Scalable Green Approach Toward Fragrant Acetates
Abstract
1. Introduction
2. Results and Discussion
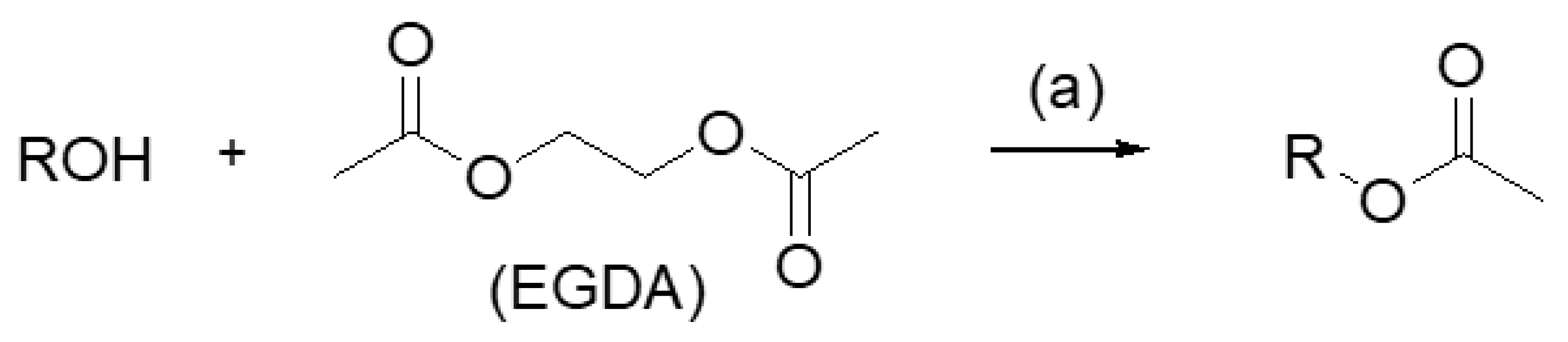
- (a)
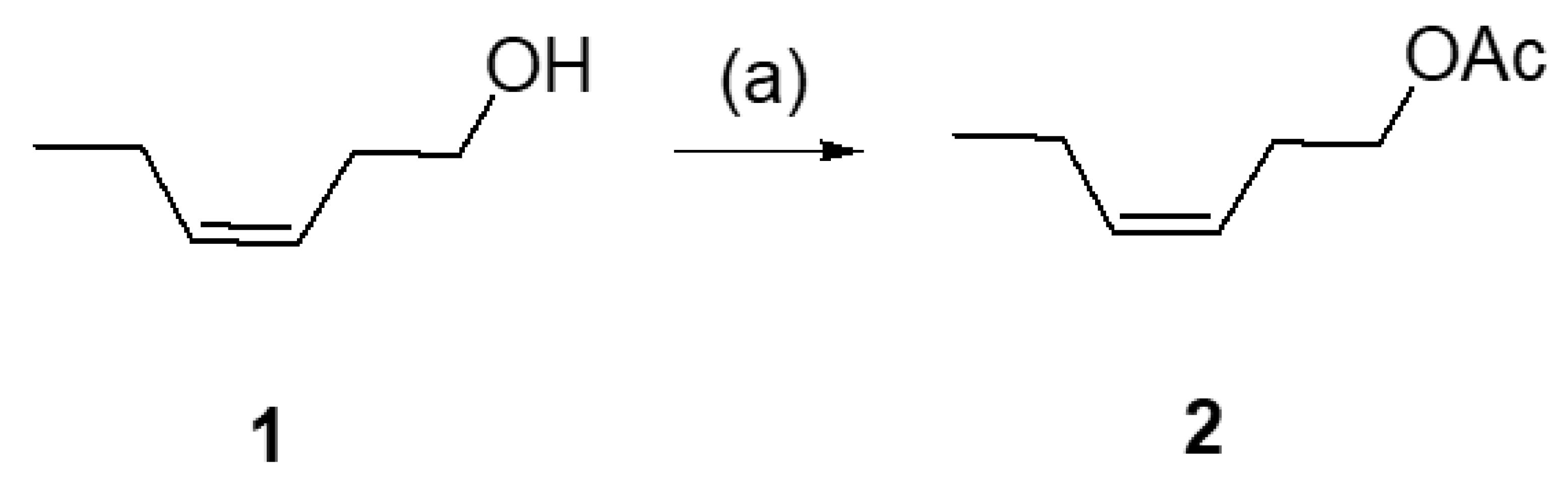
- Considering the activated substrates, allylic primary alcohols 3, 5, 7, 9, 13, and 15 (entries 2–5, 7, 8) were almost quantitatively esterified (GC-FID ratios of alcohol to acetate ranging from 10:90 to 4:96) to fragrant acetates, namely geranyl acetate 4 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34], prenyl acetate 6, (E)-2-hexenyl acetate 8, cinnamyl acetate 10 [35,36,37,38,39,40,41], phytyl acetate (E/Z = 66:34) 14 [42], and (1R)-nopyl acetate 16, respectively. On the other hand, rac-linalool 11 remained almost intact with negligible acetylation only (entry 6). Furthermore, the enzyme clearly discriminates between the primary and tertiary allylic alcohol. Analogously, the primary homoallylic alcohols 1 and 17 also provided corresponding fragrant acetates (entries 1 and 9), namely (Z)-hex-3-ene-1-yl acetate 2 and (1R)-myrtenyl acetate 18.
- (b)
- Regarding the non-activated substrates, the enzyme clearly distinguishes primary alcohols 27, 29 (entries 15, 16), and/or secondary alcohols 19, 21, 23, 33, and 35 (entries 10–13, 18, 19) vs. phenolic eugenol 31 (entry 17) and/or tertiary alcohol 37 (entry 20). While the former ones were reasonably acetylated (although not as well as activated alcohols, vide supra) to their corresponding acetates 20, 22, 24, 28, 30, 34, and 36, the latter ones were fully reluctant to the transformation. In addition, the enzyme´s enantio-discrimination led to the kinetic resolution of racemic secondary alcohols 19, 21 (entries 