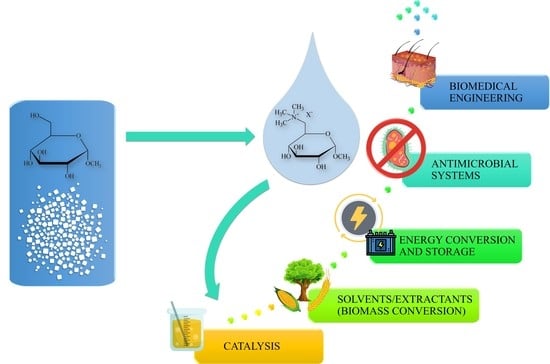
Insights into the Properties and Potential Applications of Renewable Carbohydrate-Based Ionic Liquids: A Review
Abstract
1. Introduction
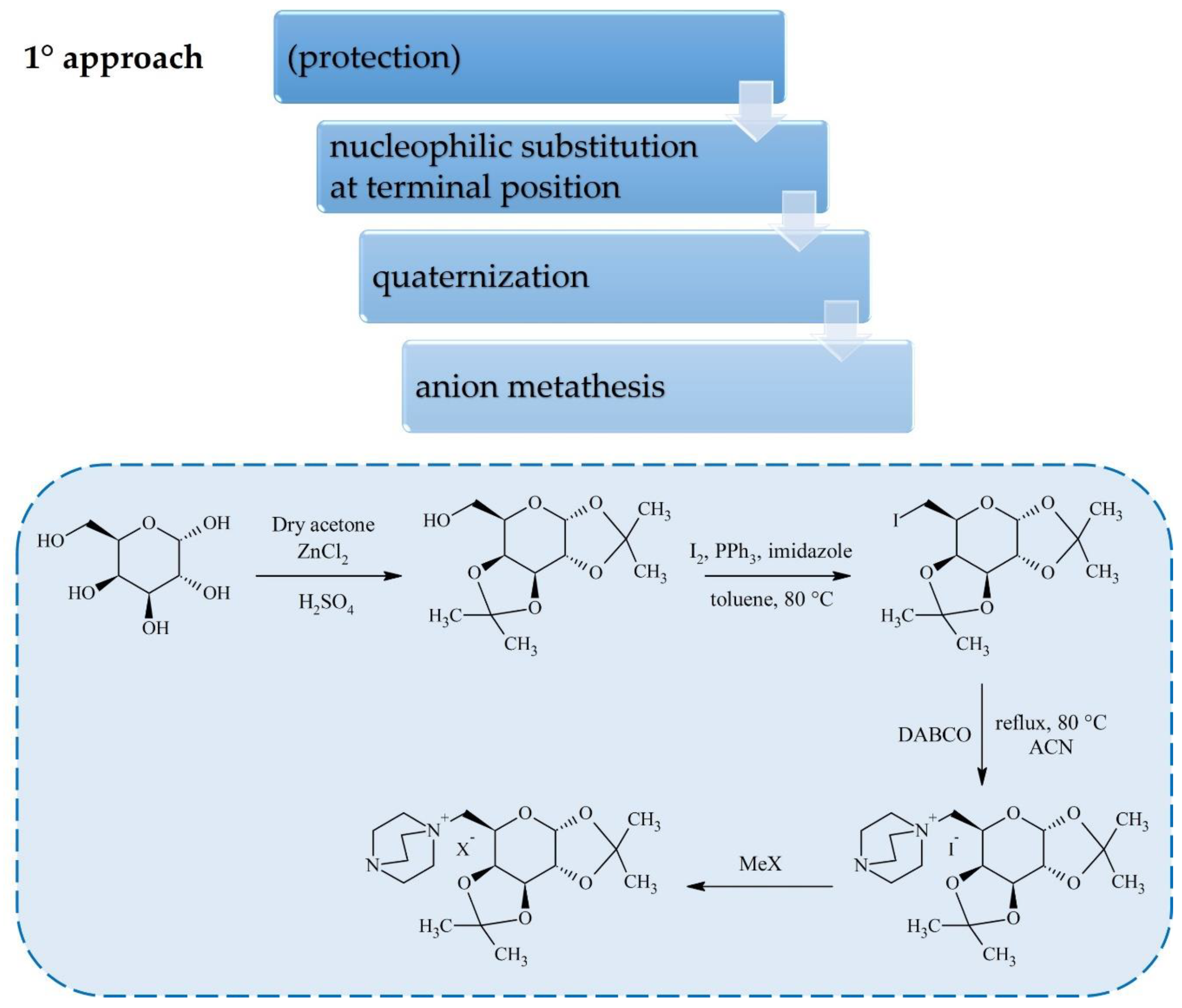
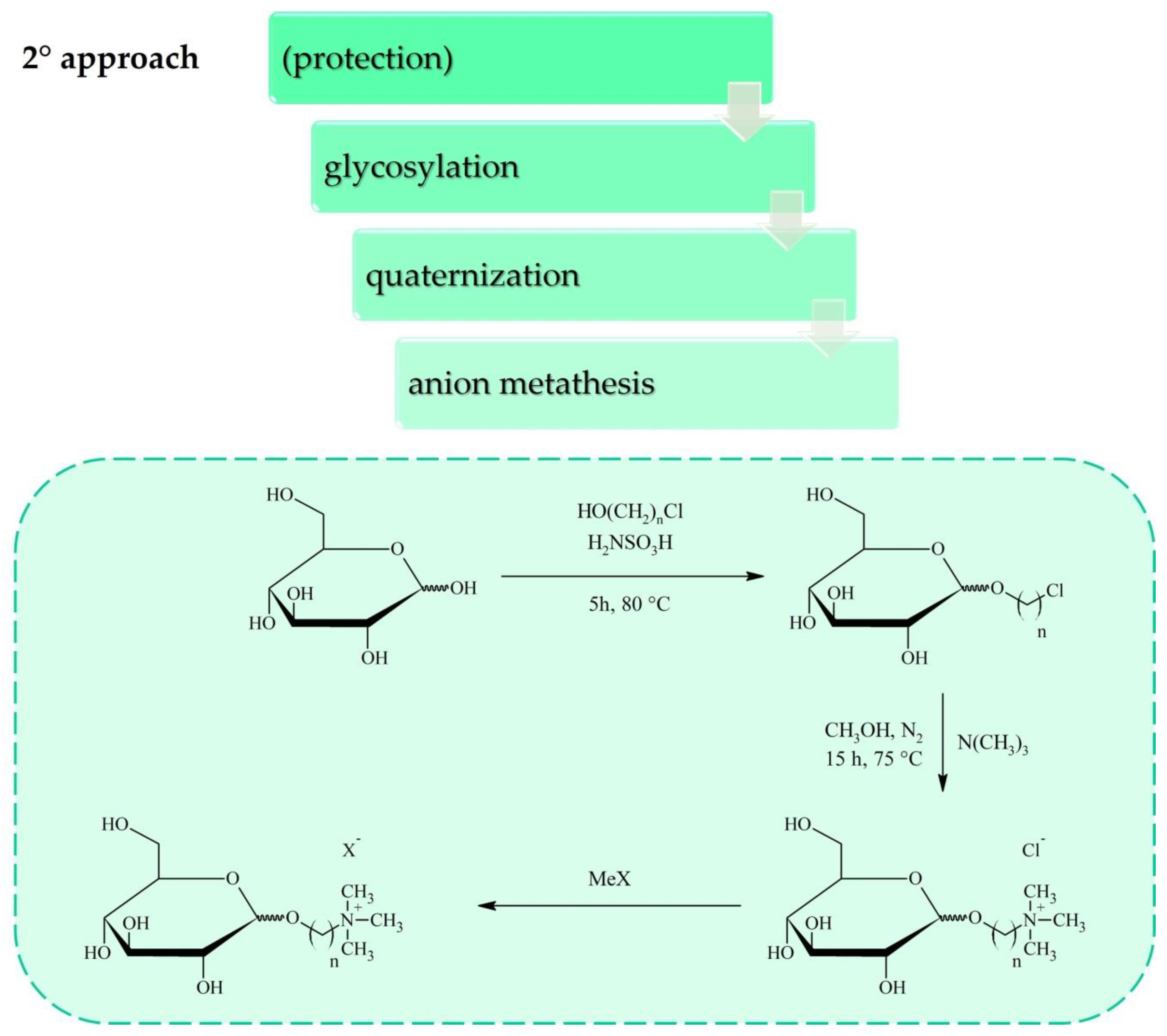
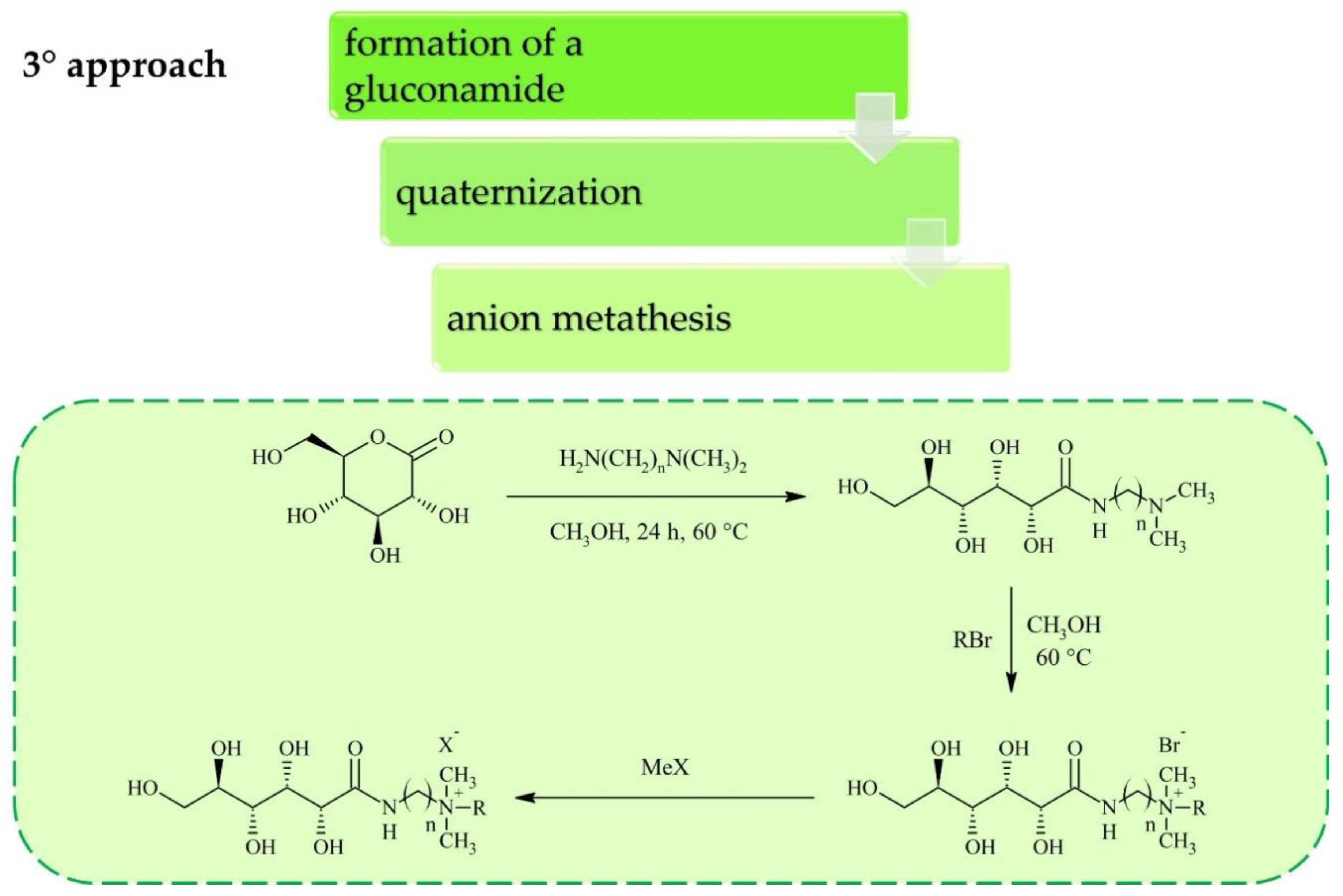
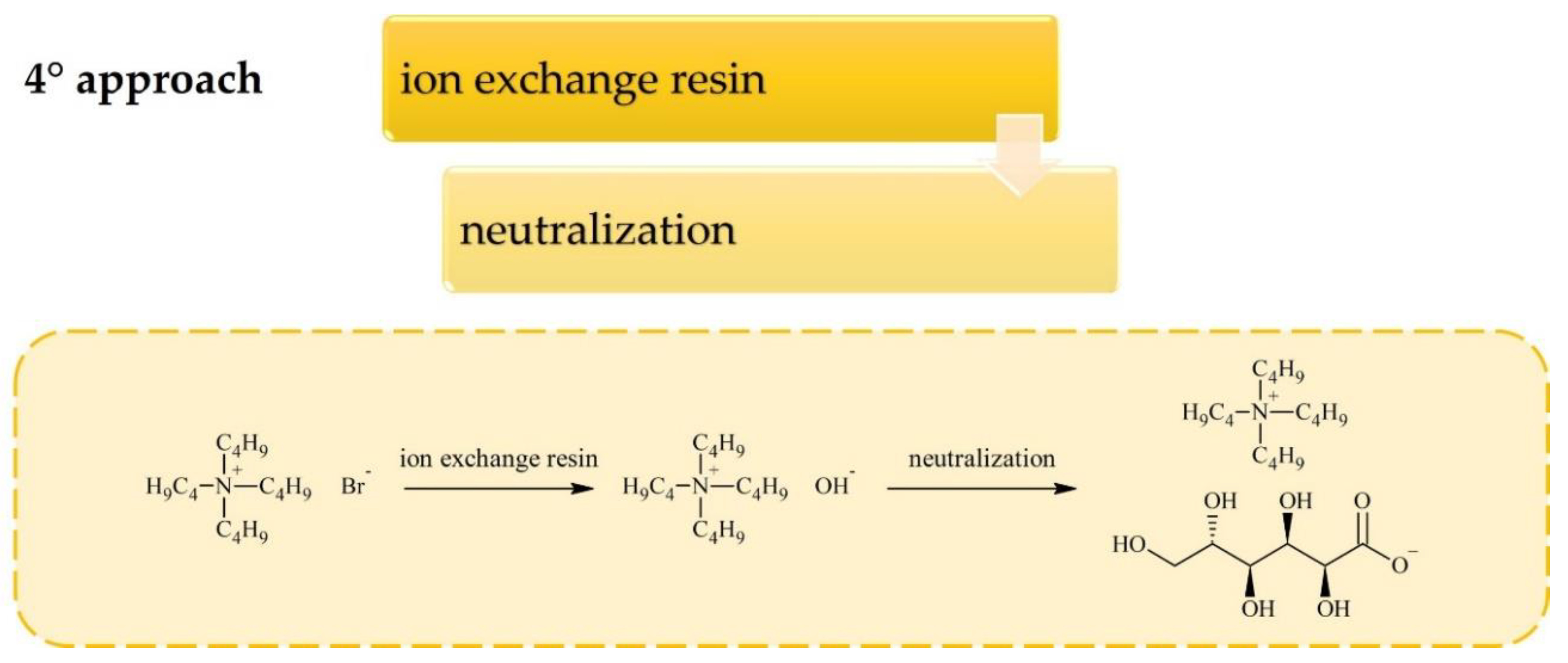
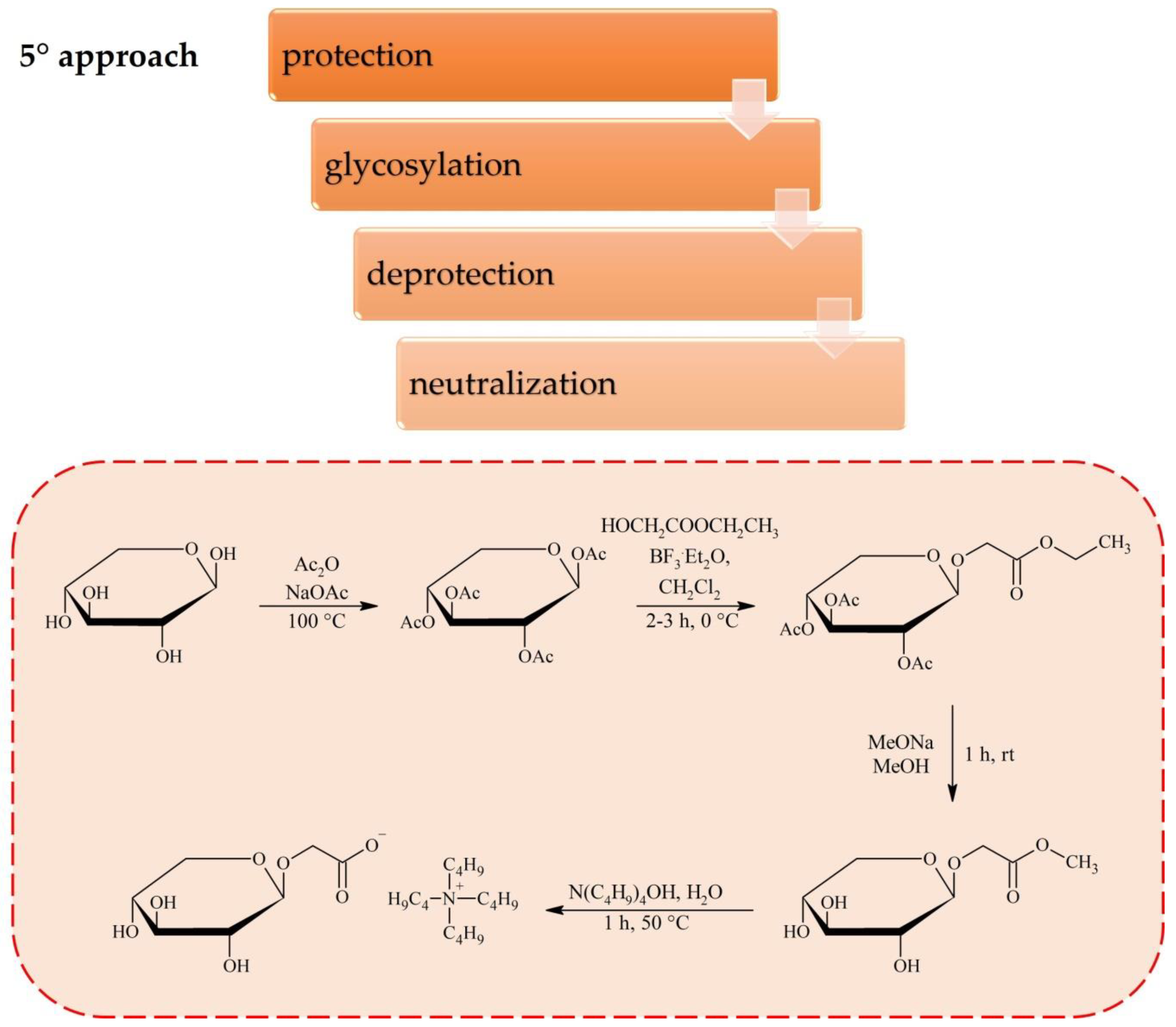
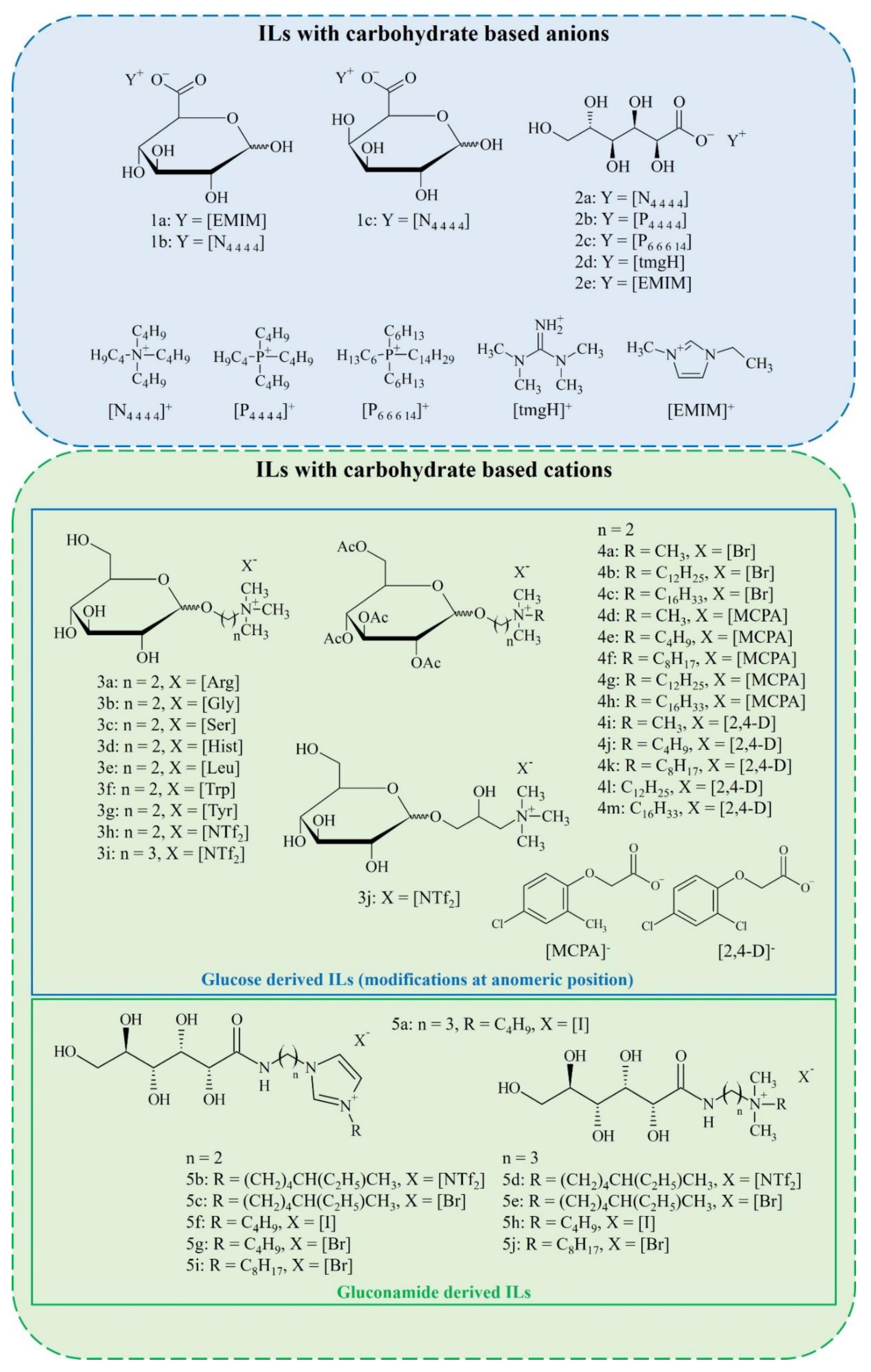
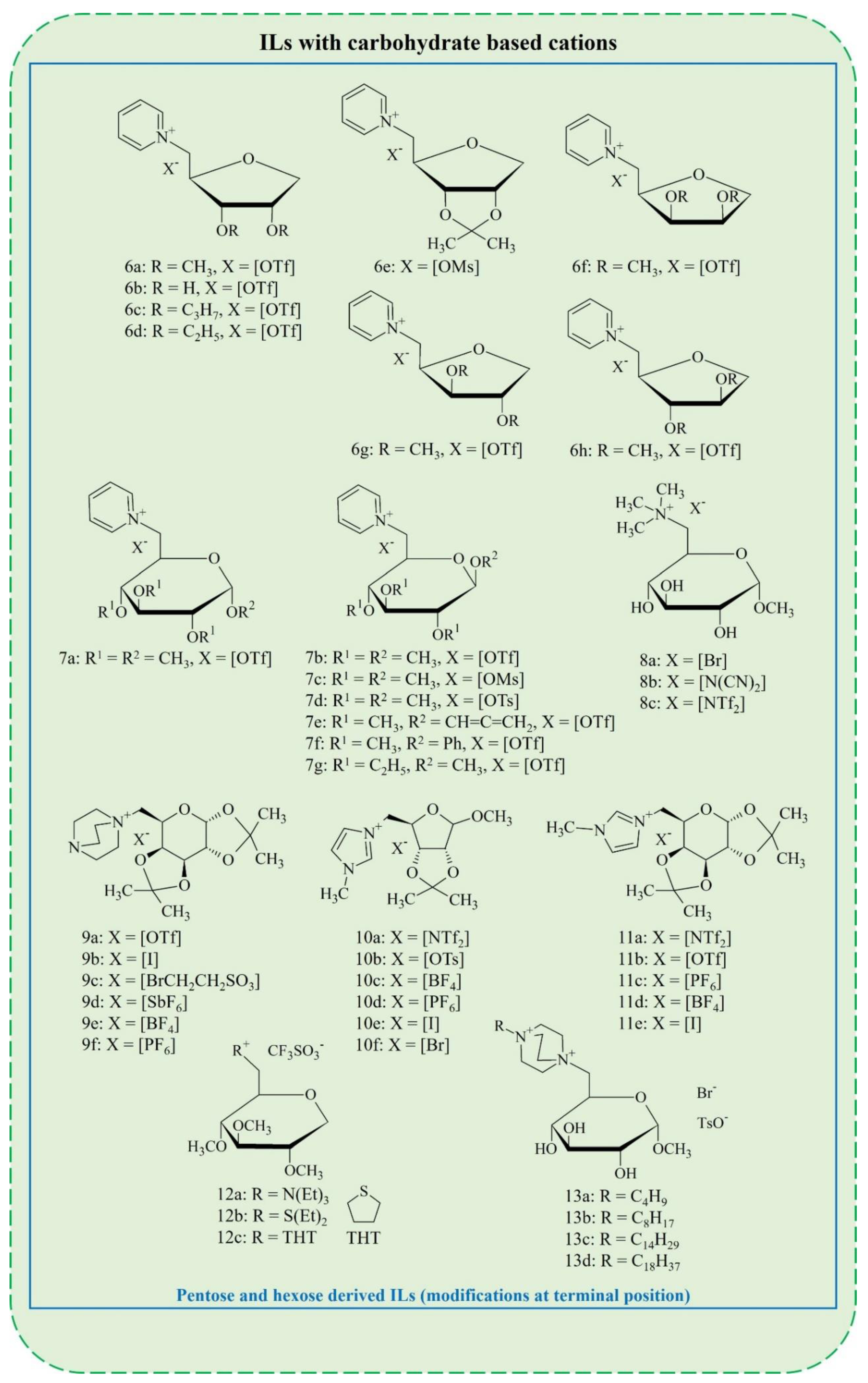
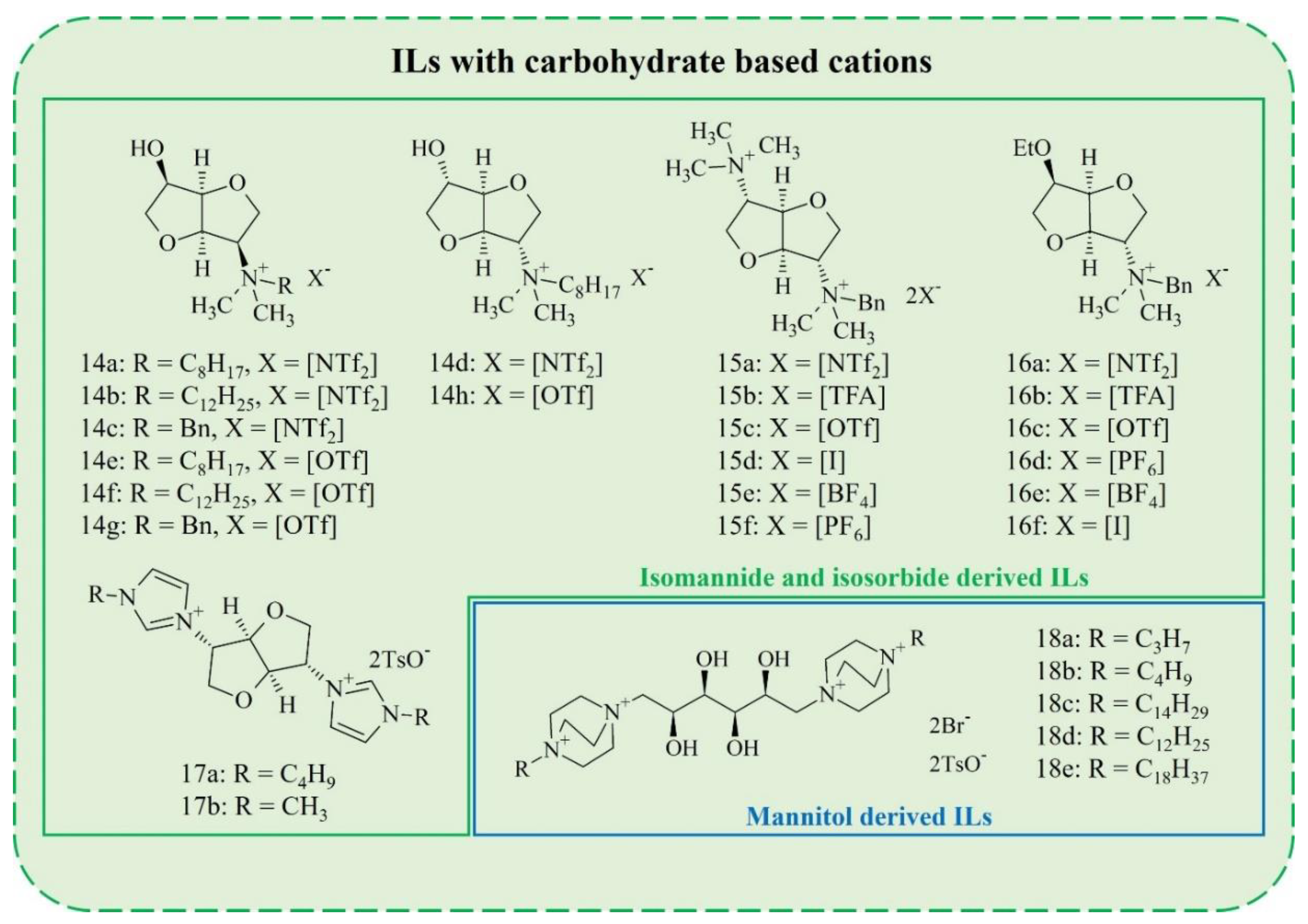
2. Synthesis of Carbohydrate-Derived ILs and Salts
3. Properties of Carbohydrate-Based ILs
3.1. Biodegradation/Toxicity
3.2. Thermal Stability and Melting Point
3.2.1. Anion
3.2.2. Cation
3.2.3. Length of the Carbon Chain
3.3. Viscosity and Glass Transition Temperature
3.4. Conductivity
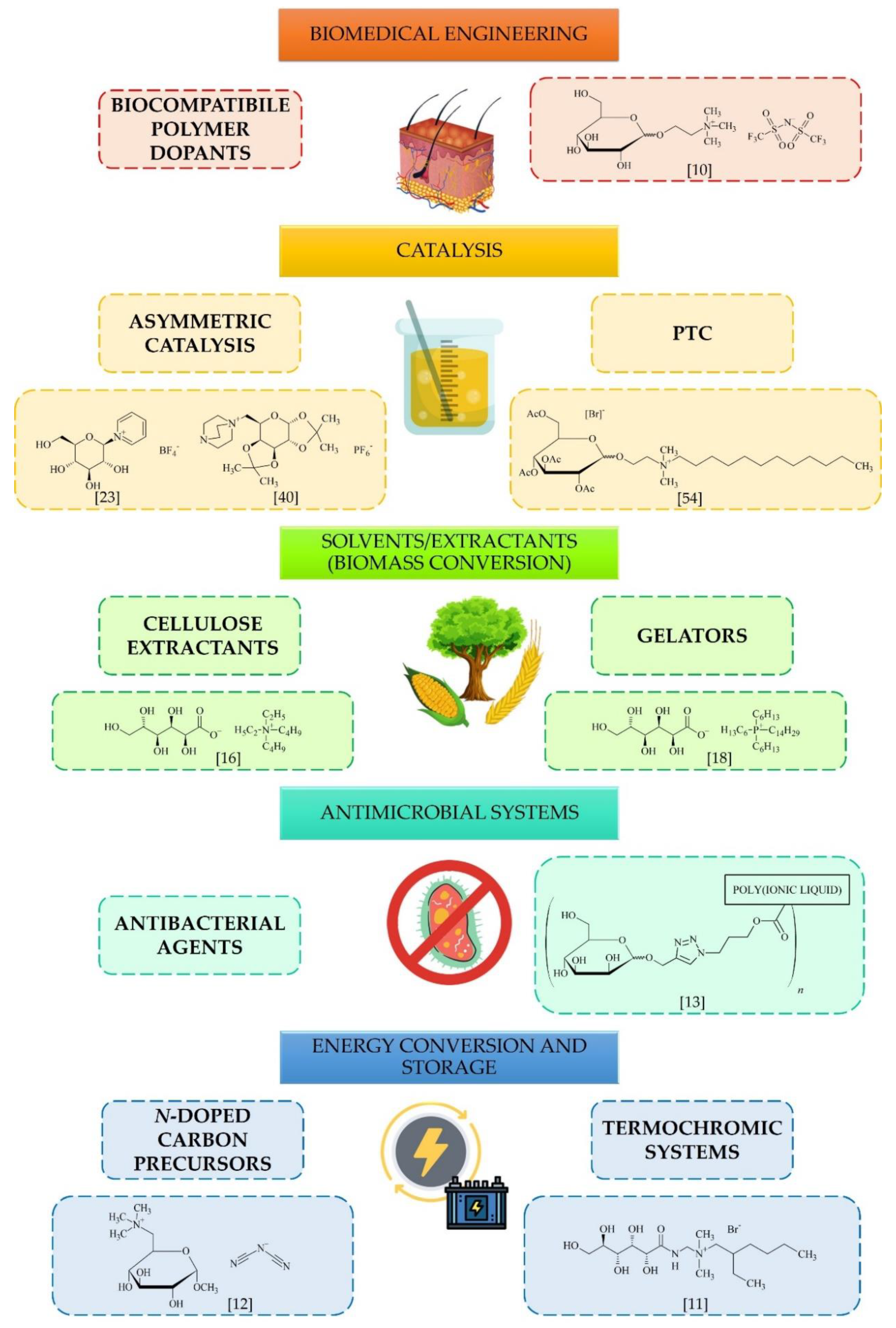
4. Applications and Future Perspectives
4.1. Organocatalysts/Chiral Catalysts
4.2. Biomedicine and Ecology
4.3. Solvents (Biomass Conversion)
4.4. Energy Conversion and Storage
5. Summary and Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Matuszek, K.; MacFarlane, D.R. Ionic Liquids. Kirk-Othmer Encycl. Chem. Technol. 2019, 1–29. [Google Scholar]
- Costa, S.P.F.; Azevedo, A.M.O.; Pinto, P.C.A.G.; Saraiva, L.M.F.S. Environmental impact of ionic liquids: An overview of recent (eco)toxicological and (bio)degradability literature. ChemSusChem 2017, 10, 2321–2347. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids—A critical review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef]
- Hulsbosch, J.; De Vos, D.E.; Binnemans, K.; Ameloot, R. Biobased ionic liquids: Solvents for a green processing industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Marra, A.; Chiappe, C.; Mele, A. Sugar-derived ionic liquids. Chim. Int. J. Chem. 2011, 65, 76–80. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, A.; Kumar Chopra, H. Exploring low-cost natural precursors as chiral building blocks in synthesis: Chiral carbohydrate-ionic liquids. Mini-Rev. Org. Chem. 2018, 15, 208–219. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Kobus, D.; Turczyn, R.; Erfurt, K.; Chrobok, A.; Biggs, M.J.P. Low resistance, highly corrugated structures based on poly(3,4-ethylenedioxythiophene) doped with a d-glucopyranoside-derived ionic liquid. Electrochem. Commun. 2020, 110, 106616. [Google Scholar] [CrossRef]
