Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer
Abstract
1. Introduction
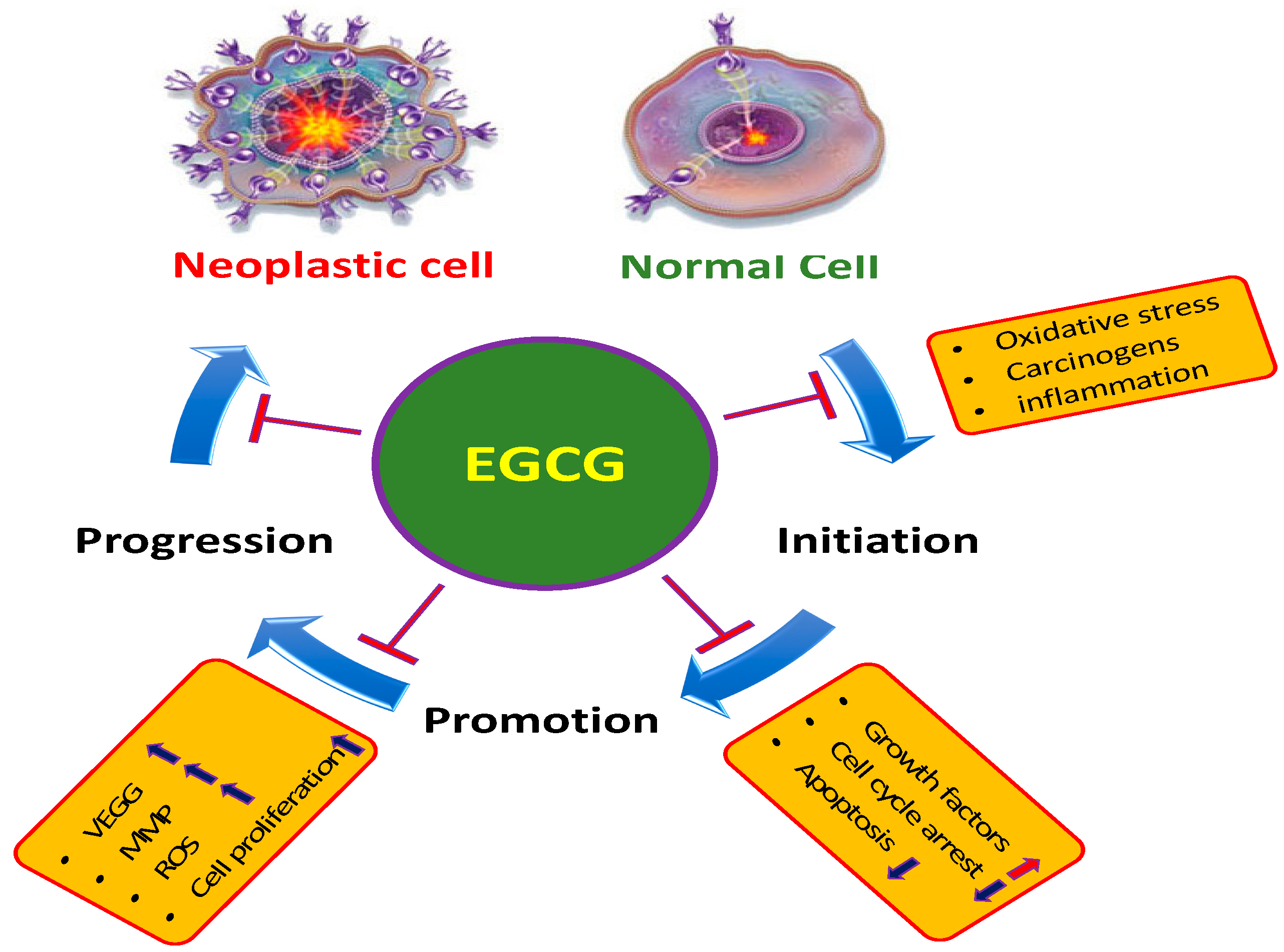
2. Main Mechanisms of EGCG in the Inhibition of Cancer
2.1. Inflammation
2.2. Reactive Oxygen Species (ROS)
2.3. Angiogenesis
2.4. Apoptosis
2.5. Tumor Suppressor Genes
2.6. Cell Cycle
| Pathogenesis | Types of Genes | Mechanism | Refs. |
|---|---|---|---|
| Inflammation | Necrosis factor (TNF)-α/intercellular adhesion molecule-1 expression | EGCG protected against tumor necrosis factor-α-mediated lung inflammation through down-regulation of oxidative stress and intercellular adhesion molecule-1 expression | [5] |
| Breast cancer | Vascular endothelial growth factor | EGCG have been proven to reduce vascular endothelial growth factor production | [14] |
| Breast cancer | HIF-1α and NFκB | EGCG inhibited the activation of HIF-1α and NFκB and VEGF expression | [16] |
| Laryngeal carcinoma cells/ Colon carcinoma cells/Cervical carcinoma cells | Apoptosis | EGCG was found to induce apoptosis in cells of the examined neoplastic cell lines in a dose-related manner | [20] |
| Hepatocellular carcinoma | Bcl-2 and NF-κB | EGCG-induced apoptosis of cancer cells was linked with a substantial decrease in Bcl-2 and NF-κB expression | [21] |
| Human prostate cancer | p53 | Epigallocatechin-3-gallate, activate p53 via acetylation at the Lys373 and Lys382 residues through inhibiting class I HDACs | [22] |
| Lung cancer | p53 | EGCG play crucial role in the inhibition of anchorage-independent growth of human lung cancer cells through upregulating p53 expression | [23] |
| Pancreatic cancer | Pten | EGCG is capable of decreasing proliferation and induce the apoptosis linked with the expression of PTEN. | [24] |
| Pancreatic cancer | Pten | EGCG upregulate PTEN expression and downregulate the expression of pAKT and p-mTOR | [28] |
| Pancreatic cancer | PI3K/Akt/mTOR pathway | EGCG subdue the expression of p-Akt and p-mTOR through PTEN to regulate the PI3K/Akt/mTOR pathway | [24] |
| Biliary tract cancer | p21 | EGCG reduced the mRNA levels of various cell cycle-related genes, but enhanced the expression of the cell cycle inhibitor p21 | [26] |
2.7. Phosphatidylinositide-3-Kinases (PI3Ks)/AKT Pathways
2.8. Signal Transducer and Activator of Transcription 3 (STAT3)
2.9. Epidermal Growth Factor Receptor (EGFR)
2.10. Activating Protein-1 (AP-1)
2.11. Phase II Detoxifying Enzymes
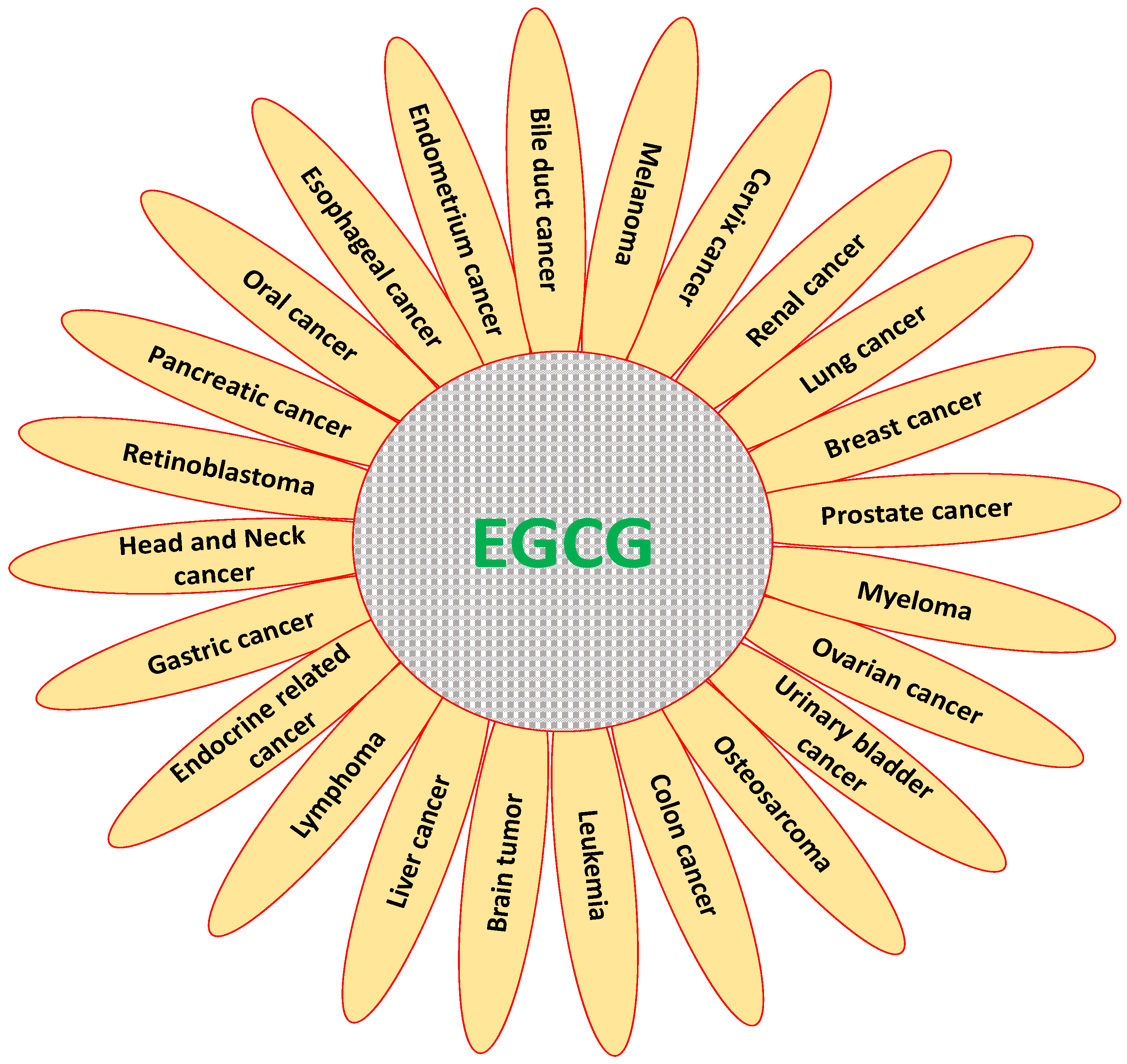
3. Role of EGCG in Inhibition and Prevention of Various Types of Cancer
3.1. Cervix Cancer
3.2. Breast Cancer
3.3. Ovarian Cancer
3.4. Endometrium Cancer
3.5. Pancreatic Cancer
3.6. Gastric Cancer
3.7. Liver Cancer
3.8. Colon Cancer
3.9. Bile Duct Cancer
3.10. Renal Cancer
3.11. Prostate Cancer
3.12. Urinary Bladder Cancer
3.13. Leukemia
3.14. Lymphoma
3.15. Head and Neck Cancer
3.16. Oral Cancer
3.17. Oesophagus Cancer
3.18. Melanoma
3.19. Lung Cancer
3.20. Myeloma
3.21. Osteosarcoma
3.22. Brain Tumor
3.23. Endocrine Related Cancer
3.24. Retinoblastoma
4. In Vivo Efficacy of Epigallocatechin-Gallate (EGCG) in the Management of Cancer
5. Measurement of Safety, Efficacy and Tolerability of EGCG in Cancer Based on Human Clinical Trials
5.1. Prostate Cancer
5.2. Urinary Bladder Cancer
5.3. Head and Neck Cancer
5.4. Breast Cancer
5.5. Ovarian Cancer
5.6. Lung Cancer
6. Effect of EGCG Alone and in Combination with Other Anticancer Compounds or Related Molecules
7. Available Concentration and Improvement of Bioavailability of Epigallocatechin Gallate (EGCG)
7.1. Liposome/Nanoliposome
7.2. Nanoemulsion
7.3. Chitosan/Carbohydrate Based Carrier
7.4. Polymeric Nanoparticles
7.5. Serum Albumin and Caseins Used as a Carrier
7.6. Structural Modification of EGCG
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- All Cancers [Internet]. 2018. Available online: http://gco.iarc.fr/today (accessed on 15 June 2019).
