Antianhedonic and Antidepressant Effects of Affron®, a Standardized Saffron (Crocus Sativus L.) Extract
Abstract
1. Introduction
2. Results and Discussion
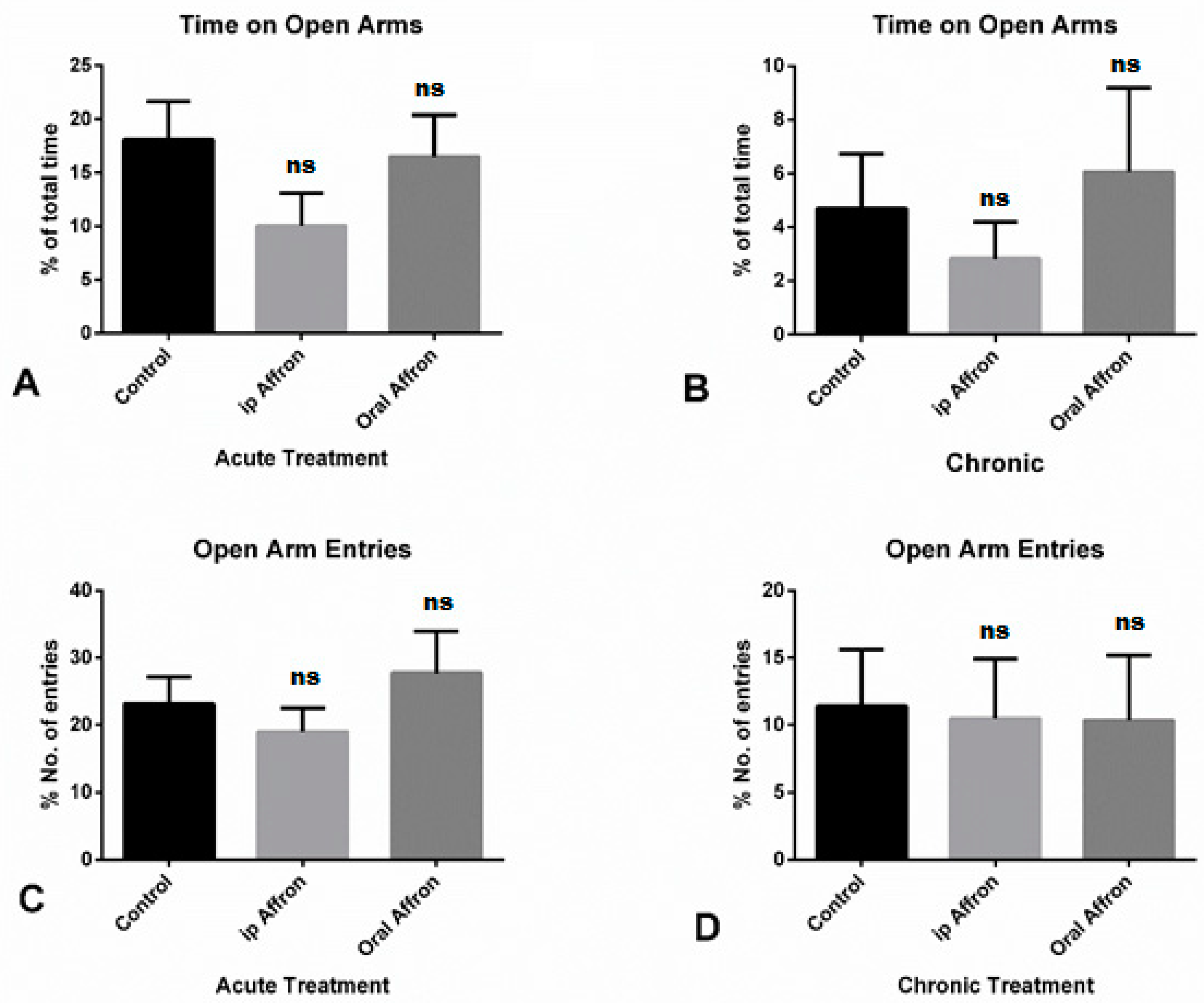
2.1. Effects of Affron® in the Elevated Plus Maze Test
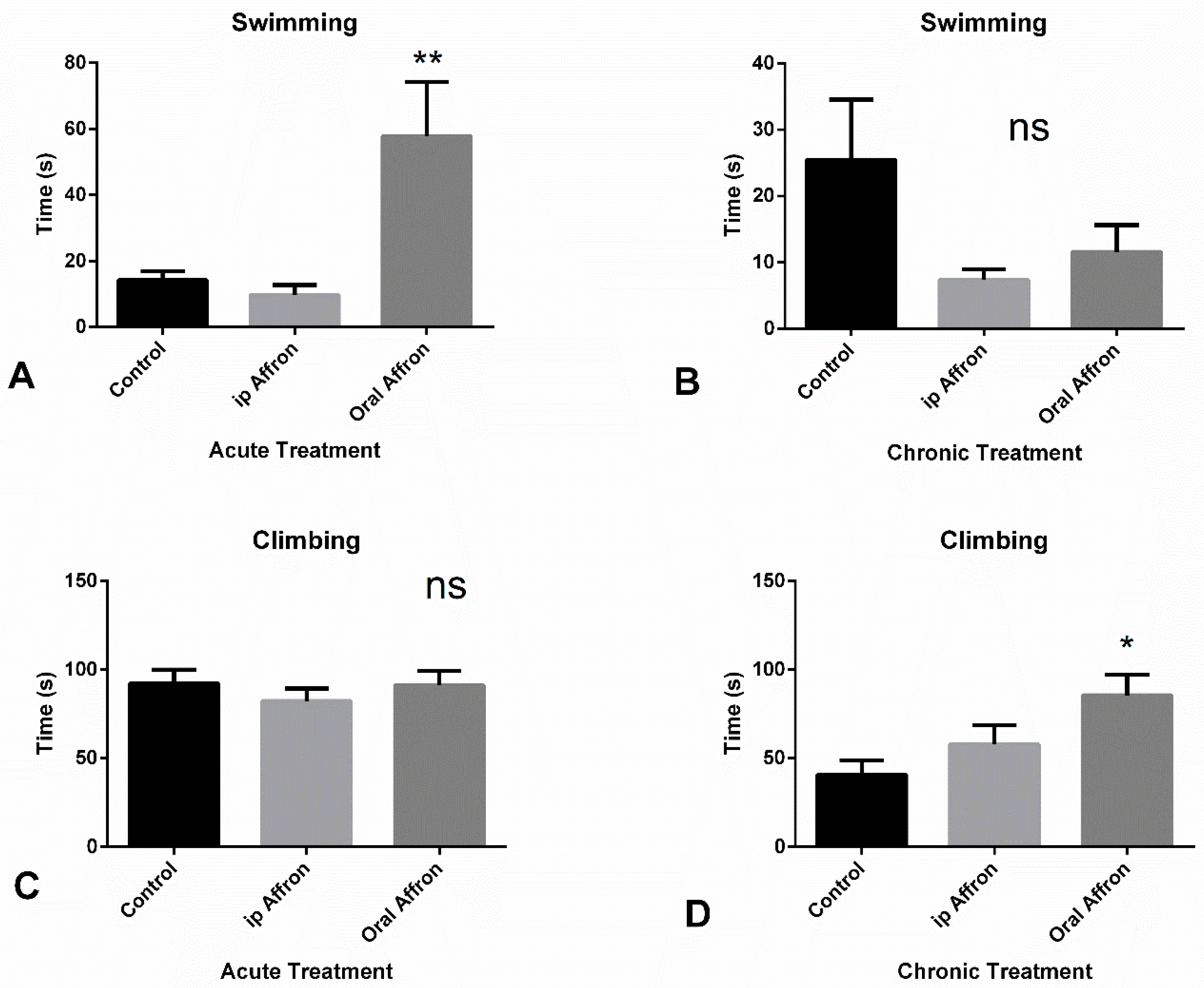
2.2. Effects of Affron® in the Forced Swimming Test
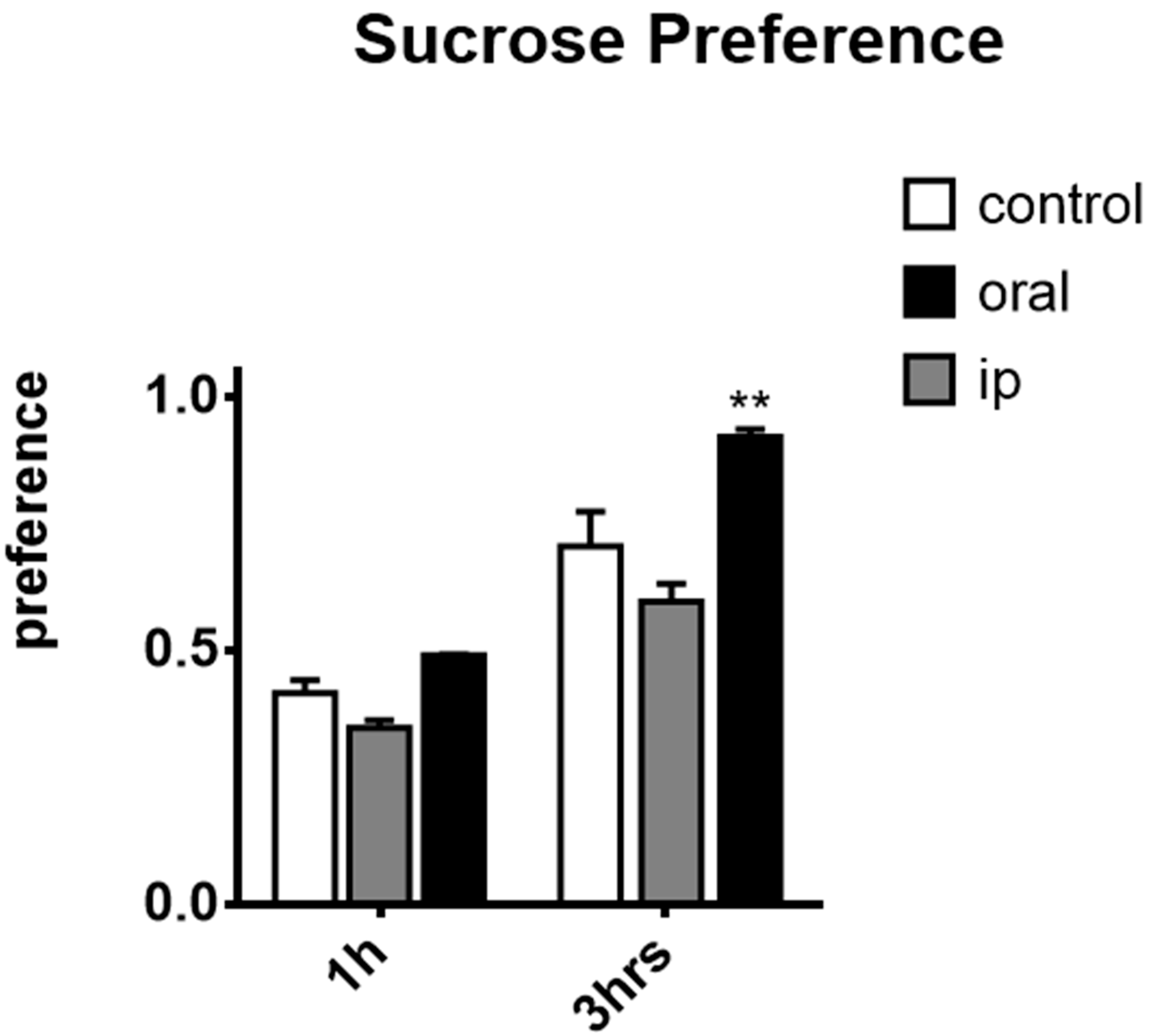
2.3. Effects of affron® in the Sucrose Preference Test
3. Material and Methods
3.1. Materials
3.2. Animals
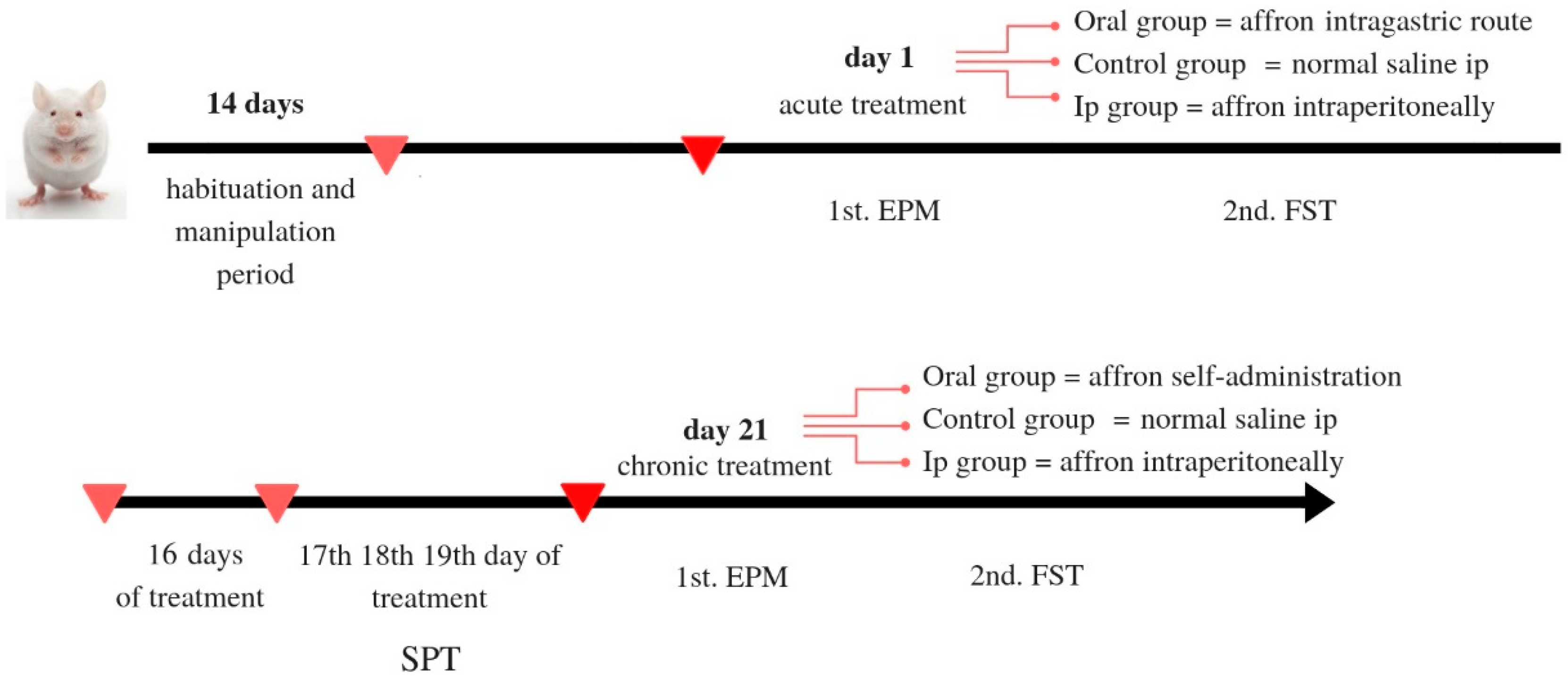
3.3. Experimental Design
3.3.1. Treatment
3.3.2. Anxiety and Depression-Like Behavior
3.3.3. Elevated Plus Maze Test
3.3.4. Forced Swim Test (Porsolt Test)
3.3.5. Sucrose Preference Test
3.4. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fajemiroye, J.O.; Galdino, P.M.; Florentino, I.F.; Da Rocha, F.F.; Ghedini, P.C.; Polepally, P.R.; Zjawiony, J.K.; Costa, E.A. Plurality of anxiety and depression alteration mechanism by oleanolic acid. J. Psychopharmacol. 2014, 28, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed]
- McManus, S.; Meltzer, H.; Brugha, T.; Bebbington, P.; Jenkins, R. Adult Psychiatric Morbidity in England, 2007. Results of a Household Survey; The Health and Social Care Information Centre; National Centre for Social Research: Leeds, UK, 2009; p. 12. [Google Scholar]
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision). 2018. Available online: https://icd.who.int/browse11/l-m/en (accessed on 1 July 2020).
- Newby, J.M.; McKinnon, A.; Kuyken, W.; Gilbody, S.; Dalgleish, T. Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood. Clin. Psychol. Rev. 2015, 40, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.C.; Nesse, R.M. Is low mood an adaptation? Evidence for subtypes with symptoms that match precipitants. J. Affect. Disord. 2005, 86, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nettle, D. Understanding of evolution may be improved by thinking about people. Evol. Psychol. 2010, 8, 205–228. [Google Scholar] [CrossRef]
- Gelenberg, A.J.; Freeman, M.P.; Markowitz, J.C.; Rosenbaum, J.F.; Thase, M.E.; Trivedi, M.H.; Van Rhoads, R.S.; Reus, V.I.; DePaulo, J.R., Jr.; Fawcett, J.A. Practice guideline for the treatment of patients with major depressive disorder third edition. Am. J. Psychiatry 2010, 167. [Google Scholar]
- Bolmont, B.; Abraini, J.H. State-anxiety and low moods: Evidence for a single concept. Physiol. Behav. 2001, 74, 421–424. [Google Scholar] [CrossRef]
