Effects of Promoter on Structural and Surface Properties of Zirconium Oxide-Based Catalyst Materials
Abstract
1. Introduction
2. Results and Discussion
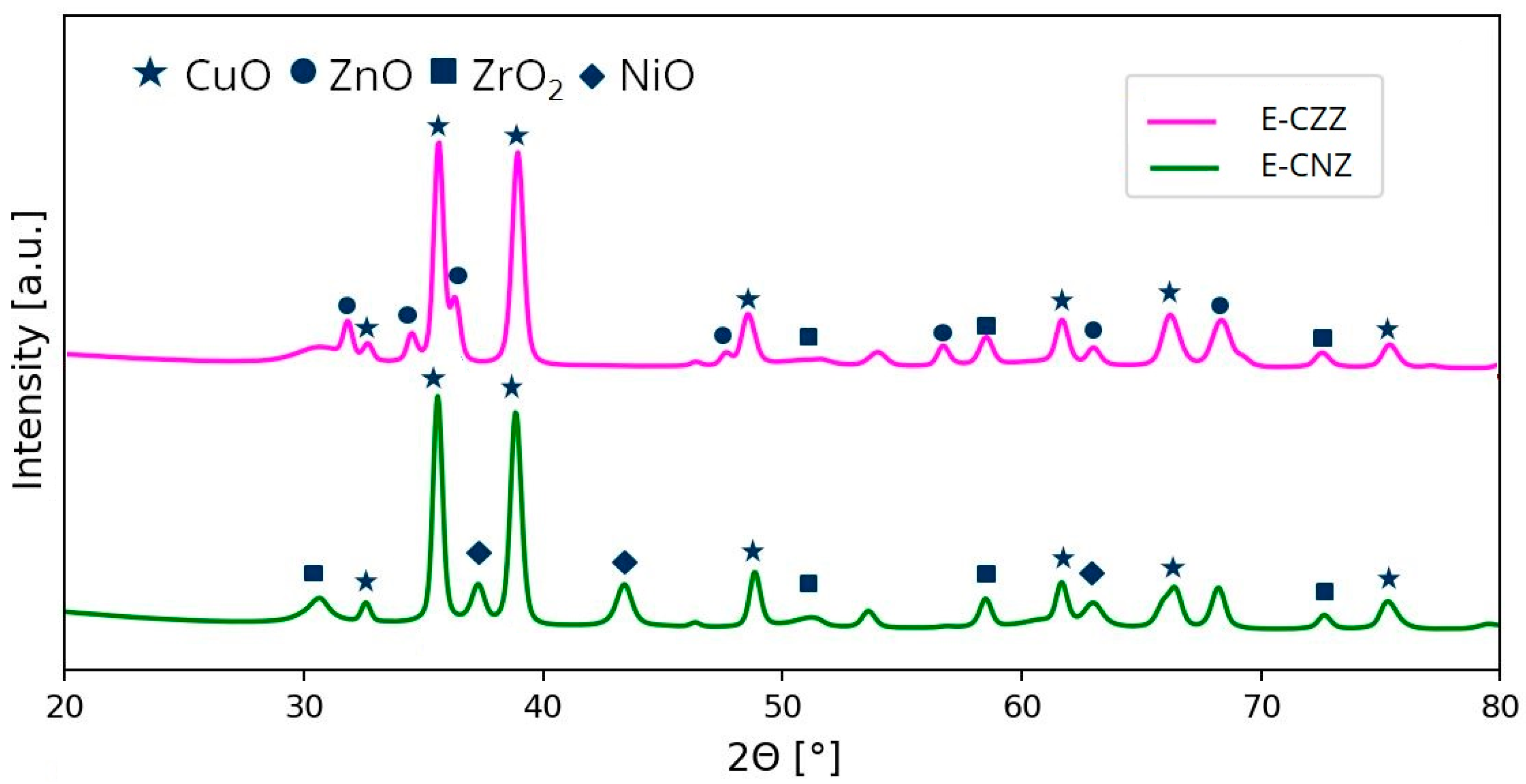
2.1. X-ray Diffraction (XRD)
2.2. N2-Physisorption
2.3. Temperature-Programmed Ammonia Desorption (TPAD)
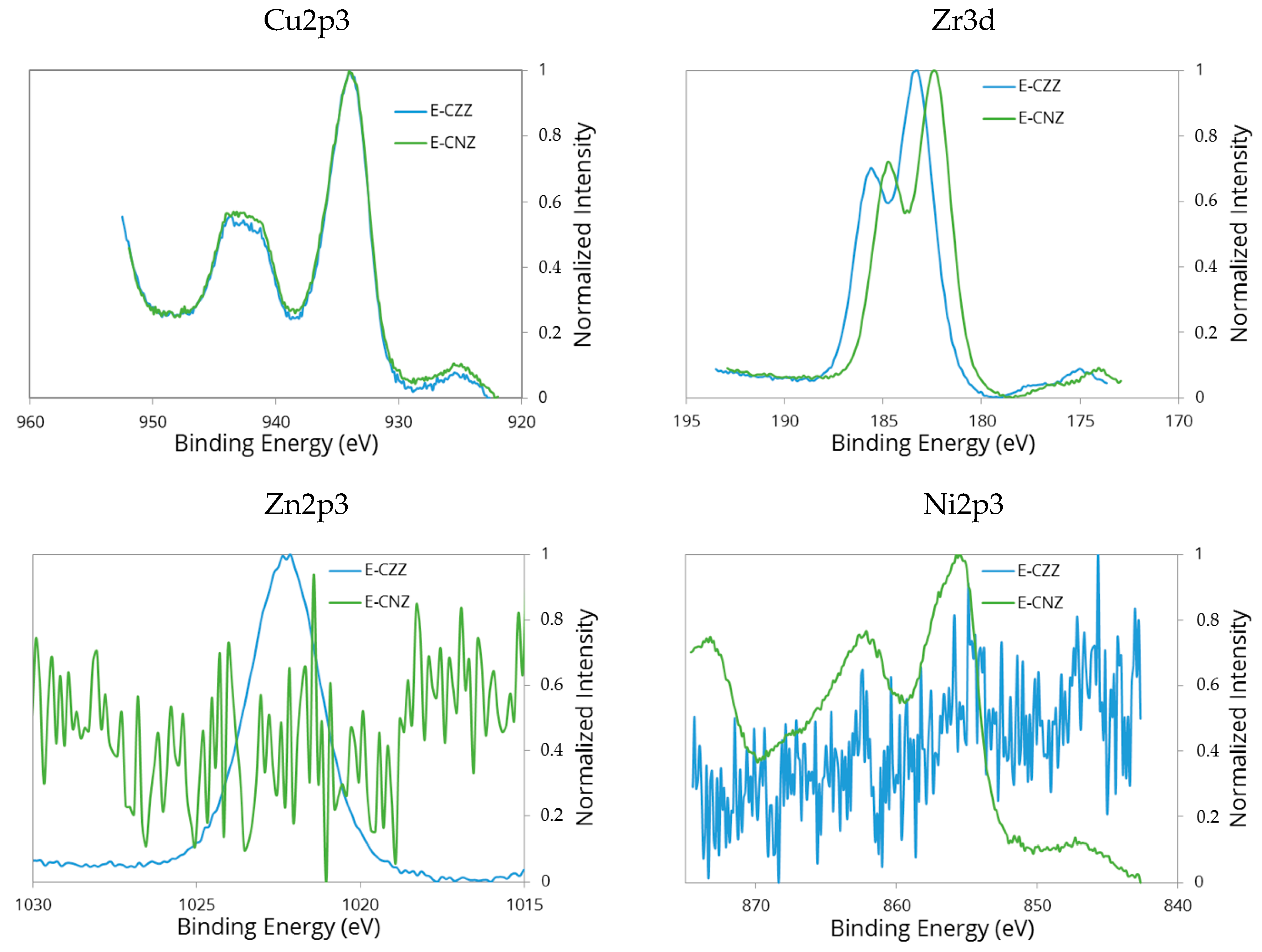
2.4. X-ray Photoelectron Spectroscopy (XPS)
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Olah, G.A.; Prakash, G.K.S.; Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 2011, 133, 12881–12898. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: From greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef]
- Reschetilowski, W. Alternative resources for the methanol economy. Russ. Chem. Rev. 2013, 82, 624–634. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. The changing paradigm in CO2 utilization. J. CO2 Util. 2013, 3, 65–73. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Jandhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Su, C.; Li, J.; He, D.; Cheng, Z.; Zhu, Q. Synthesis of isobutene from synthesis gas over nanosize zirconia catalysts. Appl. Catal. A Gen. 2000, 202, 81–89. [Google Scholar] [CrossRef]
- Liu, J.; Shi, J.; He, D.; Zhang, Q.; Wu, X.; Liang, Y.; Zhu, Q. Surface active structure of ultra-fine Cu/ZrO2 catalysts used for the CO2 + H2 to methanol reaction. Appl. Catal. A Gen. 2001, 218, 113–119. [Google Scholar] [CrossRef]
- Denise, B.; Sneeden, R.P.A. Oxide-supported copper catalysts prepared from copper formate: Differences in behaviour in methanol synthesis from CO/H2 and CO2/H2 mixtures. Appl. Catal. 1986, 28, 235–239. [Google Scholar] [CrossRef]
- Amenomiya, Y. Methanol synthesis from CO2 + H2 II. Copper-based binary and ternary catalysts. Appl. Catal. 1987, 30, 57–68. [Google Scholar] [CrossRef]