- Lin, S.R.; Fu, Y.S.; Tsai, M.J.; Cheng, H.; Weng, C.F. Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. Int. J. Mol. Sci. 2017, 18, 1412. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wu, Z.; Tan, B.; Yang, A.; Fang, Z. Emodin: Its role in prostate cancer-associated inflammation. Oncol. Rep. 2019, 42, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Lin, C.C.; Lee, C.Y.; Hsieh, P.W.; Yang, C.M. Protective effects of (−)-epigallocatechin-3-gallate against TNF-α-induced lung inflammation via ROS-dependent ICAM-1 inhibition. J. Nutr. Biochem. 2013, 24, 124–136. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Gao, W.; Wang, H.; Zhao, D.; Nie, Z.-L.; Shi, J.-Q.; Zhao, S.; Lu, X.; Wang, L.-S.; Yang, Z.-J. Green tea polyphenol epigallocatechin-3-gallate inhibits TNF-α-induced production of monocyte chemoattractant protein-1 in human umbilical vein endothelial cells. Cell Physiol. Biochem. 2014, 33, 1349–1358. [Google Scholar] [CrossRef]
- Hussain, T.; Gupta, S.; Adhami, V.M.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int. J. Cancer 2004, 113, 660–669. [Google Scholar] [CrossRef]
- Chitty, J.L.; Filipe, E.C.; Lucas, M.C.; Herrmann, D.; Cox, T.R.; Timpson, P. Recent advances in understanding the complexities of metastasis. F1000Res. 2018, 7, 1169. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.; Georgakilas, A.G.; Panagiotidis, M. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008, 266, 6–11. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef]
- Shi, X.; Ye, J.; Leonard, S.S.; Ding, M.; Vallyathan, V.; Castranova, V. Antioxidant properties of (–)-epicatechin-3-gallate and its inhibition of Cr(VI)-induced DNA damage and Cr(IV)- or TPA-stimulated NF-kappa B activation. Mol. Cell Biochem. 2000, 206, 125–132. [Google Scholar] [CrossRef]
- Park, I.J.; Lee, Y.K.; Hwang, J.T.; Kwon, D.Y.; Ha, J.; Park, O.J. Green tea catechin controls apoptosis in colon cancer cells by attenuation of H2O2-stimulated COX-2 expression via the AMPK signaling pathway at low-dose H2O2. Ann. N. Y. Acad. Sci. 2009, 1171, 538–544. [Google Scholar] [CrossRef]
- Bhimani, R.S.; Troll, W.; Grunberger, D.; Frenkel, K. Inhibition of oxidative stress in HeLa cells by chemopreventive agents. Cancer Res. 1993, 53, 4528–4533. [Google Scholar] [PubMed]
- Sartippour, M.R.; Chen, S.; Heber, D.; Beatty, P.; Zhang, L.; Liu, C.; Ellis, L.; Liu, W.; Go, V.L.; Brooks, M.N. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J. Nutr. 2002, 132, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Gingras, D.; Béliveau, R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002, 62, 381–385. [Google Scholar]
- Gu, J.W.; Makey, K.L.; Tucker, K.B.; Chinchar, E.; Mao, X.; Pei, I.; Thomas, E.Y.; Miele, L. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NF-κB, and VEGF expression. Vasc. Cell 2013, 5, 9. [Google Scholar] [CrossRef]
- Masuda, M.; Suzui, M.; Lim, J.T.; Deguchi, A.; Soh, J.W.; Weinstein, I.B. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J. Exp. Ther. Oncol. 2002, 2, 350–359. [Google Scholar] [CrossRef]
- Neuhaus, T.; Pabst, S.; Stier, S.; Weber, A.-A.; Schrör, K.; Sachinidis, A.; Vetter, H.; Ko, Y.D. Inhibition of the vascular-endothelial growth factor-induced intracellular signaling and mitogenesis of human endothelial cells by epigallocatechin-3 gallate. Eur. J. Pharmacol. 2004, 483, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yan, B.; Chen, K.; Jiang, Z.; Zhou, C.; Cao, J.; Qian, W.; Li, J.; Sun, L.; Ma, J.; et al. Resveratrol-induced downregulation of NAF-1 enhances the sensitivity of pancreatic cancer cells to gemcitabine via the ROS/Nrf2 signaling pathways. Oxid. Med. Cell. Longev. 2018, 2018, 9482018. [Google Scholar] [CrossRef]
- Borska, S.; Gebarowska, E.; Wysocka, T.; Drag-Zalesinska, M.; Zabel, M. Induction of apoptosis by EGCG in selected tumour cell lines in vitro. Folia Histochem. Cytobiol. 2003, 41, 229–232. [Google Scholar]
- Zhang, Y.; Duan, W.; Owusu, L.; Wu, D.; Xin, Y. Epigallocatechin-3-gallate induces the apoptosis of hepatocellular carcinoma LM6 cells but not noncancerous liver cells. Int. J. Mol. Med. 2015, 35, 117–124. [Google Scholar] [CrossRef]
- Thakur, V.S.; Gupta, K.; Gupta, S. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int. J. Oncol. 2012, 41, 353–361. [Google Scholar] [PubMed]
- Jin, L.; Li, C.; Xu, Y.; Wang, L.; Liu, J.; Wang, D.; Hong, C.; Jiang, Z.; Ma, Y.; Chen, Q. Epigallocatechin gallate promotes p53 accumulation and activity via the inhibition of MDM2-mediated p53 ubiquitination in human lung cancer cells. Oncol. Rep. 2013, 29, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, Z.L.; Sun, L.; Liu, Y.; Li, C.C.; Li, H.M.; Zhang, W.; Li, C.J.; Qin, W. (−)-Epigallocatechin-3-gallate induces apoptosis in human pancreatic cancer cells via PTEN. Mol. Med. Rep. 2016, 14, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.; Wagner, A.; Neureiter, D.; Pichler, M.; Jakab, M.; Illig, R.; Berr, F.; Kiesslich, T. The green tea catechin epigallocatechin gallate induces cell cycle arrest and shows potential synergism with cisplatin in biliary tract cancer cells. BMC Complement. Altern. Med. 2015, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hussain, T.; Mukhtar, H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch. Biochem. Biophys. 2003, 410, 177–185. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.J.; Liu, Y.; Cui, Y.F. PI3K/AKT/mTOR signaling is involved in (−)-Epigallocatechin-3-gallate-induced apoptosis of human pancreatic carcinoma cells. Am. J. Chin. Med. 2013, 41, 629–642. [Google Scholar] [CrossRef]
- Frezza, M.; Schmitt, S.; Dou, Q.P. Targeting the ubiquitin-proteasome pathway: An emerging concept in cancer therapy. Curr. Top. Med. Chem. 2011, 11, 2888–2905. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, D.Y.; Elliott, S.; Zhao, W.; Curiel, T.J.; Beckman, B.S.; Burow, M.E. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int. J. Oncol. 2007, 31, 705–711. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, X.; Deng, C.; Yang, L.; Yan, E.; Guo, T.; Li, Y.; Xu, M.X. Mechanism of the inhibition of the STAT3 signaling pathway by EGCG. Oncol. Rep. 2013, 30, 2691–2696. [Google Scholar] [CrossRef]
- Jin, G.; Yang, Y.; Liu, K.; Zhao, J.; Chen, X.; Liu, H.; Bai, R.; Li, X.; Jiang, Y.; Zhang, X. Combination curcumin and (−)-epigallocatechin-3-gallate inhibits colorectal carcinoma microenvironment-induced angiogenesis by JAK/STAT3/IL-8 pathway. Oncogenesis 2017, 6, e384. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Suzui, M.; Lim, J.T.; Weinstein, I.B. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin. Cancer Res. 2003, 9, 3486–3491. [Google Scholar]
- Ma, Y.-C.; Li, C.; Gao, F.; Xu, Y.; Jiang, Z.-B.; Liu, J.-X.; Jin, L.-Y. Epigallocatechin gallate inhibits the growth of human lung cancer by directly targeting the EGFR signaling pathway. Oncol. Rep. 2013, 31, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ma, W.; Huang, C.; Yang, C.S. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997, 57, 4414–4419. [Google Scholar] [PubMed]
- Hong, W.K.; Sporn, M.B. Recent Advances in Chemoprevention of Cancer. Science 1997, 278, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P. Chemoprotection against cancer by induction of phase 2 enzymes. BioFactors 2000, 12, 5–11. [Google Scholar] [CrossRef]
- Yu, R.; Jiao, J.J.; Duh, J.L.; Gudehithlu, K.; Tan, T.-H.; Kong, A.N. Activation of mitogen-activated protein kinases by green tea polyphenols: Potential signaling pathways in the regulation of antioxidant-responsive element-mediated phase II enzyme gene expression. Carcinogenesis 1997, 18, 451–456. [Google Scholar] [CrossRef]
- Chen, C.; Yu, R.; Owuor, E.D.; Kong, A.-N.T. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch. Pharm. Res. 2000, 23, 605–612. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Rahmani, A.H. Garlic and its Active Compounds: A Potential Candidate in The Prevention of Cancer by Modulating Various Cell Signalling Pathways. Anticancer Agents Med. Chem. 2019, 19, 1314–1324. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Salah, M.A.; Habeeb, A.; Babiker, A.Y.; Srikar, S.; Khan, A.A. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int. J. Clin. Exp. Med. 2014, 7, 483–491. [Google Scholar]
