Abstract
Interest in high homogenization pressure technology has grown over the years. It is a green technology with low energy consumption that does not generate high CO2 emissions or polluting effluents. Its main food applications derive from its effect on particle size, causing a more homogeneous distribution of fluid elements (particles, globules, droplets, aggregates, etc.) and favoring the release of intracellular components, and from its effect on the structure and configuration of chemical components such as polyphenols and macromolecules such as carbohydrates (fibers) and proteins (also microorganisms and enzymes). The challenges of the 21st century are leading the processed food industry towards the creation of food of high nutritional quality and the use of waste to obtain ingredients with specific properties. For this purpose, soft and nonthermal technologies such as high pressure homogenization have huge potential. The objective of this work is to review how the need to combine safety, functionality and sustainability in the food industry has conditioned the application of high-pressure homogenization technology in the last decade.
1. Introduction
In the homogenization process, a fluid is forced to pass through a gap, causing energy transformations that directly affect the dissolved, dispersed or emulsified components. The fluid undergoes mechanical (shear, hydrodynamic and cavitation effects) stress and an increase in temperature (thermal effect) of approximately 2–3 °C for every 10 MPa of homogenization pressure [1]. These affect the fluid structure and properties, and also those of its constituent elements (particles, molecules, globules, droplets, aggregates, granules, etc.). Particle sizes decrease and more homogeneous distribution is achieved, facilitating operations such as mixing and emulsification. The effects are different to those induced by HPP (High Pressure Processing), in which prepacked food is loaded into a pressure vessel and then pressurized at a range of 100–1000 MPa, with water as the pressure-transmitting medium [2].
Initially, homogenization was introduced as a manufacturing step in the dairy industry. This operation reduced the size of fat globules, increasing the stability of the emulsion and, thus, the physical and chemical stability of milk. It had a great impact on the quality of dairy products such as condensed milk, curd or ice cream. The applied pressure was less than 30 MPa and it was applied in one or two steps. However, significant technological developments have occurred since then, having an impact on the design and geometry of homogenization valves, and making it possible to work at higher pressures and with very short processing times (a few seconds) [3]. High homogenization pressures were introduced at the beginning of the 2000s as an alternative, nonthermal treatment in the food industry, and applications were extended to industries other than dairy, e.g., to fields such as textile or biotechnologic.
The existence of valves of different geometries has given rise to the design of equipment that is able to work at pressures higher than 400 MPa. Thus, a distinction is made among standard homogenization for pressures between 0 and 50 MPa, high pressure homogenization (HPH) for pressures between 50 and 300 MPa and ultrahigh pressure homogenization (UHPH) for pressures equal to or greater than 400 MPa. Processing efficiency is modulated by applying various pressure ranges or combining a pressure value with a specific number of passes through the equipment [4]. In addition, the possibility of operating continuously for a great diversity of pumpable fluids has made it possible to extend applications to the activation/inactivation of enzymes, reduction of microbial load, mixing, dispersion, emulsification or encapsulation processes, cell breakage processes and the modification of proteins or macromolecules to obtain ingredients or additives with various properties.
Nowadays, concern about food functionality and sustainability is driving research interest in increasing the bioavailability and bioaccessibility of active components and probiotics, and in the extraction of macro- and micro- molecules from food byproducts. The challenges of increasing the nutritional characteristics of food must be combined with a reduction in environmental impact and increased food security. In this context, alternative, soft and nonthermal technologies such as high pressures homogenization have huge potential. The objective of this work is to review how the need to combine safety, functionality and sustainability has conditioned the application of high pressure homogenization technology in food. Advances and applications in the last decade have been organized according to the main challenges in the food industry.
2. Evolution and Major Applications in the Last Decade
Publications in peer-reviewed journals show that the main applications of HPH in food have the following objectives:
- Conservation and safety by decreasing the microbial load and inactivating enzymes. This occurs as a consequence of the thermal effect derived from mechanical stress or from structural changes in proteins.
- Recovery and extraction of proteins, fibrous materials and bioactive compounds (mainly polyphenols) and increase of the functionality considered in terms of technological use (stabilization of emulsions and dispersions, flow capacity and viscosity modifications, emulsifying activity improvement, etc.). Mechanical stresses and hydrodynamic effects induce cell disruption, favoring the release of intracellular content or structural components of the cell wall. Moreover, dispersed particles or fat droplets can be reduced in size and modified in structure.
- Increase of functionality in terms of health effect (increase bioaccessibility, bioavailability or probiotic effect). These effects result from favoring the release of bioactive compounds, the modification of biopolymer structures and the development of novel particle interactions and networking. Micro- or nano- capsules have also been developed.
In order to numerically quantify its evolution, the increase in the number of published scientific articles (in %) was calculated, taking into account the difference in the number of items between the last two decades. The results in each of the considered areas are included in Figure 1a,b. As shown in Figure 1a, between 2000 and 2009, HPH were used mainly for the extraction of proteins, although a large number of research works focused on microorganisms and enzymes inactivation, contributing to food preservation and safety. The last decade (from 2010 until now) revealed a significant increase (74.87%) in the total number of scientific articles published. The main areas in which there was an increase greater than the total value were the use of HPH for microorganism inactivation, fiber extraction, and above all, bioactive and probiotic components. The application of HPH to extract or increase the functionality of bioactive compounds, and to improve the probiotic effect, grew by 89% and 87.9% respectively (Figure 1a). The increasing interest among consumers and the food industry in improving the organoleptic and nutritional quality of foods, along with concern for the valorization of food waste, might explain this result.
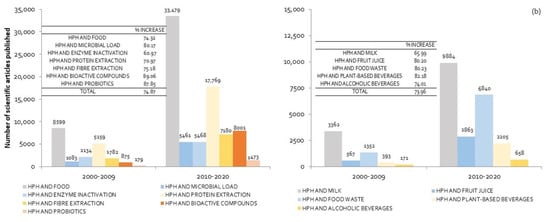
Figure 1.
Number of scientific articles published according to areas of application of HPH in the food industry (a) and the different types of food with which it has been used (b). % INCREASE was calculated as the difference in the number of articles published between 2010–2020 and 2000–2009, divided by the number of articles published in the period 2000–2009, expressed as a percentage. (Source: Science Direct. The following keywords and their combinations were used as the main search terms: high homogenization pressures, nonthermal technologies, food processing, encapsulation, functional food, bioactive components, probiotics, microbial load, enzyme inactivation, protein extraction, milk, fruit juice, food waste, plant-based beverages and alcoholic beverages).
