1. Introduction
A recently recognized disease called grapevine red blotch disease (GRBD) is of great concern among grape growers, winemakers, and researchers [
1]. Grapevine red blotch virus (GRBV), the causal agent of GRBD, was classified as a member of the
Geminiviridae family, and its presence has been confirmed in the United States, Canada, Mexico, South Korea, and Switzerland [
2,
3,
4,
5,
6]. More recently, it was found that the three-cornered alfalfa hopper (
Spissistilus festinus Say) is a vector of GRBV under laboratory conditions [
7].
Due to its recent discovery, only a small body of research regarding the impact of GRBD on grape and wine is available. The virus can impact grapevine foliar metabolism in Cabernet Franc and Cabernet Sauvignon [
8] and affect ripening in Zinfandel grapes by suppressing specific ripening events, altering the expression patterns of transcription factors and causing hormonal imbalances [
9,
10]. Fruit composition is an important factor for wine quality, which is developed during the maturation of berries and tracked by viticulturists and winemakers. Traditional fruit maturation parameters are total soluble solids (TSS), mostly sugars measured as Brix, and organic acid concentration measured as titratable acidity (TA). Additionally, the grape phenolic composition is known to have an important impact on wine [
11,
12,
13] and these parameters are affected by the presence of GRBV [
14,
15,
16]. Sugar accumulation was reduced in GRBV-infected Cabernet Sauvignon, Cabernet Franc, and Merlot grapevines compared to healthy controls [
1,
17]. Berry sugar concentration influences wine style due to its conversion in ethanol during alcoholic fermentation. Previous research has shown that ethanol facilitates extraction of phenolic compounds, such as proanthocyanidin (PA) during alcoholic fermentation [
18], and impacts the adsorption/desorption interactions between anthocyanins and solids present in wine during fermentation, such as grape cell walls, then affecting final anthocyanin concentration in wines [
19]. In addition, ethanol has a direct impact on the sensorial perception of wine aroma, taste, and mouthfeel attributes [
20].
Phenolic compounds present in the grape berry, such as flavan-3-ols, flavonols, hydroxycinnamic acids, anthocyanins, and PA, play important roles in fruit and wine quality. PA (also called condensed tannins) are oligomeric and polymeric flavan-3-ols present in grape seeds and skins and are of viticultural interest because they are the main component of astringency in red wines [
21]. The second most abundant class of phenolics in red wines, anthocyanins, are responsible for the color in red grapes and wines. Anthocyanin concentration at harvest is an important factor and can influence grape prices and winemaking decisions [
22]. In addition, hydroxycinnamic acids are usually the most abundant class of phenolics in free-run juice and, consequently, in white wines [
12]. Flavonols are less abundant, but they play an essential role in the color stability of wines through co-pigmentation with anthocyanins [
23].
Studies demonstrated that phenolic compounds present in grape berries may be affected by grapevine viral diseases, thus leading to changes in wine’s sensory attributes. For example, Merlot wines made from fruit infected with grapevine leafroll disease (GLD) presented significant decreases in anthocyanin and PA concentrations [
24]. Descriptive analysis (DA) on wines made with grapes from GLD-infected and healthy grapevines demonstrated a significant impact on the perceived color, aroma, and astringency of the wines due to disease status [
24]. However, the impacts of GRBD on wine composition and sensory properties are still unknown.
In this study, the impacts of GRBD on wine composition and sensory properties were investigated in two grapevine cultivars (Cabernet Sauvignon and Merlot) across three vineyards over two seasons. The goal was to determine potential trends due to GRBD on wine composition across site and season, and this is the follow-up study of a recently published paper regarding the impacts of GRBD on grape composition [
16].
