Royal Jelly—A Traditional and Natural Remedy for Postmenopausal Symptoms and Aging-Related Pathologies
Abstract
1. Introduction
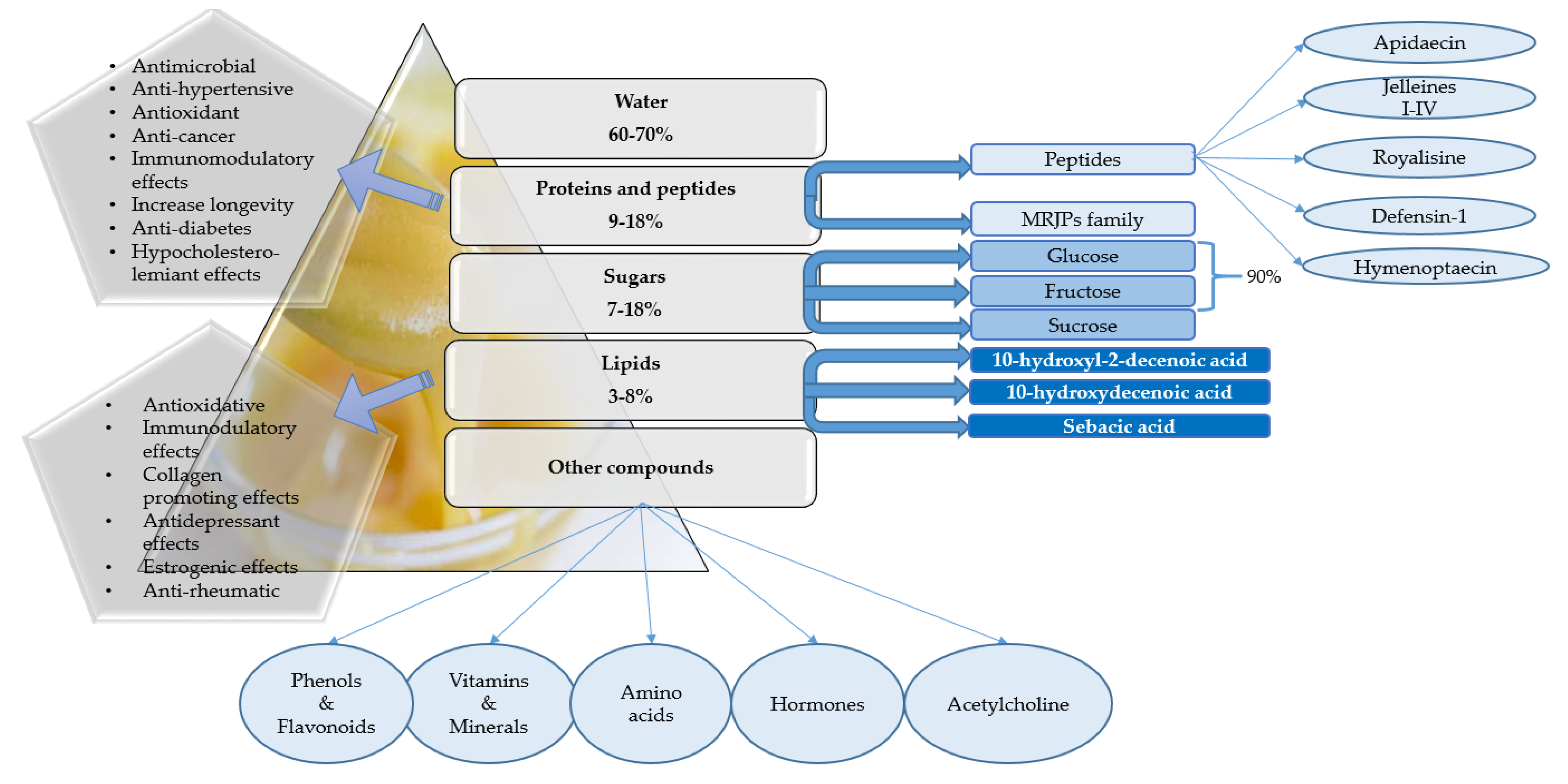
2. Chemical Composition of Royal Jelly
2.1. Proteins and Peptides
2.2. Amino Acids
2.3. Sugars
2.4. Lipids and Fatty Acids
2.5. Other Constituents: Vitamins, Minerals, Acetylcholine, Polyphenols
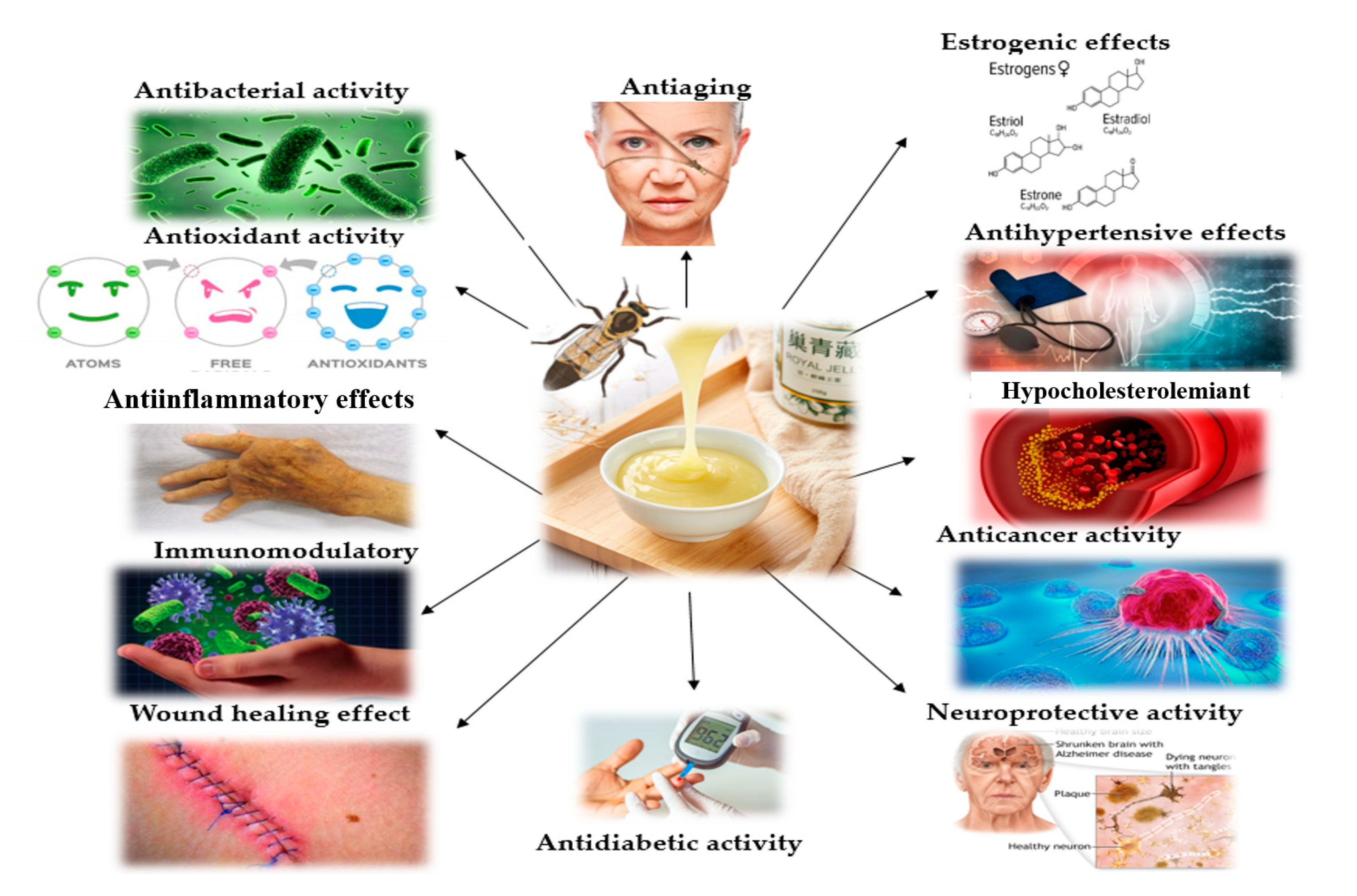
3. Studies of Royal Jelly as a Beneficial Therapeutic Agent for Postmenopausal Symptoms and Aging-Related Diseases
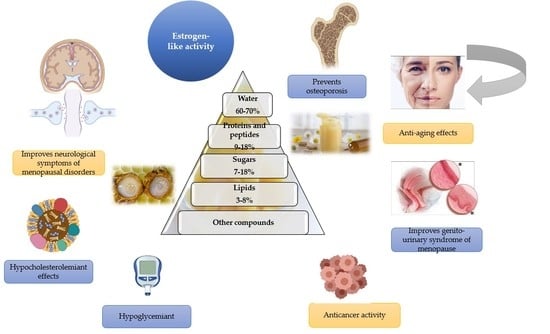
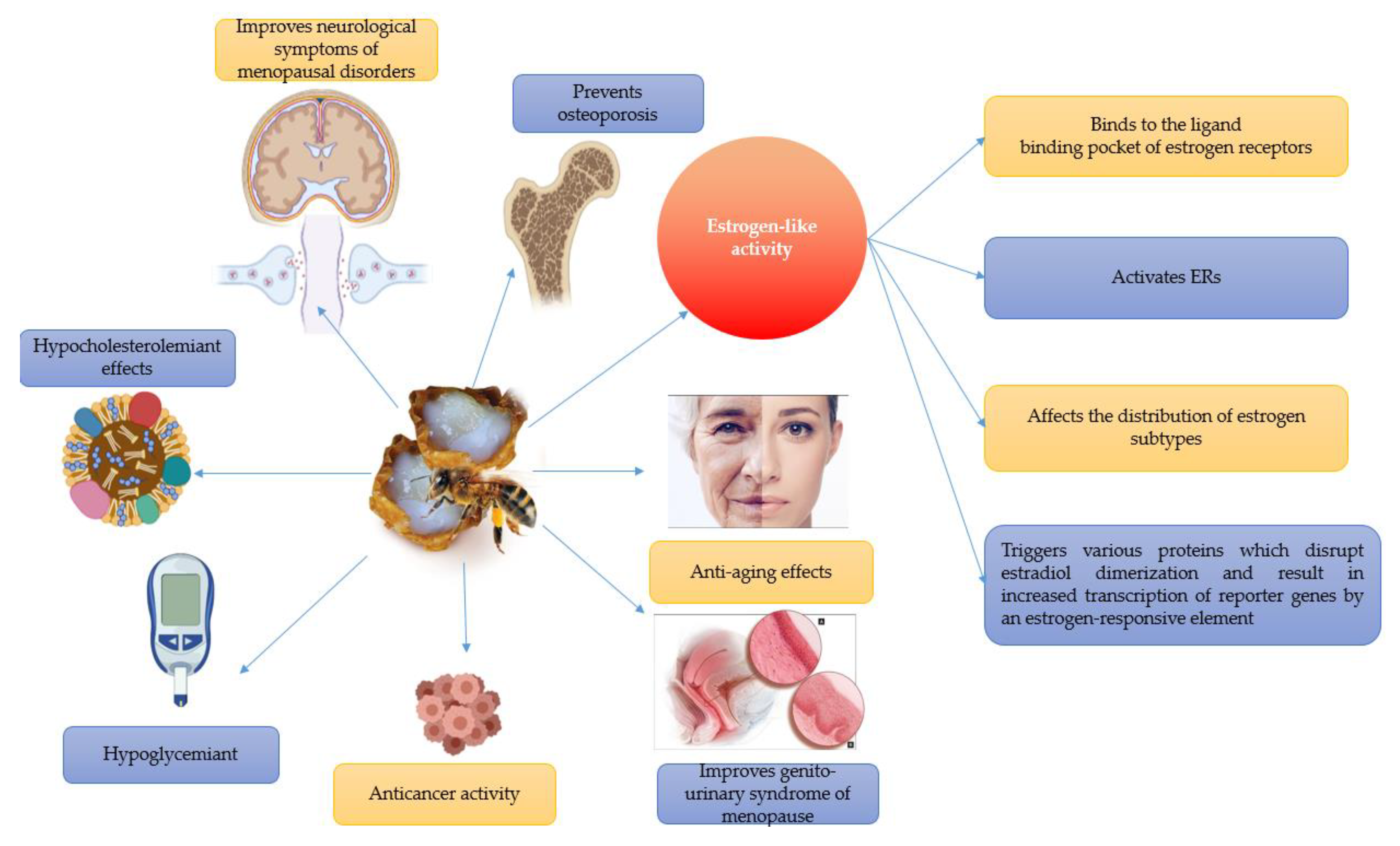
3.1. Estrogen-like Activity
3.2. Anticancer Activity
3.3. Hypocholesterolaemiant Effects
3.4. Anti-Hypertensive Effects
3.5. Effects on Bone Metabolism
3.6. Anti-Aging Effects
3.7. Neuroprotective Effects
3.8. Anti-Diabetic Effects
4. Side Effects of Royal Jelly
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- McEniery, C.M. Transitioning the Menopause. ATVB 2020, 40, 850–852. [Google Scholar] [CrossRef]
- Brzezinski, A. Menopausal symptoms: Not just estrogen deficiency. Menopause 2019, 26, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Rymer, J.; Wilson, R.; Ballard, K. Making decisions about hormone replacement therapy. BMJ 2003, 326, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.H.; Gill, S. Risks and benefits of hormone replacement therapy: The evidence speaks. Can. Med. Assoc. J. 2003, 168, 1001–1010. [Google Scholar]
- Fernandez, E.; Gallus, S.; Bosetti, C.; Franceschi, S.; Negri, E.; Vecchia, C. Hormone replacement therapy and cancer risk: A systematic analysis from a network of case-control studies. Int. J. Cancer 2003, 105, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Beck, V.; Rohr, U.; Jungbauer, A. Phytoestrogens derived from red clover: An alternative to estrogen replacement therapy? J. Steroid Biochem. Mol. Biol. 2005, 94, 499–518. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxi. Med. Cell. Long. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Li, J.; Feng, M.; Begna, D.; Fang, Y.; Zheng, A. Proteome comparison of hypopharyngeal gland development between Italian and royal jelly-producing worker honeybees (Apis mellifera L). J. Proteom. Res. 2010, 9, 6578–6594. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Cao, L.F.; Zheng, H.Q.; Pirk, C.W.; Hu, F.L.; Xu, Z.W. High Royal Jelly-Producing Honeybees (Apis mellifera ligustica) (Hymenoptera: Apidae) in China. J. Econ. Entomol. 2016, 109, 510–514. [Google Scholar] [CrossRef]
- Altaye, S.Z.; Meng, L.; Li, J. Molecular insights into the enhanced performance of royal jelly secretion by a stock of honeybee (Apis mellifera ligustica) selected for increasing royal jelly production. Apidologie 2019, 50, 436–453. [Google Scholar] [CrossRef]
- Altaye, S.Z.; Meng, L.; Lu, Y.; Li, J. The emerging proteomic research facilitates in-depth understanding of the biology of honeybees. Int. J. Mol. Sci. 2019, 20, 4252. [Google Scholar] [CrossRef] [PubMed]
- Sig, A.K.; Öz-Sığ, Ö.; Güney, M. Royal jelly: A natural therapeutic? Ortadogu Tıp Derg 2019. [Google Scholar] [CrossRef]
- Bogdanov, S. Royal Jelly, Bee Brood: Composition, Nutrition, Health. In Encyclopedia of Insects; Elesevier: Amsterdam, The Netherland, 2016. [Google Scholar]
- Kunugi, H.; Mohammed Ali, A. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed]
- Aludatt, M.; Rababah, T.; Sakandar, H.; Imran, M.; Mustafa, N.; Alhamad, M.; Mhaidat, N.; Kubow, S.; Tranchant, C.; Al Tawaha, A.R.; et al. Fermented food-derived bioactive compounds with anticarcinogenic properties: Fermented royal jelly as a novel source of compounds with health benefits. In Anticancer Plants: Properties and Application; Springer: Berlin/Heidelberg, Germany, 2018; pp. 141–165. [Google Scholar] [CrossRef]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on royal jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Khazaei, M.; Ansarian, A.; Ghanbari, E. New findings on biological actions and clinical applications of royal jelly: A review. J. Diet. Suppl. 2017, 15, 757–775. [Google Scholar] [CrossRef]