- Baumeister, H.; Knecht, A.; Hutter, N. Direct and indirect costs in persons with chronic back pain and comorbid mental disorders--a systematic review. J. Psychosom. Res. 2012, 73, 79–85. [Google Scholar] [CrossRef]
- Salum, G.A.; Isolan, L.R.; Bosa, V.L.; Tocchetto, A.G.; Teche, S.P.; Schuch, I.; Costa, J.R.; Costa Mde, A.; Jarros, R.B.; Mansur, M.A.; et al. The multidimensional evaluation and treatment of anxiety in children and adolescents: Rationale, design, methods and preliminary findings. Rev. Bra. Psiquiatria. 2011, 33, 181–195. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence. National Collaborating Centre for Mental Health. The Treatment and Management of Depression in Adults (Updated Edition); National Clinical Practice Guideline 90; National Institute for Health and Care Excellence: London, UK, 2010; pp. 466–536. [Google Scholar]
- Fajemiroye, J.O.; da Silva, D.M.; de Oliveira, D.R.; Costa, E.A. Treatment of anxiety and depression: Medicinal plants in retrospect. Fund. Clin. Pharmacol. 2016, 30, 198–215. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother. Res. 2018, 32, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Dome, P.; Tombor, L.; Lazary, J.; Gonda, X.; Rihmer, Z.J.B.R.B. Natural health products, dietary minerals and over-the-counter medications as add-on therapies to antidepressants in the treatment of major depressive disorder: A review. Brain Res. Bull. 2019, 146, 51–78. [Google Scholar] [CrossRef]
- Mischoulon, D.; Rapaport, M.H.A. Current Role of Herbal and Natural Preparations. Antidepressants 2019, 250, 225–252. [Google Scholar]
- Bathaie, S.Z.; Mousavi, S.Z. Historical uses of saffron: Identifying potential new avenues for modern Research. Avicenna, J. Phytomed. 2011, 1, 57–66. [Google Scholar]
- Fernández, J. Biology, biotechnology and biomedicine of saffron. Recent Res. Dev. Plant. Sci. 2004, 2, 127–159. [Google Scholar]
- Grigg, D.B. The Agricultural Systems of the World: An. Evolutionary Approach; Cambridge University Press: Cambridge, UK, 1974. [Google Scholar]
- Leone, S.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Leporini, L.; Brunetti, L.; Menghini, L. Phytotherapic use of the Crocus sativus L. (Saffron) and its potential applications: A brief overview. Phytother. Res. 2018, 32, 2364–2375. [Google Scholar] [CrossRef]
- Srivastava, R.; Ahmed, H.; Dixit, R.K.; Dharamveer, P.; Saraf, S.A. Crocus sativus L.: A comprehensive review. Pharmacogn. Rev. 2010, 4, 200–208. [Google Scholar] [CrossRef]
