilicates. Mol. Catal. 2017, 443, 193–202. [Google Scholar] [CrossRef]
- Sidorenko, A.; Il’Ina, I.; Kravtsova, A.; Aho, A.; Ardashov, O.V.; Li-Zhulanov, N.; Volcho, K.P.; Salakhutdinov, N.; Murzin, D.Y.; Agabekov, V. Preparation of chiral isobenzofurans from 3-carene in the presence of modified clays. Mol. Catal. 2018, 459, 38–45. [Google Scholar] [CrossRef]
- Pommier, Y.; Marchand, C.; Thibaut, L. Diamidine derivatives as inhibitors of human tyrosyl-DNA-phosphodiesterase (Tdp1). USA Patent 60/786,604, 27 March 2006. [Google Scholar]
- Zakharova, O.; Luzina, O.; Zakharenko, A.; Sokolov, D.; Filimonov, A.; Dyrkheeva, N.; Chepanova, A.; Ilina, E.; Ilyina, A.; Klabenkova, K.; et al. Synthesis and evaluation of aryliden- and hetarylidenfuranone derivatives of usnic acid as highly potent Tdp1 inhibitors. Bioorganic Med. Chem. 2018, 26, 4470–4480. [Google Scholar] [CrossRef] [PubMed]
- Antony, S.; Marchand, C.; Stephen, A.G.; Thibaut, L.; Agama, K.K.; Fisher, R.J.; Pommier, Y. Novel high-throughput electrochemiluminescent assay for identification of human tyrosyl-DNA phosphodiesterase (Tdp1) inhibitors and characterization of furamidine (NSC 305831) as an inhibitor of Tdp1. Nucleic Acids Res. 2007, 35, 4474–4484. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, P.; Bathula, C.; Basu, S.M.; Das, S.K.; Agarwal, R.; Hati, S.; Singh, A.; Sen, S.; Das, B.B. Design, synthesis and evaluation of thiohydantoin derivatives as potent topoisomerase I (Top1) inhibitors with anticancer activity. Eur. J. Med. Chem. 2015, 102, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Volcho, K.P.; Tatarova, L.E.; Korchagina, D.V.; Salakhutdinov, N.F.; Aul’chenko, I.S.; Ione, K.G.; Barkhash, V.A. Cycloaddition of carbonyl compounds to olefins on aluminosilicate catalysts. Russ. J. Org. Chem. 1994, 30, 641–653. [Google Scholar]
- Sidorenko, A.; Kravtsova, A.; Mäki-Arvela, P.; Aho, A.; Sandberg, T.; Il’Ina, I.; Li-Zhulanov, N.; Korchagina, D.; Volcho, K.; Salakhutdinov, N.; et al. Synthesis of isobenzofuran derivatives from renewable 2-carene over halloysite nanotubes. Mol. Catal. 2020, 490, 110974. [Google Scholar] [CrossRef]
- Zakharenko, A.; Khomenko, T.; Zhukova, S.; Koval, O.A.; Zakharova, O.; Anarbaev, R.; Lebedeva, N.; Korchagina, D.; Komarova, N.; Vasiliev, V.; et al. Synthesis and biological evaluation of novel tyrosyl-DNA phosphodiesterase 1 inhibitors with a benzopentathiepine moiety. Bioorganic Med. Chem. 2015, 23, 2044–2052. [Google Scholar] [CrossRef]
- Li, J.; Summerlin, M.; Nitiss, K.C.; Nitiss, J.L.; Hanakahi, L. TDP1 is required for efficient non-homologous end joining in human cells. DNA Repair 2017, 60, 40–49. [Google Scholar] [CrossRef]
- Brettrager, E.J.; Segura, I.A.; Van Waardenburg, R.C.; Waardenburg, V. Tyrosyl-DNA Phosphodiesterase I N-Terminal Domain Modifications and Interactions Regulate Cellular Function. Genes 2019, 10, 897. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Logan, G.; Reynisson, J. Wine Compounds as a Source for HTS Screening Collections. A Feasibility Study. Mol. Informatics 2012, 31, 847–855. [Google Scholar] [CrossRef]
- Eurtivong, C.; Reynisson, J. The Development of a Weighted Index to Optimise Compound Libraries for High Throughput Screening. Mol. Informatics 2018, 38, 1800068. [Google Scholar] [CrossRef]
- Lebedeva, N.A.; Rechkunova, N.I.; Lavrik, O.I. AP-site cleavage activity of tyrosyl-DNA phosphodiesterase 1. FEBS Lett. 2011, 585, 683–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; A. Scott, D.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- QikProp: Schrödinger, LLC, New York NY. 2020. Available online: https://www.schrodinger.com/citations (accessed on 11 May 2020).