- Furusawa, T.; Rakwal, R.; Nam, H.W.; Shibato, J.; Agrawal, G.K.; Kim, Y.S.; Ogawa, Y.; Yoshida, Y.; Kouzuma, Y.; Masuo, Y.; et al. Comprehensive royal jelly (RJ) proteomics using one- and two-dimensional proteomics platforms reveals novel RJ proteins and potential phospho/glycoproteins. J. Proteome Res. 2008, 7, 3194–3229. [Google Scholar] [CrossRef]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef]
- Zhang, L.; Han, B.; Li, R.; Lu, X.; Nie, A.; Guo, L.; Fang, Y.; Feng, M.; Li, J. Comprehensive identification of novel proteins and N-glycosylation sites in royal jelly. BMC Genomics 2014, 15, 135. [Google Scholar] [CrossRef]
- Han, B.; Fang, Y.; Feng, M.; Lu, X.; Huo, X.; Meng, L.; Wu, B.; Li, J. In-depth phosphoproteomic analysis of royal jelly derived from western and eastern honeybee species. J. Proteome Res. 2014, 13, 5928–5943. [Google Scholar] [CrossRef]
- Bulet, P.; Stocklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Peptide Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wu, L.; Wang, K. Chemical composition of royal jelly. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar] [CrossRef]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R. Preparation and functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Asencot, M.; Lensky, Y. The effect of sugars and juvenile hormone on the differentiation of the female honeybee larvae (Apismellifera L.) to queens. Life Sci. 1976, 18, 693–699. [Google Scholar] [CrossRef]
- Terada, Y.; Narukawa, M.; Watanabe, T. Specific hydroxy fatty acids in royal jelly activate TRPA1. J. Agric. Food Chem. 2011, 59, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Bakier, S.; Grzech, I. Gas chromatographic–mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J. Chromat. 2012, 885–886, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Šedivá, M.; Laho, M.; Kohútová, L.; Mojžišová, A.; Majtán, J.; Klaudiny, J. 10-HDA, a major fatty acid of royal jelly, exhibits pH dependent growth-inhibitory activity against different strains of Paenibacillus larvae. Molecules 2018, 23, 3236. [Google Scholar] [CrossRef]
- Suzuki, K.-M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid. Complement. Alternat. Med. 2008, 5, 295–302. [Google Scholar] [CrossRef]
- Stocker, A.; Schramel, P.; Kettrup, A.; Bengsch, E. Trace and mineral elements in royal jelly and homeostatic effects. J. Trace Elem. Med. Biol. 2005, 19, 183–189. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. AMP N1-Oxide, a unique compound of royal jelly, induces neurite outgrowth from PC12 Vells via signaling by protein kinase a independent of that by mitogen-activated protein kinase. Evid. Complement. Alternat. Med. 2010, 7, 970174. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Mishima, S.; Inagaki, S.; Goto, M.; Sako, M.; Furukawa, S. Identification of AMP N1-oxide in royal jelly as a component neurotrophic toward cultured rat pheochromocytoma PC12 cells. Biosci. Biotechnol. Biochem. 2006, 70, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wei, M.; Kang, X.; Deng, H.; Lu, Z. A novel method developed for acetylcholine detection in royal jelly by using capillary electrophoresis coupled with electrogenerated chemiluminescence based on a simple reaction. Electrophoresis 2009, 30, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- López-Gutiérrez, N.; Aguilera-Luiz, M.d.M.; Romero-González, R.; Vidal, J.L.M.; Garrido Frenich, A. Fast analysis of polyphenols in royal jelly products using automated TurboFlow™-liquid chromatography–Orbitrap high resolution mass spectrometry. J. Chromat. B 2014, 973, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Koya-Miyata, S.; Okamoto, I.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci. Biotechnol. Biochem. 2004, 68, 767–773. [Google Scholar] [CrossRef]