- McDonnell, A.M.; Pyles, H.M.; Diaz-Cruz, E.S.; Barton, C.E. Enoxacin and Epigallocatechin Gallate (EGCG) Act Synergistically to Inhibit the Growth of Cervical Cancer Cells in Culture. Molecules 2019, 24, 1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Lu, J.L.; Liang, Y.R.; Li, Q.S. Suppressive effects of egcg on cervical cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef]
- Pal, D.; Sur, S.; Roy, R.; Mandal, S.; Kumar, P.C. Epigallocatechin gallate in combination with eugenol or amarogentin shows synergistic chemotherapeutic potential in cervical cancer cell line. J. Cell. Physiol. 2018, 234, 825–836. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (−)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, O.; Soritau, O.; Balacescu, O.; Balacescu, L.; Braicu, C.; Rus, M.; Gherman, C.; Virag, P.; Irimie, F.; Berindan-Neagoe, I. Early transcriptional pattern of angiogenesis induced by EGCG treatment in cervical tumour cells. J. Cell. Mol. Med. 2012, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Mao, L.; Xu, P.; Zheng, X.; Hackman, R.M.; MacKenzie, G.G.; Wang, Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018, 9, 5682–5696. [Google Scholar] [CrossRef]
- Huang, C.Y.; Han, Z.; Li, X.; Xie, H.H.; Zhu, S.S. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol. Lett. 2017, 14, 3623–3627. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, B.; Song, Z.; Han, S.; Wang, M. Estrogen receptor-α36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. J. Pharmacol. Sci. 2016, 130, 85–93. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef]
- Luo, H.-Q.; Xu, M.; Zhong, W.-T.; Cui, Z.-Y.; Liu, F.-M.; Zhou, K.-Y.; Li, X.-Y. EGCG decreases the expression of HIF-1α and VEGF and cell growth in MCF-7 breast cancer cells. J. BUON 2014, 19, 435–439. [Google Scholar] [PubMed]
- Yunos, N.M.; Beale, P.; Yu, J.Q.; Huq, F. Synergism from sequenced combinations of curcumin and epigallocatechin-3-gallate with cisplatin in the killing of human ovarian cancer cells. Anticancer Res. 2011, 31, 1131–1140. [Google Scholar] [PubMed]
- Yan, C.; Yang, J.; Shen, L.; Chen, X. Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch. Gynecol. Obstet. 2012, 285, 459–467. [Google Scholar] [CrossRef]
- Chan, M.M.; Soprano, K.J.; Weinstein, K.; Fong, D. Epigallocatechin-3-gallate delivers hydrogen peroxide to induce death of ovarian cancer cells and enhances their cisplatin susceptibility. J. Cell. Physiol. 2006, 207, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Spinella, F.; Rosano, L.; Di, C.V.; Decandia, S.; Albini, A.; Nicotra, M.R.; Natali, P.G.; Bagnato, A. Green tea polyphenol epigallocatechin-3-gallate inhibits the endothelin axis and downstream signaling pathways in ovarian carcinoma. Mol. Cancer Ther. 2006, 5, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.W.; Bae, S.M.; Kim, Y.-W.; Lee, J.M.; Namkoong, S.E.; Lee, I.P.; Kim, S.H.; Kim, C.K.; Ahn, W.S. Anticancer effects of (−)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol. Oncol. 2004, 94, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Bae, S.M.; Lee, J.M.; Namkoong, S.E.; Han, S.J.; Lee, B.R.; Lee, I.P.; Kim, S.H.; Lee, Y.J.; Kim, C.K.; et al. Activity of green tea polyphenol epigallocatechin-3-gallate against ovarian carcinoma cell lines. Cancer Res. Treat. 2004, 36, 315–323. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Sahli, M.A.; Almatroodi, S.A. Potential Antitumor Effects of Pomegranates and Its Ingredients. Pharmacogn. Rev. 2017, 11, 136–140. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Aly, S.M.; Khan, M.A.; Aldebasi, Y.H. Role of curcumin in disease prevention and treatment. Adv. Biomed. Res. 2018, 7, 38. [Google Scholar] [CrossRef]
- Almatroudi, A.; Alsahli, M.A.; Alrumaihi, F.; Allemailem, K.S.; Rahmani, A.H. Ginger: A Novel Strategy to Battle Cancer through Modulating Cell Signalling Pathways: A Review. Curr. Pharm. Biotechnol. 2019, 20, 5–16. [Google Scholar] [CrossRef]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A prodrug of green tea polyphenol (−)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef]
- Park, S.B.; Bae, J.W.; Kim, J.M.; Lee, S.G.; Han, M. Antiproliferative and apoptotic effect of epigallocatechin-3-gallate on Ishikawa cells is accompanied by sex steroid receptor downregulation. Int. J. Mol. Med. 2012, 30, 1211–1218. [Google Scholar] [CrossRef]
- Manohar, M.; Fatima, I.; Saxena, R.; Chandra, V.; Sankhwar, P.L.; Dwivedi, A. (−)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J. Nutr. Biochem. 2013, 24, 940–947. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alzohairy, M.A.; Khan, M.A.; Aly, S.M. Therapeutic Implications of Black Seed and Its Constituent Thymoquinone in the Prevention of Cancer through Inactivation and Activation of Molecular Pathways. Evid. Based Complemen. Alt. Med. 2014, 2014, 724658. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; AlZohairy, M.A.; Aly, S.M.; Khan, M.A. Curcumin: A potential candidate in prevention of cancer via modulation of molecular pathways. Biomed. Res. Int. 2014, 2014, 761608. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alsahli, M.A.; Alharbi, H.M.; Khan, A.A.; Rahmani, A.H. Epigallocatechin-3-Gallate (EGCG), An Active Constituent of Green Tea: Implications in the Prevention of Liver Injury Induced by Diethylnitrosamine (DEN) in Rats. Appl. Sci. 2019, 9, 4821. [Google Scholar] [CrossRef]
- Wei, R.; Penso, N.E.C.; Hackman, R.M.; Wang, Y.; Mackenzie, G.G. Epigallocatechin-3-Gallate (EGCG) Suppresses Pancreatic Cancer Cell Growth, Invasion, and Migration partly through the Inhibition of Akt Pathway and Epithelial-Mesenchymal Transition: Enhanced Efficacy when Combined with Gemcitabine. Nutrients 2019, 11, 1856. [Google Scholar] [CrossRef]
- Wei, R.; Hackman, R.M.; Wang, Y.; Mackenzie, G.G. Targeting Glycolysis with Epigallocatechin-3-Gallate Enhances the Efficacy of Chemotherapeutics in Pancreatic Cancer Cells and Xenografts. Cancers 2019, 11, 1496. [Google Scholar] [CrossRef]
- Bimonte, S.; Leongito, M.; Barbieri, A.; Del Vecchio, V.; Barbieri, M.; Albino, V.; Piccirillo, M.; Amore, A.; Di Giacomo, R.; Nasto, A. Inhibitory effect of (−)-epigallocatechin-3-gallate and bleomycin on human pancreatic cancer MiaPaca-2 cell growth. Infect. Agent Cancer 2015, 10, 22. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, W.T.; Hsieh, C.H.; Kuo, Y.Y.; Chao, C.Y. Thermal cycling-hyperthermia in combination with polyphenols, epigallocatechin gallate and chlorogenic acid, exerts synergistic anticancer effect against human pancreatic cancer PANC-1 cells. PLoS ONE 2019, 14, e0217676. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Zhang, L.; Yee, J.K.; Go, V.W.; Lee, W.N. Metabolic Consequences of LDHA inhibition by Epigallocatechin Gallate and Oxamate in MIA PaCa-2 Pancreatic Cancer Cells. Metabolomics 2015, 11, 71–80. [Google Scholar] [CrossRef]
- Shankar, S.; Suthakar, G.; Srivastava, R.K. Epigallocatechin-3-gallate inhibits cell cycle and induces apoptosis in pancreatic cancer. Front. Biosci. 2007, 12, 5039–5051. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-D.; Yao, J.-J.; Wang, H.; Cui, W.-G.; Leng, J.; Ding, L.-Y.; Fan, K.-Y. Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 155–161. [Google Scholar] [PubMed]
- Tang, H.; Zeng, L.; Wang, J.; Zhang, X.; Ruan, Q.; Wang, J.; Cui, S.; Yang, D. Reversal of 5-fluorouracil resistance by EGCG is mediate by inactivation of TFAP2A/VEGF signaling pathway and down-regulation of MDR-1 and P-gp expression in gastric cancer. Oncotarget 2017, 8, 82842–82853. [Google Scholar] [CrossRef]
- Yang, C.; Du, W.; Yang, D. Inhibition of green tea polyphenol EGCG((−)-epigallocatechin-3-gallate) on the proliferation of gastric cancer cells by suppressing canonical wnt/β-catenin signalling pathway. Int. J. Food Sci. Nutr. 2016, 67, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Kuribayashi, K.; Nirasawa, S.; Tsuji, N.; Tanaka, M.; Onoda, C.; Kobayashi, D. (−)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer cell lines by down-regulating survivin expression. Int. J. Oncol. 2011, 38, 1403–1408. [Google Scholar]
- Ma, J.; Shi, M.; Li, G.; Wang, N.; Wei, J.; Wang, T.; Ma, J.; Wang, Y. Regulation of Id1 expression by epigallocatechin-3-gallate and its effect on the proliferation and apoptosis of poorly differentiated AGS gastric cancer cells. Int. J. Oncol. 2013, 43, 1052–1058. [Google Scholar] [CrossRef]
- Zhu, B.-H.; Zhan, W.-H.; Li, Z.-R.; Wang, Z.; He, Y.-L.; Peng, J.-S.; Cai, S.-R.; Ma, J.-P.; Zhang, C.-H. (−)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J. Gastroenterol. 2007, 13, 1162–1169. [Google Scholar] [CrossRef]