- Sampathu, S.; Shivashankar, S.; Lewis, Y.; Wood, A. Saffron (Crocus sativus Linn.)—Cultivation, processing, chemistry and standardization. Crit. Rev. Food Sci. Nutr. 1984, 20, 123–157. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Ordoudi, S.; Roldan-Medina, M.; Tsimidou, M. Saffron, a Functional Spice. Austin J. Nutr. Food Sci. 2015, 3, 1051–1059. [Google Scholar]
- Farahmand, S.K.; Samini, F.; Samini, M.; Samarghandian, S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology 2013, 14, 63–71. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Inamdar, M.N. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl. Biochem. Biotechnol. 2010, 162, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Ardebili, D.; Hosseinzadeh, H.; Abnous, K.; Hasani, F.V.; Robati, R.Y.; Razavi, B.M. Involvement of brain-derived neurotrophic factor (BDNF) on malathion induced depressive-like behavior in subacute exposure and protective effects of crocin. Iran. J. Basic Med. Sci. 2015, 18, 958. [Google Scholar]
- Pitsikas, N.; Boultadakis, A.; Georgiadou, G.; Tarantilis, P.A.; Sakellaridis, N. Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomedicine 2008, 15, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Moosavi, M.; Zarifkar, A.; Rastegar, K. The interplay of Akt and ERK in Aβ toxicity and insulin-mediated protection in primary hippocampal cell culture. J. Mol. Neurosci. 2015, 57, 325–334. [Google Scholar] [CrossRef]
- Nam, K.N.; Park, Y.M.; Jung, H.J.; Lee, J.Y.; Min, B.D.; Park, S.U.; Jung, W.S.; Cho, K.H.; Park, J.H.; Kang, I.; et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010, 648, 110–116. [Google Scholar] [CrossRef]
- Rathore, B.; Jaggi, K.; Thakur, S.K.; Mathur, A.; Mahdi, F. Anti-inflammatory activity of Crocus sativus extract in experimental arthritis. Int. J. Pharm. Sci. Res. 2015, 6, 1473. [Google Scholar]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Parvardeh, S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine 2004, 11, 56–64. [Google Scholar] [CrossRef]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-Garcia, A.; Prodanov, M. affron((R)) a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
- Abe, K.; Sugiura, M.; Shoyama, Y.; Saito, H. Crocin antagonizes ethanol inhibition of NMDA receptor-mediated responses in rat hippocampal neurons. Brain Res. 1998, 787, 132–138. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Pharmacokinetic Properties of Saffron and its Active Components. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Gotlib, I.H. 13 Molecular Foundations of the Symptoms of Major Depressive Disorder. Oxf. Handb. Mol. Psychol. 2015, 258. [Google Scholar]
