Synthesis and Antitumor Activity of Doxycycline Polymeric Nanoparticles: Effect on Tumor Apoptosis in Solid Ehrlich Carcinoma
Abstract
1. Introduction
2. Results
2.1. Estimation of Encapsulation Efficiency and Loading Capacity of DOXY-PNPs
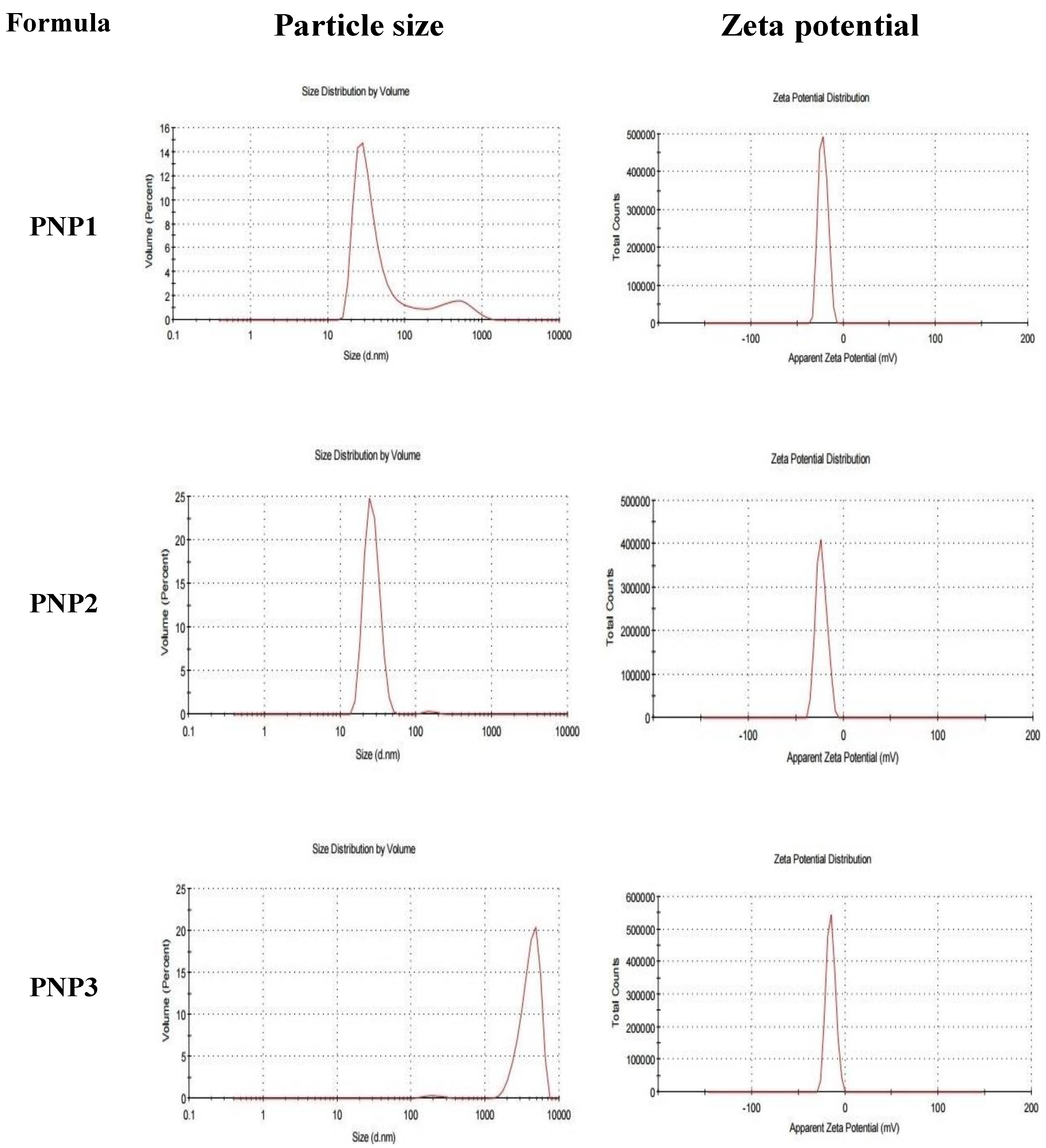
2.2. The Particle Size, ZP and PDI of DOXY-PNPs
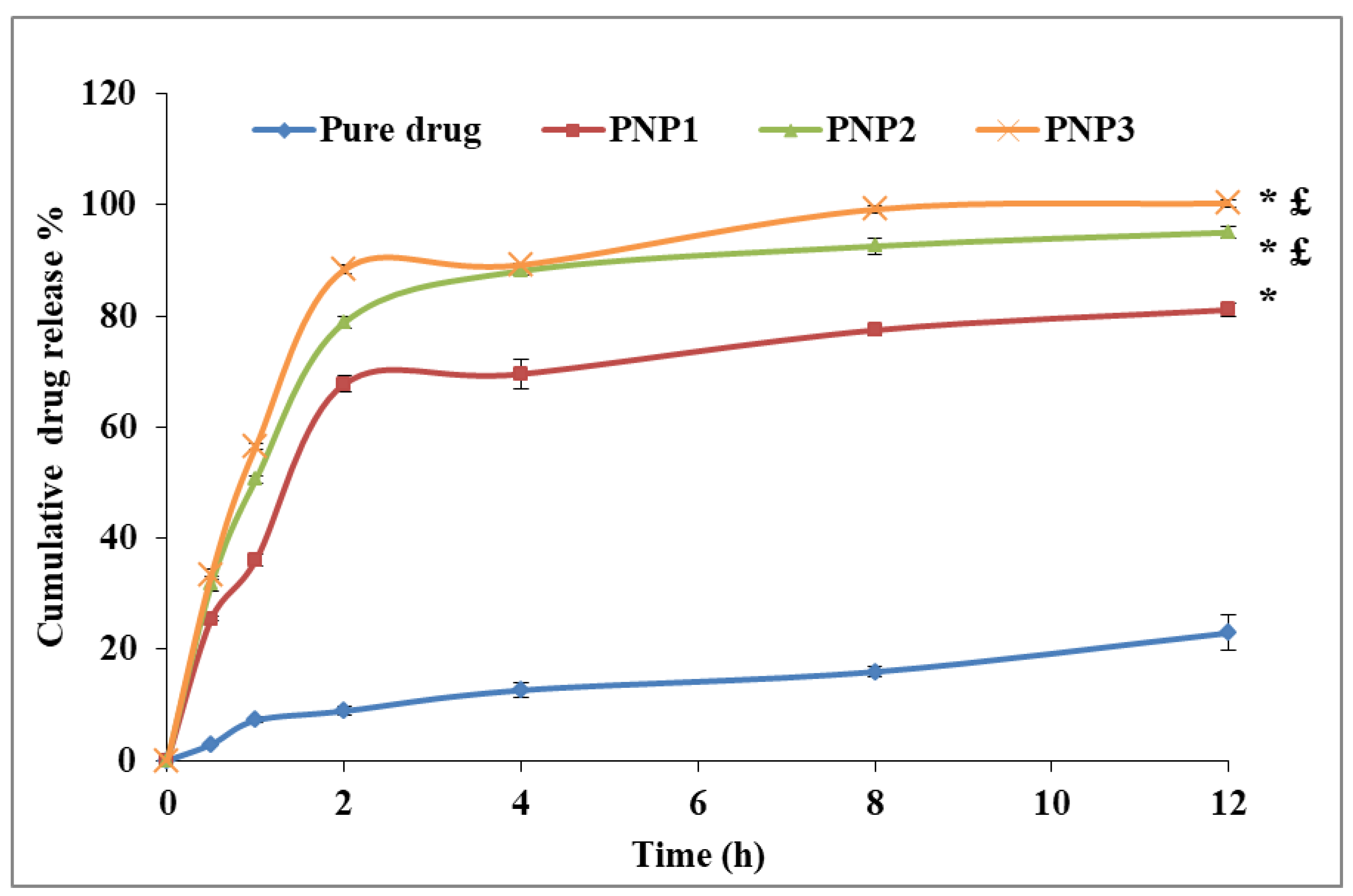
2.3. The In-Vitro Release of DOXY from the Prepared PNPs
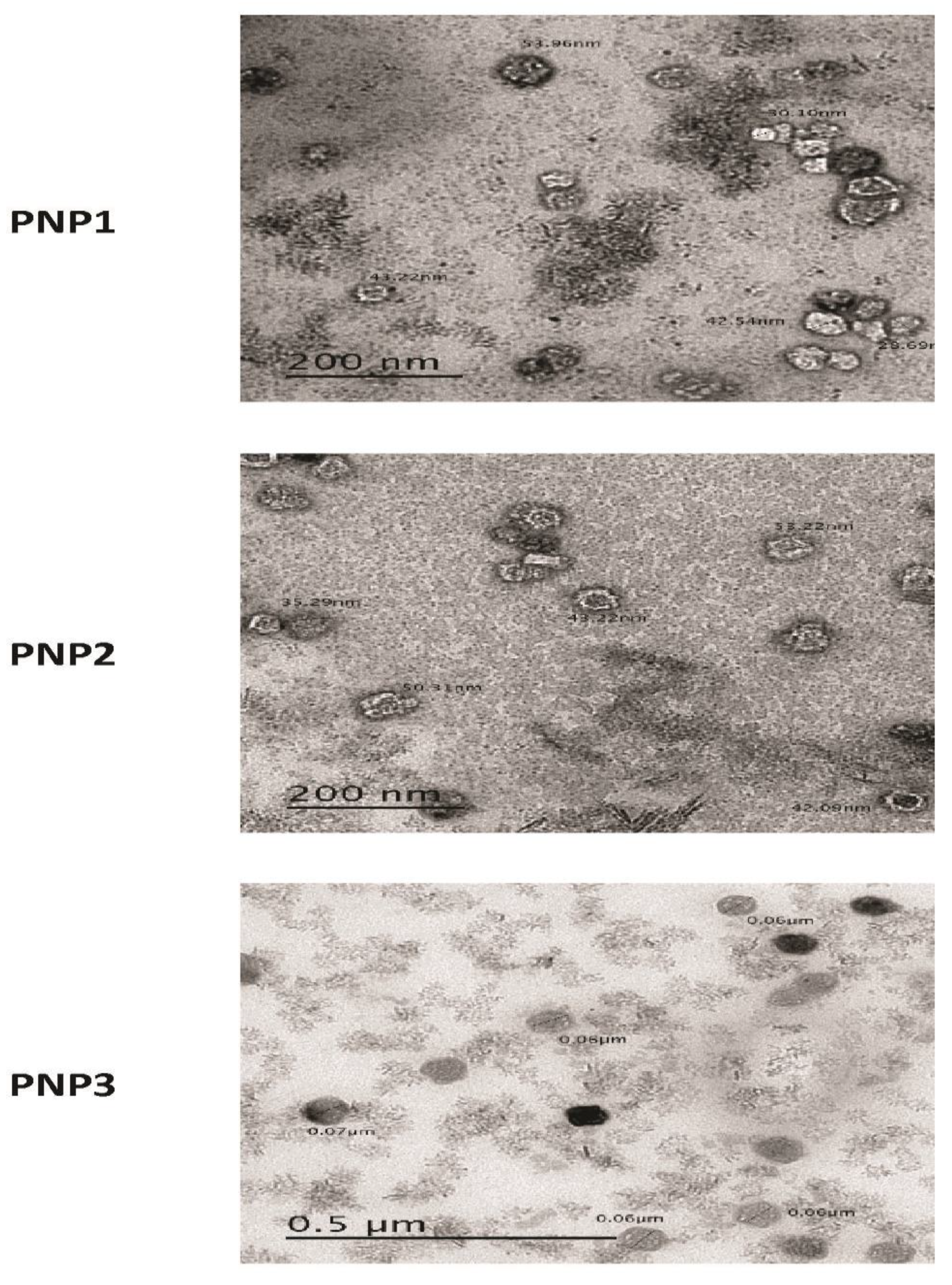
2.4. The Surface Morphology of the Prepared DOXY-PNPs
2.5. Selecting the Best Formulation