- Zhu, B.H.; Zhan, W.H.; He, Y.L.; Cai, S.R.; Wang, Z.; Zhang, C.H. Epigallocatechin-3-gallate inhibits growth and angiogenesis of gastric cancer and its molecular mechanism. Zhonghua Wei Chang Wai Ke Za Zhi 2009, 12, 82–85. [Google Scholar]
- Chen, J.; Chen, L.; Lu, T.; Xie, Y.; Li, C.; Jia, Z.; Cao, J. ERα36 is an effective target of epigallocatechin-3-gallate in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 3222–3234. [Google Scholar]
- Sur, S.; Pal, D.; Mandal, S.; Roy, A.; Panda, C.K. Tea polyphenols epigallocatechin gallete and theaflavin restrict mouse liver carcinogenesis through modulation of self-renewal Wnt and hedgehog pathways. J. Nutr. Biochem. 2016, 27, 32–42. [Google Scholar] [CrossRef]
- Park, H.J.; Shin, D.H.; Chung, W.J. Epigallocatechin gallate reduces hypoxia-induced apoptosis in human hepatoma cells. Life Sciences. 2006, 78, 2826–2832. [Google Scholar] [CrossRef]
- Darweish, M.M.; Abbas, A.; Ebrahim, M.A.; Al-Gayyar, M.M. Chemopreventive and hepatoprotective effects of Epigallocatechin-gallate against hepatocellular carcinoma: Role of heparan sulfate proteoglycans pathway. J. Pharm. Pharmacol. 2014, 66, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Moseley, V.R.; Morris, J.; Knackstedt, R.W.; Wargovich, M.J. Green tea polyphenol epigallocatechin 3-gallate, contributes to the degradation of DNMT3A and HDAC3 in HCT 116 human colon cancer cells. Anticancer Res. 2013, 33, 5325–5333. [Google Scholar] [PubMed]
- Saldanha, S.N.; Kala, R.; Tollefsbol, T.O. Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp. Cell Res. 2014, 324, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Shimizu, M.; Shirakami, Y.; Yamauchi, J.; Natsume, H.; Matsushima-Nishiwaki, R.; To, S.; Weinstein, I.B.; Moriwaki, H.; Kozawa, O. (−)-Epigallocatechin gallate downregulates EGF receptor via phosphorylation at Ser1046/1047 by p38 MAPK in colon cancer cells. Carcinogenesis 2009, 30, 1544–1552. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Lim, J.T.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef]
- Wang, X.; Ye, T.; Chen, W.-J.; Lv, Y.; Hao, Z.; Chen, J.; Zhao, J.; Wang, H.-P.; Cai, Y. Structural shift of gut microbiota during chemo-preventive effects of epigallocatechin gallate on colorectal carcinogenesis in mice. World J. Gastroenterol. 2017, 23, 8128–8139. [Google Scholar] [CrossRef]
- Yuan, J.H.; Li, Y.Q.; Yang, X.Y. Inhibition of epigallocatechin gallate on orthotopic colon cancer by upregulating the Nrf2-UGT1A signal pathway in nude mice. Pharmacology 2007, 80, 269–278. [Google Scholar] [CrossRef]
- Kwak, T.W.; Park, S.B.; Kim, H.J.; Jeong, Y.I.; Kang, D.H. Anticancer activities of epigallocatechin-3-gallate against cholangiocarcinoma cells. Oncol. Targets Ther. 2016, 10, 137–144. [Google Scholar] [CrossRef]
- Senggunprai, L.; Kukongviriyapan, V.; Prawan, A.; Kukongviriyapan, U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother. Res. 2014, 28, 841–848. [Google Scholar] [CrossRef]
- Kwak, T.W.; Kim, H.; Chung, C.-W.; Lee, H.M.; Kim, C.H.; Jeong, Y.-I.; Kang, D.H. Synergistic Anticancer Effects of Vorinostat and Epigallocatechin-3-Gallate against HuCC-T1 Human Cholangiocarcinoma Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 185158. [Google Scholar] [CrossRef]
- Lang, M.; Henson, R.; Braconi, C.; Patel, T. Epigallocatechin-gallate modulates chemotherapy-induced apoptosis in human cholangiocarcinoma cells. Liver Int. 2009, 29, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Ding, Q.; Xia, G.; Fang, Z. EGCG inhibits growth and induces apoptosis in renal cell carcinoma through TFPI-2 overexpression. Oncol. Rep. 2009, 21, 635–640. [Google Scholar] [PubMed]
- Chen, S.-J.; Yao, X.-D.; Peng, B.; Xu, Y.-F.; Wang, G.-C.; Huang, J.; Liu, M.; Zheng, J. Epigallocatechin-3-gallate inhibits migration and invasion of human renal carcinoma cells by downregulating matrix metalloproteinase-2 and matrix metalloproteinase-9. Exp. Ther. Med. 2016, 11, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Jerónimo, C.; Valentão, P.; Andrade, P.B.; Silva, B.M. Green tea: A promising anticancer agent for renal cell carcinoma. Food Chem. 2010, 122, 49–54. [Google Scholar] [CrossRef]
- Sato, A.; Sekine, M.; Kobayashi, M.; Virgona, N.; Ota, M.; Yano, T. Induction of the connexin 32 gene by epigallocatechin-3-gallate potentiates vinblastine-induced cytotoxicity in human renal carcinoma cells. Chemotherapy 2013, 59, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-G.; Lee, Y.-H.; Kwak, J.; Choi, H.-K.; Choi, K.-C.; Kim, S.; Lee, J.; Jun, W.; Park, H.-J. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int. J. Mol. Med. 2012, 30, 69–74. [Google Scholar] [CrossRef]
- Tang, S.N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010, 5, 14. [Google Scholar] [CrossRef]
- Eom, D.-W.; Lee, J.H.; Kim, Y.-J.; Hwang, G.S.; Kim, S.-N.; Kwak, J.H.; Cheon, G.J.; Kim, K.H.; Jang, H.-J.; Ham, J.; et al. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 2015, 48, 461–466. [Google Scholar] [CrossRef]
- Kim, M.H.; Chung, J. Synergistic cell death by EGCG and ibuprofen in DU-145 prostate cancer cell line. Anticancer Res. 2007, 27, 3947–3956. [Google Scholar]
- Albrecht, D.S.; Clubbs, E.A.; Ferruzzi, M.; Bomser, J.A. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem. Biol. Interact. 2008, 171, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Paschka, A.G.; Butler, R.; Young, C.Y. Induction of apoptosis in prostate cancer cell lines by the green tea component, (−)-epigallocatechin-3-gallate. Cancer Lett. 1998, 130, 1–7. [Google Scholar] [CrossRef]
- Luo, K.-W.; Chen, W.-; Lung, W.-Y.; Wei, X.-Y.; Cheng, B.-H.; Cai, Z.-M.; Huang, W.-R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chen, N.-G.; Lu, C.-C.; Shen, W.-C.; Lai, C.-H.; Ho, Y.-J.; Chung, J.-G.; Yang, J.-S. Proteomic approaches to study epigallocatechin gallate-provoked apoptosis of TSGH-8301 human urinary bladder carcinoma cells: Roles of AKT and heat shock protein 27-modulated intrinsic apoptotic pathways. Oncol. Rep. 2011, 26, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Rieger-Christ, K.M.; Hanley, R.; Lodowsky, C.; Bernier, T.; Vemulapalli, P.; Roth, M.; Kim, J.; Yee, A.S.; Le, S.M.; Marie, P.J.; et al. The green tea compound, (−)-epigallocatechin-3-gallate downregulates N-cadherin and suppresses migration of bladder carcinoma cells. J. Cell. Biochem. 2007, 102, 377–388. [Google Scholar] [CrossRef]
- Jankun, J.; Keck, R.W.; Selman, S.H. Epigallocatechin-3-gallate prevents tumor cell implantation/growth in an experimental rat bladder tumor model. Int. J. Oncol. 2014, 44, 147–152. [Google Scholar] [CrossRef]
- Borutinskaitė, V.; Virkšaitė, A.; Gudelytė, G.; Navakauskienė, R. Green tea polyphenol EGCG causes anti-cancerous epigenetic modulations in acute promyelocytic leukemia cells. Leuk. Lymphoma 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Shi, X.; Gao, H.Y.; Yan, W.; He, X.W.; Yang, W. Effects of EGCG on Proliferation, Cell Cycle and DAPK1 Gene Methylation of Acute Promyelocytic Leukemia NB4 Cell Line. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018, 26, 1288–1293. [Google Scholar]
- Moradzadeh, M.; Roustazadeh, A.; Tabarraei, A.; Erfanian, S.; Sahebkar, A. Epigallocatechin-3-gallate enhances differentiation of acute promyelocytic leukemia cells via inhibition of PML-RARα and HDAC1. Phytother. Res. 2018, 32, 471–479. [Google Scholar] [CrossRef]
- Gan, L.; Zhong, L.; Shan, Z.; Xiao, C.; Xu, T.; Song, H.; Li, L.; Yang, R.; Liu, B. Epigallocatechin-3-gallate induces apoptosis in acute promyelocytic leukemia cells via a SHP-1-p38α MAPK-Bax cascade. Oncol. Lett. 2017, 14, 6314–6320. [Google Scholar] [CrossRef][Green Version]
- Yao, S.; Zhong, L.; Chen, M.; Zhao, Y.; Li, L.; Liu, L.; Xu, T.; Xiao, C.; Gan, L.; Shan, Z.; et al. Epigallocatechin-3-gallate promotes all-trans retinoic acid-induced maturation of acute promyelocytic leukemia cells via PTEN. Int. J. Oncol. 2017, 51, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Kim, J.H. Difference in growth suppression and apoptosis induction of EGCG and EGC on human promyelocytic leukemia HL-60 cells. Arch. Pharm. Res. 2009, 32, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, Y.; Feng, Y.; Zhang, L.; Huang, X.; Shen, X.; Luo, X. (−)-Epigallocatechin gallate induces apoptosis in B lymphoma cells via caspase-dependent pathway and Bcl-2 family protein modulation. Int. J. Oncol. 2015, 46, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.F.; Shen, J.Z.; Chen, Z.Z.; Fan, L.P.; Lin, F.A. Demethylation and Transcription of p16 Gene in Malignant Lymphoma Cell Line CA46 Induced by EGCG. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2008, 16, 1073–1078. [Google Scholar]