Figure 1b shows the evolution in the number of published research works related to the application of HPH according to food type. Although the majority of works focused on fruit juices, the largest growth occurred in plant-based beverages and food waste. The huge increase in the consumption of plant-based beverages [5] and general concern about food waste-related issues are responsible for this increase. HPH technology is recognized as a green technology due to short processing times, low energy consumption, low CO2 emissions and the fact that it does not require polluting solvents.
This increase in research works based on HPH technology is also due to the development of new homogenization equipment that works at elevated pressures (i.e., up to 400 MPa) and supports specific conditions. Since the invention of adjustable valves in 1930 [6], the potential of homogenization technology has increased. The geometry and design of the valve determines the mechanical effect on the treated fluid. In 1982, the invention of the Gaulin Micro-Gap valve [7] greatly boosted the efficiency of the process, making high homogenization pressures possible in subsequent years and leading, more recently, to the development of ultrahigh homogenization pressure technology. In general, improvements have been obtained in all fields of application, making HPH an efficient tool with great potential for use in the food industry [8].
3. Preservation and Safety
HPH treatment for enzyme and microbial inactivation has been used in recent years as an alternative to thermal processes that, in most cases, cause undesirable effects such as nonenzymatic browning, cooked flavor or degradation of valuable components (see Table 1 and Table 2). It has been demonstrated that HPH treatment at processing pressures higher than 100 MPa contributes to microbial load reduction and enzyme inactivation. As previously stated, the heating that occurs in homogenization (an increase of about 2.5 °C per 10 MPa), together with structural modifications of cell walls, are the main phenomena responsible for the inactivation of microorganisms or enzymes. The final impact of HPH on microorganism viability or enzyme activity depends on several factors such as processing pressure, microbial strain or enzyme and food matrix.

Table 1.
Research works evaluating the decrease in microbial load in different food products by HPH.

Table 2.
Research works evaluating enzyme activity modulation by HPH.
In general, it has been verified that gram-negative bacteria exhibit greater susceptibility to inactivation by HPH than gram-positive ones, due to the reduced content of peptidoglycan in the cell wall that makes it thinner, and therefore, easier to disrupt. Fungi and yeasts seem to have a susceptibility that is intermediate between gram-positive and gram-negative bacteria, probably due to their wall structure, which is thicker but more complex than that of gram-positive bacteria [9]. For the inactivation of bacterial spores, pressures up to 400 MPa and additional steps are required [8].
Significant work has been undertaken on the use of HPH to reduce microbial load in fruit juices. In this type of food, it has been observed that the presence of some aroma compounds and essential oils can greatly influence the effect of HPH treatment. Patrignani et al. [12] studied the effect of the number of passes and citral addition on the spoilage microbiota of apricot juice when subjected to HPH at 100 MPa. Their results showed that yeast cell viability decreased with the increase of passes, and the relationship between both variables followed a linear trend. Moreover, the citral addition enhanced the effect of HPH, increasing the storage time by 6–8 days. To analyze the effect of the food matrix, the same authors compared the effect of HPH treatment at 100 MPa on the viability loss of S. cerevisiae 635 inoculated at a level of about 6.0 Log10 cfu/mL in apricot juice and carrot juice. In apricot juice, a significant decrease in the viability of 2.2 logarithmic cycles per mL was obtained with only four repeated passes at 100 MPa. A further increase of the number of passes at 100 MPa did not significantly increase the effectiveness of HPH treatment. Concerning carrot juice, eight repeated passes at 100 MPa were unable to completely inactivate the inoculated cells. They concluded that because of the higher viscosity and sugar content, apricot juice required more passes in HPH treatment to reduce yeast load [26]. In contrast, Zygosaccharomyces bailii 45 exhibited the same susceptibility to HPH treatment in both juices. Eight passes at 100 MPa resulted in a yeast inactivation higher than 2.5 log CFU/mL, regardless of the juice considered [27]. Nevertheless, Benjamin and Gamrasni [16] showed that HPH treatment at 100 and 150 MPa was not sufficient to reduce total bacteria and yeast count in pomegranate juice; rather, it needed to be combined with a thermal treatment at 65 °C for 15 s to achieve the same effect as pasteurization at 75 °C.
Besides fruit juices, plant-based beverages are complex dispersions with suspended proteins and oil droplets that require a homogenization stage to stabilize them and extend their commercial life. HPH can be applied at pressures higher than 100 MPa using multiple passes for these purposes, along with microbial cells destruction [21,22]. Valencia-Flores et al. [20] compared the effect on bacterial growth of HPH at 200–300 MPa and soft temperature inlet (55–75 °C) with conventional pasteurization treatment (90 °C, 90 s) in an almond beverage. They showed that 200 MPa and an inlet temperature of 55 °C yielded better results than conventional pasteurization on microbiological quality.
Beer is another beverage which may be treated with HPH. Research has established that it is possible to completely inactivate microorganisms in addition to improving the color of beer by HPH at pressures between 200–300 MPa and with 1 to 3 passes. The addition of antimicrobials such as lysozyme enzyme (50 mg/L) had a synergistic effect, reducing the required pressure to 100–150 MPa. However, HPH treatment could result in greater values for turbidity, and it would be necessary to perform another stabilization treatments to minimize the negative effects [23,24].
HPH treatment has also been used to modulate the activity of various enzymes. This treatment can increase or decrease enzyme activity depending on the processing conditions (pressure and number of passes), homogenizing valve structure, specific enzyme, pH, temperature and food matrix. Since enzymes are a complex type of globular protein, the mechanical forces and cavitation effects associated with HPH treatment result in conformational and structural changes which modify enzyme activity and stability. The main modifications in enzymes are linked to changes in the quaternary, tertiary and even secondary structures. The formation or interruption of hydrogen bonds, Van der Waals, hydrophobic and electrostatic interactions can occur, increasing the number of hydrophobic sites, revealing amino acid and sulfhydryl groups, and thus, accelerating, delaying or impeding enzyme–substrate interactions [24]. Furthermore, the magnitude of the changes induced by the HPH treatment will determine their reversibility or irreversibility. Aguilar et al. [28] noted that protein denaturation can be reversible at 100 MPa, but that it is irreversible above 200 MPa.