- Roy, A.; Roy, R.N. Stress and Major Depression: Neuroendocrine and Biopsychosocial Mechanisms. In Stress Neuroendocrinology Neurobiology: Handbook of Stress Series 2; Elsevier: San Diego, CA, USA, 2017; pp. 173–184. [Google Scholar]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom. Med. 2014, 76, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.; Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. R. 2012, 36, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Jones, K. Review of neuroplasticity and depression: Evidence for the neurotrophic or neuroplasticity theory of depression pathophysiology and systematic review of the neurophysiological implications of long-term antidepressant treatment. 2016. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Rocks, T.; Ruusunen, A.; Loughman, A.; Lopresti, A.; Marshall, S.; Berk, M.; Jacka, F.; Dean, O.M. Effect of saffron supplementation on symptoms of depression and anxiety: A systematic review and meta-analysis. Nutr. Rev. 2019. [Google Scholar] [CrossRef]
- Tóth, B.; Hegyi, P.; Lantos, T.; Szakács, Z.; Kerémi, B.; Varga, G.; Tenk, J.; Pétervári, E.; Balaskó, M.; Rumbus, Z. The efficacy of saffron in the treatment of mild to moderate depression: A meta-analysis. Planta Med. 2019, 85, 24–31. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Hood, S.D.; Drummond, P.D. Efficacy of a standardised saffron extract (affron(R)) as an add-on to antidepressant medication for the treatment of persistent depressive symptoms in adults: A randomised, double-blind, placebo-controlled study. J. Psychopharmacol. 2019, 33, 1415–1427. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. J. Med. Plants 2004, 3, 48–58. [Google Scholar]
- Hassani, F.V.; Naseri, V.; Razavi, B.M.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. DARU 2014, 22, 16. [Google Scholar] [CrossRef]
- Razavi, B.M.; Sadeghi, M.; Abnous, K.; Hasani, F.V.; Hosseinzadeh, H. Study of the role of CREB, BDNF, and VGF neuropeptide in long term antidepressant activity of crocin in the rat cerebellum. DARU 2017, 16, 1452. [Google Scholar]
- Bostan, H.B.; Mehri, S.; Hosseinzadeh, H. Toxicology effects of saffron and its constituents: A review. Irn. J. Basic Med. Sci. 2017, 20, 110–121. [Google Scholar] [CrossRef]
- Lymperopoulou, C.; Lamari, F.J.M.A.P. Saffron safety in humans: Lessons from the animal and clinical studies. Med. Aromat Plants 2015, 4, e164. [Google Scholar]