- Tsai, C.-Y.; Chen, C.-Y.; Chiou, Y.-H.; Shyu, H.-W.; Lin, K.-H.; Chou, M.-C.; Huang, M.-H.; Wang, Y.-F. Epigallocatechin-3-Gallate Suppresses Human Herpesvirus 8 Replication and Induces ROS Leading to Apoptosis and Autophagy in Primary Effusion Lymphoma Cells. Int. J. Mol. Sci. 2017, 19, 16. [Google Scholar] [CrossRef]
- Shin, Y.S.; Kang, S.U.; Park, J.K.; Kim, Y.E.; Kim, Y.S.; Baek, S.J.; Lee, S.-H.; Kim, C.-H. Anti-cancer effect of (−)-epigallocatechin-3-gallate (EGCG) in head and neck cancer through repression of transactivation and enhanced degradation of β-catenin. Phytomedicine 2016, 23, 1344–1355. [Google Scholar] [CrossRef]
- Lee, S.H.; Nam, H.J.; Kang, H.J.; Kwon, H.W.; Lim, Y.C. Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. Eur. J. Cancer 2013, 49, 3210–3218. [Google Scholar] [CrossRef]
- Haque, A.; Rahman, M.A.; Chen, Z.G.; Saba, N.F.; Khuri, F.R.; Shin, D.M.; Amin, A.R. Combination of erlotinib and EGCG induces apoptosis of head and neck cancers through posttranscriptional regulation of Bim and Bcl-2. Apoptosis 2015, 20, 986–995. [Google Scholar] [CrossRef]
- Amin, A.R.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Synergistic growth inhibition of squamous cell carcinoma of the head and neck by erlotinib and epigallocatechin-3-gallate: The role of p53-dependent inhibition of nuclear factor-kappaB. Cancer Prev. Res. (Phila). 2009, 2, 538–545. [Google Scholar] [CrossRef]
- Yoshimura, H.; Yoshida, H.; Matsuda, S.; Ryoke, T.; Ohta, K.; Ohmori, M.; Yamamoto, S.; Kiyoshima, T.; Kobayashi, M.; Sano, K. The therapeutic potential of epigallocatechin-3-gallate against human oral squamous cell carcinoma through inhibition of cell proliferation and induction of apoptosis: In vitro and in vivo murine xenograft study. Mol. Med. Rep. 2019, 20, 1139–1148. [Google Scholar] [CrossRef]
- Yuan, C.-H.; Horng, C.-T.; Lee, C.-F.; Chiang, N.-N.; Tsai, F.-J.; Lu, C.-C.; Chiang, J.-H.; Hsu, Y.-M.; Yang, J.-S.; Chen, F.-A. Epigallocatechin gallate sensitizes cisplatin-resistant oral cancer CAR cell apoptosis and autophagy through stimulating AKT/STAT3 pathway and suppressing multidrug resistance 1 signaling. Environ. Toxicol. 2017, 32, 845–855. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Shieh, P.-C.; Lin, Y.-C.; Chen, Y.-J.; Lin, Y.-H.; Kuo, D.-H.; Liu, J.-Y.; Kao, J.-Y.; Kao, M.-C.; Way, T.-D. Indoleamine 2,3-dioxygenase, an immunomodulatory protein, is suppressed by (−)-epigallocatechin-3-gallate via blocking of gamma-interferon-induced JAK-PKC-delta-STAT1 signaling in human oral cancer cells. J. Agric. Food Chem. 2010, 58, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.W.; Choi, E.C.; Kang, S.U.; Hwang, H.S.; Lee, M.H.; Pyun, J.; Park, R.; Lee, Y.; Kim, C.-H. Green tea (−)-epigallocatechin-3-gallate inhibits HGF-induced progression in oral cavity cancer through suppression of HGF/c-Met. J. Nutr. Biochem. 2011, 22, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.N.; Chu, S.C.; Kuo, W.H.; Chou, M.Y.; Lin, J.K.; Hsieh, Y.S. Epigallocatechin-3 gallate inhibits invasion, epithelial-mesenchymal transition, and tumor growth in oral cancer cells. J. Agric. Food Chem. 2011, 59, 3836–3844. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ju, Y.; Wang, J.; Zhou, R. Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells. Pathol. Res. Pract. 2017, 213, 1242–1250. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, G.H.; Guan, B.X.; Xu, X.C. Suppression of esophageal cancer cell growth using curcumin, (−)-epigallocatechin-3-gallate and lovastatin. World J. Gastroenterol. 2012, 18, 126–135. [Google Scholar] [CrossRef]
- Liu, L.; Hou, L.; Gu, S.Z.; Zuo, X.; Meng, D.; Luo, M.; Zhang, X.; Huang, S.; Zhao, X. Molecular mechanism of epigallocatechin-3-gallate in human esophageal squamous cell carcinoma in vitro and in vivo. Oncol. Rep. 2015, 33, 297–303. [Google Scholar] [CrossRef]
- Gao, Y.; Li, W.; Jia, L.; Li, B.; Chen, Y.C.; Tu, Y. Enhancement of (−)-epigallocatechin-3-gallate and theaflavin-3-3′-digallate induced apoptosis by ascorbic acid in human lung adenocarcinoma SPC-A-1 cells and esophageal carcinoma Eca-109 cells via MAPK pathways. Biochem. Biophys. Res. Commun. 2013, 438, 370–374. [Google Scholar] [CrossRef]
- Nihal, M.; Roelke, C.T.; Wood, G.S. Anti-melanoma effects of vorinostat in combination with polyphenolic antioxidant (−)-epigallocatechin-3-gallate (EGCG). Pharm. Res. 2010, 27, 1103–1114. [Google Scholar] [CrossRef]
- Nihal, M.; Ahsan, H.; Siddiqui, I.A.; Mukhtar, H.; Ahmad, N.; Wood, G.S. (−)-Epigallocatechin-3-gallate (EGCG) sensitizes melanoma cells to interferon induced growth inhibition in a mouse model of human melanoma. Cell Cycle 2009, 8, 2057–2063. [Google Scholar] [CrossRef]
- Ellis, L.Z.; Liu, W.; Luo, Y.; Okamoto, M.; Qu, D.; Dunn, J.H.; Fujita, M. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochem. Biophys. Res. Commun. 2011, 414, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.D.; Chen, S.H.; Lin, C.L.; Tsai, S.H.; Liang, Y.C. Inhibition of melanoma growth and metastasis by combination with (−)-epigallocatechin-3-gallate and dacarbazine in mice. J. Cell. Biochem. 2001, 83, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, Y.; Liu, H.; Li, J. Inhibition of invasion and up-regulation of E-cadherin expression in human malignant melanoma cell line A375 by (−)-epigallocatechin-3-gallate. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Jiao, Y.; Xue, J.; Zhang, Q.; Yang, H.; Xing, L.; Chen, G.; Wu, J.; Zhang, S.; Zhu, W. Metformin Sensitizes Non-small Cell Lung Cancer Cells to an Epigallocatechin-3-Gallate (EGCG) Treatment by Suppressing the Nrf2/HO-1 Signaling Pathway. Int. J. Biol. Sci. 2017, 13, 1560–1569. [Google Scholar] [CrossRef]
- Jiang, P.; Xu, C.; Chen, L.; Chen, A.; Wu, X.; Zhou, M.; Haq, I.U.; Mariyam, Z.; Feng, Q. Epigallocatechin-3-gallate inhibited cancer stem cell-like properties by targeting hsa-mir-485-5p/RXRα in lung cancer. J. Cell. Biochem. 2018, 119, 8623–8635. [Google Scholar] [CrossRef]
- Li, M.; Li, J.-J.; Gu, Q.-H.; An, J.; Cao, L.-M.; Yang, H.-P.; Hu, C. EGCG induces lung cancer A549 cell apoptosis by regulating Ku70 acetylation. Oncol. Rep. 2016, 35, 2339–2347. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/β-catenin pathway mediates (−)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef]
- Kim, K.C.; Lee, C. Reversal of Cisplatin resistance by epigallocatechin gallate is mediated by downregulation of axl and tyro 3 expression in human lung cancer cells. Korean J. Physiol. Pharmacol. 2014, 18, 61–66. [Google Scholar] [CrossRef]
- Zhou, C.G.; Hui, L.M.; Luo, J.M. Epigallocatechin gallate inhibits the proliferation and induces apoptosis of multiple myeloma cells via inactivating EZH2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2093–2098. [Google Scholar]
- Wang, Q.; Li, J.; Gu, J.; Huang, B.; Zhao, Y.; Zheng, N.; Ding, Y.; Zeng, L. Potentiation of (−)-epigallocatechin-3-gallate-induced apoptosis by bortezomib in multiple myeloma cells. Acta Biochim. Biophys. Sin. (Shanghai) 2009, 41, 1018–1026. [Google Scholar] [CrossRef]
- Shao, J.; Chen, Z.C.; Li, Q.B.; Lü, J. Inhibitory Effect of EGCG on Angiogenesis Induced by Multiple Myeloma Cell Line KM3 and Its Mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2007, 15, 973–977. [Google Scholar] [PubMed]
- Shammas, M.A.; Neri, P.; Koley, H.; Batchu, R.B.; Bertheau, R.C.; Munshi, V.; Prabhala, R.; Fulciniti, M.; Tai, Y.T.; Treon, S.P.; et al. Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: Biologic activity and therapeutic implications. Blood 2006, 108, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. 2016, 37, 4373–4382. [Google Scholar] [CrossRef]
- Jiang, L.; Tao, C.; He, A.; He, X. Overexpression of miR-126 sensitizes osteosarcoma cells to apoptosis induced by epigallocatechin-3-gallate. World J. Surg. Oncol. 2014, 12, 383. [Google Scholar] [CrossRef]