2.6. In-Vivo Pharmacological Activity for DOXY-PNP3
2.6.1. Survival Data
2.6.2. Mass of the Solid Tumors
2.6.3. Quantitative Polymerase Chain Reaction for Tumoral mRNA Expression of BAX and Caspase 3
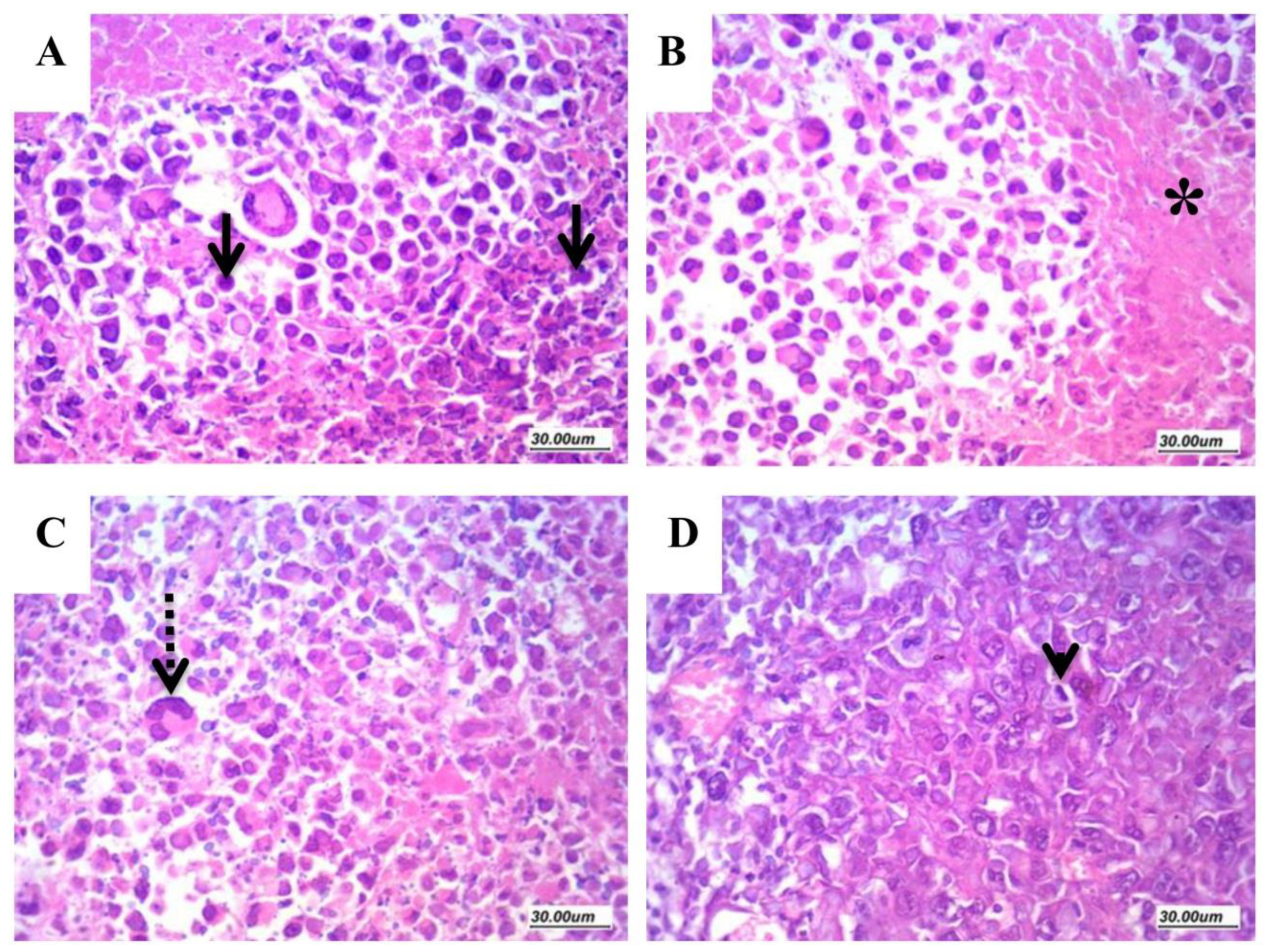
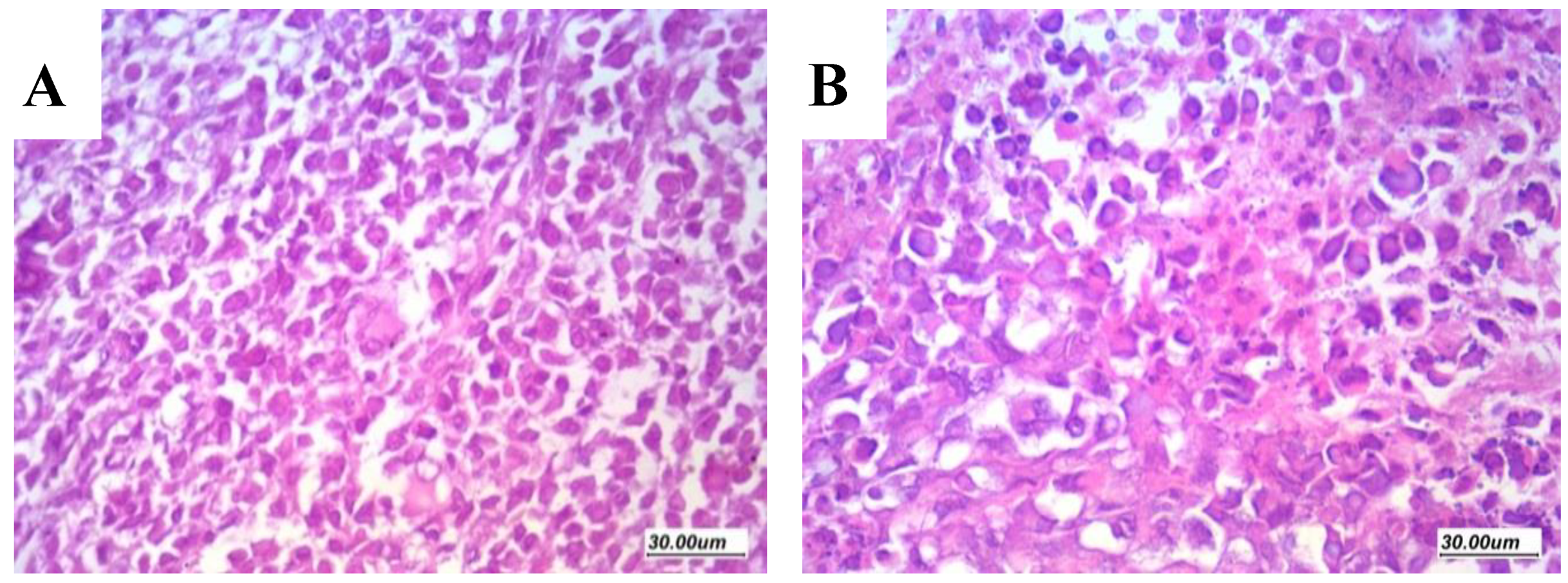
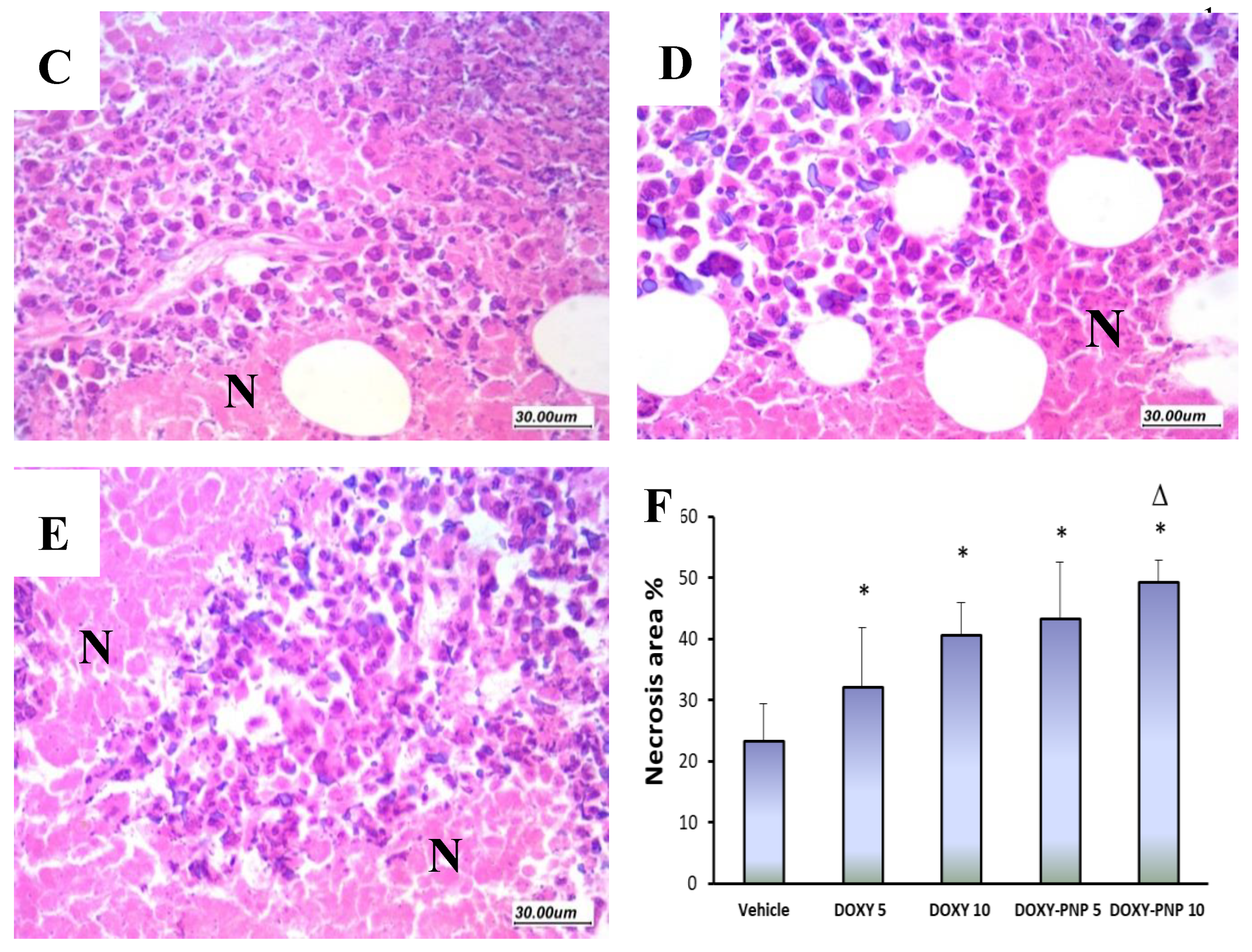
2.6.4. Assessment of Histologic Features and Tumor Necrosis in Sections Stained with H&E
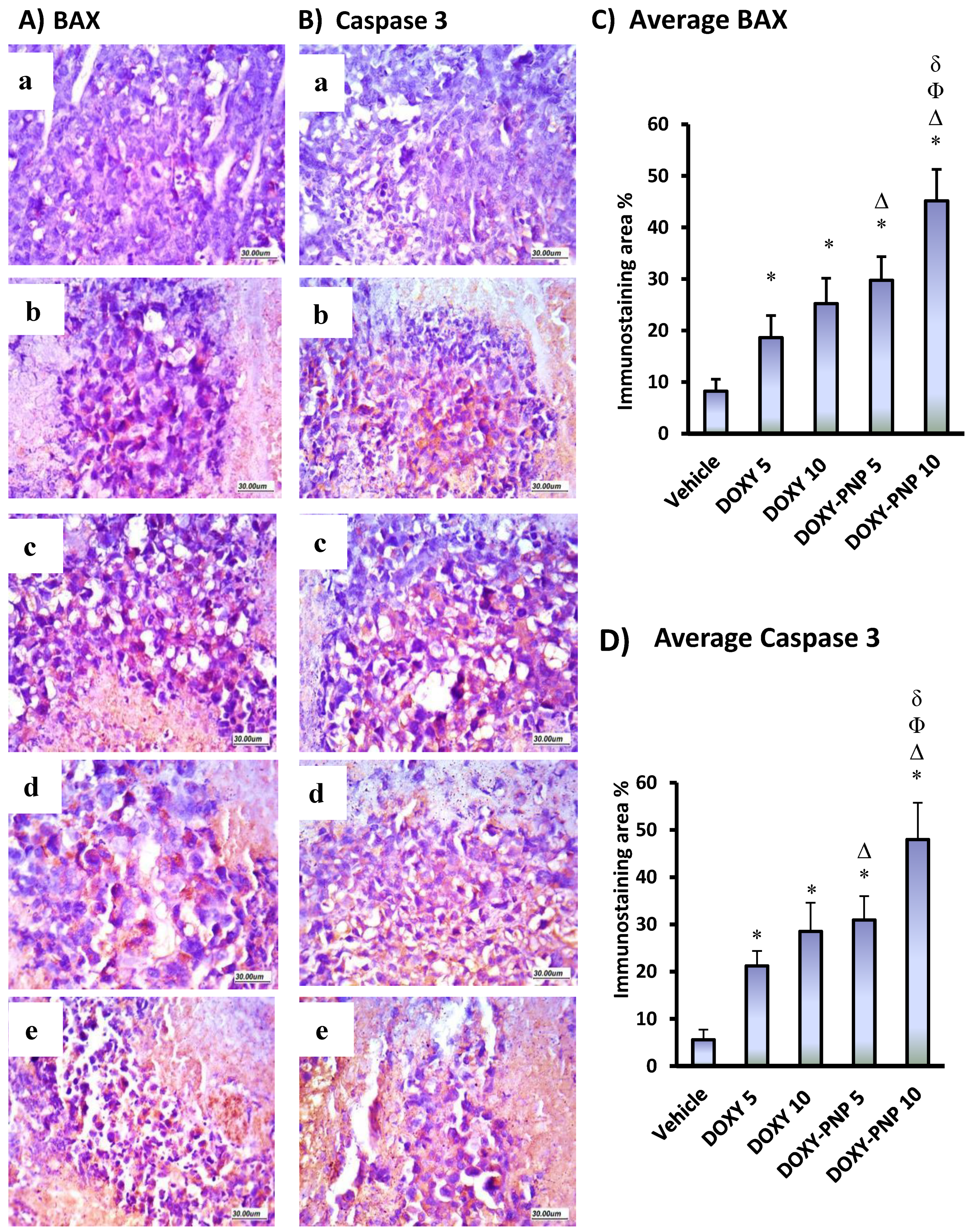
2.6.5. BAX and Caspase 3 Immunostaining in the Solid Tumors
2.6.6. Simple Linear Regression Analysis for the Tumor Data
3. Discussion