- Agha-Hosseini, M.; Kashani, L.; Aleyaseen, A.; Ghoreishi, A.; Rahmanpour, H.; Zarrinara, A.R.; Akhondzadeh, S. Crocus sativus L.(saffron) in the treatment of premenstrual syndrome: A double-blind, randomised and placebo-controlled trial. BJOG: Int. J. Obstet. Gy. 2008, 115, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpanah, M.; Ramezanshams, F.; Ghaleiha, A.; Akhondzadeh, S.; Bahmani, D.S.; Brand, S. Crocus Sativus, L.(saffron) versus sertraline on symptoms of depression among older people with major depressive disorders–a double-blind, randomized intervention study. Psychiat. Res. 2019, 282, 112–613. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Fallah-Pour, H.; Afkham, K.; Jamshidi, A.-H.; Khalighi-Cigaroudi, F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial [ISRCTN45683816]. BMC Complem. Altern. M. 2004, 4, 12. [Google Scholar] [CrossRef]
- Basti, A.A.; Moshiri, E.; Noorbala, A.-A.; Jamshidi, A.-H.; Abbasi, S.H.; Akhondzadeh, S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: A pilot double-blind randomized trial. Prog. Neuro-Psychoph. 2007, 31, 439–442. [Google Scholar] [CrossRef]
- Ghajar, A.; Neishabouri, S.M.; Velayati, N.; Jahangard, L.; Matinnia, N.; Haghighi, M.; Ghaleiha, A.; Afarideh, M.; Salimi, S.; Meysamie, A. Crocus sativus L. versus citalopram in the treatment of major depressive disorder with anxious distress: A double-blind, controlled clinical trial. Pharmacopsychiatry 2017, 50, 152–160. [Google Scholar] [CrossRef]
- Jelodar, G.; Javid, Z.; Sahraian, A.; Jelodar, S. Saffron improved depression and reduced homocysteine level in patients with major depression: A Randomized, double-blind study. Avicenna, J. Phytomed. 2018, 8, 43. [Google Scholar]
- Kashani, L.; Eslatmanesh, S.; Saedi, N.; Niroomand, N.; Ebrahimi, M.; Hosseinian, M.; Foroughifar, T.; Salimi, S.; Akhondzadeh, S. Comparison of saffron versus fluoxetine in treatment of mild to moderate postpartum depression: A double-blind, randomized clinical trial. Pharmacopsychiatry 2017, 50, 64–68. [Google Scholar] [CrossRef]
- Noorbala, A.A.; Akhondzadeh, S.H.; Tahmacebi-Pour, N.; Jamshidi, A.H. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. J. Ethnopharmacol. 2005, 97, 281–284. [Google Scholar] [CrossRef]