- Chinchen, G.C.; Waugh, K.C.; Whan, D.A. The activity and state of the copper surface in methanol synthesis catalysts. Appl. Catal. 1986, 25, 101–107. [Google Scholar] [CrossRef]
- Borovinskaya, E.; Trebbin, S.; Alscher, F.; Breitkopf, C. Synthesis, modification and characterization of CuO/ZnO/ZrO2 mixed oxide catalysts for CO2/H2 conversion. Catalysts 2019, 9, 1037. [Google Scholar] [CrossRef]
- Grabow, L.C.; Mavrikakis, M. Mechanism of methanol synthesis on Cu through CO2 and CO hydrogenation. ACS Catal. 2011, 1, 365–384. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Lekse, J.W.; Baltrus, J.P.; Ohodnicki, P.R.; Howard, B.H.; Deng, X.; Matranga, C. Active sites and structure-activity relationships of copper-based catalysts for carbon dioxide hydrogenation to methanol. ACS Catal. 2012, 2, 1667–1676. [Google Scholar] [CrossRef]
- Upare, P.P.; Jeong, M.-G.; Hwang, Y.K.; Kim, D.H.; Kim, Y.D.; Hwang, D.W.; Lee, U.-H.; Chang, J.-S. Nickel-promoted copper-silica nanocomposite catalysts for hydrogenation of levulinic acid to lactones using formic acid as a hydrogen feeder. Appl. Catal. A Gen. 2015, 491, 127–135. [Google Scholar] [CrossRef]
- Wang, Y.; Kattel, S.; Gao, W.; Li, K.; Liu, P.; Chen, J.G.; Wang, H. Exploring the ternary interactions in Cu–ZnO–ZrO2 catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Arena, F.; Barbera, K.; Italiano, G.; Bonura, G.; Spadaro, L.; Frusteri, F. Synthesis, characterization and activity pattern of Cu–ZnO/ZrO2 catalysts in the hydrogenation of carbon dioxide to methanol. J. Catal. 2007, 249, 185–194. [Google Scholar] [CrossRef]