- Ito, S.; Nitta, Y.; Fukumitsu, H.; Soumiya, H.; Ikeno, K.; Nakamura, T.; Furukawa, S. Antidepressant-like activity of 10-hydroxy-trans-2-decenoic Acid, a unique unsaturated Fatty Acid of royal jelly, in stress-inducible depression-like mouse model. Evid. Complement. Alternat. Med. 2012, 2012, 139140. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Sugimoto, H.; Aida, M. A Theoretical insight into the interaction of fatty acids involved in royal jelly with the human estrogen receptor β. Bull. Chem. Soc. Jpn. 2008, 81, 1258–1266. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.P.; Chinou, I.; Karshikoff, A.; et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef]
- Yang, X.Y.; Yang, D.S.; Wei, Z.; Wang, J.M.; Li, C.Y.; Hui, Y.; Lei, K.F.; Chen, X.F.; Shen, N.H.; Jin, L.Q.; et al. 10-Hydroxy-2-decenoic acid from royal jelly: A potential medicine for RA. J. Ethnopharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structure of properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef]
- Taebi, M.; Abdolahian, S.; Ozgoli, G.; Ebadi, A.; Kariman, N. Strategies to improve menopausal quality of life: A systematic review. J. Edu. Health Prom. 2018, 7, 93. [Google Scholar]
- Mishima, S.; Suzuki, K.-M.; Isohama, Y.; Kuratsu, N.; Araki, Y.; Inoue, M.; Miyata, T. Royal jelly has estrogenic effects in vitro and in vivo. J. Ethnopharmacol. 2005, 101, 215–220. [Google Scholar] [CrossRef]
- Eshtiyaghi, M.; Deldar, H.; Pirsaraei, Z.A.; Shohreh, B. Royal jelly may improve the metabolism of glucose and redox state of ovine oocytes matured in vitro and embryonic development following in vitro fertilization. Theriogenology 2016, 86, 2210–2221. [Google Scholar] [CrossRef]
- Husein, M.Q.; Kridli, R.T. Reproductive responses following royal jelly treatment administered orally or intramuscularly into progesterone-treated Awassi ewes. Anim. Reprod. Sci. 2002, 74, 45–53. [Google Scholar] [CrossRef]
- Imai, M.; Qin, J.; Yamakawa, N.; Miyado, K.; Umezawa, A.; Takahashi, Y. Molecular alterations during female reproductive aging: Can aged oocytes remind youth? J. Embriol. 2012. [Google Scholar] [CrossRef]
- Taavoni, S.; Barkhordari, F.; Goushegir, A.; Haghani, H. Effect of royal jelly on premenstrual syndrome among Iranian medical sciences students: A randomized, triple-blind, placebo-controlled study. Complement. Ther. Med. 2014, 22, 601–606. [Google Scholar] [CrossRef]
- Seyyedi, F.; Rafiean-Kopaei, M.; Miraj, S. Comparison of the effects of vaginal royal jelly and vaginal estrogen on quality of life, sexual and urinary function in postmenopausal women. J. Clin. Diagn. Res. 2016, 10, QC01. [Google Scholar] [CrossRef]
- Sharif, S.N.; Darsareh, F. Effect of royal jelly on menopausal symptoms: A randomized placebo-controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 37, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Asama, T.; Matsuzaki, H.; Fukushima, S.; Tatefuji, T.; Hashimoto, K.; Takeda, T. Royal jelly supplementation improves menopausal symptoms such as backache, low back pain, and anxiety in postmenopausal Japanese women. Evid. Complement. Alternat. Med. 2018, 2018, 4868412. [Google Scholar] [CrossRef] [PubMed]
