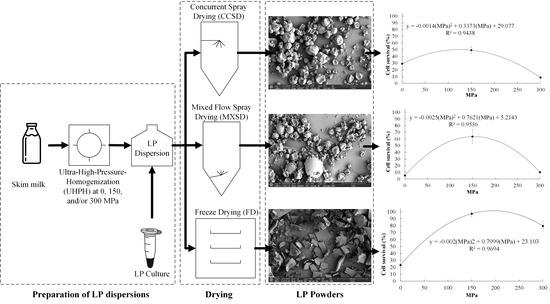
Microencapsulation of Lactobacillus plantarum NRRL B-1927 with Skim Milk Processed via Ultra-High-Pressure Homogenization
Abstract
1. Introduction
2. Results and Discussion
2.1. Survibability of LP Cells after Drying
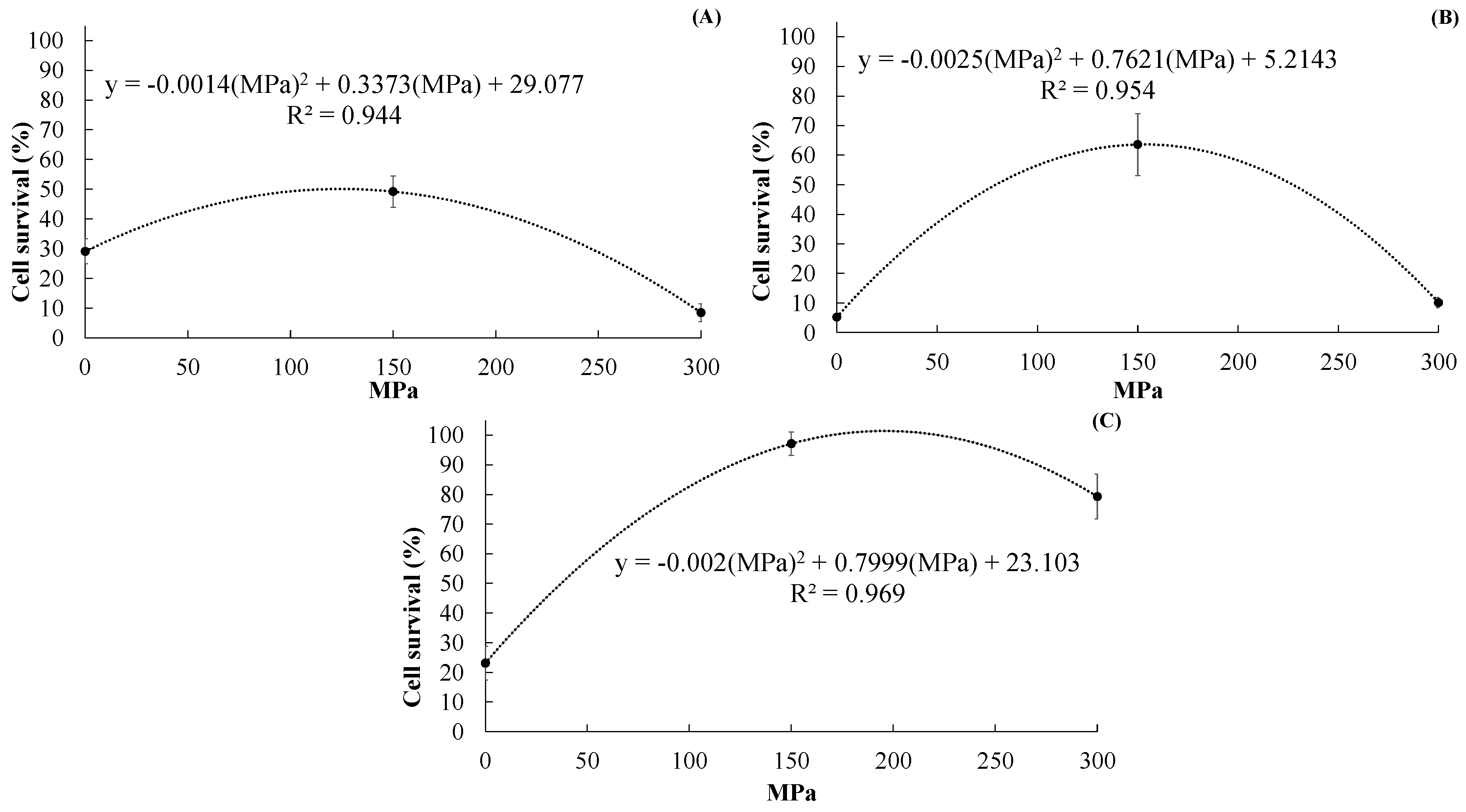
2.1.1. Effect of UHPH Treatment on Cell Survival of LP
- a.