3. Discussion
Grapes from diseased grapevines had lower sugar content when compared to healthy grapes as a result of delayed grape ripening due to GRBV infection as demonstrated in previous studies [
10,
15,
16] which resulted in RB (+) wines with lower ethanol content when compared to RB (−) wines. However, the extent of the disease’s impact on sugar accumulation was site and seasonal dependent. A larger difference was observed between RB (−) and RB (+) wines for CS1 in comparison to CS2 and ME sites in the 2014 season. In 2015, the difference between CS2 RB (−) and CS2 RB (+) wines was more significant when compared to the previous year (2014), which suggests that the impact of GRBD is variable depending on the season and thus environmental influences. The 2015 season was warmer than 2014 (
Supplemental Table S1) indicating that temperature may influence the impact of GRBD on grapevines. Previous work has demonstrated that plant defense mechanisms, such as short-interfering RNAs (siRNAs), against geminiviruses infection are influenced by temperature and can significantly reduce symptom severity at 25 °C when compared to 30 °C in cassava (
Manihot esculenta, Crantz) and tobacco (
Nicotiana benthamiana) [
27]. Therefore, we suggest that due to the warmer 2015 season, the impact of GRBD was greater than in 2014, resulting in larger differences between RB (−) and RB (+) wines in 2015.
The higher pH in CS2 RB (−) wines for both years studied may be partially explained by the potassium (K) content, which is known to be positively correlated to pH in wines [
28]. Unfortunately, K concentrations were not measured in RB (−) and RB (+) wines. However, an overall grape sample taken from CS2 vineyard at harvest in 2015 demonstrated that RB (−) and RB (+) grapes had 2110 mg/L and 1970 mg/L of K, respectively. With respect to wine acidity, even though TA was adjusted prior to fermentation, CS2 RB (−) and CS2 RB (+) wines were significantly different in 2014 and 2015, which also may have contributed to the higher pH values in CS2 RB (−) wines when compared to CS2 RB (+) wines (
Table 2). However, this difference was small enough that it will not impact the wines’ sensory perception [
29].
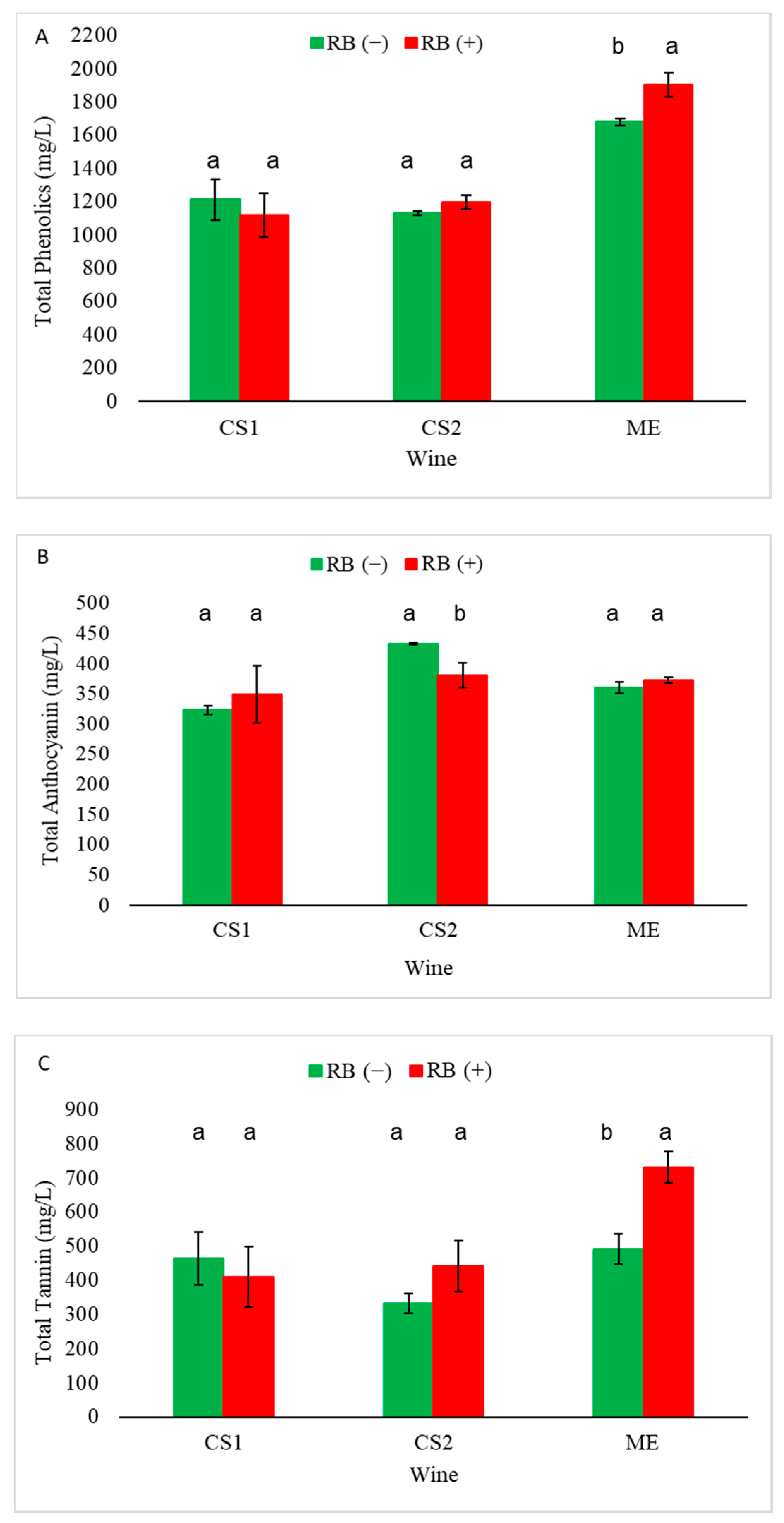
Differences in phenolic composition between RB (−) and RB (+) wines varied depending on the site and season. In 2014, the impact of GRBD depended on the site with only CS2 showing a significant decrease in anthocyanin concentration in RB (+) wines compared to RB (−) wines. Additionally, CS1 and CS2 exhibited significant decreases in polymeric phenols (tannin) due to RB disease status whereas ME was not impacted for that variable. GRBD did decrease anthocyanin concentration in the CS2 wines in both seasons studied. However, catechin and total flavan-3-ols were only significantly decreased in CS2 RB (+) wines in one season.