- Ioakimidis, L.; Thoukydidis, L.; Mirza, A.; Naeem, S.; Reynisson, J. Benchmarking the Reliability of QikProp. Correlation between Experimental and Predicted Values. QSAR Comb. Sci. 2008, 27, 445–456. [Google Scholar] [CrossRef]
- Liu, S.; Kurzrock, R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat. Rev. 2014, 40, 883–891. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds of structural types 11 and 12 are available from the authors. |


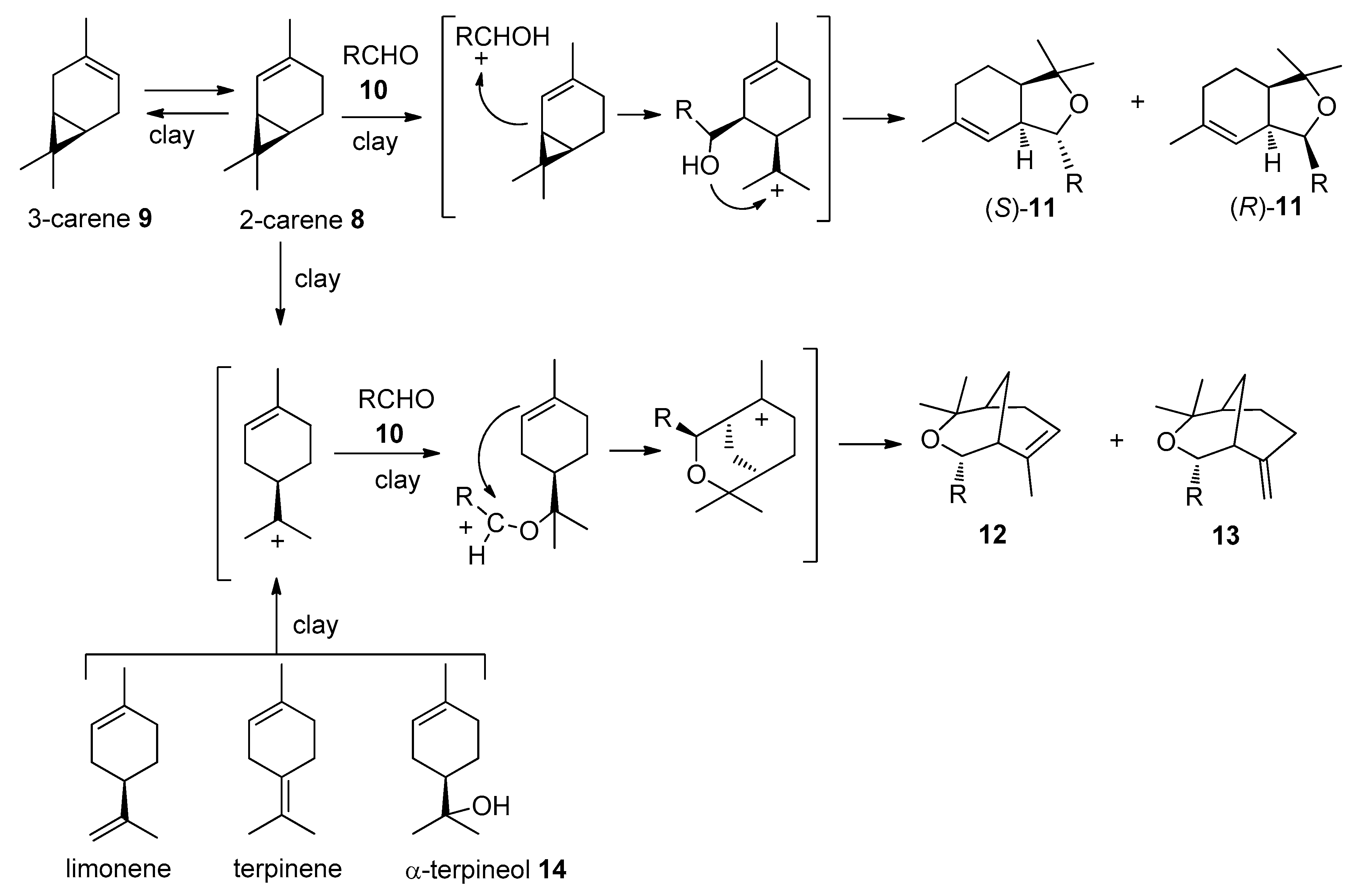




| R | RCHO | Product 11 (Yield, (S)-/(R) Ratio) | Products 12 and 13 (Total Yield, %, 12/13) |
|---|---|---|---|
| 4-Hydroxy-3-methoxyphenyl | 10a | 11a (81%, (S)-/(R) = 1:1) | 12a (11%) |
| (E)-prop-1-en-1-yl | 10c | 11c (89%, (S)-/(R) = 1.3:1) | |
| Thiophen-2-yl | 10d | 11d (78%, (S)-isomer) | 12d, 13d (5%, 7:1) |
| 3-Methylthiophen-2-yl | 10e | 11e (86%, (S)-isomer) | 12e (2%) |
| 5-Methylthiophen-2-yl | 10f | 11f (85%, (S)-isomer) | 12f, 13f (2%, 3:1) |
| 4-Bromothiophen-2-yl | 10g | - | 12g (20%) |
| 5-Bromothiophen-2-yl | 10h | 11h (73%, (S)-isomer) | 12h (15%) |
| 5-Nitrothiophen-2-yl | 10i | - | 12i, 13i (18%, 5:1) |
| 5-Nitrofuran-2-yl | 10j | - | 12j (18%) |
| Thiophen-3-yl | 10k | 11k (91%, (S)-/(R) = 1.5:1) | 12k (21%) |
| R | Compound 11 | IC501, μM | Compound 12 | IC501, μM |
|---|---|---|---|---|
| 4-Hydroxy-3-methoxyphenyl | 11a | >20 | 12a | >20 |
| (E)-prop-1-en-1-yl | 11c | >20 | - | - |