3.1. Characterization of DOXY-PNPs
3.2. In-Vivo Pharmacological Activity of DOXY-PNPs
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Doxycycline Polymeric Nanoparticles
4.2.1. Doxycycline Polymeric Nanoparticle Preparation
4.2.2. Estimation of DOXY-PNPs Encapsulation Efficiency and Loading Capacity
4.2.3. Determination of Particle Size, Zeta Potential and Polydispersity Index of the Prepared DOXY-PNPs
4.2.4. The Surface Morphology Determination Using Transmission Electron Microscopy
4.2.5. The In-vitro Release Study of DOXY from Prepared PNPs
4.2.6. The Selection of the Best Formulation of DOXY-PNPs
4.3. The Pharmacological Activity of DOXY-PNPs
4.3.1. Animals
4.3.2. Inoculation of Ehrlich Solid Tumors in Female Mice
4.3.3. Experimental Design
4.3.4. Sacrification and Separation of Solid Tumors
4.3.5. Quantitative polymerase chain reaction (PCR) of caspase 3 and BAX genes
- RNA Extraction: Homogenized tumor samples from the study groups were lysed and RNAeasy Mini Kit (Qiagen) Kit was used for isolation of total RNA which was examined for both quality and quantity with a dual spectrophotometer (Beckman, Waltham, MA, USA).