On the other hand, polymeric pigment concentrations were shown to be decreased in most of RB (+) wines (except for ME RB (+) wines (
Table 3). It is known that polymeric pigments are important for wine aging due to its role in color stability, which can account for 50% of color density within the first year of aging [
30]. Therefore, results found in this study suggest that wines made from RB (+) grapes may have lower color intensity over time than those made from RB (−) grapes due to their decreased anthocyanin and polymeric pigment concentrations.
The higher concentration of flavonols in CS2 RB (+) wines in 2014 compared to RB (−) wines could be due to either alteration in grape berry biochemistry due to GRBV infection or due to the reduced vigor of diseased grapevines, which increased sun exposure on RB (+) grape berries. It has been demonstrated that viral diseases can reduce grapevine vigor and its normal growth [
31] and studies have shown that the biosynthesis of flavonols in grape berries is highly dependent on sun exposure [
12]. In addition, it was found that GRBD decreased pruning weight in CS and shoot length in ME vines [
1,
17]. Therefore, it is possible that a decrease in leaf area and canopy volume, resulting in higher sun exposure of clusters from RB (+) grapes could partially explain these results. It has been demonstrated that CS grapes infected with GLRaV-3 had a higher content of quercetin (one of the most abundant flavonols in grapes) when compared to grapes from non-infected vines [
32].
It is important to note that the extractability of phenolic compounds from grapes into wine is not easy to predict. Many factors influence the extraction of phenolics from grape skins and seeds during winemaking, such as the degree of maturity in which the grapes were harvested, maceration conditions, and ethanol produced during fermentation [
11,
33,
34,
35]. After extraction, phenolic compounds may adsorb to yeast and grape berry cell walls, which can impact their final concentration in the wine [
36].
Vitis vinifera cultivars vary regarding their cell wall composition and morphology, and, consequently, the extractability of phenolic compounds may be different [
37,
38]. At present, the impact of GRBD on the composition and morphology of skins cell wall, where most of the phenolic compounds of winemaking interest are located, is not known.