10, 12). In the case of rac-2-heptanol 19 (entry 10), (R)-enantiomer was mostly acetylated [43,44,45,46,47,48,49] as shown by negligible formation of (S)-2-heptenyl acetate (S)-20 (see Figure S1 in the Supporting Information), and, thus, by obeying the Kazlauskas’s rule [50] (entry 11). Conversely, (1R)-endo-fenchol 25 remained practically intact. This might be due to the significant steric congestion around the hydroxyl group and/or the result of the enantiomer preference of the enzyme for the opposite enantiomer (entry 14). In addition, the enzymatic acetylation [51,52,53] of bifunctional rac-rhododendrol 21 exhibited both the chemo-selectivity (alcohol vs. phenol) and the kinetic resolution of racemate (entry 12). Thus, the secondary hydroxyl group of the (R)-enantiomer (cf. the Kazlauskas’s rule again) was mostly acetylated to furnish the ester (R)-22 along with the enantiomerically enriched (S)-rhododendrol (S)-21 (84% ee, chiral HPLC, see Figure S2 in the Supporting Information). The clear unreactivity of phenols under the reaction conditions was also exhibited by zero acetylation of eugenol 31 (entry 17).
- (c)
- Considering the achiral secondary alcohols, even minor structural differences led to a significant change in their reactivity. While isopropanol 35 (entry 19) was acetylated in a major extent (GC-FID ratio 35/36 = 15:85), slightly larger pentan-3-ol 33 (entry 18) was much less reactive (GC-FID ratio 33/34 = 45:55). The extent of acetylation of 2-indanol 23 (entry 13), being somewhere in between (GC-FID ratio 23/24 = 24:76), might indicate the high sensitivity of the respective lipase to the steric demands of the alcohols in question.
