Influence of Tethered Ions on Electric Polarization and Electrorheological Property of Polymerized Ionic Liquids
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
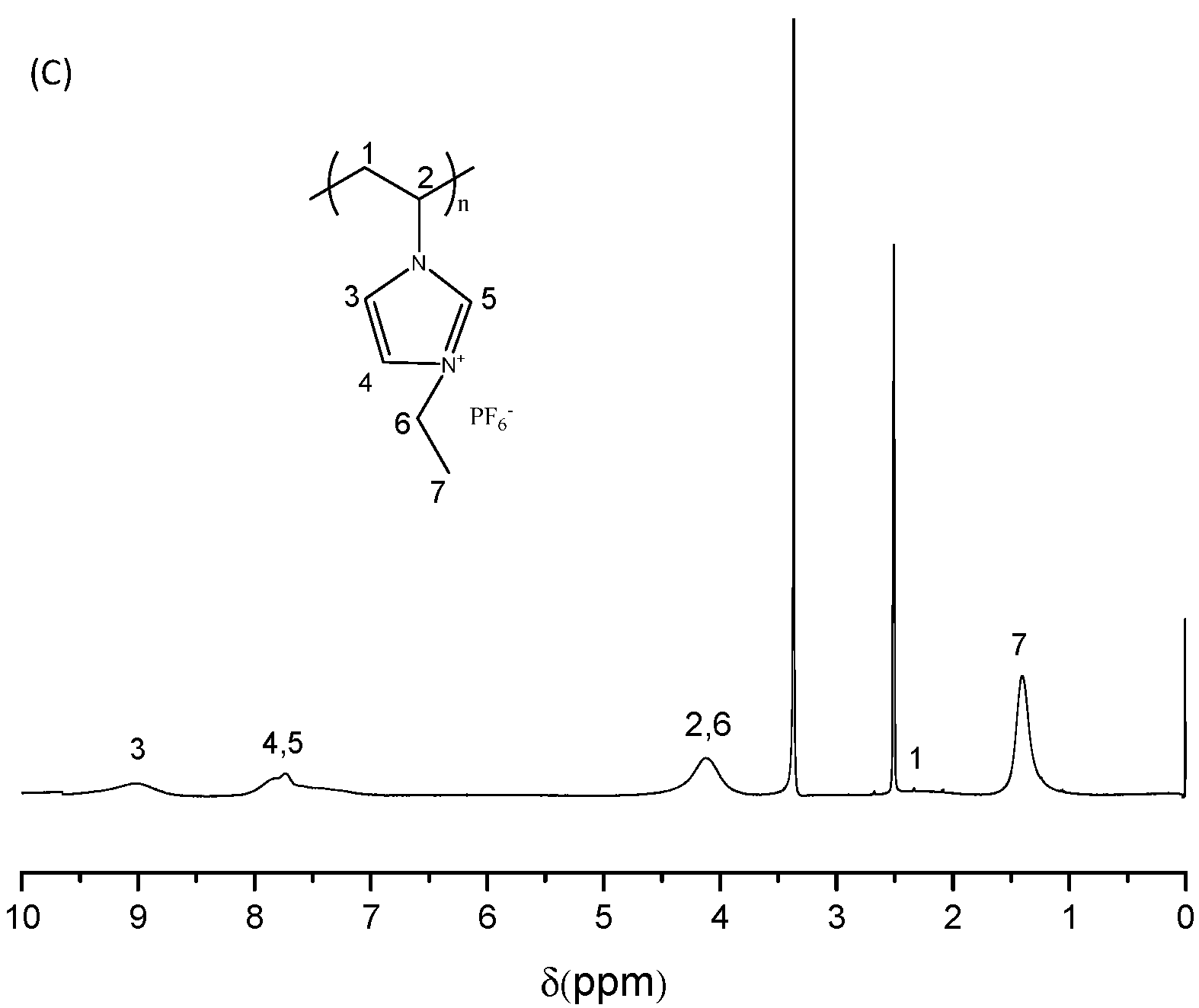
2.2. Synthesis of [C2VIm]Br
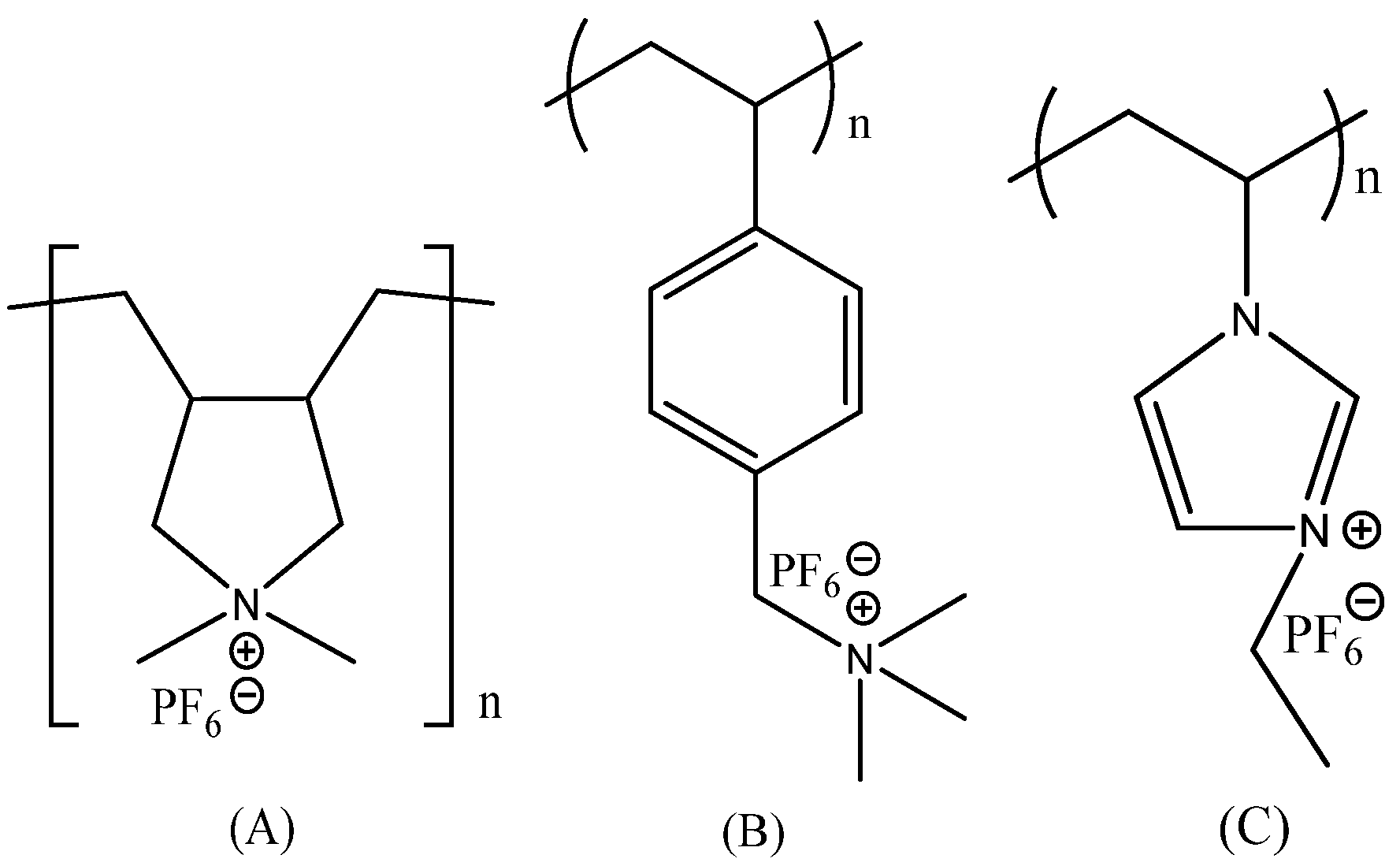
2.3. Synthesis of PILs
2.4. Preparation of ERFs
2.5. Characterization and Measurements
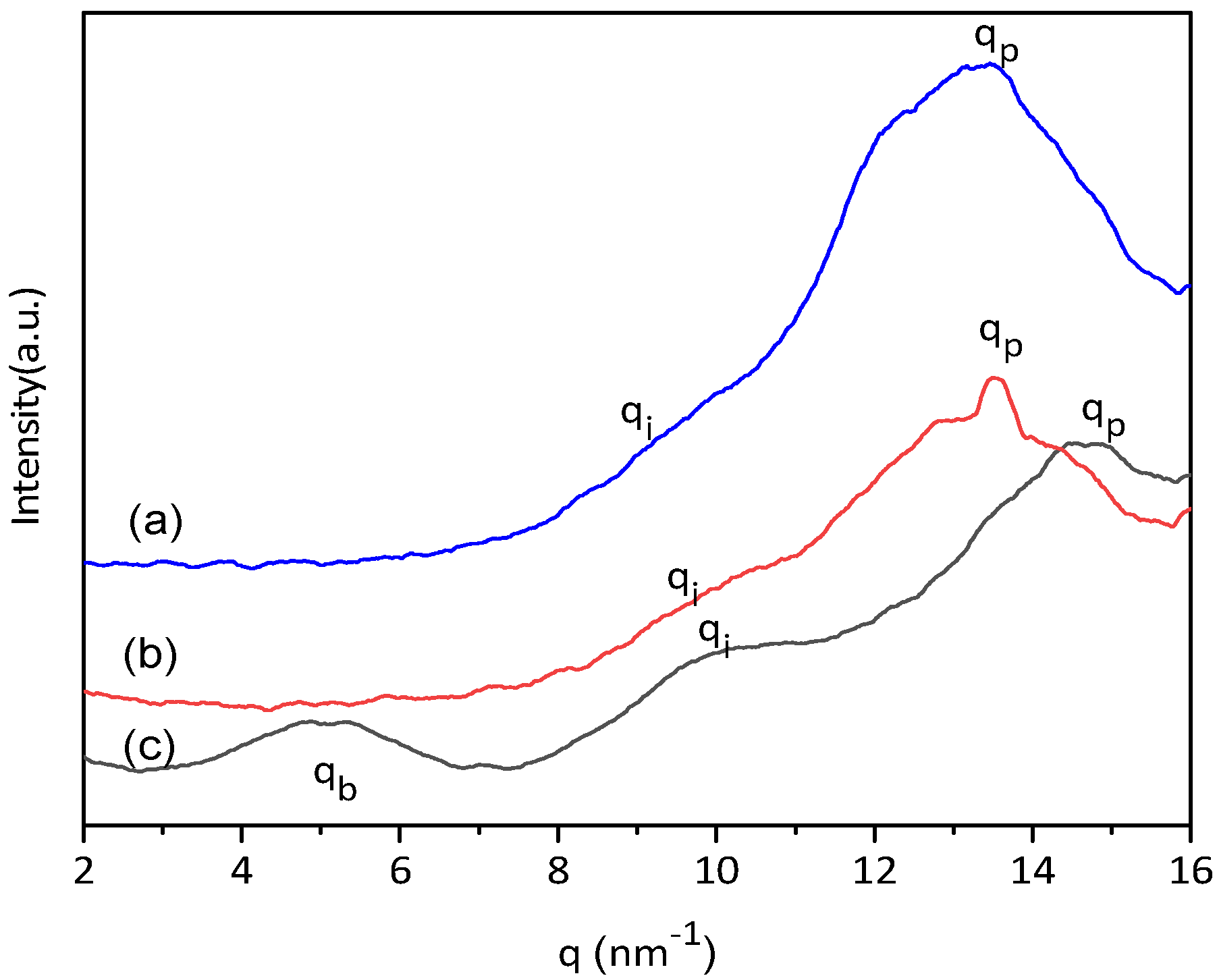
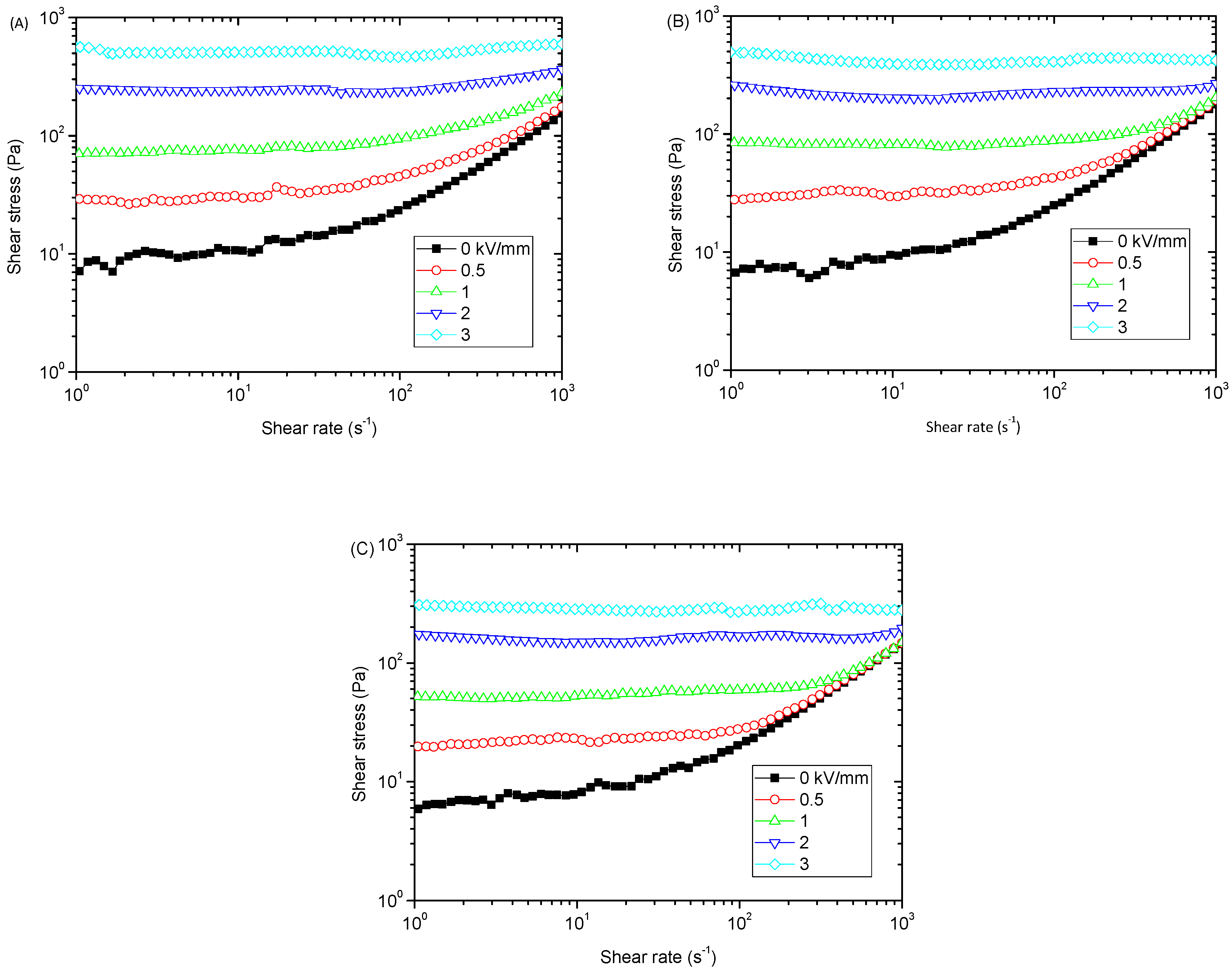
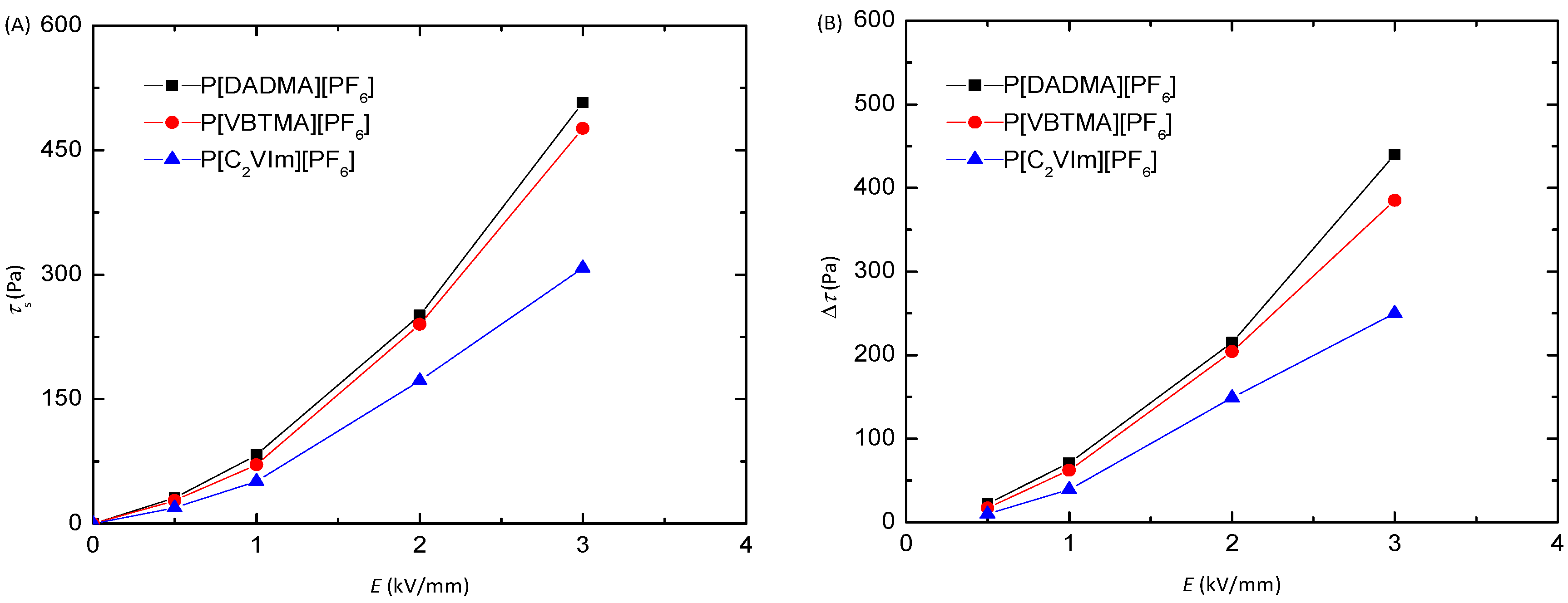
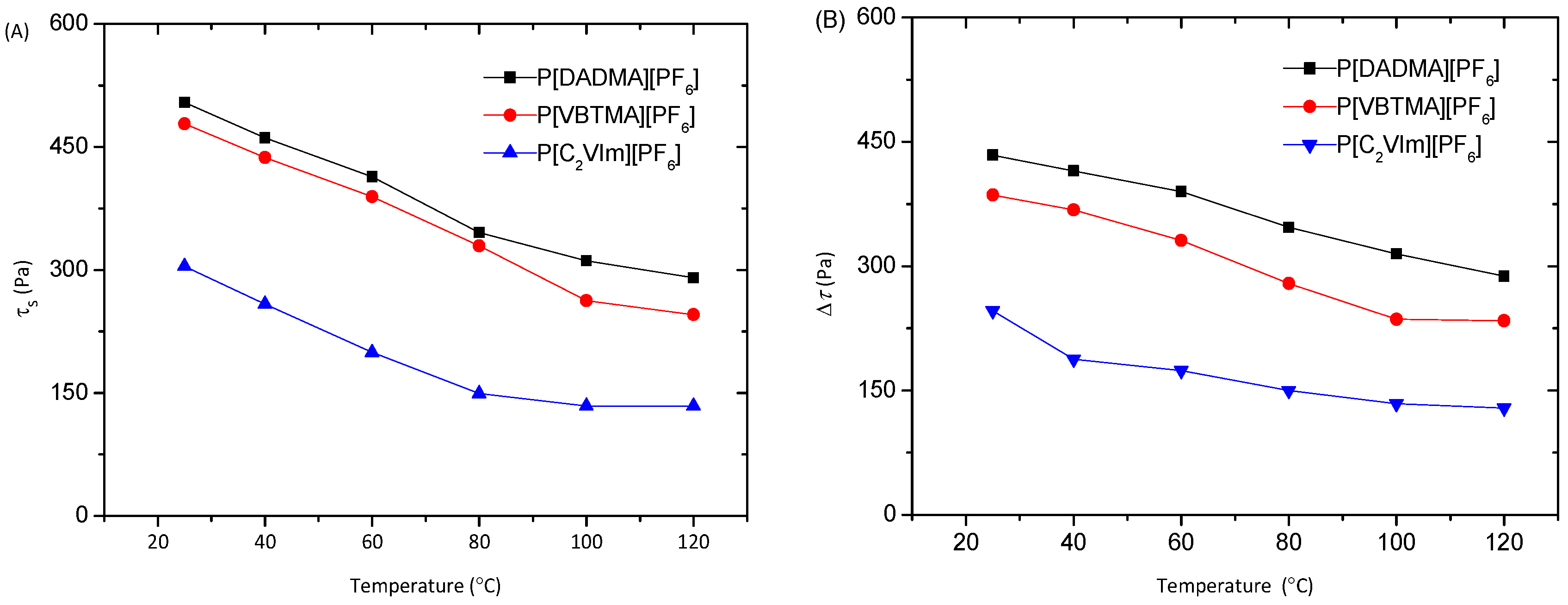
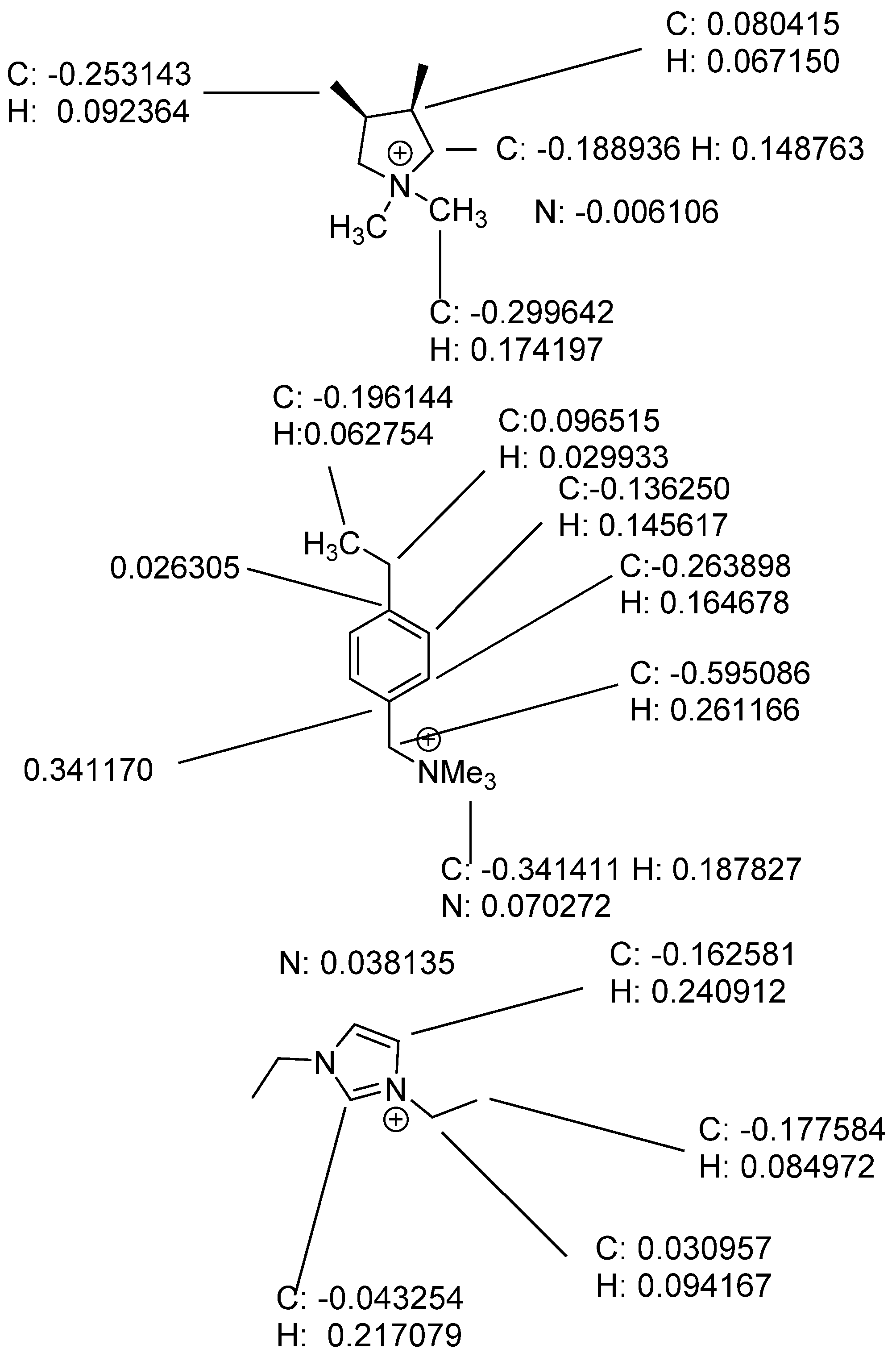
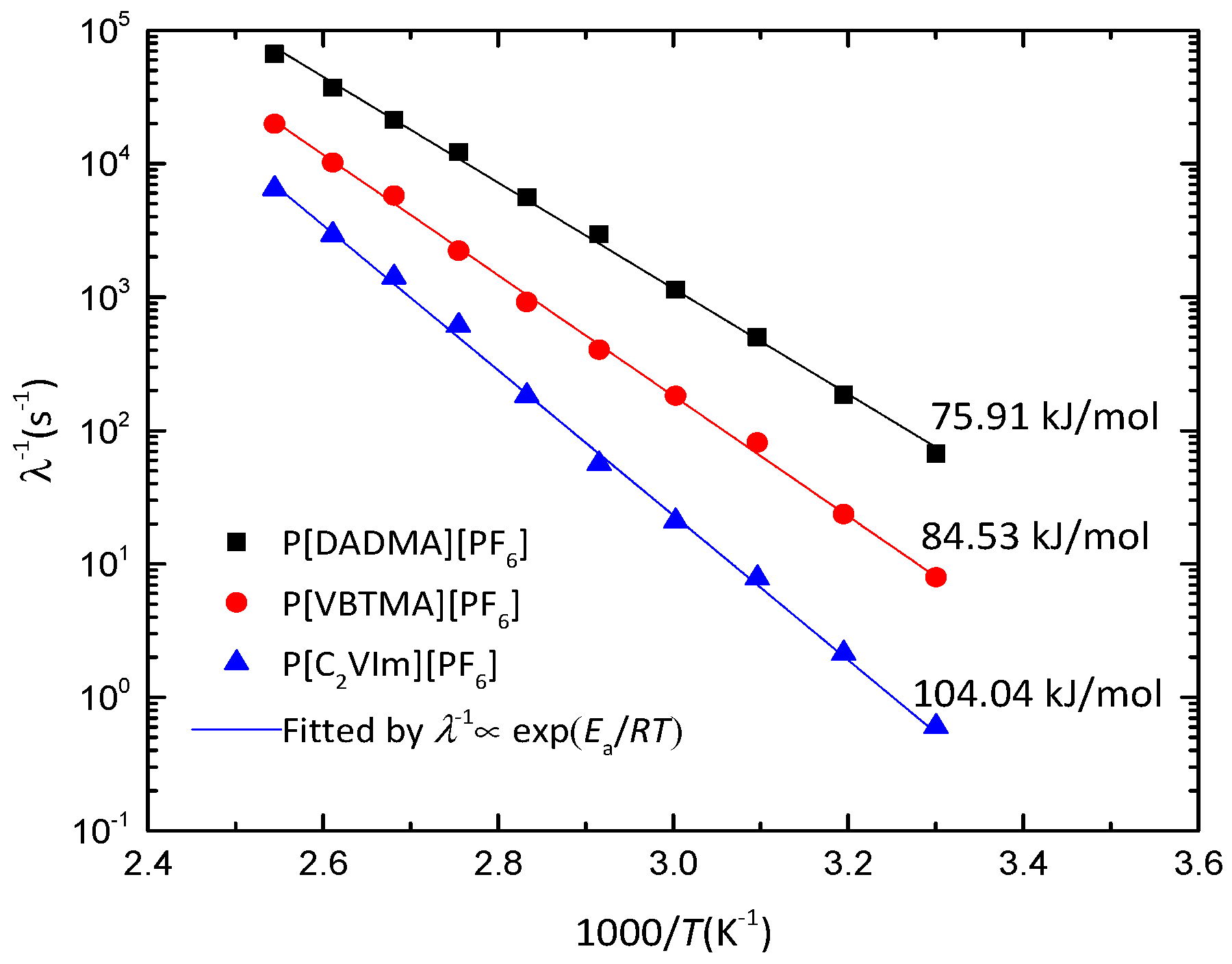
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Guo, J.; Xu, D.; Yan, F. Thermo- and pH-responsive poly(ionic liquid) membranes. Polym. Chem. 2016, 7, 1330–1336. [Google Scholar] [CrossRef]
- Zhao, Q.; Dunlop, J.W.C.; Qiu, X.; Huang, F.; Zhang, Z.; Heyda, J.; Dzubiella, J.; Antonietti, M.; Yuan, J. An instant multi-responsive porous polymer actuator driven by solvent molecule sorption. Nat. Commun. 2014, 5, 4293. [Google Scholar] [CrossRef]