- Billeci, F.; Gunaratne, H.Q.N.; D’Anna, F.; Morgan, G.G.; Seddon, K.R.; Plechkova, N.V. A magnetic self-contained thermochromic system with convenient temperature range. Green Chem. 2019, 21, 1412–1416. [Google Scholar] [CrossRef]
- Brzęczek-Szafran, A.; Erfurt, K.; Blacha-Grzechnik, A.; Krzywiecki, M.; Boncel, S.; Chrobok, A. Carbohydrate ionic liquids and salts as all-in-one precursors for n-doped carbon. ACS Sustain. Chem. Eng. 2019, 7, 19880–19888. [Google Scholar] [CrossRef]
- Hong, M.; Miao, Z.; Xu, X.; Zhang, Q. Magnetic Iron Oxide Nanoparticles immobilized with sugar-containing poly(ionic liquid) brushes for efficient trapping and killing of bacteria. ACS Appl. Bio Mater. 2020, 3, 3664–3672. [Google Scholar] [CrossRef]
- Chen, J.; Li, D.; Bao, C.; Zhang, Q. Controlled synthesis of sugar-containing poly(ionic liquid)s. Chem. Commun. 2020, 56, 3665–3668. [Google Scholar] [CrossRef]
- Pernak, J.; Czerniak, K.; Biedziak, A.; Marcinkowska, K.; Praczyk, T.; Erfurt, K.; Chrobok, A. Herbicidal ionic liquids derived from renewable sources. RSC Adv. 2016, 6, 52781–52789. [Google Scholar] [CrossRef]
- Javed, F.; Ullah, F.; Akil, H.M. Synthesis, characterization and cellulose dissolution capabilities of ammonium-based room temperature ionic liquids (RTILs). Pure Appl. Chem. 2018, 90, 1019–1034. [Google Scholar] [CrossRef]
- Billeci, F.; D’Anna, F.; Feroci, M.; Cancemi, P.; Feo, S.; Forlino, A.; Tonnelli, F.; Seddon, K.R.; Gunaratne, H.Q.N.; Plechkova, N.V. When functionalization becomes useful: Ionic liquids with a “sweet” appended moiety demonstrate drastically reduced toxicological effects. ACS Sustain. Chem. Eng. 2020, 8, 926–938. [Google Scholar] [CrossRef]
- Billeci, F.; D’Anna, F.; Gunaratne, H.Q.N.; Plechkova, N.V.; Seddon, K.R. “Sweet” ionic liquid gels: Materials for sweetening of fuels. Green Chem. 2018, 20, 4260–4276. [Google Scholar] [CrossRef]
- Handy, S.T.; Okello, M.; Dickenson, G. Solvents from biorenewable sources: Ionic liquids based on fructose. Org. Lett. 2003, 5, 2513–2515. [Google Scholar] [CrossRef]
- Pellowska-Januszek, L.; Dmochowska, B.; Skorupa, E.; Chojnacki, J.; Wojnowski, W.; Wiśniewski, A. New class of quaternary ammonium salts, derivatives of methyl d-glucopyranosides. Carbohydr. Res. 2004, 339, 1537–1544. [Google Scholar] [CrossRef]
- Poletti, L.; Chiappe, C.; Lay, L.; Pieraccini, D.; Polito, L.; Russo, G. Glucose-derived ionic liquids: Exploring low-cost sources for novel chiral solvents. Green Chem. 2007, 9, 337–341. [Google Scholar] [CrossRef]