- After CCSD
- b.
- After MXSD
- c.
- After FD
2.1.2. Effect of Drying Method on Cell Survival
2.2. Moisture Content and Water Activity
2.3. Particle Size Distribution
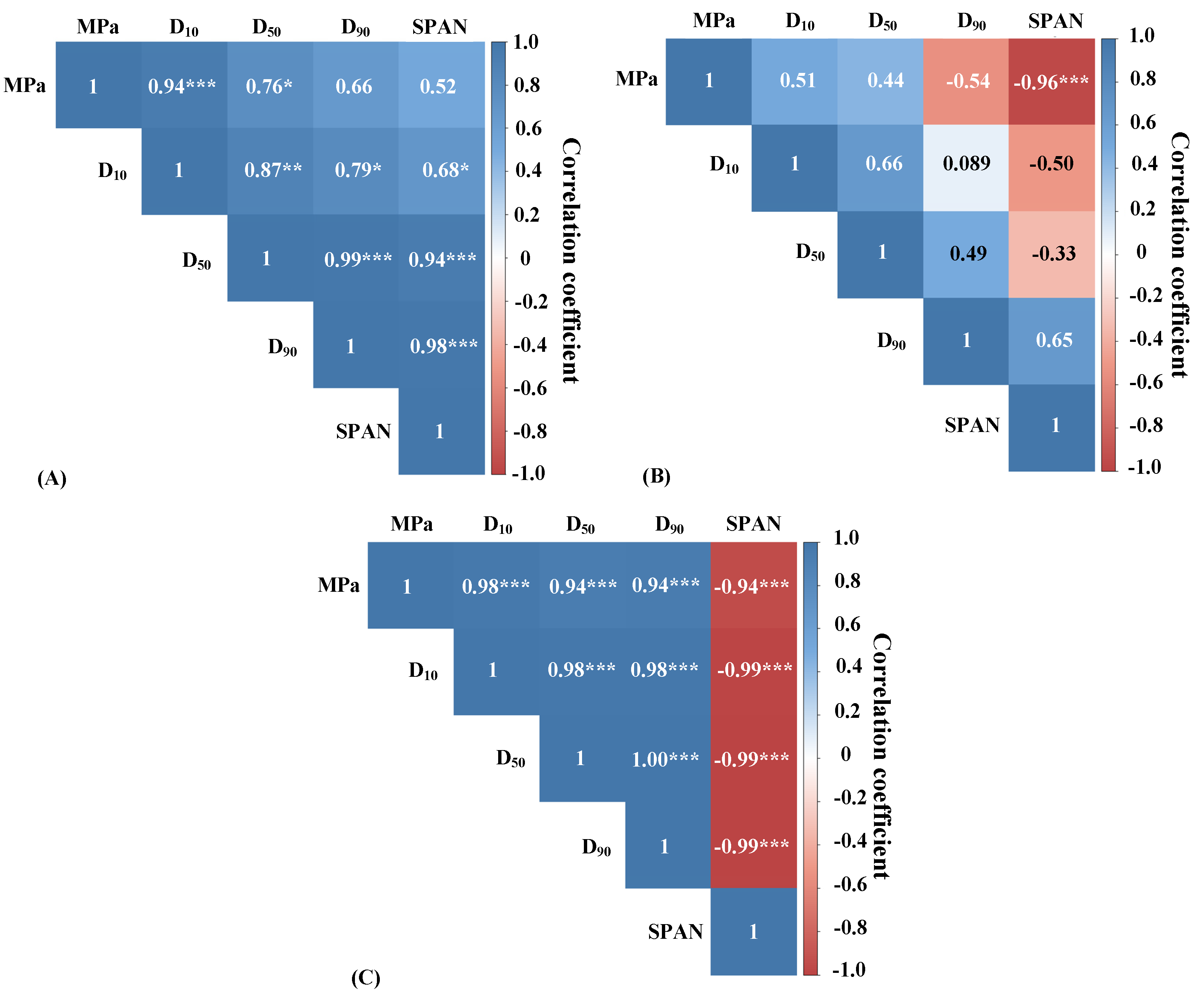
2.3.1. Effect of UHPH Processing on Particle Size of LP Powders
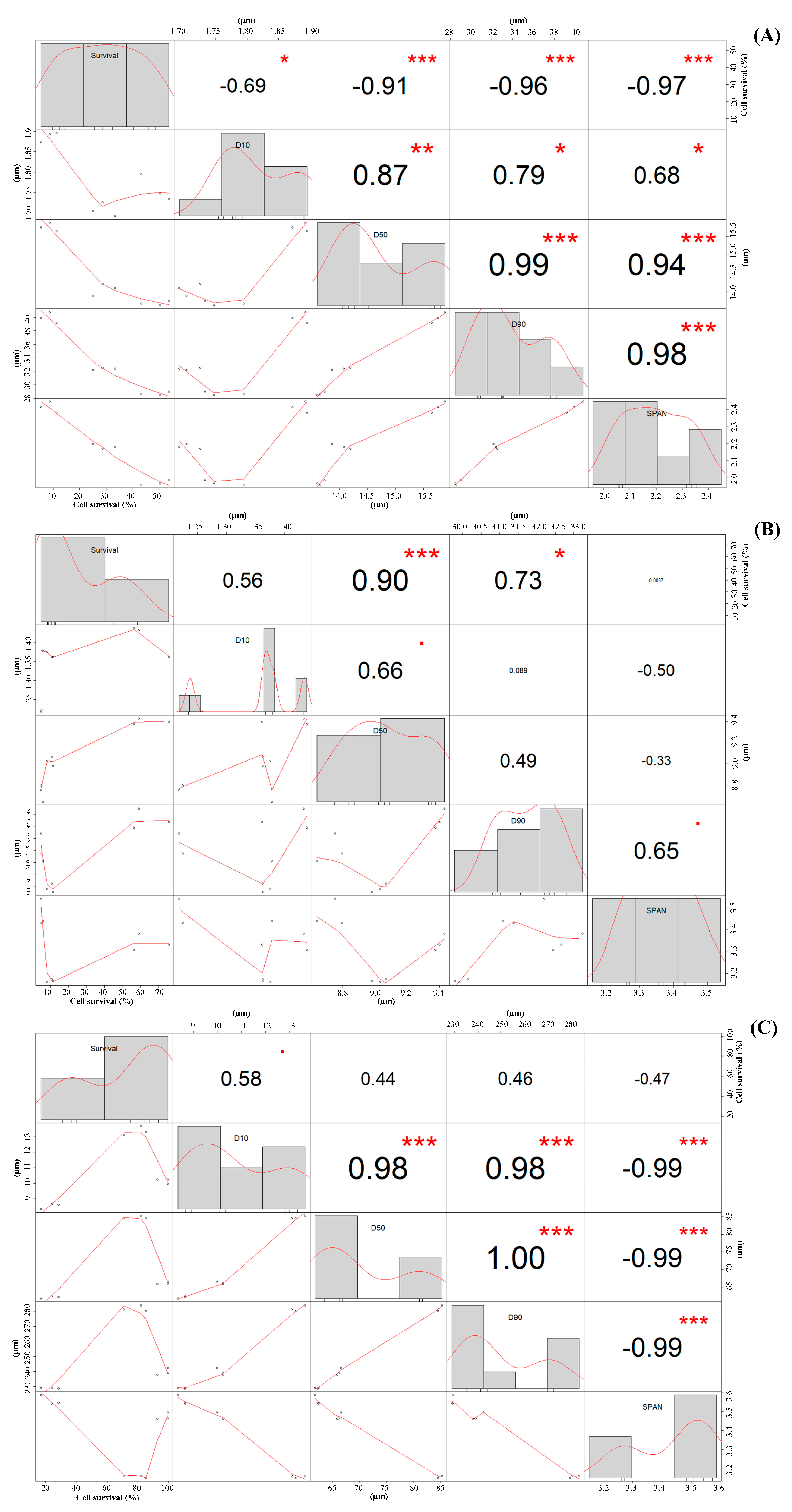
2.3.2. Correlation between Particle Size of LP Powders and Cell Survival
2.4. Morphology of LP Powders
3. Materials and Methods
3.1. Materials
3.2. Preparation of LP Cultures
3.3. Preparation of LP Dispersions
3.4. Drying of LP Dispersions
3.5. Enumeration of LP Cultures
3.6. Physical Properties of LP Powders
3.6.1. Moisture Content and Water Activity (aw)
3.6.2. Particle Size Distribution
3.6.3. SEM