- Mofid, B.; Rezaeizadeh, H.; Termos, A.; Rakhsha, A.; Mafi, A.R.; Taheripanah, T.; Ardakani, M.M.; Taghavi, S.M.E.; Moravveji, S.A.; Kashi, A.S.Y. Effect of processed honey and royal jelly on cancer-related fatigue: A double-blind randomized clinical trial. Electr. Phys. 2016, 8, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Onda, H.; Sasaki, K.; Yukiyoshi, A.; Tachibana, H.; Yamada, K. Effect of royal jelly on bisphenol a-induced proliferation of human breast cancer cells. Biosci. Biotechnol. Biochem. 2007, 71, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Olivo, L.A.; Paz-González, V. Screening of biological activities present in honeybee (Apis mellifera) royal jelly. Toxicol. Vitro. 2005, 19, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Shirzad, M.; Kordyazdi, R.; Shahinfard, N.; Nikokar, M. Does royal jelly affect tumor cells? J. HerbMed. Pharmacol. 2013, 2, 45–48. [Google Scholar]
- Kamakura, M.; Moriyama, T.; Sakaki, T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J. Pharm. Pharmacol. 2006, 58, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Augoulea, A.; Rizos, D.; Politi, M.; Tsoltos, N.; Moros, M.; Chinou, I.; Graikou, K.; Kouskouni, E.; Kambani, S.; et al. Greek-origin royal jelly improves the lipid profile of postmenopausal women. Gynecol. Endocrinol. 2016, 32, 835–839. [Google Scholar] [CrossRef]
- Guo, H.; Saiga, A.; Sato, M.; Miyazawa, I.; Shibata, M.; Takahata, Y.; Morimatsu, F. Royal jelly supplementation improves lipoprotein metabolism in humans. J. Nutr. Sci. Vitaminol. 2007, 53, 345–348. [Google Scholar] [CrossRef]
- Hadi, A.; Najafgholizadeh, A.; Aydenlu, E.S.; Shafiei, Z.; Pirivand, F.; Golpour, S.; Pourmasoumi, M. Royal jelly is an effective and relatively safe alternative approach to blood lipid modulation: A meta-analysis. J. Funct. Food. 2018, 41, 202–209. [Google Scholar] [CrossRef]
- Tokunaga, K.-H.; Yoshida, C.; Suzuki, K.-M.; Maruyama, H.; Futamura, Y.; Araki, Y.; Mishima, S. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol. Pharm. Bull. 2004, 27, 189–192. [Google Scholar] [CrossRef]
- Pan, Y.; Rong, Y.; You, M.; Ma, Q.; Chen, M.; Hu, F. Royal jelly causes hypotension and vasodilation induced by increasing nitric oxide production. Food Sci. Nutr. 2019, 7, 1361–1370. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Crandall, C.J. Bisphosphonates for postmenopausal osteoporosis. JAMA 2019, 322, 2017–2018. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Matsushita, H.; Minami, A.; Kanazawa, H.; Suzuki, T.; Watanabe, K.; Wakatsuki, A. Royal jelly does not prevent bone loss but improves bone strength in ovariectomized rats. Climacteric 2018, 21, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, S.; Okamoto, Y.; Uchiyama, S.; Nakatsuma, A.; Hashimoto, K.; Ohnishi, T.; Yamaguchi, M. Royal jelly prevents osteoporosis in rats: Beneficial effects in ovariectomy model and in bone tissue culture model. Evid. Complement. Alternat. Med. 2006, 3, 580958. [Google Scholar] [CrossRef]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T.J.N. Royal jelly delays motor functional impairment during aging in genetically heterogeneous male mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef]
- Xin, X.-X.; Chen, Y.; Chen, D.; Xiao, F.; Parnell, L.D.; Zhao, J.; Liu, L.; Ordovas, J.M.; Lai, C.-Q.; Shen, L.-R. Supplementation with major royal-jelly proteins increases lifespan, feeding, and fecundity in drosophila. J. Agric. Food Chem. 2016, 64, 5803–5812. [Google Scholar] [CrossRef] [PubMed]
- Detienne, G.; De Haes, W.; Ernst, U.R.; Schoofs, L.; Temmerman, L. Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling. Exp. Gerontol. 2014, 60, 129–135. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.; Wei, W.; Hu, F. The in vivo antiaging effect of enzymatic hydrolysate from royal jelly in d-galactose induced aging mouse. J. Chin. Inst. Food Sci. Technol. 2016, 16, 18–25. [Google Scholar] [CrossRef]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-decenoic Acid, the major lipid component of royal jelly, extends the lifespan of caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef]