- Sahraian, A.; Jelodar, S.; Javid, Z.; Mowla, A.; Ahmadzadeh, L. Study the effects of saffron on depression and lipid profiles: A double blind comparative study. Asian J. Psychiatr. 2016, 22, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Shahmansouri, N.; Farokhnia, M.; Abbasi, S.-H.; Kassaian, S.E.; Tafti, A.-A.N.; Gougol, A.; Yekehtaz, H.; Forghani, S.; Mahmoodian, M.; Saroukhani, S. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J. Affect. Dis. 2014, 155, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, M.; Moazen-Zadeh, E.; Noorbala, A.A.; Jafarinia, M.; Divsalar, P.; Kashani, L.; Shahmansouri, N.; Tafakhori, A.; Bayat, H.; Akhondzadeh, S. Saffron (Crocus sativus) versus duloxetine for treatment of patients with fibromyalgia: A randomized double-blind clinical trial. Avicenna J. Phytomed. 2018, 8, 513. [Google Scholar]
- Russell, V.A.; Sagvolden, T.; Johansen, E.B. PMC1180819; Animal models of attention-deficit hyperactivity disorder. Behav. Brain. Funct. 2005, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Djordjevic, A.; Adzic, M.; Radojcic, M.B. Effects of chronic social isolation on Wistar rat behavior and brain plasticity markers. Neuropsychobiology 2012, 66, 112–119. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. PTR 2009, 23, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Scheggi, S.; De Montis, M.G.; Gambarana, C. PMC6209858; Making Sense of Rodent Models of Anhedonia. Int. J. Neuropsychopharmacol. 2018, 21, 1049–1065. [Google Scholar] [CrossRef]
- Asai, A.; Nakano, T.; Takahashi, M.; Nagao, A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J. Agric. Food. Chem. 2005, 53, 7302–7306. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Du, P.; Fu, J. Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine 2007, 14, 633–636. [Google Scholar] [CrossRef]
- Lautenschlager, M.; Sendker, J.; Huwel, S.; Galla, H.J.; Brandt, S.; Dufer, M.; Riehemann, K.; Hensel, A. Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier. Phytomedicine 2015, 22, 36–44. [Google Scholar] [CrossRef]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000. [Google Scholar]
- Khazdair, M.R.; Boskabady, M.H.; Hosseini, M.; Rezaee, R.; Tsatsakis, A. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna, J. Phytomed. 2015, 5, 376. [Google Scholar]
- Hosseinzadeh, H.; Sadeghnia, H.R.; Rahimi, A.J.P.m. Effect of safranal on extracellular hippocampal levels of glutamate and aspartate during kainic acid treatment in anesthetized rats. Planta Med. 2008, 74, 1441–1445. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Kubera, M.; Leunis, J.C.; Geffard, M. IgM-mediated autoimmune responses directed against multiple neoepitopes in depression: New pathways that underpin the inflammatory and neuroprogressive pathophysiology. J. Affect. Dis. 2011, 135, 414–418. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. Are Anti-inflammatory Therapies Viable Treatments for Psychiatric Disorders?: Where the Rubber Meets the Road. JAMA Psychiatry 2015, 72, 527–528. [Google Scholar] [CrossRef]