- Gao, R.; Wang, D.; Heflin, J.R.; Long, T.E. Imidazolium sulfonate-containing pentablock copolymer–ionic liquid membranes for electroactive actuators. J. Mater. Chem. 2012, 22, 13473–13476. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, J.; Zhao, X. Microwave-synthesized poly(ionic liquid) particles: A new material with high electrorheological activity. J. Mater. Chem. 2014, 2, 9812–9819. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, J.; Yuan, J.; Zhao, X. Microwave-assisted synthesis and high-performance anhydrous electrorheological characteristic of monodisperse poly(ionic liquid) particles with different size of cation/anion parts. Polymer 2016, 97, 408–417. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Seo, Y.; Choi, H.J. Recent development of electro-responsive smart electrorheological fluids. Soft Matter 2019, 15, 3473–3486. [Google Scholar] [CrossRef] [PubMed]
- Coulter, J.P.; Weiss, K.D.; Carlson, J.D. Engineering applications of electrorheological materials. J. Intel. Mater. Syst. Struct. 1993, 4, 248–259. [Google Scholar] [CrossRef]
- Zatopa, A.; Walker, S.; Menguc, Y. Fully soft 3D-pinted electroactive fluidic valve for soft hydraulic robots. Soft Robotics 2018, 5, 258–271. [Google Scholar] [CrossRef]