- Real Time PCR: For quantitative determination of mRNAs expression of caspase 3 and BAX, the following procedure was performed. A quantity of 10 ng of the total RNA was directed for the synthesis of cDNA employing the Applied Biosystems high capacity cDNA reverse transcriptase kit (USA). Amplification of the cDNA was then achieved in a 48-well plate by Syber Green I PCR Master Kit (Fermentas) using the Step One instrument (Applied Biosystems, Waltham, MA, USA). Alterations in gene expression were normalized to the mean critical threshold values obtained with GAPDH by the ΔΔCt method [82]. 1 μM of the gene primers was used in the assay and are demonstrated in Table 3.
4.3.6. Histologic Investigation of the Solid Ehrlich Carcinomas
4.3.7. Immunohistochemical Determination of BAX and Caspase 3
4.3.8. Data Analysis
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mishra, S.; Tamta, A.K.; Sarikhani, M.; Desingu, P.A.; Kizkekra, S.M.; Pandit, A.S.; Kumar, S.; Khan, D.; Raghavan, S.C.; Sundaresan, N.R. Subcutaneous Ehrlich Ascites Carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep. 2018, 8, 5599. [Google Scholar] [CrossRef] [PubMed]
- Gardouh, A.R.; Barakat, B.M.; Qushawy, M.K.E.; El-kazzaz, A.Y.; Sami, M.M.; Zaitone, S.A. Antitumor activity of a molecularly imprinted nanopreparation of 5-flurouracil against Ehrlich’s carcinoma solid tumors grown in mice: Comparison to free 5-flurouracil. Chem. Biol. Interact. 2018, 295, 52–63. [Google Scholar] [CrossRef]
- El-Far, M.; Salah, N.; Essam, A.; Abd El-Azim, A.; Karam, M.; El-Sherbiny, I.M. Potential anticancer activity and mechanism of action of nanoformulated curcumin in experimental Ehrlich ascites carcinoma-bearing animals. Nanomedicine 2019, 14, 553–573. [Google Scholar] [CrossRef]
- Elmeligie, S.; Ahmed, E.M.; Abuel-Maaty, S.M.; Zaitone, S.A.-B.; Mikhail, D.S. Design and Synthesis of Pyridazine Containing Compounds with Promising Anticancer Activity. Chem. Pharm. Bull. 2017, 65, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.O.; Ceballos, G.; Villarreal, F.J. Tetracycline compounds with non-antimicrobial organ protective properties: Possible mechanisms of action. Pharm. Res. 2011, 63, 102–107. [Google Scholar] [CrossRef]
- Scatena, C.; Roncella, M.; Di Paolo, A.; Aretini, P.; Menicagli, M.; Fanelli, G.; Marini, C.; Mazzanti, C.M.; Ghilli, M.; Sotgia, F.; et al. Doxycycline, an inhibitor of mitochondrial biogenesis, effectively reduces cancer stem cells (cscs) in early breast cancer patients: A clinical pilot study. Front. Oncol. 2018, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.; Harrison, H.; Hulit, J.; Smith, D.L.; Lisanti, M.P.; Sotgia, F. Mitochondria as new therapeutic targets for eradicating cancer stem cells: Quantitative proteomics and functional validation via MCT1/2 inhibition. Oncotarget 2014, 5, 11029. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.M.; Bonuccelli, G.; Maggiolini, M.; Sotgia, F.; Lisanti, M.P. Vitamin C and Doxycycline: A synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs). Oncotarget 2017, 8, 67269. [Google Scholar] [CrossRef]
- Lamb, R.; Fiorillo, M.; Chadwick, A.; Ozsvari, B.; Reeves, K.J.; Smith, D.L.; Clarke, R.B.; Howell, S.J.; Cappello, A.R.; Martinez-Outschoorn, U.E.; et al. Doxycycline down-regulates DNA-PK and radiosensitizes tumor initiating cells: Implications for more effective radiation therapy. Oncotarget 2015, 6, 14005. [Google Scholar] [CrossRef]
- Sloan, B.; Scheinfeld, N. The use and safety of doxycycline hyclate and other second-generation tetracyclines. Expert Opin. Drug Saf. 2008, 7, 571–577. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B 2010, 80, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545. [Google Scholar] [CrossRef]
- Alai, M.S.; Lin, W.J.; Pingale, S.S. Application of polymeric nanoparticles and micelles in insulin oral delivery. J. Food Drug Anal. 2015, 23, 351–358. [Google Scholar] [CrossRef]
- Owais, M.; Khan, A.A.; Zubair, S.; Zia, Q. Self-assembled amphotericin B-loaded polyglutamic acid nanoparticles: Preparation, characterization and in vitro potential against Candida albicans. Int. J. Nanomed. 2015, 10. [Google Scholar] [CrossRef]
- Matoba, T.; Koga, J.; Nakano, K.; Egashira, K.; Tsutsui, H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J. Cardiol. 2017, 70, 206–211. [Google Scholar] [CrossRef]
- Lim, E.-K.; Chung, B.H.; Chung, S.J. Recent advances in ph-sensitive polymeric nanoparticles for smart drug delivery in cancer therapy. Curr. Drug Targets 2018, 19, 300–317. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Babu, J.P.; Osorio, R.; Medina-Castillo, A.L.; García-Godoy, F.; Toledano, M. Modified polymeric nanoparticles exert in vitro antimicrobial activity against oral bacteria. Materials 2018, 11, 1013. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Navarro-Hortal, M.D.; Varela-López, A.; Osorio, R.; Quiles, J.L. Novel Polymeric Nanocarriers Reduced Zinc and Doxycycline Toxicity in the Nematode Caenorhabditis elegans. Antioxidants 2019, 8, 550. [Google Scholar] [CrossRef]
- Yücel, Ç.; Değim, Z.; Yilmaz, Ş. Nanoparticle and liposome formulations of doxycycline: Transport properties through Caco-2 cell line and effects on matrix metalloproteinase secretion. Biomed. Pharmacother. 2013, 67, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Agudelo, R.; Scheuermann, K.; Gala-García, A.; Monteiro, A.P.F.; Pinzón-García, A.D.; Cortés, M.E.; Sinisterra, R.D. Hybrid nanofibers based on poly-caprolactone/gelatin/hydroxyapatite nanoparticles-loaded Doxycycline: Effective anti-tumoral and antibacterial activity. Mater. Sci. Eng. C 2018, 83, 25–34. [Google Scholar] [CrossRef]