In the case of juices, the main alteration reactions are caused by the polyphenoloxidase, which is responsible for browning and oxidation reactions; it was shown that it was possible to inactivate it with homogenization pressures of 80–150 MPa [29,30]. On the other hand, α-amylase—whose use in recent years has been increasing, since it reduces the starch content in beverages, thereby avoiding turbidity and gelatinization—is resistant to HPH [31]. A similar resistance was observed on Pseudomonas fluorescens protease when HPH at 100–150 MPa was applied to reduce its proteolytic rate [32].
On the other hand, HPH can also be applied to enhance enzyme activity. Some authors have applied HPH to increase the activity of enzymes involved in the shelf life or processing of several food matrices. Lysozyme and lactoferrin in milk increased their antimicrobial activity against L. monocytogenes after HPH at 100 MPa [8,36]. Pinho et al. [17] observed an increase in the enzymatic activity of lactoperoxidase in skim milk at pressures of between 100 and 250 MPa. In contrast, if the homogenization pressure increased up to 300 MPa, a reduction of around 30% in enzyme activity was detected. In another work, defatted peanut flour was dispersed in distilled water and pH adjusted, and further subjected to HPH treatment at 40 and 80 MPa. After that, the peanut protein was recovered from the dispersed solution by an acid precipitation and redispersed in distilled water. The HPH treatment increased the extraction yield and the hydrolysis of the peanut protein isolates by endogenous enzymes. DPPH radical scavenging and hydroxyl radical scavenging activities were also increased [34].
It was shown that low cost, versatility and performance improvement of enzymatic processes can be achieved when the activity of commercial enzymes is increased by HPH. In particular, Tribst et al. [24] improved the activity of amyloglucosidase, glucose oxidase and neutral protease using HPH between 100–150 MPa and nonoptimum temperatures. Commercial enzymes derived from fungi and available as powders were diluted in acetate buffer solutions and then subjected to HPH treatment. Tribst et al. [24] observed an uneven effect of the number of passes. Only one pass was required to increase the activity of amyloglucosidase and neutral protease, and while no effect was observed in subsequent passes, successive steps continued to increase the enzyme activity of glucose oxidase; the energy involved in the molecular changes associated with the increase in enzyme activity might be responsible for this.
4. Extraction and Technological Functionality Improvement of Proteins, Fibrous Materials and Bioactive Compounds
HPH has been used in recent years to contribute to food process sustainability [5]. In this area, HPH has been applied for the valorization of agrifood byproducts with two objectives: (i) to increase the extractability of intracellular or cell wall structural components, and (ii) to improve the technological functionality of biomolecules from food byproducts. Most agri-food wastes or byproducts are rich in fibrous material and, in some cases, in proteins or bioactive compounds which are of interest to the food industry for use as food ingredients [37] or as sustainable packaging materials [38]. HPH induces cells disruption, favoring the release of structural and intracellular contents.
The main kind of products with which HPH is used to extract fibers, proteins or bioactive compounds are solid byproducts, such as pomace from fruit or vegetable juicing, fruit or vegetable peels, minimal processing waste and vegetal parts of plants or cereal seed hulls; the most important examples are included in Table 3. In these cases, solid wastes need to be fluidized by diluting them in water or another solvent. In other cases, an extraction method is applied and the extracted phase is further subjected to HPH. The authors of [39] extracted pectin form potato peel by HPH at 200 MPa. They obtained improvements in the viscosity, emulsifying properties, degree of esterification and physicochemical characteristics, and therefore, recommended the use of HPH to obtain pectin that could be used as a stabilizing agent or a thickener in food manufacturing. Similarly, Fayaz et al. [40] showed that HPH favors the release of okara proteins and soluble fiber. Soy okara was dispersed in deionized water at 10 g/100 g and prehomogenized with a high-speed blender. After that, a homogenization pressure of 150 MPa for 5 passes made it possible to extract proteins with a yield of 90%. The authors of [41] applied HPH to make edible and biodegradable films for food packaging from a type of edible fungus, i.e., Flammulina velutipes. Wu et al. [42] demonstrated the possibility of using HPH treatment to make biodegradable biopolymer films from pomelo peel.

Table 3.
Research works aimed to the extraction and improvement of technological functionality of proteins, fibers or bioactive compounds from agri-food wastes by HPH.
HPH reduces the particle size and structure of macromolecules, modifying their solubility, interaction properties, viscosity, or other physic-chemical properties. Saricaoglu et al. [43] improved the functionality of proteins from the hazelnut industry by HPH at 100 MPa and 1 pass. The homogenization pressure decreased the particle size of proteins, increasing their zeta potential and water solubility; emulsifying and sparkling properties were improved too. Hua et al. [44] demonstrated a microstructural change of tomato waste fibers by applying HPH at 100 MPa and 10 passes. The authors transformed around 8% of the insoluble fibers into soluble ones. Xu et al. [45] indicated that for the preparation of soluble peach fiber from fresh peach marc, it must be dispersed in three times the volume of deionized water, thus improving the efficiency of cellulose hydrolysis. For pectin extraction from milled dried lemon peel, variations in dilutions changed the properties of the extracted pectin, resulting in residues with different pectic characteristics [46]. Discarded external lettuce leaves were dispersed in hydroalcoholic solutions and polyphenols extracted with ethanol to obtain good phenolic extraction yields [47].