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthetic Procedures and Analytical Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials, 5th ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Anbu, N.; Nagarjun, N.; Jacob, M.; Kalaiarasi, J.M.V.K.; Dhakshinamoorthy, A. Acetylation of Alcohols, Amines, Phenols, Thiols under Catalyst and Solvent-Free Conditions. Chemistry 2019, 1, 69–79. [Google Scholar] [CrossRef]
- Stergiou, P.-Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M.; et al. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.G.A.; de Meneses, A.C.; de Araújo, P.H.H.; de Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Dhake, K.P.; Thakare, D.D.; Bhanage, B.M. Lipase: A potential biocatalyst for the synthesis of valuable flavour and fragrance ester compounds. Flavour Fragr. J. 2013, 28, 71–83. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010, 12, 1127–1138. [Google Scholar] [CrossRef]
- Díaz-Álvarez, A.E.; Francos, J.; Lastra-Barreira, B.; Crocheta, P.; Cadierno, V. Glycerol and derived solvents: new sustainable reaction media for organic synthesis. Chem. Commun. 2011, 47, 6208–6227. [Google Scholar] [CrossRef]
- García, J.I.; García-Marína, H.; Piresa, E. Glycerol based solvents: synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef]
- Wolfson, A.; Atyya, A.; Dlugy, C.; Tavor, D. Glycerol triacetate as solvent and acyl donor in the production of isoamyl acetate with Candida antarctica lipase B. Bioprocess Biosyst. Eng. 2010, 33, 363–366. [Google Scholar] [CrossRef]
- Milivojević, A.; Ćorović, M.; Carević, M.; Banjanac, K.; Vujisić, L.; Veličković, D.; Bezbradica, D. Highly efficient enzymatic acetylation of flavonoids: Development of solvent-free process and kinetic evaluation. Biochem. Eng. J. 2017, 128, 106–115. [Google Scholar] [CrossRef]
- Varga, Z.; Kmecz, I.; Szécsényi, A.; Székely, E. Neat lipase-catalysed kinetic resolution of racemic 1-phenylethanol and a straightforward modelling of the reaction. Biocatal. Biotransformation 2017, 35, 427–433. [Google Scholar] [CrossRef]
- Bayout, I.; Bouzemi, N.; Guo, N.; Mao, X.; Serra, S.; Riva, S.; Secundo, F. Natural flavor ester synthesis catalyzed by lipases. Flavour Fragr. J. 2020, 35, 209–218. [Google Scholar] [CrossRef]
- Bourg-Garros, S.; Razafindramboa, N.; Pavia, A.A. Optimization of lipase-catalyzed synthesis of (z)-3-hexen-1-yl acetate by direct esterification in hexane and a solvent-free medium. Enzym. Microb. Technol. 1998, 22, 240–245. [Google Scholar] [CrossRef]
- Bourg-Garros, S.; Razafindramboa, N.; Pavia, A.A. Large-scale preparation of (Z)-3-hexen-1-yl acetate using Candida antarctica-immobilized lipase in hexane. Biotechnol. Biochem. 1998, 59, 495–500. [Google Scholar]
- Chiang, W.D.; Chang, S.W.; Shieh, C.J. Studies on the optimized lipase-catalyzed biosynthesis of cis-3-hexen-1-yl acetate in n-hexane. Process Biochem. 2003, 38, 1193–1199. [Google Scholar] [CrossRef]
- Langrand, G.; Triantaphylides, C.; Baratti, J. Lipase catalyzed formation of flavour esters. Biotechnol. Lett. 1988, 10, 549–554. [Google Scholar] [CrossRef]