- Oh, J.S.; Choi, S.B. State of the art of medical devices featuring smart electro-rheological and magneto-rheological fluids. J. King. Saud. Univ. Sci. 2017, 29, 390–400. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Dong, Y.; Zhao, X.; Yin, J. Distinctly Different Electroresponsive Electrorheological Effect in Low-Molecular-Weight and Polymerized Ionic Liquids: Rheological and Dielectric Relaxation Studies. J. Phys. Chem. B 2018, 122, 12184–12193. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liu, Y.; Wang, B.; Xiang, L.; Zhao, X.; Yin, J. Influence of counterion type on dielectric and electrorheological responses of poly(ionic liquid)s. Polymer 2017, 132, 273–285. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Hao, B.N.; Zhang, H.; Wang, M.; Liu, Y.D. Fabrication of imidazolium-based poly(ionic liquid) microspheres and their electrorheological responses. J. Mater. Sci. 2017, 52, 5778–5787. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Zheng, C.; Liu, Y.; Zhao, X.; Yin, J. Enhanced interfacial polarization and electro-responsive characteristic of di-ionic poly(ionic liquid)s. Polymer 2019, 182, 121847. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Liu, Y.; Zhao, X.; Yin, J. Nonmonotonic Influence of Size of Quaternary Ammonium Countercations on Micromorphology, Polarization, and Electroresponse of Anionic Poly(ionic liquid)s. J. Phys. Chem B 2020, 124, 2920–2929. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, J.H.; Dong, Y.Z.; Zhao, X.P.; Yin, J.B. Enhanced temperature effect of electrorheological fluid based on cross-linked poly(ionic liquid) particles: Rheological and dielectric relaxation studies. Soft Matter 2017, 13, 1027–1039. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, B.; Xiang, L.; Liu, Y.; Zhao, X.; Yin, J. Influence of side chain sizes on dielectric and electrorheological responses of poly(ionic liquid)s. J. Phys. Chem. B 2017, 121, 6226–6237. [Google Scholar] [CrossRef] [PubMed]
- Bodo, E. Structural features of triethylammonium acetate through molecular dynamics. Molecules 2020, 25, 1432. [Google Scholar] [CrossRef]
- Maksym, P.; Tarnacka, M.; Dzienia, A.; Erfurt, K.; Chrobok, A.; Zieba, A.; Wolnica, K.; Kaminski, K.; Paluch, M. A facile route to well-defined imidazolium-based poly(ionic liquid)s of enhanced conductivity via RAFT. Polym. Chem. 2017, 8, 5433–5443. [Google Scholar] [CrossRef]