- Lachowska, M.; Skrzypek, J. Methanol synthesis from carbon dioxide and hydrogen over Mn-promoted copper/zinc/zirconia catalysts. React. Kinet. Catal. Lett. 2004, 2, 269–273. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, G.; Barbera, K.; Bordiga, S.; Bonura, G.; Spadaro, L.; Frusteri, F. Solid-state interactions, adsorption sites and functionality of Cu–ZnO/ZrO2 catalysts in the CO2 hydrogenation to CH3OH. Appl. Catal. A Gen. 2008, 350, 16–23. [Google Scholar] [CrossRef]
- Wang, Y.H.; Gao, W.G.; Wang, H.; Zheng, Y.E.; Na, W.; Li, K.Z. Structure-activity relationships of Cu–ZrO2 catalysts for CO2 hydrogenation to methanol: Interaction effects and reaction mechanism. RSC Adv. 2017, 7, 8709–8717. [Google Scholar] [CrossRef]
- Ren, J.; Qin, X.; Yang, J.-Z.; Qin, Z.-F.; Guo, H.-L.; Lin, J.-Y.; Li, Z. Methanation of carbon dioxide over Ni–M/ZrO2 (M=Fe, Co, Cu) catalysts: Effect of addition of a second metal. Fuel Process. Technol. 2015, 137, 204–211. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Mondragón Galicia, G.; Mendoza Anaya, D.; Palacios, J.; Angeles-Chavez, C.; Arenas-Alatorre, J. Synthesis and characterization of bimetallic Cu–Ni/ZrO2 nanocatalysts: H2 production by oxidative steam reforming of methanol. Int. J. Hydrog. Energy 2008, 33, 4569–4576. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Klötzer, B.; Mayr, L.; Rameshan, R.; Zemlyanov, D.; Bernardi, J.; Föttinger, K.; Rupprechter, G. Surface modification processes during methane decomposition on Cu-promoted Ni–ZrO2 catalysts. Catal. Sci. Technol. 2015, 5, 967–978. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD) (2000) Joint Committee on Powder Diffraction Standards, Diffraction Data File No. 05-0661, 12–23. Available online: https://www.icdd.com/ (accessed on 26 May 2020).