- Zheng, J.; Lai, W.; Zhu, G.; Wan, M.; Chen, J.; Tai, Y.; Lu, C. 10-Hydroxy-2-decenoic acid prevents ultraviolet A-induced damage and matrix metalloproteinases expression in human dermal fibroblasts. J. Eur. Acad. Dermatol. Venerol. 2013, 27, 1269–1277. [Google Scholar] [CrossRef]
- Park, H.M.; Hwang, E.; Lee, K.G.; Han, S.-M.; Cho, Y.; Kim, S.Y. Royal jelly protects against ultraviolet B–induced photoaging in human skin fibroblasts via enhancing collagen production. J. Med. Food. 2011, 14, 899–906. [Google Scholar] [CrossRef]
- Jiang, C.-M.; Liu, X.; Li, C.-X.; Qian, H.-C.; Chen, D.; Lai, C.-Q.; Shen, L.-R. Anti-senescence effect and molecular mechanism of the major royal jelly proteins on human embryonic lung fibroblast (HFL-I) cell line. J. Zhejiang Univ. 2018, 19, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Raine-Fenning, N.J.; Brincat, M.P.; Muscat-Baron, Y. Skin aging and menopause. Am. J. Clin. Dermatol. 2003, 4, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Cho, M.H.; Cho, Y.; Kim, S.Y. Royal jelly increases collagen production in rat skin after ovariectomy. J. Med. Food 2012, 15, 568–575. [Google Scholar] [CrossRef]
- Henderson, V.W. The neurology of menopause. Neurology 2006, 12, 149–159. [Google Scholar] [CrossRef]
- Zamani, Z.; Reisi, P.; Alaei, H.; Pilehvarian, A.A. Effect of royal jelly on spatial learning and memory in rat model of streptozotocin-induced sporadic Alzheimer’s disease. Adv. Biomed. Res. 2012, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Cihan, Y.; Arsav, V.; Göcen, E. Royal jelly in the prevention of radiation-induced brain damages. J. Neurol. Sci. 2012, 28, 475–486. [Google Scholar]
- Zhang, X.; Yu, Y.; Sun, P.; Fan, Z.; Zhang, W.; Feng, C. Royal jelly peptides: Potential inhibitors of β-secretase in N2a/APP695swe cells. Sci. Rep. 2019, 9, 168. [Google Scholar] [CrossRef]
- Pyrzanowska, J.; Piechal, A.; Blecharz-Klin, K.; Joniec-Maciejak, I.; Graikou, K.; Chinou, I.; Widy-Tyszkiewicz, E. Long-term administration of Greek Royal Jelly improves spatial memory and influences the concentration of brain neurotransmitters in naturally aged Wistar male rats. J. Ethnopharmacol. 2014, 155, 343–351. [Google Scholar] [CrossRef]
- Minami, A.; Matsushita, H.; Ieno, D.; Matsuda, Y.; Horii, Y.; Ishii, A.; Takahashi, T.; Kanazawa, H.; Wakatsuki, A.; Suzuki, T. Improvement of neurological disorders in postmenopausal model rats by administration of royal jelly. Climacteric 2016, 19, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Münstedt, K.; Bargello, M.; Hauenschild, A. Royal jelly reduces the serum glucose levels in healthy subjects. J. Med. Food. 2009, 12, 1170–1172. [Google Scholar] [CrossRef]
- Yoshida, M.; Hayashi, K.; Watadani, R.; Okano, Y.; Tanimura, K.; Kotoh, J.; Sasaki, D.; Matsumoto, K.; Maeda, A. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Sci. 2017, 79, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zamami, Y.; Takatori, S.; Goda, M.; Koyama, T.; Iwatani, Y.; Jin, X.; Takai-Doi, S.; Kawasaki, H. Royal jelly ameliorates insulin resistance in fructose-drinking rats. Biol. Pharm. Bull. 2008, 31, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, E.; Nejati, V.; Azadbakht, M. Protective effect of royal jelly against renal damage in streptozotocin induced diabetic rats. Iran J. Toxicol. 2015, 9, 1258–1263. [Google Scholar] [CrossRef]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef]
- Kaczor, M.; Koltek, A.; Matuszewski, J. Effect of royal jelly on blood lipids in atherosclerosis. Polski Tygodnik Lekarski. 1962, 17, 1140–1144. [Google Scholar]
- Mobasseri, M.; Pourmoradian, S.; Mahdavi, R.; Faramarzi, E. Effects of royal jelly supplementation on lipid profile and high-sensitivity c-reactive protein levels in type-2 diabetic women: A pilot study. Curr. Top. Nutr. Res. 2014, 12, 101–106. [Google Scholar]