- Taghavikish, M.; Subianto, S.; Dutta, N.K.; Choudhury, N.R. Facile fabrication of polymerizable ionic liquid based-gel beads via thiol−ene chemistry. Acs Appl. Mater. Interfaces 2015, 7, 17298–17306. [Google Scholar] [CrossRef] [PubMed]
- Colin, E.; Keith Izod, P.B.; Smith, J.D. The synthesis and crystal structures of rbc(sime3)3 and csc(sime3)3·3.5 c6h6: A one-dimensional ionic solid and an ionic solid with a molecular structure. Angew. Chem. Inter. Ed. 1995, 34, 687–688. [Google Scholar]
- Fei, Z.F.; Ang, W.H.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Ionic solid-state dimers and polymers derived from imidazolium dicarboxylic acids. Chem.: A Eur. J. 2006, 12, 4014–4020. [Google Scholar] [CrossRef]
- Heres, M.; Cosby, T.; Mapesa, E.U.; Liu, H.; Berdzinski, S.; Strehmel, V.; Dadmun, M.; Paddison, S.J.; Sangoro, J. Ion transport in glassy polymerized ionic liquids: Unraveling the impact of the molecular structure. Macromolecules 2018, 52, 88–95. [Google Scholar] [CrossRef]
- Buitrago, C.; Francisco, C.; Bolintineanu, D.S.; Seitz, M.E.; Opper, K.L.; Wagener, K.B.; Stevens, M.J.; Frischknecht, A.L.; Winey, K.I. Direct comparisons of X-ray scattering and atomistic molecular dynamics simulations for precise acid copolymers and ionomers. Macromolecules 2015, 48, 1210–1220. [Google Scholar] [CrossRef]