- Costa, A.; Forte, A.; Zalewska, K.; Tiago, G.; Petrovski, Z.; Branco, L.C. Novel biocompatible ionic liquids based on gluconate anion. Green Chem. Lett. Rev. 2015, 8, 8–12. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, Y.J.; Fang, Y.; Ge, W.H.; Lin, W.; Li, M.Q.; Xu, J.B.; Wan, Y.; Liu, Y.; Wu, H. The first direct synthesis of chiral Tröger’s bases catalyzed by chiral glucose-containing pyridinium ionic liquids. Chem. Eng. J. 2017, 316, 1026–1034. [Google Scholar] [CrossRef]
- Jha, A.K.; Jain, N. Synthesis of glucose-tagged triazolium ionic liquids and their application as solvent and ligand for copper(I) catalyzed amination. Tetrahedron Lett. 2013, 54, 4738–4741. [Google Scholar] [CrossRef]
- Thomas, M.; Montenegro, D.; Castaño, A.; Friedman, L.; Leb, J.; Huang, M.L.; Rothman, L.; Lee, H.; Capodiferro, C.; Ambinder, D.; et al. Polycations. 17. Synthesis and properties of polycationic derivatives of carbohydrates. Carbohydr. Res. 2009, 344, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Jayachandra, R.; Reddy, S.R. Synthesis of D-ribose and D-galactose derived chiral ionic liquids as recyclable chiral solvent for michael addition reaction. Trends Carbohydr. Res. 2015, 7, 60–67. [Google Scholar]
- Jayachandra, R.; Reddy, S.R. Natural sugars derived chiral ionic liquids for asymmetric michael addition reaction. ChemistrySelect 2016, 1, 2341–2343. [Google Scholar] [CrossRef]
- Jayachandra, R.; Reddy, S.R. A remarkable chiral recognition of racemic Mosher’s acid salt by naturally derived chiral ionic liquids using 19F NMR spectroscopy. RSC Adv. 2016, 6, 39758–39761. [Google Scholar] [CrossRef]
- Plaza, P.G.J.; Bhongade, B.A.; Singh, G. Synthesis of chiral carbohydrate ionic liquids. Synlett 2008, 6, 2973–2976. [Google Scholar]
- Kumar, V.; Olsen, C.E.; Schäffer, S.J.C.; Parmar, V.S.; Malhotra, S.V. Synthesis and applications of novel bis(ammonium) chiral ionic liquids derived from isomannide. Org. Lett. 2007, 9, 3905–3908. [Google Scholar] [CrossRef]
- Kumar, V.; Pei, C.; Olsen, C.E.; Schäffer, S.J.C.; Parmar, V.S.; Malhotra, S.V. Novel carbohydrate-based chiral ammonium ionic liquids derived from isomannide. Tetrahedron Asymmetry 2008, 19, 664–671. [Google Scholar] [CrossRef]