- Mobasseri, M.; Ghiyasvand, S.; Ostadrahimi, A.; Ghojazadeh, M.; Noshad, H.; Pourmoradian, S. Effect of fresh royal jelly ingestion on glycemic response in patients with type 2 diabetes. Iranian Red Crescent Med. J. 2015, 17, e20074. [Google Scholar] [CrossRef]
- Shidfar, F.; Jazayeri, S.; Mousavi, S.N.; Malek, M.; Hosseini, A.F.; Khoshpey, B. Does supplementation with royal jelly improve oxidative stress and insulin resistance in type 2 diabetic patients? Iran. J. Public Health. 2015, 44, 797–803. [Google Scholar]
- Chiu, H.-F.; Chen, B.-K.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharm. Biol. 2017, 55, 497–502. [Google Scholar] [CrossRef]
- Petelin, A.; Kenig, S.; Kopinč, R.; Deželak, M.; Černelič Bizjak, M.; Jenko Pražnikar, Z.; Medicine, A. Effects of royal jelly administration on lipid profile, satiety, inflammation, and antioxidant capacity in asymptomatic overweight adults. J. Evid. Complement. 2019, 2019, 4969720. [Google Scholar] [CrossRef]
- Takaki-Doi, S.; Hashimoto, K.; Yamamura, M.; Kamei, C. Antihypertensive activities of royal jelly protein hydrolysate and its fractions in spontaneously hypertensive rats. Acta Med. Okayama. 2009, 63, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kaku, M.; Rocabado, J.M.R.; Kitami, M.; Ida, T.; Uoshima, K. Royal jelly affects collagen crosslinking in bone of ovariectomized rats. J. Funct. Food. 2014, 7, 398–406. [Google Scholar] [CrossRef]
- Chen, D.; Liu, F.; Wan, J.-B.; Lai, C.-Q.; Shen, L.-R. Effect of major royal jelly proteins on spatial memory in aged rats: Metabolomics analysis in urine. J. Agric. Food Chem. 2017, 65, 3151–3159. [Google Scholar] [CrossRef]
- Sefirin, D.; Nange, A.C.; Florette, M.T.; Franklin, Z.G.; Florence, A.C.; Pierre, K.; Dieudonné, N. Royal jelly induced anxiolytic effects and prevent hot flushes in a menopausal model on wistar rat. J. Biomed. J. Sci. Tech. Res. 2019, 19, 14557–14566. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Shen, L. Protective effects of major royal jelly proteins on reproductive function of mice during perimenopausal period. Agric. Life Sci. 2019, 45, 751–759. [Google Scholar] [CrossRef]
- You, M.M.; Liu, Y.C.; Chen, Y.F.; Pan, Y.M.; Miao, Z.N.; Shi, Y.Z.; Si, J.J.; Chen, M.L.; Hu, F.L. Royal jelly attenuates nonalcoholic fatty liver disease by inhibiting oxidative stress and regulating the expression of circadian genes in ovariectomized rats. J. Food Biochem. 2020, 44, e13138. [Google Scholar] [CrossRef] [PubMed]
- Rosmilah, M.; Shahnaz, M.; Patel, G.; Lock, J.; Rahman, D.; Masita, A.; Noormalin, A. Characterization of major allergens of royal jelly Apis mellifera. J. Trop. Biomed. 2008, 25, 243–251. [Google Scholar]
- Yonei, Y.; Shibagaki, K.; Tsukada, N.; Nagasu, N.; Inagaki, Y.; Miyamoto, K.; Suzuki, O.; Kiryu, Y. Case report: Haemorrhagic colitis associated with royal jelly intake. Gastroenterol. Hepatol. 1997, 12, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Aoki, M.; Kawana, S. Case of anaphylaxis caused by ingestion of royal jelly. J. Dermatol. 2008, 35, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Paola, F.; Pantalea, D.D.; Gianfranco, C.; Antonio, F.; Angelo, V.; Eustachio, N.; Elisabetta, D.L. Oral allergy syndrome in a child provoked by royal jelly. Case Rep. Med. 2014, 2014, 941248. [Google Scholar] [CrossRef]
- Lee, N.J.; Fermo, J.D. Warfarin and royal jelly interaction. Pharmacotherapy 2006, 26, 583–586. [Google Scholar] [CrossRef] [PubMed]
| Author, Reference | Beneficial Effect | Study Design | Number of Subjects | Dose and Administration | Results |
|---|---|---|---|---|---|