- Kumar, A.; Cover, N.F.; Lai-Yuen, S.; Parsons, A. Synergetic effects of doxycycline-loaded chitosan nanoparticles for improving drug delivery and efficacy. Int. J. Nanomed. 2012, 2411. [Google Scholar] [CrossRef] [PubMed]
- Parasaram, V.; Nosoudi, N.; LeClair, R.J.; Binks, A.; Vyavahare, N. Targeted drug delivery to emphysematous lungs: Inhibition of MMPs by doxycycline loaded nanoparticles. Pulm. Pharm. Ther. 2016, 39, 64. [Google Scholar] [CrossRef][Green Version]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.A.; Jayarama, S.; Salimath, B.P.; Rangappa, K.S. Pro-apoptotic activity of imidazole derivatives mediated by up-regulation of Bax and activation of CAD in Ehrlich Ascites Tumor cells. Investig. New Drugs 2007, 25, 343–350. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Nour, S.; Hosay, R.E.; Shalaby, S. Preparation and in-vitro Evaluation of Poly-ε-Caprolactone Nanoparticles Containing Atorvastation Calcium. In Proceedings of the Proceedings of the 5th International Conference on Nanotechnology: Fundamentals and Applications, Prague, Czech Republic, 11–13 August 2014; p. 202. [Google Scholar]
- Panyam, J.; Williams, D.; Dash, A.; Leslie-Pelecky, D.; Labhasetwar, V. Solid-state Solubility Influences Encapsulation and Release of Hydrophobic Drugs from PLGA/PLA Nanoparticles. J. Pharm. Sci. 2004, 93, 1804–1814. [Google Scholar] [CrossRef]
- Jawahar, N.; Venkatesh, D.N.; Sureshkumar, R.; Senthil, V.; Ganesh, G.N.K.; Vinoth, P.; Sood, S.; Samanta, M.K. Development and characterization of PLGA-nanoparticles containing carvedilol. J. Pharm. Sci. Res. 2009, 1, 123–128. [Google Scholar]
- Dora, C.P.; Singh, S.K.; Kumar, S.; Datusalia, K.; Deep, A. Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol. Pharm. 2010, 67, 283–290. [Google Scholar] [PubMed]
- Suksiriworapong, J.; Sripha, K.; Kreuter, J.; Junyaprasert, V.B. Comparative Study of Ibuprofen and Indomethacin Loaded Poly(caprolactone) Nanoparticles: Physicochemical Properties. J. Magn. Magn. Mater. 2010, 17–27. [Google Scholar]
- Qushawy, M.; Prabahar, K.; Abd-Alhaseeb, M.; Swidan, S.; Nasr, A. Preparation and evaluation of carbamazepine solid lipid nanoparticle for alleviating seizure activity in pentylenetetrazole-kindled mice. Molecules 2019, 24, 3971. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.; Qushawy, M.; Swidan, S. Spray dried lactose based proniosomes as stable provesicular drug delivery carriers: Screening, formulation, and physicochemical characterization. Int. J. Appl. Pharm. 2018, 10, 125–137. [Google Scholar] [CrossRef]
- López-López, M.; Fernández-Delgado, A.; Moyá, M.L.; Blanco-Arévalo, D.; Carrera, C.; de la Haba, R.R.; Ventosa, A.; Bernal, E.; López-Cornejo, P. Optimized preparation of levofloxacin loaded polymeric nanoparticles. Pharmaceutics 2019, 11, 57. [Google Scholar] [CrossRef]
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. 2016, 11, 404–416. [Google Scholar] [CrossRef]
- Behera, A.; Sahoo, S. Preparation and evaluation of glibenclamide-loaded biodegradable nanoparticles. Trop. J. Pharm. Res. 2012, 11, 345–350. [Google Scholar] [CrossRef]
- Lamb, R.; Ozsvari, B.; Lisanti, C.L.; Tanowitz, H.B.; Howell, A.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: Treating cancer like an infectious disease. Oncotarget 2015, 6, 4569. [Google Scholar] [CrossRef]
- Cianfrocca, M.; Cooley, T.P.; Lee, J.Y.; Rudek, M.A.; Scadden, D.T.; Ratner, L.; Pluda, J.M.; Figg, W.D.; Krown, S.E.; Dezube, B.J. Matrix metalloproteinase inhibitor COL-3 in the treatment of AIDS-related Kaposi’s sarcoma: A phase I AIDS malignancy consortium study. J. Clin. Oncol. 2002, 20, 153–159. [Google Scholar]
- Rudek, M.A.; Figg, W.D.; Dyer, V.; Dahut, W.; Turner, M.L.; Steinberg, S.M.; Liewehr, D.J.; Kohler, D.R.; Pluda, J.M.; Reed, E. Phase I clinical trial of oral COL-3, a matrix metalloproteinase inhibitor, in patients with refractory metastatic cancer. J. Clin. Oncol. 2001, 19, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jiang, W.; Ding, J.; Li, M.; Cheng, Y.; Sun, S.; Fu, C.; Liu, Y. Polymer nanoparticle-based chemotherapy for spinal malignancies. J. Nanomater. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Pieper, S.; Onafuye, H.; Mulac, D.; Cinatl Jr, J.; Wass, M.N.; Michaelis, M.; Langer, K. Incorporation of doxorubicin in different polymer nanoparticles and their anticancer activity. Beilstein J. Nanotechnol. 2019, 10, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Nasr, M.; Elkhatib, W.F.; Eltayeb, W.N. In vitro evaluation of antimicrobial activity and cytotoxicity of different nanobiotics targeting multidrug resistant and biofilm forming Staphylococci. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Zaitone, S.A.; Moustafa, Y.M. Boswellic acids synergize antitumor activity and protect against the cardiotoxicity of doxorubicin in mice bearing Ehrlich’s carcinoma. Can. J. Physiol. Pharm. 2015, 93, 695–708. [Google Scholar] [CrossRef]
- Elmeligie, S.; Khalil, N.A.; Ahmed, E.M.; Emam, S.H.; Zaitone, S.A.-B. Synthesis of new N1-Substituted-5-aryl-3-(3,4,5-trimethoxyphenyl)-2-pyrazoline derivatives as antitumor agents targeting the colchicine site on tubulin. Biol. Pharm. Bull. 2016, 39, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- The biochemistry of apoptosis, Nature. Available online: https://www.nature.com/articles/35037710 (accessed on 29 January 2020).