- Chulalaknanukul, W.; Condoret, J.; Combes, D. Kinetics of geranyl acetate synthesis by lipase-catalysed transesterification in n-hexane. Enzyme Microb. Technol. 1992, 14, 293–298. [Google Scholar] [CrossRef]
- Akoh, C.C.; Claon, P.A. Enzymatic synthesis of geranyl acetate in n-hexane with Candida antarctica lipases. J. Am. Chem. Soc. 1994, 71, 575–578. [Google Scholar]
- Claon, P.A.; Akoh, C.C. Lipase-catalyzed synthesis of primary terpenyl acetates by transesterification: study of reaction parameters. J. Agric. Food Chem. 1994, 42, 2349–2352. [Google Scholar] [CrossRef]
- Yee, L.N.; Akoh, C.C. Enzymatic synthesis of geranyl acetate by transesterification with acetic anhydride as acyl donor. J. Am. Oil Chem. Soc. 1996, 73, 1379–1384. [Google Scholar] [CrossRef]
- Huang, S.; Chang, H. Kinetic study on the esterification of geraniol and acetic acid in organic solvents using surfactant-coated lipase. J. Chem. Technol. Biotechnol. 1999, 74, 183–187. [Google Scholar] [CrossRef]
- Bartling, K.; Thompson, J.U.; Pfromm, P.H.; Czermak, P.; Rezac, M.E. Lipase-catalyzed synthesis of geranyl acetate in n-hexane with membrane-mediated water removal. Biotechnol. Bioeng. 2001, 75, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Peres, C.; Harper, N.; da Silva, M.D.R.G.; Barreiros, S. Effect of zeolites on lipase catalyzed esterification in nonaqueous media. Enzyme Microb. Technol. 2005, 37, 145–149. [Google Scholar] [CrossRef]
- Barahona, D.; Pfromm, P.H.; Rezac, M.E. Effect of water activity on the lipase catalyzed esterification of geraniol in ionic liquid [bmim]PF6. Biotechnol. Bioeng. 2006, 93, 318–324. [Google Scholar] [CrossRef] [PubMed]
- De la Casa, R.M.; Sinisterra, J.V.; Sánchez-Montero, J.M. Characterization and catalytic properties of a new crude lipase from C. Rugosa. Enzyme Microb. Technol. 2006, 38, 599–609. [Google Scholar] [CrossRef]
- Antoniotti, S.; Fernandez, X.; Duñach, E. Reaction design for evaluation of the substrate range of hydrolases. Biocatal. Biotransformation 2008, 26, 228–234. [Google Scholar] [CrossRef]
- Couto, R.; Vidinha, P.; Peres, C.; Ribeiro, A.S.; Ferreira, O.; Oliveira, M.V.; Macedo, E.A.; Loureiro, J.M.; Barreiros, S. Geranyl Acetate Synthesis in a Packed-Bed Reactor Catalyzed by Novozym in Supercritical Carbon Dioxide and in Supercritical Ethane. Ind. Eng. Chem. Res. 2011, 50, 1938–1946. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Navarro, A. A clean enzymatic process for producing flavour esters by direct esterification in switchable ionic liquid/solid phases. Green Chem. 2012, 14, 3026–3033. [Google Scholar] [CrossRef]
- Gupta, A.; Dhakate, S.R.; Pahwa, M.; Sinha, S.; Chand, S.; Mathur, R.B. Geranyl acetate synthesis catalyzed by Thermomyces lanuginosus lipase immobilized on electrospun polyacrylonitrile nanofiber membrane. Process Biochem. 2013, 48, 124–132. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Synthesis of geranyl acetate in non-aqueous media using immobilized Pseudomonas cepacia lipase on biodegradable polymer film: Kinetic modelling and chain length effect study. Process Biochem. 2014, 49, 1304–1313. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Gómez, C.; García-Verdugo, E.; Burguete, M.I.; Sánchez, G.; Vaultier, M.; Luis, S.V. Green bioprocesses in sponge-like ionic liquids. Catal. Today 2015, 255, 54–59. [Google Scholar] [CrossRef]