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- Mermelstein, N.H. Probing probiotics. Food Technol. 2019, 73, 82–84. [Google Scholar]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. Lwt-Food Sci. Technol. 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Jain, S.; Yadav, H.; Sinha, P.R.; Naito, Y.; Marotta, F. Dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei has a protective effect against Salmonella enteritidis infection in mice. Int. J. Immunopathol. Pharmacol. 2008, 21, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Mis Solval, K.; Chouljenko, A.; Chotiko, A.; Sathivel, S. Growth kinetics and lactic acid production of Lactobacillus plantarum NRRL B-4496, L. acidophilus NRRL B-4495, and L. reuteri B-14171 in media containing egg white hydrolysates. Lwt-Food Sci. Technol. 2019, 105, 393–399. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef]
- Meng, X.C.; Stanton, C.; Fitzgerald, G.F.; Daly, C.; Ross, R.P. Anhydrobiotics: The challenges of drying probiotic cultures. Food Chem. 2008, 106, 1406–1416. [Google Scholar] [CrossRef]
- Dianawati, D.; Mishra, V.; Shah, N.P. Stability of microencapsulated Lactobacillus acidophilus and Lactococcus lactis ssp. cremoris during storage at room temperature at low aw. Food Res. Int. 2013, 50, 259–265. [Google Scholar] [CrossRef]
- Paéz, R.; Lavari, L.; Vinderola, G.; Audero, G.; Cuatrin, A.; Zaritzky, N.; Reinheimer, J. Effect of heat treatment and spray drying on lactobacilli viability and resistance to simulated gastrointestinal digestion. Food Res. Int. 2012, 48, 748–754. [Google Scholar] [CrossRef]
- FDA. 21 Code of Federal Regulations. 2018. Available online: https://www.ecfr.gov/cgi-bin/textidx?SID=a6f2c4f5c13000a42ea50653bd22687d&mc=true&tpl=/ecfrbrowse/Title21/21tab_02.tpl (accessed on 15 July 2020).