- Moylan, S.; Eyre, H.A.; Maes, M.; Baune, B.T.; Jacka, F.N.; Berk, M. Exercising the worry away: How inflammation, oxidative and nitrogen stress mediates the beneficial effect of physical activity on anxiety disorder symptoms and behaviours. Neurosci. Biobehav. R. 2013, 37, 573–584. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Saffron (Crocus sativus) for depression: A systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Human Psychopharmacol. 2014, 29, 517–527. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Farkhondeh, T. Antiinflammatory, Antioxidant, and Immunomodulatory Effects of Crocus sativus L. and its Main Constituents. Phytother. Res. PTR 2016, 30, 1072–1094. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Chang, A.; Grigg, J.; McCluskey, P. Efficacy and Safety of Saffron Supplementation: Current Clinical Findings. Crit. Rev. Food Sci. Nutr. 2016, 56, 2767–2776. [Google Scholar] [CrossRef]
- Samarghandian, S.; Samini, F.; Azimi-Nezhad, M.; Farkhondeh, T. Anti-oxidative effects of safranal on immobilization-induced oxidative damage in rat brain. Neurosci. Lett. 2017, 659, 26–32. [Google Scholar] [CrossRef]
- Poma, A.; Fontecchio, G.; Carlucci, G.; Chichiricco, G. Anti-inflammatory properties of drugs from saffron crocus. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Wiedlocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jazdzik, M.; Janus, M.; Debowska, W.; Mosiolek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers—A meta-analysis. Prog. Neuro-Psychoph 2018, 80, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Halataei, B.A.; Khosravi, M.; Arbabian, S.; Sahraei, H.; Golmanesh, L.; Zardooz, H.; Jalili, C.; Ghoshooni, H. Saffron (Crocus sativus) aqueous extract and its constituent crocin reduces stress-induced anorexia in mice. Phytother. Res. PTR 2011, 25, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Hooshmandi, Z.; Rohani, A.H.; Eidi, A.; Fatahi, Z.; Golmanesh, L.; Sahraei, H. Reduction of metabolic and behavioral signs of acute stress in male Wistar rats by saffron water extract and its constituent safranal. Pharm. Biol. 2011, 49, 947–954. [Google Scholar] [CrossRef][Green Version]