- Adarme, C.A.A.; Leão, R.A.C.; de Souza, S.P.; Itabaiana, I., Jr.; de Souza, R.O.M.A.; Rezende, C.M. Continuous-Flow Chemo and Enzymatic Synthesis of Monoterpenic Esters with Integrated Purification. Mol. Catal. 2018, 453, 39–46. [Google Scholar] [CrossRef]
- Murcia, M.D.; Gómez, M.; Gómez, E.; Gómez, J.L.; Hidalgo, A.M.; Sánchez, A.; Vergara, P. Kinetic modelling and kinetic parameters calculation in the lipase-catalysed synthesis of geranyl acetate. Chem. Eng. Res. Des. 2018, 138, 135–143. [Google Scholar] [CrossRef]
- Perdomo, I.C.; Gianolio, S.; Pinto, A.; Romano, D.; Contente, M.L.; Paradisi, F.; Molinari, F. Efficient Enzymatic Preparation of Flavor Esters in Water. J. Agric. Food Chem. 2019, 67, 6517–6522. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.D.; Devendran, S. Lipase catalyzed synthesis of cinnamyl acetate via transesterification in non-aqueous medium. Process Biochem. 2012, 47, 496–502. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Karanet, A.; Tavor, D. A sustainable one-pot synthesis of cinnamyl acetate in triacetin. Tetrahedron Lett. 2012, 53, 4565–4567. [Google Scholar] [CrossRef]
- Geng, B.; Wang, M.; Qi, W.; Su, R.; He, Y. Cinnamyl acetate synthesis by lipase-catalyzed transesterification in a solvent-free system. Biotech. Appl. Biochem. 2012, 59, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Qi, W.; Wang, M.; Su, R.; He, Z. Lipase immobilized on novel ceramic supporter with Ni activation for efficient cinnamyl acetate synthesis. J. Mol. Catal. B Enzym. 2014, 110, 32–38. [Google Scholar] [CrossRef]
- Tomke, D.P.; Rathod, V.K. Ultrasound assisted lipase catalyzed synthesis of cinnamyl acetate via transesterification reaction in a solvent free medium. Ultrason. Sonochemistry 2015, 27, 241–246. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Sasaki, T.; Bhanage, B.M. Synthesis of lipase nano-bio-conjugates as an efficient biocatalyst: characterization and activity–stability studies with potential biocatalytic applications. RSC Adv. 2015, 5, 55238–55251. [Google Scholar] [CrossRef]
- Perdomo, I.C.; Contente, M.L.; Pinto, A.; Romano, D.; Fernandes, P.; Molinari, F. Continuous preparation of flavour-active acetate esters by direct biocatalytic esterification. Flavour Fragr. J. 2020, 35, 190–196. [Google Scholar] [CrossRef]
- Nishio, T.; Takahashi, K.; Yoshimoto, T.; Kodera, Y.; Saito, Y.; Inada, Y. Terpene alcohol ester synthesis by polyethylene glycol-modified lipase in benzene. Biotechnol. Lett. 1987, 9, 187–190. [Google Scholar] [CrossRef]
- Yanagishita, H.; Sakaki, K.; Hirata, H. Optical Resolution of 2-Alkanol by Lipase-Catalyzed Acetylation with Vinyl Acetate in Packed-Bed Reactor with Recycling System. J. Oleo Sci. 2007, 56, 137–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dlugy, C.; Wolfson, A. Lipase catalyse glycerolysis for kinetic resolution of racemates. Bioprocess Biosyst. Eng. 2007, 30, 327–330. [Google Scholar] [CrossRef]