- La Cruz, D.S.; Green, M.D.; Ye, Y.; Elabd, Y.A.; Long, T.E.; Winey, K.I. Correlating backbone-to-backbone distance to ionic conductivity in amorphous polymerized ionic liquids. J. Polym. Sci. Part. B 2012, 50, 338–346. [Google Scholar] [CrossRef]
- Weiss, K.D.; Carlson, J.D.; Coulter, J.P. Review: Material Aspects of Electrorheological Systems. J. Intell. Mater. Syst. Struct. 1993, 4, 13–34. [Google Scholar] [CrossRef]
- Ikazaki, F.; Kawai, A.; Uchida, K.; Kawakami, T.; Edamura, K.; Sakurai, K.; Anzai, H.; Asako, Y. Mechanisms of electrorheology: The effect of the dielectric property. J. Phys. D 1998, 31, 336–347. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Yin, J. Interfacial Polarization and Electro-responsive Electrorheological Effect of Anionic and Cationic Poly(ionic liquid)s. Acs Appl. Polym. Mater. 2019, 1, 2862–2874. [Google Scholar] [CrossRef]
- Coelho, R.; Aladenize, B. Les diélectriques, les propretés diélectriques des matériaux isolants; Hermes Press: New Castle, PA, USA, 1993. [Google Scholar]