One of the most important factors responsible for PA extraction during winemaking is ethanol concentration. It has been found that higher ethanol production in wines during fermentation increases the extraction of skin and seed PA significantly [
18]. However, RB (+) wines consistently contained lower ethanol concentrations when compared to RB (−) wines due to delayed ripening in RB (+) grapes. All other winemaking parameters that could impact phenolic extractability, such as maceration and pump-over regime, were standardized among different treatments. Therefore, the higher PA concentration in RB (+) wines compared to RB (−) wines was related to PA content in the grape berries due to GRBV infection. Plants have developed defense mechanisms under abiotic and biotic stresses, such as viral infections. Flavonoid biosynthesis is often enhanced under the influence of several types of stress, such as pathogens [
39]. Additionally, it has been shown that Merlot grapevines infected by GLRaV-3 had higher PA content in leaves when compared to leaves from healthy vines [
40]. The impacts of GRBD on grape composition were studied on the grapes used to produce the wines analyzed in the current study [
16]. It was found that skins from RB (+) berries had a higher concentration of PA when compared to RB (−) berries. It is well-documented that PA extractability is higher from skins than seeds during red wine fermentation [
41], especially when no extended maceration is employed. Therefore, the higher concentration of PA in RB (+) compared to RB (−) wines is likely due to increased synthesis of PA in grape skins in response to stress caused by GRBV infection. Tannins are known to be part of the defense mechanism in plants [
39].
Studies performed on
Vitis vinifera L. cv. Zinfandel grapes during one season showed that GRBV was able to inhibit ripening-associated pathways, such as a reduced metabolic flux in the central and peripheral phenylpropanoid pathways, impacting the expression of transcription factors that are part of the biosynthesis of anthocyanins [
9]. However, our results demonstrated that the impact of GRBD on wine phenolic composition is variable and depended on site and seasonal factors. Similarly to GRBD, previous studies on grapevine leafroll disease showed a seasonal impact on the effect of the disease on both basic chemical and phenolic composition of grapes and wines [
24]. In addition, it is known that mineral nutrition, yield, vigor, stage of development, environmental conditions, and biotic and abiotic factors have a strong impact on grape phenolic accumulation and composition [
42].
As demonstrated in this study, RB (−) grapes consistently had higher sugar content than RB (+) grapes. It has been shown that increasing must sugar concentration as a result of advanced grape maturity resulted in significant effects on yeast metabolism during fermentation. As the production of ethanol by yeast increased, there were concomitant increases in most yeast-derived metabolites [
43]. Amino acid levels were shown to be strongly impacted by GRBD. Amino acids present in wine originate from different sources: those indigenous to the grape that can be partially or entirely metabolized by yeasts during fermentation; and those excreted by live yeasts at the end of fermentation or released by proteolysis during the autolysis of dead yeasts [
44]. In both cases in which ethanol content differences were the largest between RB (−) and RB (+) wines (CS1, 2014 and CS2, 2015), RB (−) wines had higher levels of specific amino acids when compared to RB (+) wines (
Table 5 and
Table 6). Yeast activity and reproduction were likely extended due to the higher sugar content at the onset of fermentation and longer fermentation (as shown in
supplemental data—Figure S1) in RB (−) wines, thus resulting in higher biosynthesis and later release of amino acids during yeast autolysis. Amino acids, such as asparagine, beta-alanine, alanine, glutamine, threonine, glutamine, valine, and glycine, were shown to be present at a higher level in at least one of the RB (−) wines when compared to RB (+) wines. The same amino acids were shown to be released during yeast autolysis in model wine [
45].