- Zarcula, C.; Corîci, L.; Croitoru, R.; Ursoiu, A.; Peter, F. Preparation and properties of xerogels obtained by ionic liquid incorporation during the immobilization of lipase by the sol–gel method. J. Mol. Catal. B. Enzym. 2010, 65, 79–86. [Google Scholar] [CrossRef]
- Strohalm, H.; Dold, S.; Pendzialek, K.; Weiher, M.; Engel, K.H. Preparation of Passion Fruit-Typical 2-Alkyl Ester Enantiomers via Lipase-Catalyzed Kinetic Resolution. J. Agric. Food Chem. 2010, 58, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Tomin, A.; Weiser, D.; Hellner, G.; Bata, Z.; Corici, L.; Péter, F.; Koczka, B.; Poppe, L. Fine-tuning the second generation sol–gel lipase immobilization with ternary alkoxysilane precursor systems. Process Biochem. 2011, 46, 52–58. [Google Scholar] [CrossRef]
- Hellner, G.; Boros, Z.; Tomin, A.; Poppe, L. Novel Sol-Gel Lipases by Designed Bioimprinting for Continuous-Flow Kinetic Resolutions. Adv. Synth. Catal. 2011, 353, 2481–2491. [Google Scholar] [CrossRef]
- Schober, M.; Gadler, P.; Knaus, T.; Kayer, H.; Birner-Grünberger, R.; Gülly, C.; Macheroux, P.; Wagner, U.; Faber, K. A Stereoselective Inverting sec-Alkylsulfatase for the Deracemization of sec-Alcohols. Org. Lett. 2011, 13, 4296–4299. [Google Scholar] [CrossRef]
- Kazlauskas, R.J.; Weissfloch, A.N.E.; Rappaport, A.T.; Cuccia, L.A. A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 1991, 56, 2656–2665. [Google Scholar] [CrossRef]
- Yuasa, Y.; Shibuya, S.; Yuasa, Y. Resolution of Racemic Rhododendrol by Lipase-Catalyzed Enantioselective Acetylation. Synth. Commun. 2003, 33, 1469–1475. [Google Scholar] [CrossRef]
- Kitayama, T.; Isomori, S.; Nakamura, K. Asymmetric synthesis of enantiomerically pure zingerols by lipase-catalyzed transesterification and efficient synthesis of their analogues. Tetrahedrom Asymmetry 2013, 24, 621–627. [Google Scholar] [CrossRef]
- Karume, I.; Takahashi, M.; Hamdan, S.M.; Musa, M.M. Deracemization of Secondary Alcohols by using a Single Alcohol Dehydrogenase. ChemCatChem 2016, 8, 1459–1463. [Google Scholar] [CrossRef]
- Velusamy, S.; Borpuzari, S.; Punniyamurthy, T. Cobalt (II)-catalyzed direct acetylation of alcohols with acetic acid. Tetrahedron 2005, 61, 2011–2015. [Google Scholar] [CrossRef]
- Miyazawa, T.; Yamamoto, M.; Danjo, H. Chemoselective acylation of (hydroxyalkyl) phenols catalyzed by Candida antarctica lipase B. Biotechnol. Lett. 2013, 35, 625–630. [Google Scholar] [CrossRef]
- Song, L.; Liu, Y.; Tong, R. Cephalosporolide B Serving as a Versatile Synthetic Precursor: Asymmetric Biomimetic Total Syntheses of Cephalosporolides C, E, F, G, and (4-OMe-)G. Org. Lett. 2013, 15, 5850–5853. [Google Scholar] [CrossRef]
- Kulangiappar, K.; Anbukulandainathan, M.; Raju, T. Nuclear Versus Side-Chain Bromination of 4-Methoxy Toluene by an Electrochemical Method. Synth. Commun. 2014, 44, 2494–2502. [Google Scholar] [CrossRef]
- Boffi, A.; Cacchi, S.; Ceci, P.; Cirilli, R.; Fabrizi, G.; Prastaro, A.; Niembro, S.; Shafir, A.; Vallribera, A. The Heck Reaction of Allylic Alcohols Catalyzed by Palladium Nanoparticles in Water: Chemoenzymatic Synthesis of (R)-(–)-Rhododendrol. ChemCatChem 2011, 3, 347–353. [Google Scholar] [CrossRef]