- Karimi, R.; Mortazavian, A.M.; Da Cruz, A.G. Viability of probiotic microorganisms in cheese during production and storage: A review. Dairy Sci. Technol. 2011, 91, 283–308. [Google Scholar] [CrossRef]
- Maciel, G.M.; Chaves, K.S.; Grosso, C.R.F.; Gigante, M.L. Microencapsulation of Lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J. Dairy Sci. 2014, 97, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- García, A.H. Anhydrobiosis in bacteria: From physiology to applications. J. Biosci. 2011, 36, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Santivarangkna, C.; Higl, B.; Foerst, P. Protection mechanisms of sugars during different stages of preparation process of dried lactic acid starter cultures. Food Microbiol. 2008, 25, 429–441. [Google Scholar] [CrossRef]
- Ananta, E.; Volkert, M.; Knorr, D. Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int. Dairy J. 2005, 15, 399–409. [Google Scholar] [CrossRef]
- Gul, O. Microencapsulation of Lactobacillus casei Shirota by spray drying using different combinations of wall materials and application for probiotic dairy dessert. J. Food Process. Preserv. 2017, 41, e13198. [Google Scholar] [CrossRef]
- Golowczyc, M.A.; Silva, J.; Abraham, A.G.; De Antoni, G.L.; Teixeira, P. Preservation of probiotic strains isolated from kefir by spray drying. Lett. Appl. Microbiol. 2010, 50, 7–12. [Google Scholar] [CrossRef]
- Jiang, N.; Dev Kumar, G.; Chen, J.; Mishra, A.; Mis Solval, K. Comparison of concurrent and mixed-flow spray drying on viability, growth kinetics and biofilm formation of Lactobacillus rhamnosus GG microencapsulated with fish gelatin and maltodextrin. Lwt-Food Sci. Technol. 2020, 124, 109200. [Google Scholar] [CrossRef]
- Patrignani, F.; Lanciotti, R. Applications of high and ultra high pressure homogenization for food safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef]
- Paquin, P. Technological properties of high pressure homogenizers: The effect of fat globules, milk proteins, and polysaccharides. Int. Dairy J. 1999, 9, 329–335. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Jiang, L.; Regenstein, J.M.; Jiang, N.; Poias, V.; Zhang, X.; Qi, B.; Li, A.; Wang, Z. Interaction of soybean protein isolate and phosphatidylcholine in nanoemulsions: A fluorescence analysis. Food Hydrocoll. 2019, 87, 814–829. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Singh, R.K. Ultra high pressure homogenization of soy milk: Effect on quality attributes during storage. Beverages 2016, 2, 15. [Google Scholar] [CrossRef]
- Cruz, N.; Capellas, M.; Hernández, M.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Ultra high pressure homogenization of soymilk: Microbiological, physicochemical and microstructural characteristics. Food Res. Int. 2007, 40, 725–732. [Google Scholar] [CrossRef]
- Hebishy, E.; Zamora, A.; Buffa, M.; Blasco-Moreno, A.; Trujillo, A.-J. Characterization of whey protein oil-in-water emulsions with different oil concentrations stabilized by ultra-high pressure homogenization. Processes 2017, 5, 6. [Google Scholar] [CrossRef]
- Sandra, S.; Dalgleish, D.G. Effects of ultra-high-pressure homogenization and heating on structural properties of casein micelles in reconstituted skim milk powder. Int. Dairy J. 2005, 15, 1095–1104. [Google Scholar] [CrossRef]