- Sellers, W.R.; Fisher, D.E. Apoptosis and cancer drug targeting. Eur. J. Clin. Investig. 1999, 104, 1655–1661. [Google Scholar] [CrossRef]
- Son, K.; Fujioka, S.; Iida, T.; Furukawa, K.; Fujita, T.; Yamada, H.; Chiao, P.J.; Yanaga, K. Doxycycline induces apoptosis in PANC-1 pancreatic cancer cells. Anticancer Res. 2009, 29, 3995–4003. [Google Scholar]
- Wang, S.Q.; Zhao, B.X.; Liu, Y.; Wang, Y.T.; Liang, Q.Y.; Cai, Y.; Zhang, Y.Q.; Yang, J.H.; Song, Z.H.; Li, G.F. New application of an old drug: Antitumor activity and mechanisms of doxycycline in small cell lung cancer. Int. J. Oncol. 2016, 48, 1353–1360. [Google Scholar] [CrossRef]
- Chen, B.; Yang, J.Z.; Wang, L.F.; Zhang, Y.J.; Lin, X.J. Ifosfamide-loaded poly (lactic-co-glycolic acid) PLGA-dextran polymeric nanoparticles to improve the antitumor efficacy in Osteosarcoma. BMC Cancer 2015, 15, 752. [Google Scholar] [CrossRef]
- Nair, L.; Jagadeeshan, S.; Nair, S.A.; Kumar, G.V. Biological evaluation of 5-fluorouracil nanoparticles for cancer chemotherapy and its dependence on the carrier, PLGA. Int. J. Nanomed. 2011, 6, 1685. [Google Scholar]
- Thakur, S.; Pramod, K.; Malviya, R. Utilization of polymeric nanoparticle in cancer treatment: A review. J. Pharm. Care Health Syst. 2017, 4, 172. [Google Scholar]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Wu, X.Y. In vivo evaluation of a new polymer-lipid hybrid nanoparticle (PLN) formulation of doxorubicin in a murine solid tumor model. Eur. J. Pharm. Biopharm. 2007, 65, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Xue, H.Y.; Babakhanian, K.; Wu, X.Y. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J. Pharm. Exp. Ther. 2006, 317, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Manias, J.L.; Ramaswamy, M.; Liu, Z.; Erhan, S.Z.; Wu, X.Y. A new polymer–lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 2006, 23, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Wu, X.Y. Simultaneous delivery of doxorubicin and GG918 (Elacridar) by new Polymer-Lipid Hybrid Nanoparticles (PLN) for enhanced treatment of multidrug-resistant breast cancer. J. Control. Release 2006, 116, 275–284. [Google Scholar] [CrossRef]
- Li, F.; Zhao, X.; Wang, H.; Zhao, R.; Ji, T.; Ren, H.; Anderson, G.J.; Nie, G.; Hao, J. Multiple layer-by-layer lipid-polymer hybrid nanoparticles for improved FOLFIRINOX chemotherapy in pancreatic tumor models. Adv. Funct. Mater. 2015, 25, 788–798. [Google Scholar] [CrossRef]
- Wang, T.; Yang, S.; Mei, L.A.; Parmar, C.K.; Gillespie, J.W.; Praveen, K.P.; Petrenko, V.A.; Torchilin, V.P. paclitaxel-loaded PEG-PE–based micellar nanopreparations targeted with tumor-specific landscape phage fusion protein enhance apoptosis and efficiently reduce tumors. Mol. Cancer Ther. 2014, 13, 2864–2875. [Google Scholar] [CrossRef]
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-Step assembly of DOX/ICG loaded lipid–polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef]
- Markwalter, C.E.; Pagels, R.F.; Wilson, B.K.; Ristroph, K.D.; Prud’homme, R.K. Flash nanoprecipitation for the encapsulation of hydrophobic and hydrophilic compounds in polymeric nanoparticles. JoVE -J. Vis. Exp. 2019, 58757. [Google Scholar] [CrossRef]
- Bramosanti, M.; Chronopoulou, L.; Grillo, F.; Valletta, A.; Palocci, C. Microfluidic-assisted nanoprecipitation of antiviral-loaded polymeric nanoparticles. Colloids Surf. A Phys. Eng. Asp. 2017, 532, 369–376. [Google Scholar] [CrossRef]
- El-Ghorab, A.H.; El-Massry, K.F.; Marx, F.; Fadel, H.M. Antioxidant activity of Egyptian Eucalyptus camaldulensisvar. brevirostrisleaf extracts. Food/Nahrung 2003, 47, 41–45. [Google Scholar] [CrossRef]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ghourab, M.; Gad, S.; Qushawy, M. The application of Plackett-Burman design and response surface methodology for optimization of formulation variables to produce Piroxicam niosomes. Int. J. Drug Dev. Res. 2013, 5, 121–130. [Google Scholar]
- Canchi, A.; Khosa, A.; Singhvi, G.; Banerjee, S.; Dubey, S.K. Design and characterization of polymeric nanoparticles of pioglitazone hydrochloride and study the effect of formulation variables using QbD approach. CNM 2018, 2, 162–168. [Google Scholar] [CrossRef]
- Badran, M.M.; Mady, M.M.; Ghannam, M.M.; Shakeel, F. Preparation and characterization of polymeric nanoparticles surface modified with chitosan for target treatment of colorectal cancer. Int. J. Biol. Macromol. 2017, 95, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Kiafar, F.; Jelvehgari, M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into Eudragit RL nanoparticles. Res. Pharm. Sci. 2017, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Qushawy, M.; Nasr, A. Solid lipid nanoparticles (slns) as nano drug delivery carriers: Preparation, characterization and application. Int. J. Appl. Pharm. 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Prabahar, K.; Udhumansha, U.; Qushawy, M. Optimization of thiolated chitosan nanoparticles for the enhancement of in vivo hypoglycemic efficacy of sitagliptin in streptozotocin-induced diabetic rats. Pharmaceutics 2020, 12, 300. [Google Scholar] [CrossRef]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. Antibacterial efficacy of inhalable levofloxacin-loaded polymeric nanoparticles against e. coli biofilm cells: The effect of antibiotic release profile. Pharm. Res. 2010, 27, 1597–1609. [Google Scholar] [CrossRef]