In general, RB (−) wines contained higher levels of monosaccharides and oligosaccharides compared to RB (+) wines. For instance, trehalose is known for its role as a reserve carbohydrate in yeast, but it is also associated with the protection of cells against many environmental stressors, including ethanol stress [
46]. Therefore, it is possible that RB (−) wines contained higher levels of this compound after yeast autolysis due to the larger biomass of yeast produced during fermentation (due to higher sugar contents at the onset of fermentation in RB (−) grapes) and also due to the higher ethanol content (
Table 6), which potentially induced trehalose biosynthesis for yeast protection against ethanol. Another example is that RB (−) wines also exhibited higher levels of cellobiose compared to RB (+) wines at all the sites. Cellobiose, a disaccharide of glucose, is the repeating unit of cellulose, which is known to be part of grape berry cell walls [
47]. Previous studies have demonstrated that GRBD delays grape ripening [
1,
10,
15,
16], which potentially alters cell wall modifications in grapes. During ripening, a substantial weakening of primary cell walls and degradative changes to cell wall polysaccharides occur. These changes in cell wall architecture, combined with the increased pore size, make the cell wall a much more open structure, increasing the accessibility of enzymes responsible for cell wall degradation at later ripening stages, and decreasing limitation to cell wall disassembly [
48]. Therefore, it is possible that RB (−) wines had higher levels of cellobiose than RB (+) wines due to the advanced stage of ripening of RB (−) grapes, which may have facilitated cellulose hydrolysis during fermentation.
The delayed grape ripening caused by GRBD could also potentially impact polysaccharide content in wines. It has been shown that wines made with grapes harvested at seven weeks after veraison had higher amounts of polysaccharides than wines made with grapes harvested three weeks after veraison [