- Benzaria, A.; Maresca, M.; Taieb, N.; Dumay, E. Interaction of curcumin with phosphocasein micelles processed or not by dynamic high-pressure. Food Chem. 2013, 138, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Chevalier-Lucia, D.; Blayo, C.; Gràcia-Julià, A.; Picart-Palmade, L.; Dumay, E. Processing of phosphocasein dispersions by dynamic high pressure: Effects on the dispersion physico-chemical characteristics and the binding of α-tocopherol acetate to casein micelles. Innov. Food Sci. Emerg. Technol. 2011, 12, 416–425. [Google Scholar] [CrossRef]
- Burns, P.; Patrignani, F.; Serrazanetti, D.; Vinderola, G.C.; Reinheimer, J.A.; Lanciotti, R.; Guerzoni, M.E. Probiotic crescenza cheese containing Lactobacillus casei and Lactobacillus acidophilus manufactured with high-pressure homogenized milk. J. Dairy Sci. 2008, 91, 500–512. [Google Scholar] [CrossRef]
- Mis-Solval, K.; Jiang, N.; Yuan, M.; Joo, K.H.; Cavender, G. The effect of the ultra-high-pressure homogenization of protein encapsulants on the survivability of probiotic cultures after spray drying. Foods 2019, 8, 689. [Google Scholar] [CrossRef]
- Ying, D.; Sun, J.; Sanguansri, L.; Weerakkody, R.; Augustin, M.A. Enhanced survival of spray-dried microencapsulated Lactobacillus rhamnosus GG in the presence of glucose. J. Food Eng. 2012, 109, 597–602. [Google Scholar] [CrossRef]
- Desrumaux, A.; Marcand, J. Formation of sunflower oil emulsions stabilized by whey proteins with high-pressure homogenization (up to 350 MPa): Effect of pressure on emulsion characteristics. Int. J. Food Sci. Technol. 2002, 37, 263–269. [Google Scholar] [CrossRef]
- Hayes, M.G.; Kelly, A.L. High pressure homogenisation of raw whole bovine milk (a) effects on fat globule size and other properties. J. Dairy Res. 2003, 70, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, A.J.; Roig-Sagués, A.X.; Zamora, A.; Ferragut, V. 12-High-Pressure Homogenization for Structure Modification. In Innovative Food Processing Technologies; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 315–344. [Google Scholar]
- McMahon, D.J.; Oommen, B.S. Casein micelle structure, functions, and interactions. In Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects, 4th ed.; McSweeney, P.L.H., Fox, P.F., Eds.; Springer US: Boston, MA, USA, 2013; pp. 185–209. [Google Scholar]
- Corzo-Martínez, M.; Mohan, M.; Dunlap, J.; Harte, F. Effect of ultra-high pressure homogenization on the interaction between bovine casein micelles and ritonavir. Pharm. Res. 2015, 32, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Roach, A.; Dunlap, J.; Harte, F. Association of triclosan to casein proteins through solvent-mediated high-pressure homogenization. J. Food Sci. 2009, 74, N23–N29. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, B.M.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J. Appl. Microbiol. 2004, 96, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Chen, X.D. Towards a maximal cell survival in convective thermal drying processes. Food Res. Int. 2011, 44, 1127–1149. [Google Scholar] [CrossRef]
- Anekella, K.; Orsat, V. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. Lwt-Food Sci. Technol. 2013, 50, 17–24. [Google Scholar] [CrossRef]