- Gomes Da Silva, M.D.R.; Pereira, M.M.A. New chiral imidazolium ionic liquids from isomannide. Carbohydr. Res. 2011, 346, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Van Buu, O.N.; Vo-Thanh, G. Synthesis of novel chiral ammonium-based ionic liquids derived from isosorbide and their applications in an asymmetric aza diels-alder reaction. Lett. Org. Chem. 2007, 4, 158–167. [Google Scholar] [CrossRef]
- Van Buu, O.N.; Aupoix, A.; Hong, N.D.T.; Vo-Thanh, G. Chiral ionic liquids derived from isosorbide: Synthesis, properties and applications in asymmetric synthesis. New J. Chem. 2009, 33, 2060–2072. [Google Scholar] [CrossRef]
- Dmochowska, B.; Skorupa, E.; Pellowska-Januszek, L.; Czarkowska, M.; Sikorski, A.; Wiśniewski, A. Preparation, single-crystal X-ray diffraction and high-resolution NMR spectroscopic analyses of N-[(1,4-anhydro-5-deoxy-2,3-O-isopropylidene-d,l-ribitol)-5-yl]trimethylammonium iodide. Carbohydr. Res. 2006, 341, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Chiappe, C.; Marra, A.; Mele, A. Synthesis and applications of ionic liquids derived from natural sugars. In Carbohydrates in Sustainable Development II; Springer: Berlin/Heidelberg, Germany, 2010; pp. 177–195. ISBN 0002-9297. [Google Scholar]
- Ferlin, N.; Courty, M.; Gatard, S.; Spulak, M.; Quilty, B.; Beadham, I.; Ghavre, M.; Haiß, A.; Kümmerer, K.; Gathergood, N.; et al. Biomass derived ionic liquids: Synthesis from natural organic acids, characterization, toxicity, biodegradation and use as solvents for catalytic hydrogenation processes. Tetrahedron 2013, 69, 6150–6161. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Q.; Chen, J.; Bai, G.; Zhuo, K. Interaction of gluconate-based ionic liquids with common solvents: A study of volumetric, viscosity and conductivity properties. J. Mol. Liq. 2016, 223, 1013–1020. [Google Scholar] [CrossRef]
- Reiß, M.; Brietzke, A.; Eickner, T.; Stein, F.; Villinger, A.; Vogel, C.; Kragl, U.; Jopp, S. Synthesis of novel carbohydrate based pyridinium ionic liquids and cytotoxicity of ionic liquids for mammalian cells. RSC Adv. 2020, 10, 14299–14304. [Google Scholar] [CrossRef]
- Kaur, N.; Chopra, H.K. Synthesis and applications of carbohydrate based chiral ionic liquids as chiral recognition agents and organocatalysts. J. Mol. Liq. 2020, 298, 111994. [Google Scholar] [CrossRef]
- Erfurt, K.; Wandzik, I.; Walczak, K.; Matuszek, K.; Chrobok, A. Hydrogen-bond-rich ionic liquids as effective organocatalysts for Diels-Alder reactions. Green Chem. 2014, 16, 3508–3514. [Google Scholar] [CrossRef]