49]. One hypothesis is that because GRBD delays grape ripening, lower levels of polysaccharides are being released into the wine during fermentation/maceration. Since part of the monosaccharides and oligosaccharides in wines originate from hydrolyzed polysaccharides during fermentation and aging, RB (+) wines ended up, in general, with lower levels of monosaccharides and oligosaccharides than RB (−) wines.
No clear GRBD impact was observed for carboxylic acids in wine, with vineyard site and season having a larger influence. Many carboxylic acids present in wines, such as citric acid, succinic acid, malic acid, and pyruvic acid, are formed as products of alcoholic fermentation during the glycolysis process through the Krebs cycle, which uses hexose sugars as substrate [
13]. Thus, musts with fewer hexose sugars (mainly glucose, fructose, and galactose in grapes) at the start of fermentation could result in wines with less carboxylic acids. A trend of increased carboxylic acid levels was observed for wines made from grapes which had the largest differences between RB (−) and RB (+) must TSS and thus wine ethanol content (CS1, 2014 and CS2, 2015).
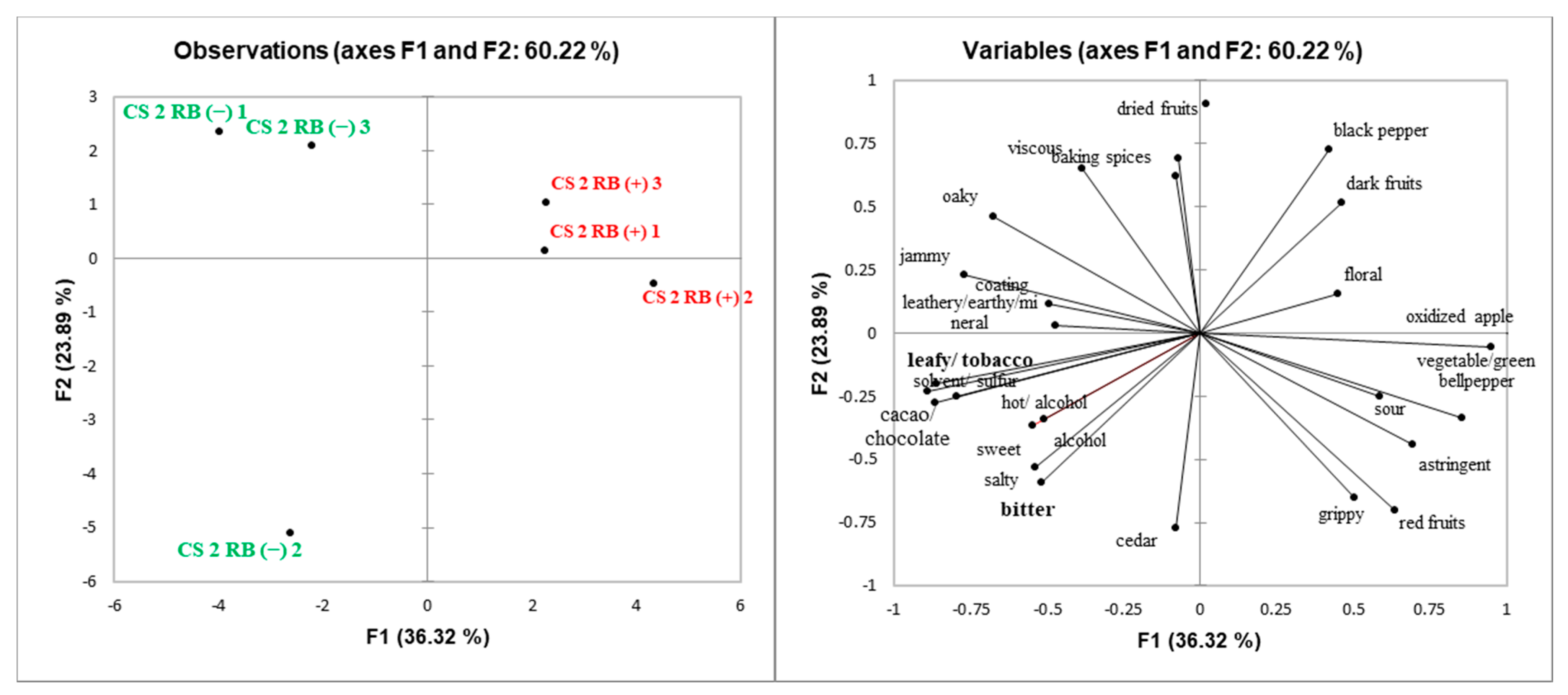
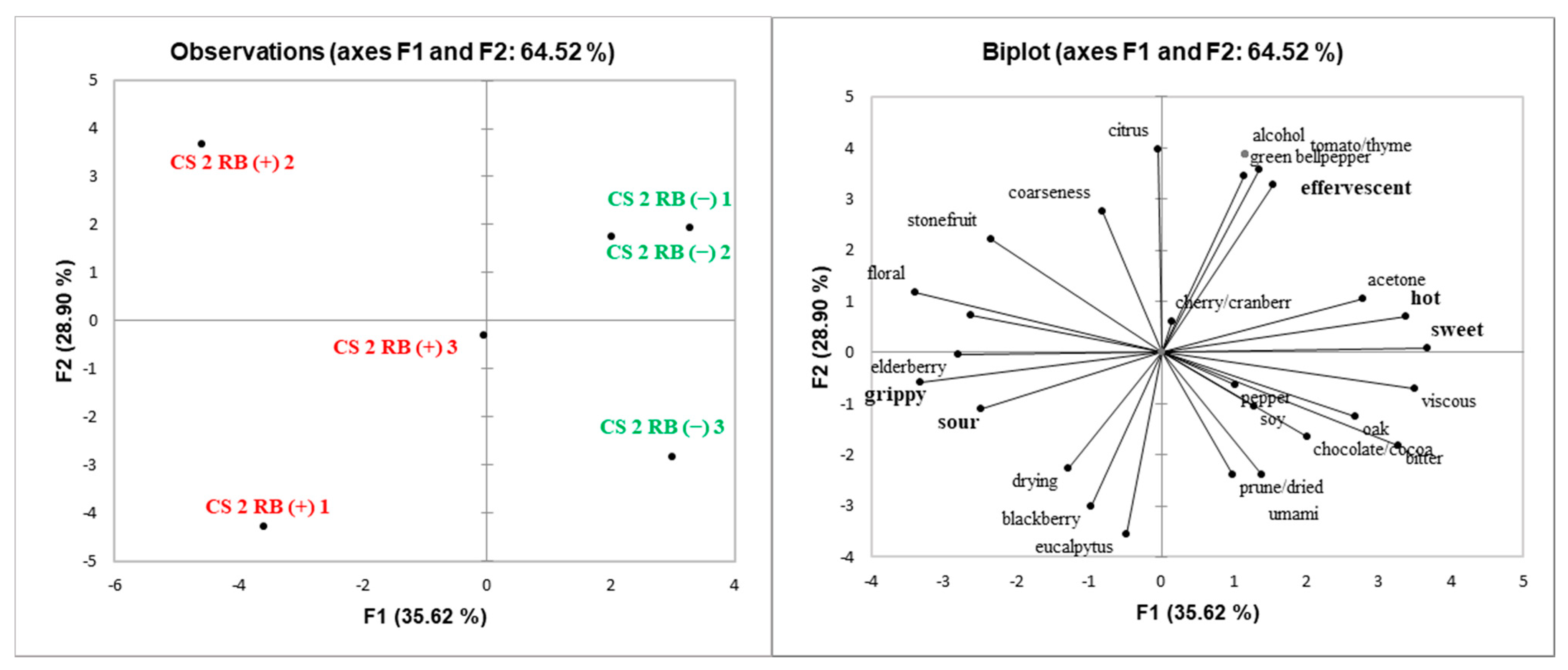
The sensorial differences between RB (−) and RB (+) wines demonstrated by DA strongly correlated with the chemical analysis of the wines. The chemical composition of CS1 wines indicates that the difference in ethanol concentration between RB (−) and RB (+) (14.6% and 13.0%, respectively—
Table 2) was large enough to be perceived by panelist regarding “alcohol” aroma and “hot” mouthfeel. In a study with Riesling wines, judges rated the attribute “heat” higher in wines with 13.6% (
v/
v) ethanol than in wines with 12.6% (
v/
v), demonstrating that a difference of 1% (
v/
v) in ethanol content in wines is large enough to be perceived by a trained consumer panel [
50]. Ethanol also has been shown to play a synergistic role with catechin, which enhanced differences between RB (−) and RB (+) wines regarding “bitter” taste. Chemical and phenolic analysis showed that CS2 RB (−) wines had higher ethanol content and catechin concentration when compared to CS2 RB (+) wines. Studies found that a higher concentration of catechin and ethanol enhances bitterness in wine [