5. Increase of Bioavailability and Encapsulation of Bioactive Compounds
In the last decade, many studies have been carried out to demonstrate that the application of HPH to liquid foods can modify the bioaccessibility (i.e., the fraction of an ingested nutrient that is released from the food matrix and made available for intestinal absorption) or bioavailability (i.e., the fraction of an ingested nutrient that is absorbed by the intestine and incorporated into the bloodstream) of its bioactive compounds. In most studies, an increase in the bioaccessibility of phytochemicals was observed due to their release within the structure of the food in which they were found. In other cases, a modification of biological functionality occurs due to a change in its chemical structure. Zhou [62] carried out an interesting review that demonstrated these effects in three bioactive components: carotenoids, phenolic compounds and vitamin C. The review showed that fruit juices (carrot, tomato, orange, apple and berries) are the most common food in which HPH increases the bioaccessibility of bioactive compounds. HPH decreases the particle size of suspended pulp and increases cloud stability and, thus, the availability of bioactive components. Treatment with HPH in mandarin juices increased the bioaccessibility of total carotenoids by five times, although in the case of flavonoids, no such drastic changes were observed. Therefore, HPH treatment was recommended for the production of tangerine juices that promote health, mainly through the improvement of the bioaccessibility of the carotenoids contained therein [63]. Quan et al. [64] established that the improvement of bioaccessibility could be conditioned by the food matrix. They observed that HPH at 250 MPa favored the release from cell walls and increased the content of total phenolic compounds in kiwi and pomelo juices, but that it had a negative effect on its bioaccessibility (in vitro) as a consequence of the major degradation that occurred in the digestion process. Conversely, the addition of skimmed or whole milk to the juices had no significant effect on total phenolic content, but increased their bioaccessibility in kiwi juice and pomelo juice from 21.6% to 37.8% and 60.1% to 63.3%, respectively. Similarly, Alongi et al. [65] showed (in vitro) that the bioaccessibility of chlorogenic acids increased from nearly 25% to more than 50% by adding milk with different fat contents to coffee and applying HPH (50–150 MPa). Alongi et al. [65] observed that the pressure required was lower the lower the fat percentage; they attributed this effect to the micellarization of chlorogenic acids, a phenomenon that reduced their susceptibility to degradation during digestion. Sometimes, positive effects of HPH were observed after storage. Betoret et al. [66] found in low pulp mandarin juice that, despite the increase in suspended pulp after HPH and trehalose addition, flavonoid hesperidin initially decreased but resulted in less flavonoid degradation during storage.
HPHs have also been applied, albeit with a much smaller number of published articles, to nondairy, vegetable-based beverages. Although, in some cases, no significant improvements in the nutritional characteristics were detected, in others, such as almond or soy beverages, a reduction in antinutrients was achieved [67]. The denaturation, aggregation and chemical modification of proteins may change their allergic potential. Toro- Funes et al. [68] demonstrated an increase of 40% in the extractability of phytosterols from almond milk subjected to a HPH of 300 MPa (6 passes). However, the content of tocopherol and polyamines such as spermidine were reduced by up to 90%. The application of HPH for kefir production from a hazelnut beverage achieved improvements in the total content of phenolic compounds and antioxidant capacity, causing a reduction in the content of lactic and citric acid [69].
Improvements in the bioavailability of bioactive components by HPH are also possible in solid foods. HPH (10–20 cycles and 100–200 MPa) was used to fabricate an aqueous nanosuspension of fermented soybean powder, favoring in vitro release of isoflavones from nanosuspension [70].
The other way in which HPH can contribute to improvements in the nutritional properties of foods is by using this technology for the encapsulation of bioactive components (see Table 4). In this way, it is possible to increase the stability, conservation and controlled delivery to target sites, thereby increasing food functionality. HPH produces intense disruptive forces that break up particles into smaller sizes, favoring the encapsulation of specific components in a suitable media. Mechanical stress and heating associated and emulsifier interactions can affect the effectiveness of the process, and therefore, the activity and bioaccessibility of the bioactive compound.

Table 4.
Research works in which HPH treatment was applied to encapsulate.
Many studies have investigated the nanoencapsulation of curcumin by HPH at 40–100 MPa. The results showed that the emulsifier type had an influence on the bioaccessibility of curcumin [71]. Frank et al. [72] studied the degradation of anthocyanins from bilberry extract by subjecting them, by HPH, to temperature and mechanical stresses similar to those involved in the process of emulsification and encapsulation. HPH was applied with a simple pass and in a pressure range between 30 and 150 MPa. Thereafter, the samples were immediately cooled to 298 K. The results showed no significant influence of mechanical stresses associated with HPH on anthocyanin stability, even at high-pressure treatment, i.e., up to 150 MPa. The combination temperature–time was the main parameter affecting the degradation of anthocyanin.
A great level of interest has been shown in applying HPH as an encapsulation technique in bacteria with probiotic effects. Patrignani et al. [73] underlined the potential of the HPH microencapsulation of probiotic microorganisms to produce fermented milk with improved functionality and enhanced sensory properties. They established 50 MPa and 5 passes as being adequate conditions to produce stable microcapsules of Lactobacilli with high yield and excellent viability during storage. Moreover, the microencapsulation of adjunct bacteria reduced the acidity of fermented milk. Calabuig-Jiménez et al. [74] microencapsulated L. salivarius spp. salivarius in alginate coatings using HPH at 70 MPa. A positive effect of microcapsules was observed when evaluating the survival of the probiotic strain on simulated gastrointestinal conditions.
HPH treatments at pressure levels below 100 MPa, considered sublethal pressures, were applied to microbial cultures, i.e., initiators, co-initiators, or probiotics and yeasts, in order to produce cultures with improved functional, technological and sensory properties (some examples are included in Table 5). The use of strains belonging to the genus Bifidobacterium and Lactobacillus predominated as probiotics, and to a lesser extent Enterococcus, Streptococcus and Saccharomyces [8]. The bacterial cells responded to mechanical stress induced by HPH, modifying their metabolic activity and membrane composition. Therefore, technological and functional properties such as fermentation kinetic, enzymatic activities, hydrophobicity or resistance to gastrointestinal digestion can be improved.

Table 5.
Research works in which HPH treatment was applied to probiotic cells.
Siroli et al. [83] reported that the main regulatory mechanism that probiotic lactobacilli adopt to counteract pressure stress is the modification of the composition of membrane fatty acids. Specifically, they observed an increase in unsaturated fatty acids when HPH at 100 and 150 MPa was applied to Lactobacillus paracasei A13. Considering that the increase of the unsaturation level is a key mechanism to compensate for the oxidative damages induced by physico-chemical stressors in microbial cells, they concluded that HPH at sublethal pressures is useful to improve the activity of some Lactobacillus species.