| Thiophen-2-yl | 11d | 4.85 ± 1.06 | 12d | 3.35 ± 1.06 |
| 3-Methylthiophen-2-yl | 11e | 3.6 ± 1.7 | 12e | 2.25 ± 0.63 |
| 5-Methylthiophen-2-yl | 11f | 4.7 ± 2.0 | - | - |
| 4-Bromothiophen-2-yl | - | - | 12g | 0.65 ± 0.22 |
| 5-Bromothiophen-2-yl | 11h | 0.75 ± 0.07 | 12h | 1.75 ± 0.78 |
| 5-Nitrothiophen-2-yl | - | - | 12i | 14 ± 1 |
| 5-Nitrofuran-2-yl | - | - | 12j | 28 ± 10 |
| Thiophen-3-yl | 11k | 1.60 ± 0.56 | 12k | 1.20 ± 0.14 |
| Furamidine | 1.2 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Il’ina, I.V.; Dyrkheeva, N.S.; Zakharenko, A.L.; Sidorenko, A.Y.; Li-Zhulanov, N.S.; Korchagina, D.V.; Chand, R.; Ayine-Tora, D.M.; Chepanova, A.A.; Zakharova, O.D.; et al. Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1. Molecules 2020, 25, 3496. https://doi.org/10.3390/molecules25153496
Il’ina IV, Dyrkheeva NS, Zakharenko AL, Sidorenko AY, Li-Zhulanov NS, Korchagina DV, Chand R, Ayine-Tora DM, Chepanova AA, Zakharova OD, et al. Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1. Molecules. 2020; 25(15):3496. https://doi.org/10.3390/molecules25153496
Chicago/Turabian StyleIl’ina, Irina V., Nadezhda S. Dyrkheeva, Alexandra L. Zakharenko, Alexander Yu. Sidorenko, Nikolay S. Li-Zhulanov, Dina V. Korchagina, Raina Chand, Daniel M. Ayine-Tora, Arina A. Chepanova, Olga D. Zakharova, and et al. 2020. "Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1" Molecules 25, no. 15: 3496. https://doi.org/10.3390/molecules25153496