- Kumar, H.M.; Joyasawal, S.; Reddy, B.V.S.; Chakravarthy, P.P.; Krishna, A.D.; Yadav, J.S. Reaction of orthoesters with alcohols in the presence of acidic catalysts: a study. Indian J. Chem. 2005, 44, 1686–1692. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
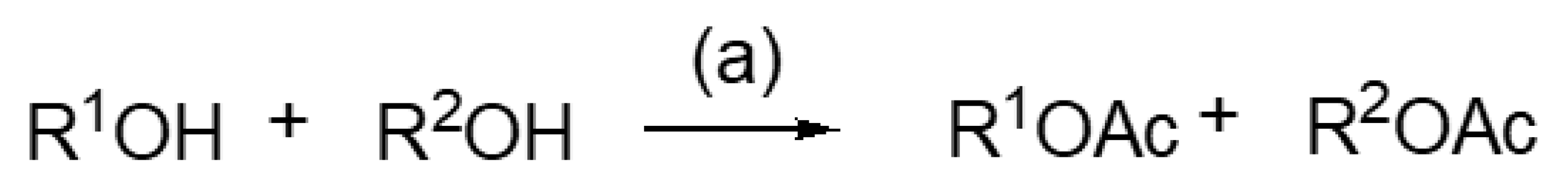


| Entry | Alcohol | Acetate | Time (h) | GC-FID Ratio a (%) |
|---|---|---|---|---|
| 1 | 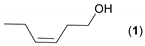 | 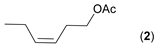 | 19 | 6:94 |
| 2 | 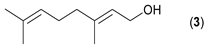 |  | 24 | 5:95 |
| 3 | 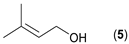 | 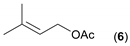 | 23 | 4:96 |
| 4 | 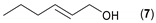 | 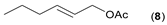 | 24 | 5:95 |
| 5 |  | 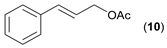 | 48 | 8:92 |
| 6 |  | 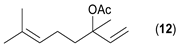 | 72 | 91:9 |
| 7 | 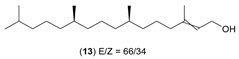 | 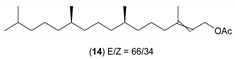 | 24 | 4:96 |
| 8 | 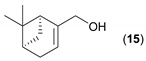 | 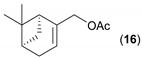 | 24 | 10:90 |
| 9 | 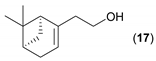 | 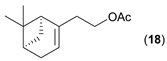 | 24 | 6:94 |
| 10 | 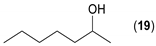 | 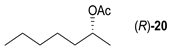 | 20 | 48:52 |
| 11 | 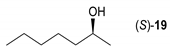 | 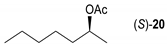 | 72 | 91:9 |
| 12 |  | 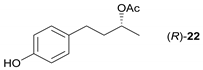 | 48 | 52:48 |
| 13 |  | 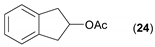 | 72 | 24:76 |
| 14 | 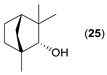 |  | 48 | 98:2 |
| 15 |  | 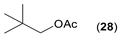 | 72 | 14:86 |
| 16 |  | 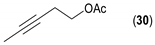 | 42 | 11:89 |
| 17 |  |  | 48 | 100:0 |
| 18 | 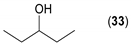 | 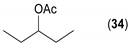 | 51 | 45:55 |
| 19 | 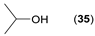 | 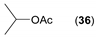 | 48 | 15:85 |
| 20 | 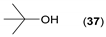 |  | 48 | 100:0 |
| Entry | Substrates R1OH + R2OH | Products R1OAc + R2OAc | Time (h) | R1OH/R1OAc (GC-FID Ratio %) a | R2OH/R2OAc (GC-FID Ratio %) a |
|---|---|---|---|---|---|
| 1 |  | 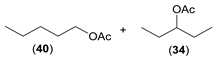 | 8 | 39/40 (6:94) | 33/34 (96.5:3.5) |
| 2 |  |  | 48 | 41/42 (19:81) | 33/34 (80:20) |
| 3 | 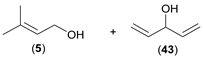 |  | 24 | 5/6 (9:91) | 43/44 (81:19) |
| 4 | 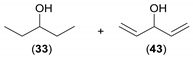 | 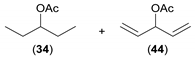 | 56 | 33/34 (66:34) | 43/44 (51:49) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchl’ová, E.; Szolcsányi, P. Scalable Green Approach Toward Fragrant Acetates. Molecules 2020, 25, 3217. https://doi.org/10.3390/molecules25143217
Puchl’ová E, Szolcsányi P. Scalable Green Approach Toward Fragrant Acetates. Molecules. 2020; 25(14):3217. https://doi.org/10.3390/molecules25143217
Chicago/Turabian StylePuchl’ová, Eva, and Peter Szolcsányi. 2020. "Scalable Green Approach Toward Fragrant Acetates" Molecules 25, no. 14: 3217. https://doi.org/10.3390/molecules25143217
APA StylePuchl’ová, E., & Szolcsányi, P. (2020). Scalable Green Approach Toward Fragrant Acetates. Molecules, 25(14), 3217. https://doi.org/10.3390/molecules25143217