- Kremer, F.; Schönhals, A. Broadband dielectric spectroscopy; Springer Science & Business Media: Berlin, Germany, 2002. [Google Scholar]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Klein, R.J.; Zhang, S.; Dou, S.; Jones, B.H.; Colby, R.H.; Runt, J. Modeling electrode polarization in dielectric spectroscopy: Ion mobility and mobile ion concentration of single-ion polymer electrolytes. J. Chem. Phys. 2006, 124, 144903. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Saiwaki, T.; Fukao, K. Dielectric Relaxation Behavior of Polymerized Ionic Liquid. Macromolecules 2010, 43, 6092–6098. [Google Scholar] [CrossRef]
- Nakamura, K.; Fukao, K.; Inoue, T. Dielectric Relaxation and Viscoelastic Behavior of Polymerized Ionic Liquids with Various Counteranions. Macromolecules 2012, 45, 3850–3858. [Google Scholar] [CrossRef]
- Kisliuk, A.; Bocharova, V.; Popov, I.; Gainaru, C.; Sokolov, A.P. Fundamental parameters governing ion conductivity in polymer electrolytes. Electrochim. Acta 2019, 299, 191–196. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds P[DADMA][PF6], P[VBTMA][PF6] and P[C2VIm][PF6] are available from the authors. |



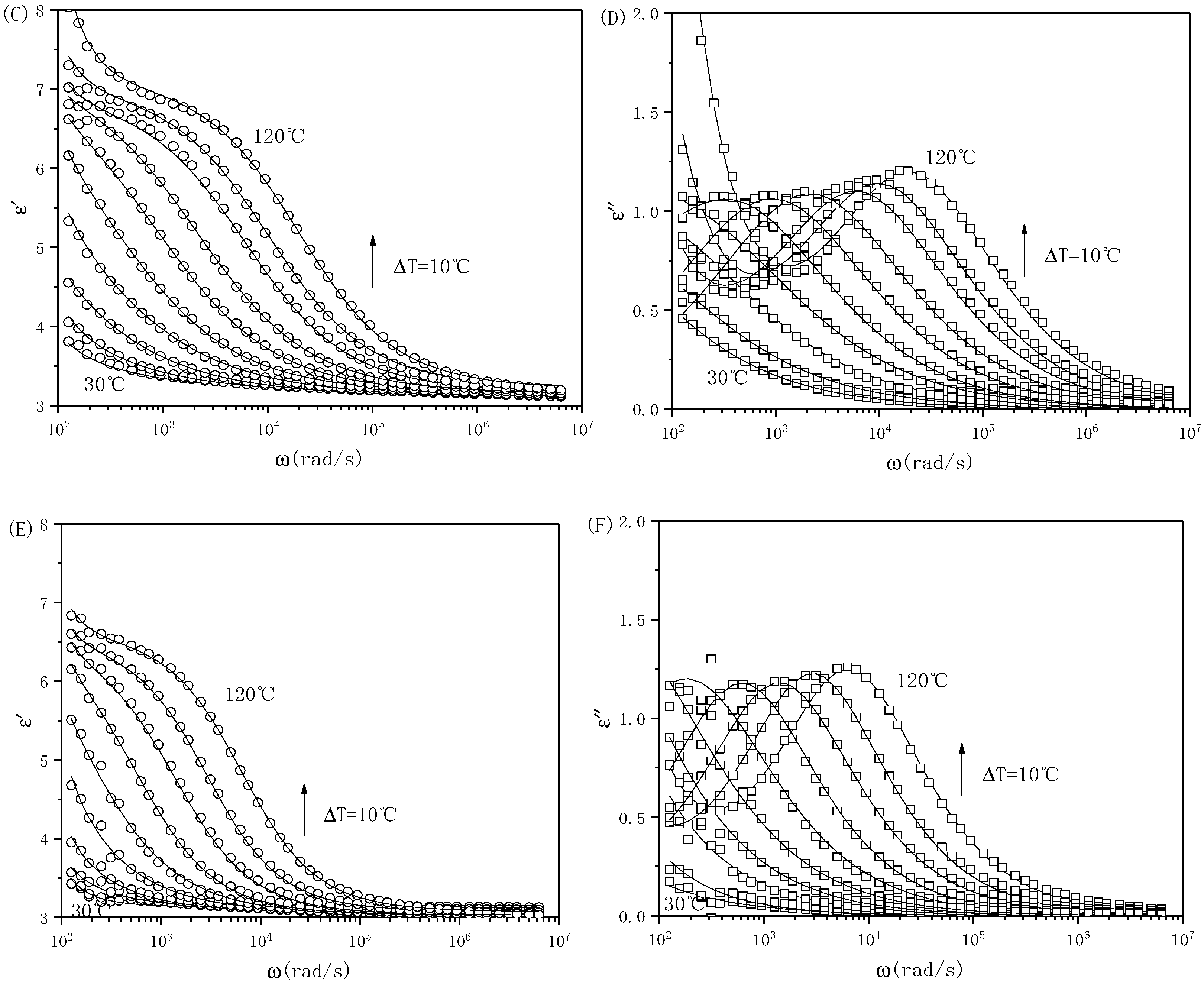
| Sample | ε′0 | ε′∞ | ∆ε′ | λ(s) | σp(S/m) |
|---|---|---|---|---|---|