- Hönicke, A.S.; Ender, S.A.; Radons, J. Combined administration of EGCG and IL-1 receptor antagonist efficiently downregulates IL-1-induced tumorigenic factors in U-2 OS human osteosarcoma cells. Int. J. Oncol. 2012, 41, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Xu, Y.-M.; Wu, Y.; Yu, K.-K.; Zhang, C.; Ji, Y.-H.; Ding, G.; Chen, F. Epigallocatechin-3-gallate induces apoptosis, inhibits proliferation and decreases invasion of glioma cell. Neurosci. Bull. 2014, 30, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Siegelin, M.D.; Habel, A.; Gaiser, T. Epigalocatechin-3-gallate (EGCG) downregulates PEA15 and thereby augments TRAIL-mediated apoptosis in malignant glioma. Neurosci. Lett. 2008, 448, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jia, J. Green tea extract, epigallocatechin-3-gallate, inhibits the growth and invasive ability of human glioma cells. Mol. Med. Rep. 2008, 1, 735–739. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grube, S.; Ewald, C.; Kögler, C.; McLean, A.L.; Kalff, R.; Walter, J. Achievable Central Nervous System Concentrations of the Green Tea Catechin EGCG Induce Stress in Glioblastoma Cells in Vitro. Nutr. Cancer 2018, 70, 1145–1158. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Li, J.; Zhang, Q.; Zhong, P.; Teng, T.; Chen, M.; Xie, Z.-W.; Ji, A.; Li, Y. Epigallocatechin-3-gallate inhibits the growth and increases the apoptosis of human thyroid carcinoma cells through suppression of EGFR/RAS/RAF/MEK/ERK signaling pathway. Cancer Cell Int. 2019, 19, 43. [Google Scholar] [CrossRef]
- Li, T.; Zhao, N.; Lu, J.; Zhu, Q.; Liu, X.; Hao, F.; Jiao, X. Epigallocatechin gallate (EGCG) suppresses epithelial-Mesenchymal transition (EMT) and invasion in anaplastic thyroid carcinoma cells through blocking of TGF-β1/Smad signaling pathways. Bioengineered 2019, 10, 282–291. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Perri, A.; Vizza, N.; Russo, A.; Panno, M.L.; Bonofiglio, D.; Giordano, C.; Mauro, L.; Aquila, S.; Tramontano, D.; et al. Epigallocatechin gallate inhibits growth and epithelial-to-mesenchymal transition in human thyroid carcinoma cell lines. J. Cell. Physiol. 2013, 228, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Adhami, V.M.; Gupta, S.; Cheng, P.; Mukhtar, H. Role of the retinoblastoma (pRb)-E2F/DP pathway in cancer chemopreventive effects of green tea polyphenol epigallocatechin-3-gallate. Arch. Biochem. Biophys. 2002, 398, 125–131. [Google Scholar] [CrossRef]
- Li, S.; Wu, L.; Feng, J.; Li, J.; Liu, T.; Zhang, R.; Xu, S.; Cheng, K.; Zhou, Y.; Zhou, S.; et al. In vitro and in vivo study of epigallocatechin-3-gallate-induced apoptosis in aerobic glycolytic hepatocellular carcinoma cells involving inhibition of phosphofructokinase activity. Sci. Rep. 2016, 6, 28479. [Google Scholar] [CrossRef]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef]
- Liang, G.; Tang, A.; Lin, X.; Li, L.; Zhang, S.; Huang, Z.; Tang, H.; Li, Q.Q. Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int. J. Oncol. 2010, 37, 111–123. [Google Scholar]
- Kumar, N.B.; Pow-Sang, J.; Egan, K.M.; Spiess, P.E.; Dickinson, S.; Salup, R.; Helal, M.; McLarty, J.; Williams, C.R.; Schreiber, F.; et al. Randomized, Placebo-Controlled Trial of Green Tea Catechins for Prostate Cancer Prevention. Cancer Prev. Res. 2015, 8, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.B.; Pow-Sang, J.; Spiess, P.E.; Park, J.; Salup, R.; Williams, C.R.; Parnes, H.; Schell, M.J. Randomized, placebo-controlled trial evaluating the safety of one-year administration of green tea catechins. Oncotarget 2016, 7, 70794–70802. [Google Scholar] [CrossRef]
- Gee, J.; Saltzstein, D.R.; Kim, K.; Kolesar, J.; Huang, W.; Havighurst, T.C.; Wollmer, B.W.; Stublaski, J.; Downs, T.; Mukhtar, H.; et al. A Phase II Randomized, Double-blind, Presurgical Trial of Polyphenon E in Bladder Cancer Patients to Evaluate Pharmacodynamics and Bladder Tissue Biomarkers. Cancer Prev. Res. 2017, 10, 298–307. [Google Scholar] [CrossRef]
- Zhu, W.; Mei, H.; Jia, L.; Zhao, H.; Li, X.; Meng, X.; Zhao, X.; Xing, L.; Yu, J. Epigallocatechin-3-gallate mouthwash protects mucosa from radiation-induced mucositis in head and neck cancer patients: A prospective, non-randomized, phase 1 trial [published online ahead of print, 2019]. Investig. New Drugs 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Jia, L.; Sun, X.; Chen, G.; Zhao, X.; Li, X.; Meng, X.; Kong, L.; Xing, L.; et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br. J. Radiol. 2016, 89, 20150665. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Labbe, D.P.; Araya-Farias, M.; Doyen, A.; Bazinet, L.; Duchesne, T.; Plante, M.; Gregoire, J.; Renaud, M.C.; Bachvarov, D.; et al. A two-stage, single-arm, phase II study of EGCG-enriched green tea drink as a maintenance therapy in women with advanced stage ovarian cancer. Gynecol. Oncol. 2013, 131, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, W.; Xie, P.; Li, H.; Zhang, X.; Sun, X.; Yu, J.; Xing, L. A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer. Radiother. Oncol. 2014, 110, 132–136. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Su, J.; Li, B.; Chen, T.; Wong, Y.-S. Synergistic apoptosis-inducing effects on A375 human melanoma cells of natural borneol and curcumin. PLoS ONE 2014, 9, e101277. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Zhu, K. SOX2OT variant 7 contributes to the synergistic interaction between EGCG and Doxorubicin to kill osteosarcoma via autophagy and stemness inhibition. J. Exp. Clin. Cancer Res. 2018, 37, 37. [Google Scholar] [CrossRef]
- Hu, F.; Wei, F.; Wang, Y.; Wu, B.; Fang, Y.; Xiong, B. EGCG synergizes the therapeutic effect of cisplatin and oxaliplatin through autophagic pathway in human colorectal cancer cells. J. Pharmacol. Sci. 2015, 128, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Ohkura, Y.; Okabe, S.; Fujiki, H. Combination cancer chemoprevention with green tea extract and sulindac shown in intestinal tumor formation in Min mice. J. Cancer Res. Clin. Oncol. 2001, 127, 69–72. [Google Scholar] [CrossRef]
- Suganuma, M.; Kurusu, M.; Suzuki, K.; Tasaki, E.; Fujiki, H. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by upregulation of GADD153 gene. Int. J. Cancer. 2006, 119, 33–40. [Google Scholar] [CrossRef]
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp. Cell Res. 2013, 319, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, K.; Bray, B.J.; Rosengren, R.J. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells. Anticancer Drugs. 2004, 15, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Härdtner, C.; Multhoff, G.; Falk, W.; Radons, J. (−)-Epigallocatechin-3-gallate, a green tea-derived catechin, synergizes with celecoxib to inhibit IL-1-induced tumorigenic mediators by human pancreatic adenocarcinoma cells Colo357. Eur. J. Pharmacol. 2012, 684, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Hebbar, V.; Shen, G.; Gopalakrishnan, A.; Khor, T.O.; Yu, S.; Xu, C.; Kong, A.-N. Synergistic effects of a combination of dietary factors sulforaphane and (−) epigallocatechin-3-gallate in HT-29 AP-1 human colon carcinoma cells. Pharm. Res. 2008, 25, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Scandlyn, M.J.; Stuart, E.C.; Somers-Edgar, T.J.; Menzies, A.R.; Rosengren, R.J. A new role for tamoxifen in oestrogen receptor-negative breast cancer when it is combined with epigallocatechin gallate. Br. J. Cancer. 2008, 99, 1056–1063. [Google Scholar] [CrossRef]
- Stearns, M.E.; Wang, M. Synergistic Effects of the Green Tea Extract Epigallocatechin-3-gallate and Taxane in Eradication of Malignant Human Prostate Tumors. Transl. Oncol. 2011, 4, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Stearns, M.E.; Amatangelo, M.D.; Varma, D.; Sell, C.; Goodyear, S.M. Combination therapy with epigallocatechin-3-gallate and doxorubicin in human prostate tumor modeling studies: Inhibition of metastatic tumor growth in severe combined immunodeficiency mice. Am. J. Pathol. 2010, 177, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol and gamma-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells. Int. J. Oncol. 2008, 33, 851–859. [Google Scholar] [PubMed]
- Morré, D.J.; Morré, D.M.; Sun, H.; Cooper, R.; Chang, J.; Janle, E.M. Tea catechin synergies in inhibition of cancer cell proliferation and of a cancer specific cell surface oxidase (ECTO-NOX). Pharmacol. Toxicol. 2003, 92, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Okabe, S.; Kai, Y.; Sueoka, N.; Sueoka, E.; Fujiki, H. Synergistic effects of (--)-epigallocatechin gallate with (--)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999, 59, 44–47. [Google Scholar]