- Singh, Y.; Srinivas, A.; Gangwar, M.; Meher, J.G.; Misra-Bhattacharya, S.; Chourasia, M.K. Subcutaneously administered ultrafine PLGA nanoparticles containing doxycycline hydrochloride target lymphatic filarial parasites. Mol. Pharm. 2016, 13, 2084–2094. [Google Scholar] [CrossRef]
- Ahmed, A.; Ghourab, M.; Shedid, S.; Qushawy, M. Optimization of piroxicam niosomes using central composite design. Int. J. Pharm. Pharm. Sci. 2013, 5, 229–236. [Google Scholar]
- Dutta, R.S.; Hauzel, L.; Roy, P.K.; Kalita, P.; Devi, T.B.; Deka, D.; Pachuau, L. Nanoprecipitated ethylcellulose-curcumin particles for controlled release and enhanced antioxidant activity. Curr. Nanosci. 2018, 14, 298–306. [Google Scholar] [CrossRef]
- Misra, R.; Acharya, S.; Dilnawaz, F.; Sahoo, S.K. Sustained antibacterial activity of doxycycline-loaded poly(D, L -lactide-co-glycolide) and poly(ε-caprolactone) nanoparticles. Nanomedicine 2009, 4, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Skanjeti, A.; Miranti, A.; Yabar, G.M.D.; Bianciotto, D.; Trevisiol, E.; Stasi, M.; Podio, V. A simple and accurate dosimetry protocol to estimate activity for hyperthyroidism treatment. Nucl. Med. Rev. 2015, 18, 13–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albus, U. Guide for the Care and Use of Laboratory Animals, 8th ed.; SAGE Publications Sage UK: London, England, 2012. [Google Scholar]
- Bahr, H.I.; Toraih, E.A.; Mohammed, E.A.; Mohammad, H.M.F.; Ali, E.A.I.; Zaitone, S.A. Chemopreventive effect of leflunomide against Ehrlich’s solid tumor grown in mice: Effect on EGF and EGFR expression and tumor proliferation. Life Sci. 2015, 141, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.; Tegeler, W.; Mazzone, H.M.; Leroy, J.G.; Boone, B.A.; Foley, G.E. Determination of sensitivity of individual biopsy specimens to potential inhibitory agents: Evaluation of some explant culture methods as assay systems. Cancer Chemother. Rep. 1966, 50, 543–555. [Google Scholar]
- Abd-Alhaseeb, M.M.; Zaitone, S.A.; Abou-El-Ela, S.H.; Moustafa, Y.M. Olmesartan potentiates the anti-angiogenic effect of sorafenib in mice bearing ehrlich’s ascites carcinoma: Role of angiotensin (1–7). PLoS ONE 2014, 9, e85891. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; Zaitone, S.A.; Shouman, S.A.; Moustafa, Y.M. Dorzolamide synergizes the antitumor activity of mitomycin C against Ehrlich’s carcinoma grown in mice: Role of thioredoxin-interacting protein. Naunyn Schmiedebergs Arch Pharm. 2015, 388, 1271–1282. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method Methods. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- O’Brien, R.G.; Kaiser, M.K. MANOVA method for analyzing repeated measures designs: An extensive primer. Psychol. Bull. 1985, 97, 316. [Google Scholar] [CrossRef]
- MacInnes, J. An Introduction to Secondary Data Analysis with IBM SPSS Statistics; Sage: London, UK, 2016. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



| Formula Code | Drug: Polymer Mass Ratio | HPMC Concentration | Tween 80 Concentration | Organic: Aqueous Phase |
|---|---|---|---|---|
| PNP1 | 1:2 | 0.8 g% | 1% | 1:8 |
| PNP2 | 1:1 | |||
| PNP3 | 2:1 |
| Formula Code | EE % | Drug Loading % | Particle Size (nm) | ZP (mv) | PDI |
|---|---|---|---|---|---|
| PNP1 | 42.15 ± 0.84 | 30.29 ± 0.15 | 203.6 ± 1.4 | −21.8 ± 4.89 | 0.431 ± 0.02 |
| PNP2 | 56.78 ± 0.52 £ | 49.57 ± 0.11 £ | 489.7 ± 6.7 £ | −23 ± 5.68 | 0.851 ± 0.05 £ |
| PNP3 | 84.65 ± 0.93 £,& | 66.13 ± 0.36 £,& | 615.3 ± 8.3 £,& | −15.1 ± 4.84 | 1.00 ± 0.04 £,& |
| Target Gene | Primer Sequence: 5′-3′ |
|---|---|
| Caspase-3 | F: ATGTCAGCTCGCAATGG R: AAGAAATTATGGAATTG |
| BAX | F: CAGATCATGAAGACAGG R: GTGGATACAGACTCCCC |
| GAPDH | F: TAGGTATATGTTAAATTT R: GCTGACATTTAGGTAGAA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardouh, A.R.; Attia, M.A.; Enan, E.T.; Elbahaie, A.M.; Fouad, R.A.; El-Shafey, M.; Youssef, A.M.; Alomar, S.Y.; Ali, Z.A.-E.; Zaitone, S.A.; et al. Synthesis and Antitumor Activity of Doxycycline Polymeric Nanoparticles: Effect on Tumor Apoptosis in Solid Ehrlich Carcinoma. Molecules 2020, 25, 3230. https://doi.org/10.3390/molecules25143230
Gardouh AR, Attia MA, Enan ET, Elbahaie AM, Fouad RA, El-Shafey M, Youssef AM, Alomar SY, Ali ZA-E, Zaitone SA, et al. Synthesis and Antitumor Activity of Doxycycline Polymeric Nanoparticles: Effect on Tumor Apoptosis in Solid Ehrlich Carcinoma. Molecules. 2020; 25(14):3230. https://doi.org/10.3390/molecules25143230
Chicago/Turabian StyleGardouh, Ahmed R., Mohammed A. Attia, Eman T. Enan, Alaaeldeen M. Elbahaie, Rania A. Fouad, Mohamed El-Shafey, Amal M. Youssef, Suliman Y. Alomar, Zinab Abd-Elhady Ali, Sawsan A. Zaitone, and et al. 2020. "Synthesis and Antitumor Activity of Doxycycline Polymeric Nanoparticles: Effect on Tumor Apoptosis in Solid Ehrlich Carcinoma" Molecules 25, no. 14: 3230. https://doi.org/10.3390/molecules25143230
APA StyleGardouh, A. R., Attia, M. A., Enan, E. T., Elbahaie, A. M., Fouad, R. A., El-Shafey, M., Youssef, A. M., Alomar, S. Y., Ali, Z. A.-E., Zaitone, S. A., & Qushawy, M. K. E. (2020). Synthesis and Antitumor Activity of Doxycycline Polymeric Nanoparticles: Effect on Tumor Apoptosis in Solid Ehrlich Carcinoma. Molecules, 25(14), 3230. https://doi.org/10.3390/molecules25143230