| Kaczor et al., 1962 [88] | Anti-atherosclerosis | Randomized, placebo-controlled study | 27 patients and 12 controls | Seven patients: 100 mL subcutaneously every day for 2 weeks, and 4 weeks on alternate days 20 patients: same scheme for 3 weeks Controls: placebo—3 weeks | In supplemented patients
|
| Guo et al., 2007 [60] | Improves lipoprotein metabolism | Randomized, placebo-controlled study | 15 patients | Seven cases received 6 g of royal jell daily, for 4 weeks Eight cases were the control group |
|
| Mobasseri et al., 2014 [89] | Hypolipemiant | Randomized, placebo-controlled study | 50 women (25 cases and 25 controls) with type 2 diabetes | 1000 mg/day of royal jelly or placebo—8 weeks | In supplemented patients
|
| Mobasseri et al., 2015 [90] | Hypoglycemiant | Randomized, placebo-controlled study | 50 patients (20 cases and 20 controls) with type 2 diabetes | 10 g of fresh royal jelly or placebo after 12 h fasting |
|
| Shidfar et al., 2015 [91] | Anti-diabetic | Randomized, placebo-controlled study | 46 type 2 diabetic patients | 1000 mg royal jelly or placebo—3 times/day for 8 weeks |
|
| Mofid et al., 2016 [53] | Anti-fatigue | Randomized, double-blind placebo-controlled study | 52 cancer patients | 26 patients received 5 mL of processed honey and royal jelly twice/day—4 weeks 26 controls received 5 mL of pure honey twice/day—4 weeks |
|
| Chiu et al., 2016 [92] | Hypocholesterolemiant Decreases the risk of cardiovascular disease | Randomized, placebo-controlled study | 40 patients with mild hypercholesterolemia | Nine capsules of royal jelly or placebo, daily—3 months (350 mg of royal jelly or placebo /capsule) |
|
| Lambrinoudaki et al., 2016 [59] | Improves the lipid profile | Prospective study | 36 postmenopausal women | 150 mg/day, 3 months |
|
| Seyyedi et al., 2016 [50] | Improves sexual and urinary function | Randomized, placebo-controlled study | 90 postmenopausal women | Group 1—vaginal cream of royal jelly 15%, 3 months Group 2—lubricant, 3 months Group 3—conjugated estrogens, 3 months |
|
| Asama et al., 2018 [52] | Improves backache, low back pain and anxiety | Randomized, placebo-controlled study | 42 postmenopausal women | 800 mg of enzyme-treated royal jelly or 800 mg of dextrin—3 months |
|
| Sharif et al., 2019 [51] | Improves menopause-related symptoms | Randomized, double-blind placebo-controlled study | 200 postmenopausal women | 1000 mg royal jelly or placebo/day—8 weeks |
|
| Petelin et al., 2019 [93] | Improves the lipid profile, satiety and antioxidant capacity | Randomized, double-blind placebo-controlled study | 60 obese patients (30 cases and 30 controls) | Two capsules of lyophilized royal jelly (333 mg/capsule) or placebo—8 weeks |
|
| Author, Reference | Beneficial Effect | Study Design | Subjects | Dose and Administration | Results |
|---|---|---|---|---|---|
| Hidaka et al., 2006 [66] | Prevents osteoporosis | Prospective study | 48 female Sprague-Dawley rats (6 controls and 42 ovariectomized rats) with low tibial bone mineral density | The rats were divided into 8 groups and royal jelly was mixed with MF powdered pellets, in order to be orally administered (0.5 g of royal jelly mixed with 100 g MF pellets or 2 g of royal jelly mixed with 100 g MF pellets). |
|
| Zamami et al., 2008 [85] | Improves insulin resistance | Randomized, Placebo-controlled study | Male Winstar rats | 100 or 300 mg/kg, po—8 weeks or placebo |
|
| Takaki-Doi et al., 2009 [94] | Anti-hypertensive | Prospective study | Hypertensive rats | Seven peptide fractions of royal jelly protein hydrolysate were administered separately in doses of 10, 30, 100 mg/kg i.v. or 1000 mg/kg po |
|
| Park et al., 2012 [76] | Anti-aging effects on the skin | Prospective study | Ovariectomized virgin female of Sprague-Dawley rats | The rats were fed with a dietary supplement containing 1% royal jelly extract |