- Chung, S.S.; Vadgama, J.V. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3–NFκB signaling. Anticancer Res. 2015, 35, 39–46. [Google Scholar]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Ullmann, U.; Haller, J.; Decourt, J.P.; Girault, N.; Girault, J.; Richard-Caudron, A.S.; Pineau, B.; Weber, P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 2003, 31, 88–101. [Google Scholar] [CrossRef]
- Klinski, E.; Semov, A.; Yan, X.; Alakhov, V.; Muyzhnek, E.; Kiselev, V. Block copolymer based composition of epigallocatechin-3-gallate with improved oral bioavailability as a way to increase its therapeutic activity. J. Nanomed. Biother. Discov. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front. Biosci. 2012, 4, 111–131. [Google Scholar] [CrossRef]
- Law, F.C.P.; Yao, M.; Bi, H.C.; Lam, S. Physiologically based pharmacokinetic modelling of tea catechin mixture in rats and humans. Pharmacol. Res. Perspect. 2017, 5, e00305. [Google Scholar] [CrossRef]
- Nakagawa, K.; Miyazawa, T. Chemiluminescence-high-performance liquid chromatographic determination of tea catechin, (−)-epigallocatechin 3-gallate, at picomole levels in rat and human plasma. Anal. Biochem. 1997, 248, 41–49. [Google Scholar] [CrossRef]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Catterall, F.; King, L.J.; Clifford, M.N.; Ioannides, C. Bioavailability of dietary doses of 3H-labelled tea antioxidants (+)-catechin and (−)-epicatechin in rat. Xenobiotica 2003, 33, 743–753. [Google Scholar] [CrossRef]
- Jia, Z.; Shufang, N.; Shu, W. Nanoencapsulation enhances epigallocatechin-3-gallate stability and its antiatherogenic bioactivities in macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar]
- Chu, K.O.; Pang, C.C. Pharmacokinetics and Disposition of Green Tea Catechins Pharmacokinetics and Adverse Effects of Drugs - Mechanisms and Risks Factors, Ntambwe Malangu. In Tech. Open. 2018, 58, 17–36. [Google Scholar] [CrossRef][Green Version]
- Sesink, A.L.; Arts, I.C.; Faassen-Peters, M.; Hollman, P.C. Intestinal uptake of quercetin-3- glucoside in rats involves hydrolysis by lactase phlorizin hydrolase. J. Nutr. 2003, 133, 773–776. [Google Scholar] [CrossRef]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lee, M.J.; Sheng, S.; Meng, X.; Prabhu, S.; Winnik, B.; Huang, B.; Chung, J.Y.; Yan, S.; Ho, C.T.; et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem. Res. Toxicol. 2000, 13, 177–184. [Google Scholar] [CrossRef]
- Mulder, T.P.; Rietveld, A.G.; van Amelsvoort, J.M. Consumption of both black tea and green tea results in an increase in the excretion of hippuric acid into urine. Am. J. Clin. Nutr. 2005, 81, 256S–260S. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nanodelivery: A review. J. Func. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Fang, J.Y.; Lee, W.R.; Shen, S.C.; Huang, Y.L. Effect of liposome encapsulation of tea catechins on their accumulation in basal cell carcinomas. J. Dermatol. Sci. 2006, 42, 101–109. [Google Scholar] [CrossRef]
- Zou, L.Q.; Peng, S.F.; Liu, W.; Gan, L.; Liu, W.L.; Liang, R.H.; Liu, C.M.; Niu, J.; Cao, Y.L.; Liu, Z.; et al. Improved in vitro, digestion stability of (−)-epigallocatechin gallate through nanoliposome encapsulation. Food Res. Int. 2014, 64, 492–499. [Google Scholar] [CrossRef]
- Hsieh, D.-S.; Wang, H.; Tan, S.-W.; Huang, Y.-H.; Tsai, C.-Y.; Yeh, M.-K.; Wu, C.-J. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials 2011, 32, 7633–7640. [Google Scholar] [CrossRef]
- Sanna, V.; Singh, C.K.; Jashari, R.; Adhami, V.M.; Chamcheu, J.C.; Rady, I.; Sechi, M.; Mukhtar, H.; Siddiqui, I.A. Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci. Rep. 2017, 7, 41573. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR e_ect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Chen, B.-H.; Hsieh, C.-H.; Tsai, S.-Y.; Wang, C.-Y.; Wang, C.-C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, Q.; Zhou, J.; Chen, B.; Xi, J.; Long, P.; Zhang, L.; Hou, R. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chem. 2018, 242, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Mohammed, J.T.; Syeda, M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, S.; Feng, Y.; Zou, P.; Wang, Y.; Qin, P.; Yue, J.; Liang, Y.; Wang, H.; Liu, L. Preparation of chitosan-Epigallocatechin-3-O-gallate nanoparticles and their inhibitory effect on the growth of breast cancer cells. J. Innov. Opt. Health Sci. 2018, 11, 1850018. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J. Enhanced oral bioavailability of EGCG using pH-sensitive polymeric nanoparticles: Characterization and in vivo investigation on nephrotic syndrome rats. Drug Des. Dev. Ther. 2018, 12, 2509–2518. [Google Scholar] [CrossRef]
- Haratifar, S.; Meckling, K.A.; Corredig, M. Antiproliferative activity of tea catechins associated with casein micelles, using HT29 colon cancer cells. J. Dairy Sci. 2014, 97, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Meena, R.; Paulraj, R. Fabrication of BSA-green tea polyphenols-chitosan nanoparticles and its role in radioprotection: A molecular and biochemical approach. J. Agric. Food Chem. 2016, 64, 6024–6034. [Google Scholar] [CrossRef] [PubMed]
- Kurita, I.; Maeda-Yamamoto, M.; Tachibana, H.; Kamei, M. Antihypertensive effect of Benifuuki tea containing O-methylated EGCG. J. Agric. Food Chem. 2010, 58, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, Y.; Li, Z.; Ma, C.; Lou, Z.; Yokoyama, W.; Wang, H. Lipase-catalyzed synthesis of acetylated EGCG and antioxidant properties of the acetylated derivatives. Food Res. Int. 2014, 56, 279–286. [Google Scholar] [CrossRef]


| Cancer Types | Study Type | Finding of the Study | Refs. |
|---|---|---|---|
| Cervix cancer | In vitro | EGCG with eugenol amrogentin greatly inhibit the cellular proliferation and colony formation | [44] |
| Cervix cancer | In vitro | EGCG treatment causes down regulation of genes involved in the stimulation of proliferation and motility and invasion processes. | [46] |
| Breast cancer | In vitro | EGCG reduced breast cancer cell growth in a concentration- and time dependent manner | [48] |
| Breast cancer | In vitro | Epigallocatechin gallate powerfully inhibited the growth of cancer stem/progenitor cells. | [49] |
| Breast cancer | In vitro | Protein expression of HIF-1α and VEGF dropped in cancer cells pre-treated with increasing concentrations of | [51] |
| Ovarian cancer | In vitro | EGCG improved the toxicity of cisplatin and epigallocatechin-3-gallate increased cisplatin strength | [52] |
| Ovarian cancer | In vitro | EGCG plays an important role in decreasing ovarian cancer cell growth. Correspondingly, Epigallocatechin gallate showed growth inhibitory effects in each cell line in a dose-dependent approach and induced apoptosis and cell cycle arrest | [56] |
| Ovarian cancer | In vitro | Epigallocatechin-3-gallate causes a substantial task in decreasing cancer cell growth, showed dose dependent growth inhibitory effects | [57] |
| Endometrial cancer | In vitro | EGCG caused the arrest of cells in the G0/G1 phase of the cell cycle | [62] |
| Endometrial cancer | In vitro | EGCG was established to inhibit proliferation of adenocarcinoma cells | [63] |
| Pancreatic cancer | In vitro | EGCG decreased pancreatic cancer cell migration, growth and invasion | [67] |
| Pancreatic cancer | In vitro | EGCG reduced pancreatic cancer cell growth in a concentration-dependent manner | [68] |
| Pancreatic cancer | In vitro | The synergistic activity was credited to the cell cycle arrest and the induction of the reactive oxygen species-dependent mitochondria mediated apoptosis | [70] |
| Pancreatic cancer | In vitro | EGCG caused growth arrest at G1 stage of cell cycle, and induced apoptosis | [72] |
| Gastric cancer | In vitro | EGCG was accomplished to inhibit vascular endothelial growth factor secretion and expression | [74] |
| Gastric cancer | In vitro | EGCG significantly inhibited proliferation and increased apoptosis of cancer cells in vitro. | [75] |
| Gastric cancer | In vitro | EGCG meaningfully promoted apoptosis and inhibited the proliferation | [77] |
| Gastric cancer | In vitro | EGCG treatment reduced vascular endothelial growth factor protein level | [78] |
| Gastric cancer | In vitro | Microvessel density in tumor tissues receiving epigallocatechin-3-gallate treatment was also evidently reduced and markedly reduced VEGF protein level | [79] |