- Brzęczek-Szafran, A.; Więcek, P.; Guzik, M.; Chrobok, A. Combining amino acids and carbohydrates into readily biodegradable, task specific ionic liquids. RSC Adv. 2020, 10, 18355–18359. [Google Scholar] [CrossRef]
- Ferlin, N.; Gatard, S.; Van Nhien, A.N.; Courty, M.; Bouquillon, S. Click reactions as a key step for an efficient and selective synthesis of D-xylose-based ILs. Molecules 2013, 18, 11512–11525. [Google Scholar] [CrossRef]
- McElroy, C.R.; Constantinou, A.; Jones, L.C.; Summerton, L.; Clark, J.H. Towards a holistic approach to metrics for the 21st century pharmaceutical industry. Green Chem. 2015, 17, 3111–3121. [Google Scholar] [CrossRef]
- Fukaya, Y.; Iizuka, Y.; Sekikawa, K.; Ohno, H. Bio ionic liquids: Room temperature ionic liquids composed wholly of biomaterials. Green Chem. 2007, 9, 1155–1157. [Google Scholar] [CrossRef]
- Tao, D.J.; Cheng, Z.; Chen, F.F.; Li, Z.M.; Hu, N.; Chen, X.S. Synthesis and thermophysical properties of biocompatible cholinium-based amino acid ionic liquids. J. Chem. Eng. Data 2013, 58, 1542–1548. [Google Scholar] [CrossRef]
- Marcinkowska, K.; Praczyk, T.; Gawlak, M.; Niemczak, M.; Pernak, J. Efficacy of herbicidal ionic liquids and choline salt based on 2,4-D. Crop. Prot. 2017, 98, 85–93. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, L.; Wen, P.; Ye, X.; Dong, R.; Sun, W.; Fan, M.; Yang, D.; Zhou, F.; Liu, W. The ecotoxicity and tribological properties of choline amino acid ionic liquid lubricants. Tribol. Int. 2018, 121, 435–441. [Google Scholar] [CrossRef]
- Gatard, S.; Plantier-Royon, R.; Remond, C.; Muzard, M.; Kowandy, C. Preparation of new β-D-xyloside- and β-D-xylobioside-based ionic liquids through chemical and/or enzymatic reactions. Carbohydr. Res. 2017, 451, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Thuy Pham, T.P.; Cho, C.W.; Yun, Y.S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Coleman, D.; Gathergood, N. Biodegradation studies of ionic liquids. Chem. Soc. Rev. 2010, 39, 600–637. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, F.; Zeng, L.; Wang, C.; Yang, Y.; Tan, Z. Assessment of the toxicity and biodegradation of amino acid-based ionic liquids. RSC Adv. 2019, 9, 10100–10108. [Google Scholar] [CrossRef]