|
| Zamani et al., 2012 [78] | Neuroprotective role | Placebo-controlled study | Rats bilaterally intracerebroventricular infused with streptozocin |
|
|
| Shirzad et al., 2013 [57] | Decreases the sizes of malignant tumors | Prospective study | 28 male Balb/c mice subcutaneously injected with tumor cells | Group 1 received 100 mg/kg of royal jelly Group 2 received 200 mg/kg of royal jelly Group 3 received 300 mg/kg of royal jelly Group 4 received a vehicle |
|
| Pyrzanowska et al., 2014 [81] | Improves spatial memory | Randomized, placebo-controlled study | 18-month old male Winstar rats | 50 and 100 mg of royal jelly powder/kg/day by gastric gavage—8 weeks |
|
| Kaku et al., 2014 [95] | Improves bone quality | Prospective study | Ovariectomized rats | Royal jelly was administered to the rats for 12 weeks, orally |
|
| Minami et al., 2016 [82] | Improves neurological symptoms of menopausal disorders | Randomized, placebo-controlled study | Ovariectomized rats | Royal jelly was administered to the rats for 82 days |
|
| Yoshida et al., 2016 [84] | Hypoglicemiant | Randomized, placebo-controlled study | Obese/diabetic KK-Ay mice | 10 mg/kg of royal jelly daily, by oral gavage—4 weeks |
|
| Chen et al., 2017 [96] | Improves spatial memory | Randomized, placebo-controlled study | Male aged rats | The rats were supplemented for 14 weeks with royal jelly. Controls received distilled water |
|
| Shimizu et al., 2018 [65] | Improves bone quality | Randomized, placebo-controlled study | 12 weeks old ovariectomized rats | Royal jelly was administered daily, for 3 months |
|
| Jiang et al., 2018 [74] | Anti-senescence activity | Prospective study | Human embryonic lung fibroblast cells (HFL-I) | HFL-I cells were cultured in media containing various concentrations of MRJPs |
|
| Sefirin et al., 2019 [97] | Anxiolytic effectsPrevents hot flushes | Randomized, placebo-controlled study | Ovariectomized Winstar rats | 100, 200 and 300 mg/kg of royal jelly |
|
| Liu et al., 2019 [98] | Protects reproductive function | Randomized, placebo-controlled study | 50 female mice | Group 1: 125, 250, 500 mg/kg MRJPs daily Group 2: 125 mg/kg casein daily Group 3: saline solution dailyIntragastric administration for seven weeks |
|
| Pan et al., 2019 [63] | Antihypertensive effects | Randomized, placebo-controlled study | Hypertensive rats | Group 1: 1g/kg of royal jelly, by oral administration, for 4 weeks Group 2: control group |
|
| You et al., 2020 [99] | Attenuates nonalcoholic fatty liver disease | Prospective study | Ovariectomized rats | 150, 300 or 450 mg/kg/day for 2 months |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălan, A.; Moga, M.A.; Dima, L.; Toma, S.; Elena Neculau, A.; Anastasiu, C.V. Royal Jelly—A Traditional and Natural Remedy for Postmenopausal Symptoms and Aging-Related Pathologies. Molecules 2020, 25, 3291. https://doi.org/10.3390/molecules25143291
Bălan A, Moga MA, Dima L, Toma S, Elena Neculau A, Anastasiu CV. Royal Jelly—A Traditional and Natural Remedy for Postmenopausal Symptoms and Aging-Related Pathologies. Molecules. 2020; 25(14):3291. https://doi.org/10.3390/molecules25143291
Chicago/Turabian StyleBălan, Andreea, Marius Alexandru Moga, Lorena Dima, Sebastian Toma, Andrea Elena Neculau, and Costin Vlad Anastasiu. 2020. "Royal Jelly—A Traditional and Natural Remedy for Postmenopausal Symptoms and Aging-Related Pathologies" Molecules 25, no. 14: 3291. https://doi.org/10.3390/molecules25143291
APA StyleBălan, A., Moga, M. A., Dima, L., Toma, S., Elena Neculau, A., & Anastasiu, C. V. (2020). Royal Jelly—A Traditional and Natural Remedy for Postmenopausal Symptoms and Aging-Related Pathologies. Molecules, 25(14), 3291. https://doi.org/10.3390/molecules25143291