| P[DADMA][PF6] | 6.93 | 3.20 | 3.73 | 0.015 | 8.3 × 10−9 |
| P[VBTMA][PF6] | 6.63 | 3.18 | 3.45 | 0.125 | 4.8 × 10−9 |
| P[C2VIm][PF6] | 6.53 | 3.15 | 3.38 | 1.657 | 2.6 × 10−9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.; Wang, B.; Zhao, J.; Zhao, X.; Yin, J. Influence of Tethered Ions on Electric Polarization and Electrorheological Property of Polymerized Ionic Liquids. Molecules 2020, 25, 2896. https://doi.org/10.3390/molecules25122896
He F, Wang B, Zhao J, Zhao X, Yin J. Influence of Tethered Ions on Electric Polarization and Electrorheological Property of Polymerized Ionic Liquids. Molecules. 2020; 25(12):2896. https://doi.org/10.3390/molecules25122896
Chicago/Turabian StyleHe, Fang, Bo Wang, Jia Zhao, Xiaopeng Zhao, and Jianbo Yin. 2020. "Influence of Tethered Ions on Electric Polarization and Electrorheological Property of Polymerized Ionic Liquids" Molecules 25, no. 12: 2896. https://doi.org/10.3390/molecules25122896
APA StyleHe, F., Wang, B., Zhao, J., Zhao, X., & Yin, J. (2020). Influence of Tethered Ions on Electric Polarization and Electrorheological Property of Polymerized Ionic Liquids. Molecules, 25(12), 2896. https://doi.org/10.3390/molecules25122896