- Erfurt, K.; Markiewicz, M.; Siewniak, A.; Zalewski, M.; Stolte, S.; Chrobok, A. Biodegradable surface active D-glucose based quaternary ammonium ionic liquids in the solventless synthesis of chloroprene. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Markiewicz, M.; Maszkowska, J.; Nardello-Rataj, V.; Stolte, S. Readily biodegradable and low-toxic biocompatible ionic liquids for cellulose processing. RSC Adv. 2016, 6, 87325–87331. [Google Scholar] [CrossRef]
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Synthesis and characterization of choline–fatty-acid-based ionic liquids: A new biocompatible surfactant. J. Colloid Interface Sci. 2019, 551, 72–80. [Google Scholar] [CrossRef]
- Mena, I.F.; Diaz, E.; Palomar, J.; Rodriguez, J.J.; Mohedano, A.F. Cation and anion effect on the biodegradability and toxicity of imidazolium– and choline–based ionic liquids. Chemosphere 2020, 240, 124947. [Google Scholar] [CrossRef]
- Ranke, J.; Müller, A.; Bottin-Weber, U.; Stock, F.; Stolte, S.; Arning, J.; Störmann, R.; Jastorff, B. Lipophilicity parameters for ionic liquid cations and their correlation to in vitro cytotoxicity. Ecotoxicol. Environ. Saf. 2007, 67, 430–438. [Google Scholar] [CrossRef]
- Pretti, C.; Renzi, M.; Ettore Focardi, S.; Giovani, A.; Monni, G.; Melai, B.; Rajamani, S.; Chiappe, C. Acute toxicity and biodegradability of N-alkyl-N-methylmorpholinium and N-alkyl-DABCO based ionic liquids. Ecotoxicol. Environ. Saf. 2011, 74, 748–753. [Google Scholar] [CrossRef]
- Frade, R.F.M.; Rosatella, A.A.; Marques, C.S.; Branco, L.C.; Kulkarni, P.S.; Mateus, N.M.M.; Afonso, C.A.M.; Duarte, C.M.M. Toxicological evaluation on human colon carcinoma cell line (CaCo-2) of ionic liquids based on imidazolium, guanidinium, ammonium, phosphonium, pyridinium and pyrrolidinium cations. Green Chem. 2009, 11, 1660–1665. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Del Sesto, R.E.; McCleskey, T.M.; Macomber, C.; Ott, K.C.; Koppisch, A.T.; Baker, G.A.; Burrell, A.K. Limited thermal stability of imidazolium and pyrrolidinium ionic liquids. Thermochim. Acta 2009, 491, 118–120. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, P.; Pałasiński, M.; Wiejak, S. The Thermal Decomposition of Carbohydrates. Part I. The Decomposition of Mono-, Di-, and Oligo-Saccharides. Adv. Carbohydr. Chem. Biochem. 1989, 47, 203–278. [Google Scholar]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Jayachandra, R.; Lakshmipathy, R.; Reddy, S.R. Hydrophobic d-galactose based ionic liquid for the sequestration of Pb2+ ions from aqueous solution. J. Mol. Liq. 2016, 219, 1172–1178. [Google Scholar] [CrossRef]