Lanciotti et al. [84] studied the effect of HPH between 50 MPa and 100 MPa on the fermentation kinetics, metabolic profile and enzymatic activity of four species of Lactobacilli involved in dairy product fermentation and ripening. Although the results varied according to the species, they documented no significant effect on cell viability, an increased proteolytic activity and positive changes in fermentation dynamics. The resistance to simulated gastric conditions, hydrophobicity and auto-aggregation capacity were strain-dependent for L. acidophilus Dru and L. paracasei A13 when subjected to HPH at 50 MPa. HPH increased the three properties for L. paracasei A13 but reduced them for L. acidophilus Dru; the authors attributed this to the compositional and structural differences in the cellular outer structures, thus suggesting that the HPH effects on macromolecules and their interactions with the gut immune cells play a key role in the probiotic effect. The same authors noted that HPH treated L. paracasei cells modified their interaction with the small intestine of mice, inducing a higher IgA response compared to untreated L. paracasei cells [12,79]. Betoret et al. [66] demonstrated an improvement in the hydrophobicity of Lactobacillus salivarius spp. salivarius added to mandarin juice with trehalose when HPH at 0, 20 and 100 MPa was applied.
6. Conclusions
Although in the beginning, the application of high homogenizing pressures was aimed at more efficient homogenizing and increasing the stability of emulsions such as milk, advances in valve design have allowed for an increase in working pressure extending the scope of possible applications.
In the last decade, the number of research works related to the implementation of HPH—for extracting bioactive components from agri-food wastes, to improve the bioavailability and probiotic properties of bioactive components and microorganisms, and also as an encapsulation technique—has grown by more than 80%. At the same time, progress has been made in the application of HPH to reduce the microbial load or modulate the activity of some enzymes.
The general mechanisms responsible for the effect of HPH are known, but the final effect is largely conditioned by the type of valve, pressure applied, number of passes, the nature of the components and macromolecules, and the food matrix. For this reason, research is needed for each specific application.
Results published in the last decade have shown HPH to be a nonthermal technology which is able to accomplish the food industry’s objectives of quality and safety, functionality and sustainability.
Author Contributions
Conceptualization, N.B. and E.B.; methodology and search, J.M., L.I.H.-C. and N.B.; writing—original draft preparation, J.M. and N.B.; writing—review and editing, E.B., L.S. and C.B.; funding acquisition, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research and APC were funded by Generalitat Valenciana, Project AICO/2017/’049-
Acknowledgments
The authors thank the research project “Fortalecimiento de los Encadenamientos Productivos de las Subregiones del Chocó” BPIN 2013000100284 Tecnológica del Chocó (in Spanish) by financial support to Leidy Indira Hinestroza-Córdoba.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Augusto, P.E.; Tribst, A.A.; Cristianini, M. High Hydrostatic Pressure and High-Pressure Homogenization Processing of Fruit Juices. In Fruit Juices; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 393–421. [Google Scholar]
- Koutchma, T. Fundamentals of HPP Technology. In Adapting High Hydrostatic Pressure for Food Processing Operations; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 5–10. [Google Scholar]
- Osorio-Arias, J.C.; Vega-Castro, O.; Martínez-Monteagudo, S.I. Fundamentals of High-Pressure Homogenization of Foods. In Reference Module in Food Science; Elsevier BV: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Two Nonthermal Technologies for Food Safety and Quality—Ultrasound and High-Pressure Homogenization: Effects on Microorganisms, Advances, and Possibilities: A Review. J. Food Prot. 2019, 82, 2049–2064. [Google Scholar] [CrossRef] [PubMed]
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.-P.; Marchesseau, S. Potentialities and Limits of Some Non-thermal Technologies to Improve Sustainability of Food Processing. Front. Nutr. 2019, 5, 130. [Google Scholar] [CrossRef]
- McClatchie, J.M. The Borden Company. Valve Homog. Dev. 1930, 28, 131–134. [Google Scholar]
- Pandolfe, W.D. Development of the New Gaulin Micro-Gap™ Homogenizing Valve. J. Dairy Sci. 1982, 65, 2035–2044. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Braschi, G.; Lanciotti, R. Combined use of natural antimicrobial based nanoemulsions and ultra-high-pressure homogenization to increase safety and shelf-life of apple juice. Food Control. 2020, 111, 107051. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Barbosa-Cánovas, G.; Lelieveld, H. High Pressure Processing of Food-Principles, Technology and Application; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-1-4939-3233-7. [Google Scholar]
- Tribst, A.A.L.; Franchi, M.A.; de Massaguer, P.R.; Cristianini, M. Quality of mango nectar processed by high-pressure homogenization with optimized heat treatment. J. Food Sci. 2011, 76, M106–M110. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, S.; Foschia, M.; Bartolomeoli, I.; Maifreni, M.; Manzocco, L. Study on the applicability of high-pressure homogenization to produce banana juices. LWT 2012, 45, 117–121. [Google Scholar] [CrossRef]
- Tabanelli, G.; Patrignani, F.; Vinderola, G.; Reinheimer, J.; Gardini, F.; Lanciotti, R. Effect of sub-lethal high-pressure homogenization treatments on the in vitro functional and biological properties of lactic acid bacteria. LWT 2013, 53, 580–586. [Google Scholar] [CrossRef]