- Perdana, J.; Bereschenko, L.; Fox, M.B.; Kuperus, J.H.; Kleerebezem, M.; Boom, R.M.; Schutyser, M.A. Dehydration and thermal inactivation of Lactobacillus plantarum WCFS1: Comparing single droplet drying to spray and freeze drying. Food Res. Int. 2013, 54, 1351–1359. [Google Scholar] [CrossRef]
- Abe, F.; Miyauchi, H.; Uchijima, A.; Yaeshima, T.; Iwatsuki, K. Effects of storage temperature and water activity on the survival of bifidobacteria in powder form. Int. J. Dairy Technol. 2009, 62, 234–239. [Google Scholar] [CrossRef]
- Chávez, B.E.; Ledeboer, A.M. Drying of Probiotics: Optimization of Formulation and Process to Enhance Storage Survival. Dry. Technol. 2007, 25, 1193–1201. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. Process Intensif. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Avila-Reyes, S.V.; Garcia-Suarez, F.J.; Jiménez, M.T.; San Martín-Gonzalez, M.F.; Bello-Perez, L.A. Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the viability. Carbohydr. Polym. 2014, 102, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire Djantou, E.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Pinto, M.; Kemp, I.; Bermingham, S.; Hartwig, T.; Bisten, A. Development of an axisymmetric population balance model for spray drying and validation against experimental data and CFD simulations. Chem. Eng. Res. Des. 2014, 92, 619–634. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray drying technique. I: Hardware and process parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Donsì, F.; Wang, Y.; Li, J.; Huang, Q. Preparation of curcumin sub-micrometer dispersions by high-pressure homogenization. J. Agric. Food Chem. 2010, 58, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Müller, R.H. Spray-drying of solid lipid nanoparticles (SLNTM). Eur. J. Pharm. Biopharm. 1998, 46, 145–151. [Google Scholar] [CrossRef]
- Paredes, A.J.; Llabot, J.M.; Sánchez Bruni, S.; Allemandi, D.; Palma, S.D. Self-dispersible nanocrystals of albendazole produced by high pressure homogenization and spray-drying. Drug Dev. Ind. Pharm. 2016, 42, 1564–1570. [Google Scholar] [CrossRef]
- Würth, R.; Foerst, P.; Kulozik, U. Effects of skim milk concentrate dry matter and spray drying air temperature on formation of capsules with varying particle size and the survival microbial cultures in a microcapsule matrix. Dry. Technol. 2018, 36, 93–99. [Google Scholar] [CrossRef]
- Alves, N.N.; Messaoud, G.B.; Desobry, S.; Costa, J.M.C.; Rodrigues, S. Effect of drying technique and feed flow rate on bacterial survival and physicochemical properties of a non-dairy fermented probiotic juice powder. J. Food Eng. 2016, 189, 45–54. [Google Scholar] [CrossRef]
- Walton, D.E. The morphology of spray-dried particles a qualitative view. Dry. Technol. 2000, 18, 1943–1986. [Google Scholar] [CrossRef]
- Vergara, C.; Saavedra, J.; Sáenz, C.; García, P.; Robert, P. Microencapsulation of pulp and ultrafiltered cactus pear (Opuntia ficus-indica) extracts and betanin stability during storage. Food Chem. 2014, 157, 246–251. [Google Scholar] [CrossRef] [PubMed]
- USDA-ARS. Lactobacillus plantarum NRRL B-1927; USDA-ARS: Peoria, IL, USA, 2019. [Google Scholar]
- Mis Solval, K.; Bankston, J.D.; Bechtel, P.J.; Sathivel, S. Physicochemical Properties of Microencapsulated ω-3 Salmon Oil with Egg White Powder. J. Food Sci. 2016, 81, E600–E609. [Google Scholar] [CrossRef] [PubMed]