| Liver tumour | In vitro | The epigallocatechin gallate reduced hypoxia-incited apoptosis in HepG2 cells as well as enhanced cell survival | [82] |
| Liver cancer | In vitro | Epigallocatechin gallate reduced expression of MMP-9, syndecan-1 and FGF-2 | [83] |
| Colorectal cancer | In vitro | Epigallocatechin gallate and sodium butyrate combination treatment induced apoptosis and cell cycle arrest | [85] |
| Colon cancer | In vitro | EGCG-induced downregulation of epidermal growth factor receptor cancer cells | [86] |
| Colon cancer | In vitro | Both Epigallocatechin-3-gallate and Poly E initiated a decrease in the phosphorylated forms of EGFR | [87] |
| Bile duct cancer | In vitro | JAK/STAT pathway activation through pro-inflammatory cytokine in cancer cells was decreased via pre-treatment with quercetin and epigallocatechin-3-gallate | [91] |
| Bile duct cancer | In vitro | The combination of vorinostat and epigallocatechin-3-gallate revealed synergistic growth inhibitory effects and caused induction of apoptosis in tumor cells. | [92] |
| Renal Cell Carcinoma | In vitro | Epigallocatechin-3-gallate inhibits growth and induces apoptosis | [94] |
| Renal Cell Carcinoma | In vitro | EGCG showed potentiality to inhibit the proliferation, and induce apoptosis | [95] |
| Renal Cell Carcinoma | In vitro | EGCG treatment provoked important upregulation of Cx32 in cancer cells | [97] |
| Prostate Cancer | In vitro | EGCG induces apoptosis through triggering caspase and preventing the expression of Bcl-2 | [99] |
| Prostate Cancer | In vitro | Epigallocatechin-3-gallate demonstrated low inhibitory effect on cancer cell proliferation | [100] |
| Prostate Cancer | In vitro | EGCG showed anticancer effects and it was proved that epigallocatechin-3-gallate inhibited cancer cell proliferation | [102] |
| Urinary bladder cancer | In vitro | Treatment of EGCG caused in important inhibition of cell proliferation via induction of apoptosis and inhibited cancer cell migration | [104] |
| Urinary bladder cancer | In vitro | Epigallocatechin-3-gallate increased growth inhibition in a dose- and time-dependent manner | [105] |
| Leukemia | In vitro | Proliferation and cell cycle progression of cancer cells treated with epigallocatechin-3-gallate were inhibited | [109] |
| Leukemia | In vitro | Epigallocatechin-3-gallate treatment induced apoptosis and increased the levels of Bax protein expression | [111] |
| Leukemia | In vitro | EGCG showed higher growth suppression and induced apoptosis demonstrated by nuclei fragmentation and nuclear fragmentation | [113] |
| Lymphoma | In vitro | EGCG induced growth inhibition and apoptosis in a dose- and time-dependent way | [114] |
| Lymphoma | In vitro | Epigallocatechin-3-gallate were able to inhibit the growth of malignancy cell lines | [115] |
| Lymphoma | In vitro | EGCG caused induction of cell death and reactive oxygen species generation | [116] |
| Head and neck cancer | In vitro | EGCG inhibits the self-renewal capacity and reduces the expression of stem cell markers | [118] |
| Head and neck cancer | In vitro | EGCG induces apoptosis of cancer cells via regulating Bim and Bcl-2 | [119] |
| Head and neck cancer | In vitro | Combined treatment with erlotinib and EGCG inhibited the protein level of p65 subunit of nuclear factor-kappaB | [120] |
| Oral cancer | In vitro | EGCG inhibited cell viability in a time- and concentration-dependent manner | [122] |
| Oral cancer | In vitro | Epigallocatechin-3-gallate in inhibiting HGF-induced tumor growth and invasion | [124] |
| Oral cancer | In vitro | EGCG caused an inhibitory effect on cell migration, motility, spread, and adhesion | [125] |
| Oesophagus cancer | In vitro | Epigallocatechin-3-gallate considerably reduced the invasion and viability capacity of cancer cells | [127] |
| Oesophagus cancer | In vitro | Epigallocatechin-3-gallate inhibited proliferation of cancer cells | [128] |
| Lymphoma | In vitro | Vorinostat alone or in combination with epigallocatechin-3-gallate imparts anti-proliferative effects | [130] |
| Lymphoma | In vitro | EGCG-induced inhibition of tumor cell proliferation | [132] |
| Lung cancer | In vitro | EGCG decrease the expression of both Axl and Tyro 3 receptor tyrosine kinases | [139] |
| Myeloma | In vitro | The treatment of the cancer cell line with epigallocatechin-3-gallate inhibits cell proliferation as well induces apoptosis | [141] |
| Myeloma | In vitro | EGCG inhibited the effect of endothelial cell migration induced and the numbers of migrated cells and numbers of migrated cells | [142] |
| Osteosarcoma | In vitro | EGCG has an anticancer effect on cancer cells | [144] |
| Osteosarcoma | In vitro | EGCG showed role in the suppression of proliferation of cancer cells in a concentration-dependent and time-dependent manner | [145] |
| Brain tumor | In vitro | EGCG induced apoptosis in glioma cells. | [147] |
| Brain tumor | In vitro | EGCG treatment leads to a decrease in cell viability and the S-phase cell fraction | [149] |
| Thyroid cancer | In vitro | EGCG decreased the migration and invasion, | [151] |
| Thyroid cancer | In vitro | EGCG considerably suppresses invasion and migration in anaplastic cancer cells | [152] |
| Retinoblastoma | In vitro | EGCG treatment of cancer cells resulted in a dose- and time-dependent decrease in the total pRb | [154] |
| EGCG + Anticancer Compound | Type of Cancer | Outcome of the Study | Refs. |
|---|---|---|---|
| EGCG + Doxorubicin | Osteosarcoma | Epigallocatechin-3-gallate reduce the Doxorubicin-induced pro-survival autophagy | [166] |
| EGCG + Cisplatin or oxaliplatin | Colorectal cancer | Treatment of colorectal cancer cells with Epigallocatechin-3-gallate and cisplatin or oxaliplatin confirmed a synergistic effect on inhibition of cell proliferation and induction of cell death. | [167] |
| EGCG + Sulindac | Intestinal nepoplasia | Treatment with both green tea extract and sulindac significantly decrease the number of per mouse | [168] |
| EGCG + Celecoxib | Prostate cancer | Co-treatment with epigallocatechin-3-gallate and celecoxib powerfully induced the expression of both GADD153 mRNA level and protein | [169] |
| EGCG + Sulforaphane | Ovarian cancer | Sulforaphane inhibits cell viability of cancer cell and epigallocatechin-3-gallate enhance the inhibiting effect of sulforaphane | [170] |
| EGCG + Hydroxytamoxifen | Breast cancer | The combination of EGCG and 4-hydroxytamoxifen provokes synergistic cytotoxicity in cancer | [171] |
| EGCG + Celecoxib | Pancreatic cancer | Co-incubation of cancer cells with celecoxib and epigallocatechin-3-gallate synergistically reduced metabolic activity through induction of apoptosis | [172] |
| EGCG + SU5416 | Neuroblastoma | Combination of drugs can be a promising therapeutic strategy for controlling the growth of neuroblastoma cells. | [173] |
| EGCG + Sulforaphane | Colon cancer | Low and high dose combinations of Sulforaphane and epigallocatechin-3-gallate attenuated the cellular senescence induced by epigallocatechin-3-gallate alone | [174] |
| EGCG + Tamoxifen | Breast cancer | Tamoxifen at realistic dose suppress the growth of ER-negative breast cancer when combined with Epigallocatechin-3-gallate. | [175] |
| EGCG + Taxane | Prostate cancer | Epigallocatechin-3-gallate in combination with taxane may provide a novel therapeutic treatment of prostate cancer | [176] |
| EGCG + Doxorubicin | Prostate cancer | Epigallocatechin-3-gallate combined with Doxorubicin may have significant clinical application in the treatment of metastatic prostate cancer | [177] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. https://doi.org/10.3390/molecules25143146
Almatroodi SA, Almatroudi A, Khan AA, Alhumaydhi FA, Alsahli MA, Rahmani AH. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules. 2020; 25(14):3146. https://doi.org/10.3390/molecules25143146
Chicago/Turabian StyleAlmatroodi, Saleh A., Ahmad Almatroudi, Amjad Ali Khan, Fahad A. Alhumaydhi, Mohammed A. Alsahli, and Arshad Husain Rahmani. 2020. "Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer" Molecules 25, no. 14: 3146. https://doi.org/10.3390/molecules25143146
APA StyleAlmatroodi, S. A., Almatroudi, A., Khan, A. A., Alhumaydhi, F. A., Alsahli, M. A., & Rahmani, A. H. (2020). Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules, 25(14), 3146. https://doi.org/10.3390/molecules25143146