- Guan, Y.; Zhou, L.; Bi, J.; Yi, J.; Liu, X.; Chen, Q.; Wu, X.; Zhou, M. Change of microbial and quality attributes of mango juice treated by high pressure homogenization combined with moderate inlet temperatures during storage. Innov. Food Sci. Emerg. Technol. 2016, 36, 320–329. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Zou, B. Effect of High-Pressure Homogenization and Dimethyl Dicarbonate (DMDC) on Microbial and Physicochemical Qualities of Mulberry Juice. J. Food Sci. 2016, 81, M702–M708. [Google Scholar] [CrossRef]
- Xia, X.; Dai, Y.; Wu, H.; Liu, X.; Wang, Y.; Cao, J.; Zhou, J. Effects of pressure and multiple passes on the physicochemical and microbial characteristics of lupin-based beverage treated with high-pressure homogenization. J. Food Process. Preserv. 2019, 43, e13912. [Google Scholar] [CrossRef]
- Benjamin, O.; Gamrasni, D. Microbial, nutritional, and organoleptic quality of pomegranate juice following high-pressure homogenization and low-temperature pasteurization. J. Food Sci. 2020, 85, 592–599. [Google Scholar] [CrossRef]
- Pinho, C.R.; Franchi, M.A.; Tribst, A.A.; Cristianini, M. Effect of Ultra High-Pressure Homogenization on Alkaline Phosphatase and Lactoperoxidase Activity in Raw Skim Milk. Procedia Food Sci. 2011, 1, 874–878. [Google Scholar] [CrossRef]
- Amador-Espejo, G.G.; Hernández-Herrero, M.M.; Juan, B.; Trujillo, A.-J. Inactivation of Bacillus spores inoculated in milk by Ultra High-Pressure Homogenization. Food Microbiol. 2014, 44, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Mercan, E.; Sert, D.; Akın, N. Determination of powder flow properties of skim milk powder produced from high-pressure homogenization treated milk concentrates during storage. LWT 2018, 97, 279–288. [Google Scholar] [CrossRef]
- Valencia-Flores, D.C.; Hernández-Herrero, M.M.; Guamis, B.; Ferragut, V. Comparing the Effects of Ultra-High-Pressure Homogenization and Conventional Thermal Treatments on the Microbiological, Physical, and Chemical Quality of Almond Beverages. J. Food Sci. 2013, 78, E199–E205. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Casanova, F.P.; Petruzzi, L.; Sinigaglia, M.; Corbo, M. Using physical approaches for the attenuation of lactic acid bacteria in an organic rice beverage. Food Microbiol. 2016, 53, 1–8. [Google Scholar] [CrossRef]
- Codina-Torrella, I.; Guamis, B.; Zamora, A.; Quevedo, J.; Trujillo, A.-J. Microbiological stabilization of tiger nuts’ milk beverage using ultra-high-pressure homogenization. A preliminary study on microbial shelf-life extension. Food Microbiol. 2018, 69, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.A.; Tribst, A.A.L.; Cristianini, M. Inactivation of Lactobacillus brevis in Beer Utilizing a Combination of High-Pressure Homogenization and Lysozyme Treatment. J. Inst. Brew. 2011, 117, 634–638. [Google Scholar] [CrossRef]
- Franchi, M.A.; Tribst, A.A.L.; Cristianini, M. High-pressure homogenization: A non-thermal process applied for inactivation of spoilage microorganisms in beer. J. Inst. Brew. 2013, 119, 237–241. [Google Scholar] [CrossRef]
- Comuzzo, P.; Calligaris, S.; Iacumin, L.; Ginaldi, F.; Paz, A.E.P.; Zironi, R. Potential of high-pressure homogenization to induce autolysis of wine yeasts. Food Chem. 2015, 185, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Capra, M.L.; Patrignani, F.; Quiberoni, A.D.L.; Reinheimer, J.A.; Lanciotti, R.; Guerzoni, M.E. Effect of high-pressure homogenization on lactic acid bacteria phages and probiotic bacteria phages. Int. Dairy J. 2009, 19, 336–341. [Google Scholar] [CrossRef]
- Patrignani, F.; Vannini, L.; Kamdem, S.L.S.; Lanciotti, R.; Guerzoni, M.E. Potentialities of High-Pressure Homogenization to Inactivate Zygosaccharomyces bailii in Fruit Juices. J. Food Sci. 2010, 75, M116–M120. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.G.D.S.; Cristianini, M.; Sato, H.H.; dos Santos, J.G. Modification of enzymes by use of high-pressure homogenization. Food Res. Int. 2018, 109, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Bot, F.; Calligaris, S.; Cortella, G.; Plazzotta, S.; Nocera, F.; Anese, M. Study on high pressure homogenization and high-power ultrasound effectiveness in inhibiting polyphenoloxidase activity in apple juice. J. Food Eng. 2018, 221, 70–76. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L. High-pressure homogenisation combined with blanching to turn lettuce waste into a physically stable juice. Innov. Food Sci. Emerg. Technol. 2019, 52, 136–144. [Google Scholar] [CrossRef]
- Tribst, A.A.L.; Cristianini, M. High pressure homogenization of a fungi α-amylase. Innov. Food Sci. Emerg. Technol. 2012, 13, 107–111. [Google Scholar] [CrossRef]
- De Oliveira, M.M.; Júnior, B.R.D.C.L.; Tribst, A.A.L.; Cristianini, M. Use of high-pressure homogenization to reduce milk proteolysis caused by Pseudomonas fluorescens protease. LWT 2018, 92, 272–275. [Google Scholar] [CrossRef]
- Tribst, A.A.L.; Cristianini, M. Changes in commercial glucose oxidase activity by high pressure homogenization. Innov. Food Sci. Emerg. Technol. 2012, 16, 355–360. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, M.; Shi, J.; Yang, B.; Li, J.; Luo, D.; Jiang, G.; Jiang, Y. Effects of combined high-pressure homogenization and enzymatic treatment on extraction yield, hydrolysis and function properties of peanut proteins. Innov. Food Sci. Emerg. Technol. 2011, 12, 478–483. [Google Scholar] [CrossRef]
- Tribst, A.A.L.; Ribeiro, L.R.; Cristianini, M. Comparison of the effects of high-pressure homogenization and high pressure processing on the enzyme activity and antimicrobial profile of lysozyme. Innov. Food Sci. Emerg. Technol. 2017, 43, 60–67. [Google Scholar] [CrossRef]
- Iucci, L.; Patrignani, F.; Vallicelli, M.; Guerzoni, M.E.; Lanciotti, R. Effects of high-pressure homogenization on the activity of lysozyme and lactoferrin against Listeria monocytogenes. Food Control. 2007, 18, 558–565. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, Y.; Chen, P.; Peng, P.; Liu, S.; Li, D.; Ruan, R. Effect of alkaline and high-pressure homogenization on the extraction of phenolic acids from potato peels. Innov. Food Sci. Emerg. Technol. 2016, 37, 91–97. [Google Scholar] [CrossRef]