- Ieraci, A.; Mallei, A.; Popoli, M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016, 33, 58–64. [Google Scholar] [CrossRef]
- Cryan, J.F.; Markou, A.; Lucki, I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol. Sci. 2002, 23, 238–245. [Google Scholar] [CrossRef]
- Bogdanova, O.V.; Kanekar, S.; D’Anci, K.E.; Renshaw, P.F. Factors influencing behavior in the forced swim test. Physiol. Behav. 2013, 118, 227–239. [Google Scholar] [CrossRef]
- Zhang, J.C.; Wu, J.; Fujita, Y.; Yao, W.; Ren, Q.; Yang, C.; Li, S.X.; Shirayama, Y.; Hashimoto, K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2014, 18. [Google Scholar] [CrossRef]
- Eagle, A.L.; Mazei-Robison, M.; Robison, A.J. Sucrose preference test to measure stress-induced anhedonia. Bio. Protoc. 2016, 6, 1822. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Lucki, I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol. 1997, 8, 523–532. [Google Scholar] [CrossRef]
- Warner-Schmidt, J.L.; Duman, R.S. VEGF as a potential target for therapeutic intervention in depression. Curr. Opin. Pharmacol. 2008, 8, 14–19. [Google Scholar] [CrossRef]
- Pothion, S.; Bizot, J.C.; Trovero, F.; Belzung, C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav. Brain Res. 2004, 155, 135–146. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound affron® are available from Pharmactive Biotech Products SL. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orio, L.; Alen, F.; Ballesta, A.; Martin, R.; Gomez de Heras, R. Antianhedonic and Antidepressant Effects of Affron®, a Standardized Saffron (Crocus Sativus L.) Extract. Molecules 2020, 25, 3207. https://doi.org/10.3390/molecules25143207
Orio L, Alen F, Ballesta A, Martin R, Gomez de Heras R. Antianhedonic and Antidepressant Effects of Affron®, a Standardized Saffron (Crocus Sativus L.) Extract. Molecules. 2020; 25(14):3207. https://doi.org/10.3390/molecules25143207
Chicago/Turabian StyleOrio, Laura, Francisco Alen, Antonio Ballesta, Raquel Martin, and Raquel Gomez de Heras. 2020. "Antianhedonic and Antidepressant Effects of Affron®, a Standardized Saffron (Crocus Sativus L.) Extract" Molecules 25, no. 14: 3207. https://doi.org/10.3390/molecules25143207
APA StyleOrio, L., Alen, F., Ballesta, A., Martin, R., & Gomez de Heras, R. (2020). Antianhedonic and Antidepressant Effects of Affron®, a Standardized Saffron (Crocus Sativus L.) Extract. Molecules, 25(14), 3207. https://doi.org/10.3390/molecules25143207