- Donhowe, E.G.; Flores, F.P.; Kerr, W.L.; Wicker, L.; Kong, F. Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. Lwt-Food Sci. Technol. 2014, 57, 42–48. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| UHPH | Drying Method | Log CFU/g Solids | Log Reduction | |
|---|---|---|---|---|
| Before Drying | After Drying | |||
| 0 MPa | CCSD | 8.81 ± 0.08 | 8.27 ± 0.06 *** | 0.54 ± 0.06 c |
| MXSD | 9.23 ± 0.16 | 7.95 ± 0.04 *** | 1.28 ± 0.04 a | |
| FD | 9.23 ± 0.16 | 8.58 ± 0.11 ** | 0.65 ± 0.11 bc | |
| 150 MPa | CCSD | 9.07 ± 0.05 | 8.76 ± 0.05 *** | 0.31 ± 0.05 d |
| MXSD | 9.07 ± 0.05 | 8.87 ± 0.07 ** | 0.20 ± 0.07 de | |
| FD | 9.07 ± 0.05 | 9.06 ± 0.02 | 0.01 ± 0.02 f | |
| 300 MPa | CCSD | 9.28 ± 0.14 | 8.19 ± 0.16 *** | 1.09 ± 0.16 ab |
| MXSD | 9.28 ± 0.14 | 8.28 ± 0.08 *** | 1.00 ± 0.08 ab | |
| FD | 9.28 ± 0.14 | 9.18 ± 0.04 | 0.10 ± 0.04 e | |
| UHPH | Drying Method | Moisture (g/100 g, w. b.) | aw |
|---|---|---|---|
| (Non-treated) 0 MPa | CCSD | 3.27 ± 0.08 c | 0.28 ± 0.01 b |
| MXSD | 4.16 ± 0.04 b | 0.33 ± 0.02 a | |
| FD | 6.69 ± 0.06 a | 0.13 ± 0.01 e | |
| 150 MPa | CCSD | 2.17 ± 0.14 e | 0.24 ± 0.00 c |
| MXSD | 3.38 ± 0.03 c | 0.32 ± 0.02 a | |
| FD | 0.88 ± 0.04 g | 0.06 ± 0.01 f | |
| 300 MPa | CCSD | 2.46 ± 0.07 d | 0.23 ± 0.00 c |
| MXSD | 3.23 ± 0.05 c | 0.25 ± 0.00 bc | |
| FD | 1.36 ± 0.19 f | 0.17 ± 0.00 d |
| UHPH | Drying Method | Particle Size Distribution Values | |||
|---|---|---|---|---|---|
| D10 (µm) | D50 (µm) | D90 (µm) | Span | ||
| Non-treated (0 Mpa) | CCSD | 1.71 ± 0.02 def | 14.05 ± 0.16 e | 32.36 ± 0.17 e | 2.18 ± 0.01 f |
| MXSD | 1.27 ± 0.09 g | 8.73 ± 0.08 g | 31.56 ± 0.58 ef | 3.47 ± 0.06 b | |
| FD | 8.55 ± 0.17 c | 62.04 ± 0.32 c | 229.12 ± 0.27 c | 3.56 ± 0.02 a | |
| 150 Mpa | CCSD | 1.76 ± 0.03 de | 13.66 ± 0.07 e | 28.68 ± 0.27 f | 1.97 ± 0.01 g |
| MXSD | 1.41 ± 0.04 efg | 9.40 ± 0.03 f | 32.78 ± 0.41 e | 3.34 ± 0.04 c | |
| FD | 10.15 ± 0.15 b | 66.12 ± 0.36 b | 239.73 ± 2.37 b | 3.47 ± 0.02 ab | |
| 300 Mpa | CCSD | 1.89 ± 0.01 d | 15.76 ± 0.12 d | 39.95 ± 0.79 d | 2.42 ± 0.03 e |
| MXSD | 1.37 ± 0.01 fg | 9.03 ± 0.04 fg | 29.94 ± 018 ef | 3.17 ± 0.01 d | |
| FD | 13.34 ± 0.28 a | 84.88 ± 0.42 a | 281.71 ± 1.99 a | 3.16 ± 0.01 d | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mis Solval, K.E.; Cavender, G.; Jiang, N.; Chen, J.; Singh, R. Microencapsulation of Lactobacillus plantarum NRRL B-1927 with Skim Milk Processed via Ultra-High-Pressure Homogenization. Molecules 2020, 25, 3863. https://doi.org/10.3390/molecules25173863
Mis Solval KE, Cavender G, Jiang N, Chen J, Singh R. Microencapsulation of Lactobacillus plantarum NRRL B-1927 with Skim Milk Processed via Ultra-High-Pressure Homogenization. Molecules. 2020; 25(17):3863. https://doi.org/10.3390/molecules25173863
Chicago/Turabian StyleMis Solval, Kevin E., George Cavender, Nan Jiang, Jinru Chen, and Rakesh Singh. 2020. "Microencapsulation of Lactobacillus plantarum NRRL B-1927 with Skim Milk Processed via Ultra-High-Pressure Homogenization" Molecules 25, no. 17: 3863. https://doi.org/10.3390/molecules25173863
APA StyleMis Solval, K. E., Cavender, G., Jiang, N., Chen, J., & Singh, R. (2020). Microencapsulation of Lactobacillus plantarum NRRL B-1927 with Skim Milk Processed via Ultra-High-Pressure Homogenization. Molecules, 25(17), 3863. https://doi.org/10.3390/molecules25173863