- Flôres, S.H.; Rios, A.D.O.; Iahnke, A.O.; de Campo, C.; Vargas, C.G.; Santos, C.D.; Caetano, K.D.S.; Stoll, L.; Crizel, T.D.M. Films for Food from Ingredient Waste. In Reference Module in Food Science; Elsevier BV: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of high hydrostatic pressure and high-pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, G.; Plazzotta, S.; Calligaris, S.; Manzocco, L.; Nicoli, M.C. Impact of high-pressure homogenization on physical properties, extraction yield and biopolymer structure of soybean okara. LWT 2019, 113, 108324. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, K.; Li, C.; Cheng, S.; Zhou, J.; Wu, Z. A novel biodegradable film from edible mushroom (F. velutipes) by product: Microstructure, mechanical and barrier properties associated with the fiber morphology. Innov. Food Sci. Emerg. Technol. 2018, 47, 153–160. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, D.; Lu, J.; Jiao, C.; Li, S.; Lei, Y.; Liu, D.; Wang, J.; Zhang, Z.; Liu, Y.; et al. Effect of high-pressure homogenization on microstructure and properties of pomelo peel flour film-forming dispersions and their resultant films. Food Hydrocoll. 2020, 102, 105628. [Google Scholar] [CrossRef]
- Sarıcaoğlu, F.T.; Atalar, I.; Yilmaz, V.A.; Odabas, H.I.; Gul, O. Application of multi pass high pressure homogenization to improve stability, physical and bioactive properties of rosehip (Rosa canina L.) nectar. Food Chem. 2019, 282, 67–75. [Google Scholar] [CrossRef]
- Hua, X.; Xu, S.; Wang, M.; Chen, Y.; Yang, H.; Yang, R. Effects of high-speed homogenization and high-pressure homogenization on structure of tomato residue fibers. Food Chem. 2017, 232, 443–449. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, Q.; Yuan, F.; Gao, Y. In vitro binding capacities and physicochemical properties of soluble fiber prepared by microfluidization pretreatment and cellulase hydrolysis of peach pomace. LWT 2015, 63, 677–684. [Google Scholar] [CrossRef]
- Willemsen, K.L.; Panozzo, A.; Moelants, K.; de Bon, S.J.; Desmet, C.; Cardinaels, R.; Moldenaers, P.; Wallecan, J.; Hendrickx, M. Physico-chemical and viscoelastic properties of high pressure homogenized lemon peel fiber fraction suspensions obtained after sequential pectin extraction. Food Hydrocoll. 2017, 72, 358–371. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L. Effect of ultrasounds and high-pressure homogenization on the extraction of antioxidant polyphenols from lettuce waste. Innov. Food Sci. Emerg. Technol. 2018, 50, 11–19. [Google Scholar] [CrossRef]
- Huang, X.; Tu, Z.; Xiao, H.; Li, Z.; Zhang, Q.; Wang, H.; Hu, Y.; Zhang, L. Dynamic high pressure microfluidization-assisted extraction and antioxidant activities of sweet potato (Ipomoea batatas L.) leaves flavonoid. Food Bioprod. Process. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Rommi, K.; Rahikainen, J.; Vartiainen, J.; Holopainen, U.; Lahtinen, P.; Honkapää, K.; Lantto, R. Potato peeling costreams as raw materials for biopolymer film preparation. J. Appl. Polym. Sci. 2015, 133. [Google Scholar] [CrossRef]
- Xie, Y.; Ho, S.-H.; Chen, C.-N.N.; Chen, C.-Y.; Jing, K.; Ng, I.-S.; Chen, J.; Chang, J.-S.; Lu, Y. Disruption of thermo-tolerant Desmodesmus sp. F51 in high pressure homogenization as a prelude to carotenoids extraction. Biochem. Eng. J. 2016, 109, 243–251. [Google Scholar] [CrossRef]
- Zhu, X.; Lundberg, B.; Cheng, Y.; Shan, L.; Xing, J.-J.; Peng, P.; Chen, P.; Huang, X.; Li, D.; Ruan, R. Effect of high-pressure homogenization on the flow properties of citrus peel fibers. J. Food Process. Eng. 2017, 41, e12659. [Google Scholar] [CrossRef]
- Preece, K.; Hooshyar, N.; Zuidam, N. Whole soybean protein extraction processes: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 163–172. [Google Scholar] [CrossRef]
- Sarıcaoğlu, F.T.; Gul, O.; Besir, A.; Atalar, I. Effect of high-pressure homogenization (HPH) on functional and rheological properties of hazelnut meal proteins obtained from hazelnut oil industry by-products. J. Food Eng. 2018, 233, 98–108. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, F.; Lan, X.; Gong, S.; Wang, Z. Characteristics of pectin from black cherry tomato waste modified by dynamic high-pressure microfluidization. J. Food Eng. 2018, 216, 90–97. [Google Scholar] [CrossRef]
- Otoni, C.G.; Lodi, B.D.; Lorevice, M.V.; Leitão, R.C.; Ferreira, M.D.; de Moura, M.R.; Mattoso, L.H. Optimized and scaled-up production of cellulose-reinforced biodegradable composite films made up of carrot processing waste. Ind. Crop. Prod. 2018, 121, 66–72. [Google Scholar] [CrossRef]
- Xing, J.-J.; Cheng, Y.; Chen, P.; Shan, L.; Ruan, R.; Li, D.; Wang, L. Effect of high-pressure homogenization on the extraction of sulforaphane from broccoli (Brassica oleracea) seeds. Powder Technol. 2019, 358, 103–109. [Google Scholar] [CrossRef]
- Mustafa, W.; Pataro, G.; Ferrari, G.; Donsì, F. Novel approaches to oil structuring via the addition of high-pressure homogenized agri-food residues and water forming capillary bridges. J. Food Eng. 2018, 236, 9–18. [Google Scholar] [CrossRef]
- Griffin, S.; Sarfraz, M.; Farida, V.; Nasim, M.J.; Ebokaiwe, A.P.; Keck, C.M.; Jacob, C. No time to waste organic waste: Nanosizing converts remains of food processing into refined materials. J. Environ. Manag. 2018, 210, 114–121. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.; Ishak, M.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological, and thermal behavior. Int. J. Boil. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Ferrari, G.; Imhof, A.; Donsi’, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, R.; Xu, Y.; Chen, M.; Zhang, J.; Gao, Q.; Li, J. Developing a stable high-performance soybean meal-based adhesive using a simple high-pressure homogenization technology. J. Clean. Prod. 2020, 256, 120336. [Google Scholar] [CrossRef]