20,
25].
GRBD had the smallest impact on ME as seen by chemical analysis of the wines (
Table 4,
Table 5,
Table 6 and
Table 7), which is reflected in the relatively small sensory differences. ME RB (+) wines were rated higher in “astringency/dry” mouthfeel compared to ME RB (−) wines, and this correlates with the higher concentration of tannins in ME RB (+) wines determined by protein precipitation assay compared to ME RB (−) wines (
Figure 1).
It has been shown that lower pH and lower ethanol concentration increases “sourness” in dealcoholized white wine concentrate [
25], which was the case of CS2 RB (+) wines in 2015. In addition, CS2 RB (+) wines were rated more “grippy”, a sub-term of astringency than RB (−) in 2015 (
Supplemental Table S2) [
50], which agrees with the higher concentration of tannin determined by the protein precipitation assay. The determination of tannin concentration in wines by protein precipitation has been shown to have the best correlation with astringency ratings in wines [
51]. CS2 RB (−) wines were also rated higher than RB (+) wines regarding “sweet” taste in 2015 (
Supplemental Table S2). This is partially explained because RB (−) wines have higher ethanol content than RB (+) wines. It has been found that alcohol enhanced the perception of sweetness in high alcohol wines when tasted after low alcohol wines [
20]. However, in this study, the wines were presented in a randomized order during the DA evaluations. Therefore, other factors may have contributed to these findings, such as the higher content of monosaccharides in CS2 RB (−) wines. The levels of seven out of 32 monosaccharides (xylulose, xylonic acid, xylitol, ribonic acid, ketohexose, galactonic acid, and fructose) were significantly higher in RB (−) when compared with RB (+) wines for CS2 (2015 season) (
Table 6). Monosaccharides have sweet detection thresholds of 10–50 mM, and the sweet taste of sugars increases the perception of wine body [
9,
13].