- Arellano, I.H.J.; Guarino, J.G.; Paredes, F.U.; Arco, S.D. Thermal stability and moisture uptake of 1-alkyl-3-methylimidazolium bromide. J. Therm. Anal. Calorim. 2011, 103, 725–730. [Google Scholar] [CrossRef]
- Montanino, M.; Carewska, M.; Alessandrini, F.; Passerini, S.; Appetecchi, G.B. The role of the cation aliphatic side chain length in piperidinium bis(trifluoromethansulfonyl)imide ionic liquids. Electrochim. Acta 2011, 57, 153–159. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Song, Y.; Xia, Y.; Liu, Z. Influence of cation structure on physicochemical and antiwear properties of hydroxyl-functionalized imidazolium bis(trifluoromethylsulfonyl)imide ionic liquids. Tribol. Trans. 2012, 55, 738–746. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. Viscosity of ionic liquids: An extensive database and a new group contribution model based on a feed-forward artificial neural network. J. Chem. Inf. Model. 2014, 54, 1311–1324. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Branco, L.C.; Crespo, J.G.; Nunes, M.C.; Raymundo, A.; Afonso, C.A.M. Comparison of physicochemical properties of new ionic liquids based on imidazolium, quaternary ammonium, and guanidinium cations. Chem. Eur. J. 2007, 13, 8478–8488. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Chong, A.L.; Forsyth, M.; Kar, M.; Vijayaraghavan, R.; Somers, A.; Pringle, J.M. New dimensions in salt-solvent mixtures: A 4th evolution of ionic liquids. Faraday Discuss. 2018, 206, 9–28. [Google Scholar] [CrossRef]
- Azov, V.A.; Egorova, K.S.; Seitkalieva, M.M.; Kashina, A.S.; Ananikov, V.P. “Solvent-in-salt” systems for design of new materials in chemistry, biology and energy research. Chem. Soc. Rev. 2018, 47, 1250–1284. [Google Scholar] [CrossRef]
- Bonĥte, P.; Dias, A.P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Monkos, K. Determination of the glass-transition temperature of proteins from a viscometric approach. Int. J. Biol. Macromol. 2015, 74, 1–4. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Matsumoto, H.; Tatsumi, K. Low-melting, low-viscous, hydrophobic ionic liquids: Aliphatic quaternary ammonium salts with perfluoroalkyltrifluoroborates. Chem. Eur. J. 2005, 11, 752–766. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Matsumoto, H.; Tatsumi, K. Low-melting, low-viscous, hydrophobic ionic liquids: 1-Alkyl(alkyl ether)-3-methylimidazolium perfluoroalkyltrifluoroborate. Chem. Eur. J. 2004, 10, 6581–6591. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Kang, S.; Chung, Y.G.; Kang, J.H.; Song, H. CO2 absorption characteristics of amino group functionalized imidazolium-based amino acid ionic liquids. J. Mol. Liq. 2020, 297, 111825. [Google Scholar] [CrossRef]