- Zhou, L. High-Pressure Homogenization Effect on the Stability and Bioaccessibility of Bioactive Phytochemicals and Vitamins in the Food Matrix. In Reference Module in Food Science; Elsevier BV: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sentandreu, E.; Stinco, C.M.; Vicario, I.M.; Mapelli-Brahm, P.; Navarro, J.L.; Meléndez-Martínez, A.J. High-pressure homogenization as compared to pasteurization as a sustainable approach to obtain mandarin juices with improved bioaccessibility of carotenoids and flavonoids. J. Clean. Prod. 2020, 262, 121325. [Google Scholar] [CrossRef]
- Quan, W.; Tao, Y.; Qie, X.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effects of high-pressure homogenization, thermal processing, and milk matrix on the in vitro bioaccessibility of phenolic compounds in pomelo and kiwi juices. J. Funct. Foods 2020, 64, 103633. [Google Scholar] [CrossRef]
- Alongi, M.; Calligaris, S.; Anese, M. Fat concentration and high-pressure homogenization affect chlorogenic acid bioaccessibility and α-glucosidase inhibitory capacity of milk-based coffee beverages. J. Funct. Foods 2019, 58, 130–137. [Google Scholar] [CrossRef]
- Betoret, E.; Jiménez, L.C.; Patrignani, F.; Lanciotti, R.; Rosa, M.D. Effect of high-pressure processing and trehalose addition on functional properties of mandarin juice enriched with probiotic microorganisms. LWT 2017, 85, 418–422. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, P.K.; Roohinejad, S.; Lorenzo, J.M. Effect of Innovative Food Processing Technologies on the Physicochemical and Nutritional Properties and Quality of Non-Dairy Plant-Based Beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef]
- Toro-Funes, N.; Bosch-Fusté, J.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Influence of Ultra-High-Pressure Homogenization Treatment on the Phytosterols, Tocopherols, and Polyamines of Almond Beverage. J. Agric. Food Chem. 2014, 62, 9539–9543. [Google Scholar] [CrossRef] [PubMed]
- Atalar, I. Functional kefir production from high pressure homogenized hazelnut milk. LWT 2019, 107, 256–263. [Google Scholar] [CrossRef]
- Kapoor, R.; Pathak, S.; Najmi, A.K.; Aeri, V.; Panda, B.P. Processing of soy functional food using high pressure homogenization for improved nutritional and therapeutic benefits. Innov. Food Sci. Emerg. Technol. 2014, 26, 490–497. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.; Köhler, K.; Karbstein, H.P. Stability of anthocyanins in high pressure homogenisation. Food Chem. 2012, 130, 716–719. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Braschi, G.; Betoret, E.; Reinheimer, J.A.; Lanciotti, R. Microencapsulation of functional strains by high pressure homogenization for a potential use in fermented milk. Food Res. Int. 2017, 97, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, L.C.; Ester, B.; Betoret, N.; Patrignani, F.; Barrera, C.; Seguí, L.; Lanciotti, R.; Rosa, M.D. High pressures homogenization (HPH) to microencapsulate L. salivarius spp. salivarius in mandarin juice. Probiotic survival and in vitro digestion. J. Food Eng. 2019, 240, 43–48. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.; Lim, L.-T.; Xu, W.; Xu, G. Coencapsulation of Polyphenols and Anthocyanins from Blueberry Pomace by Double Emulsion Stabilized by Whey Proteins: Effect of Homogenization Parameters. Molecules 2018, 23, 2525. [Google Scholar] [CrossRef]
- Tatar, B.C.; Sumnu, G.; Oztop, M. Microcapsule characterization of phenolic powder obtained from strawberry pomace. J. Food Process. Preserv. 2019, 43, e13892. [Google Scholar] [CrossRef]
- Ester, B.; Noelia, B.; Laura, C.-J.; Patrignani, F.; Cristina, B.; Lanciotti, R.; Marco, D.R. Probiotic survival and in vitro digestion of L. salivarius spp. salivarius encapsulated by high homogenization pressures and incorporated into a fruit matrix. LWT 2019, 111, 883–888. [Google Scholar] [CrossRef]
- Muramalla, T.; Aryana, K. Some low homogenization pressures improve certain probiotic characteristics of yogurt culture bacteria and Lactobacillus acidophilus LA-K. J. Dairy Sci. 2011, 94, 3725–3738. [Google Scholar] [CrossRef]
- Tabanelli, G.; Burns, P.; Patrignani, F.; Gardini, F.; Lanciotti, R.; Reinheimer, J.; Vinderola, G. Effect of a non-lethal High-Pressure Homogenization treatment on the in vivo response of probiotic lactobacilli. Food Microbiol. 2012, 32, 302–307. [Google Scholar] [CrossRef]
- Patrignani, F.; Serrazanetti, D.I.; Mathara, J.M.; Siroli, L.; Gardini, F.; Holzapfel, W.; Lanciotti, R. Use of homogenisation pressure to improve quality and functionality of probiotic fermented milks containing Lactobacillus rhamnosus BFE 5264. Int. J. Dairy Technol. 2015, 69, 262–271. [Google Scholar] [CrossRef]
- Burns, P.; Patrignani, F.; Tabanelli, G.; Vinderola, G.C.; Siroli, L.; Reinheimer, J.A.; Gardini, F.; Lanciotti, R. Potential of high pressure homogenisation on probiotic Caciotta cheese quality and functionality. J. Funct. Foods 2015, 13, 126–136. [Google Scholar] [CrossRef]
- Barrera, C.; Burca, C.; Betoret, E.; García-Hernández, J.; Hernández, M.; Betoret, N. Improving antioxidant properties and probiotic effect of clementine juice inoculated with Lactobacillus salivarius spp. salivarius (CECT 4063) by trehalose addition and/or sublethal homogenisation. Int. J. Food Sci. Technol. 2019, 54, 2109–2122. [Google Scholar] [CrossRef]
- Siroli, L.; Braschi, G.; Rossi, S.; Gottardi, D.; Patrignani, F.; Lanciotti, R. Lactobacillus paracasei A13 and High-Pressure Homogenization Stress Response. Microorganisms 2020, 8, 439. [Google Scholar] [CrossRef]
- Lanciotti, R.; Patrignani, F.; Iucci, L.; Saracino, P.; E Guerzoni, M. Potential of high-pressure homogenization in the control and enhancement of proteolytic and fermentative activities of some Lactobacillus species. Food Chem. 2007, 102, 542–550. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).