- Santiago, R.; Lemus, J.; Moya, C.; Moreno, D.; Alonso-Morales, N.; Palomar, J. Encapsulated ionic liquids to enable the practical application of amino acid-based ionic liquids in CO2 capture. ACS Sustain. Chem. Eng. 2018, 6, 14178–14187. [Google Scholar] [CrossRef]
- Guzmán, J.; Ortega-Guevara, C.; de León, R.G.; Martínez-Palou, R. Absorption of CO2 with amino acid-based ionic liquids and corresponding amino acid precursors. Chem. Eng. Technol. 2017, 40, 2339–2345. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, K.; Ren, S.; Hou, Y.; Wu, W. Efficient capture of low partial pressure H2S by tetraethyl ammonium amino acid ionic liquids with absorption-promoted solvents. RSC Adv. 2016, 6, 101462–101469. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, S.; Hou, Y.; Zhang, K.; Zhang, Q.; Wu, W. Highly reversible and efficient absorption of low-concentration no by amino-acid-based ionic liquids. ACS Sustain. Chem. Eng. 2020, 8, 3283–3290. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Zhang, S.; Dokko, K.; Watanabe, M. Carbon materialization of ionic liquids: From solvents to materials. Mater. Horizons 2015, 2, 168–197. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaida, B.; Brzęczek-Szafran, A. Insights into the Properties and Potential Applications of Renewable Carbohydrate-Based Ionic Liquids: A Review. Molecules 2020, 25, 3285. https://doi.org/10.3390/molecules25143285
Gaida B, Brzęczek-Szafran A. Insights into the Properties and Potential Applications of Renewable Carbohydrate-Based Ionic Liquids: A Review. Molecules. 2020; 25(14):3285. https://doi.org/10.3390/molecules25143285
Chicago/Turabian StyleGaida, Bartłomiej, and Alina Brzęczek-Szafran. 2020. "Insights into the Properties and Potential Applications of Renewable Carbohydrate-Based Ionic Liquids: A Review" Molecules 25, no. 14: 3285. https://doi.org/10.3390/molecules25143285
APA StyleGaida, B., & Brzęczek-Szafran, A. (2020). Insights into the Properties and Potential Applications of Renewable Carbohydrate-Based Ionic Liquids: A Review. Molecules, 25(14), 3285. https://doi.org/10.3390/molecules25143285