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Zavalij, P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials; Springer: New York, NY, USA, 2005; p. 108. [Google Scholar]
- Hall, B.D.; Zanchet, D.; Ugarte, D. Estimating nanoparticle size from diffraction measurements. J. Appl. Crystallogr. 2000, 33, 1335–1341. [Google Scholar] [CrossRef]
- Speakman, S.A. Estimating Crystallite Size Using XRD; MIT Centre for Materials Science and Engineering: Cambridge, MA, USA, 2014; Available online: http://prism.mit.edu/XRAY/oldsite/CrystalSizeAnalysis.pdf (accessed on 26 May 2020).
- XPS Interpretation of Copper. Available online: https://xpssimplified.com/elements/copper.php (accessed on 21 February 2020).
- XPS Interpretation of Nickel. Available online: https://xpssimplified.com/elements/nickel.php (accessed on 21 February 2020).
- Davidson, A.; Tempere, J.F.; Che, M.; Roulet, H.; Dufour, G. Spectroscopic studies of Nickel(II) and Nickel(III) species generated upon thermal treatments of nickel/ceria-supported materials. J. Phys. Chem. 1996, 100, 4919–4929. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; Mc Intyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, Z.-Y.; Chen, Z.; Zhang, L.-Z.; Zhao, X.-F.; Si, F.-Z.; Li, J.-H. Engineering mesoporous NiO with enriched electrophilic Ni3+ and O− toward efficient oxygen evolution. Catalysts 2018, 8, 310. [Google Scholar] [CrossRef]
- Kanai, Y.; Watanabe, T.; Fujitani, T.; Uchijima, T.; Nakamura, J. The synergy between Cu and ZnO in methanol synthesis catalysts. Catal. Lett. 1996, 38, 157–163. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, J. The chemical modification seen in the Cu/ZnO methanol synthesis catalysts. Appl. Catal. A Gen. 2000, 191, 111–129. [Google Scholar] [CrossRef]
- Frost, J.C. Junction effect interactions in methanol synthesis catalysts. Nature 1988, 334, 577–580. [Google Scholar] [CrossRef]
- Burch, R.; Chappell, R.J. Support and additive effects in the synthesis of methanol over copper Catalysts. Appl. Catal. 1988, 45, 131–150. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef] [PubMed]
- Koeppel, R.A.; Baiker, A.; Wokaun, A. Copper/zirconia catalysts for the synthesis of methanol from carbon dioxide: Influence of preparation variables on structural and catalytic properties of catalysts. Appl. Catal. A Gen. 1992, 84, 77–102. [Google Scholar] [CrossRef]
- Jansen, W.P.A.; Beckers, J.; Van der Heuvel, J.C.; Van der Gon, A.W.D.; Bliek, A.; Brongersma, H.H. Dynamic Behavior of the Surface Structure of Cu/ZnO/SiO2 Catalysts. J. Catal. 2002, 210, 229–236. [Google Scholar] [CrossRef]
- Saito, M.; Fujitani, T.; Takeuchi, M.; Watanabe, T. Development of copper/zinc oxide-based multicomponent catalysts for methanol synthesis from carbon dioxide and hydrogen. Appl. Catal. A Gen. 1996, 138, 311–318. [Google Scholar] [CrossRef]
- Borovinskaya, E.; Alscher, F.; Breitkopf, C. Modified zirconium oxide catalysts for effective conversion of carbon dioxide into useful liquid compounds. In Preprints of the DGMK Conference “Challenges for Petrochemicals and Fuels: Integration of Value Chains and Energy Transition”; Ernst, S., Beller, M., Eds.; German Society for Petroleum and Coal Science and Technology: Berlin, Germany, 2018; pp. 197–202. [Google Scholar]
- Zurbel, A.; Kraft, M.; Kavurucu-Schubert, S.; Bertau, M. Methanol Synthesis by CO2 Hydrogenation over Cu/ZnO/Al2O3 Catalysts under Fluctuating Conditions. Chem. Ing. Tech. 2018, 90, 721–724. [Google Scholar] [CrossRef]
- Scotti, N.; Bossola, F.; Zaccheria, F.; Ravasio, N. Copper-Zirconia Catalysts: Powerful Multifunctional Catalytic Tools to Approach Sustainable Processes. Catalysts 2020, 10, 168. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Sample | CuO Crystal Size [nm] | SBET [m²/g] | Total Acidity [mmol·NH3/g] | Acid Site Density [μmol/m²] |
|---|---|---|---|---|
| E-CZZ | 8.6 | 18.5 | 0.963 | 52.05 |
| E-CNZ | 8.3 | 23.1 | 0.671 | 29.05 |
| Sample | O1s | Ni2p3 | Cu2p3 | Zn2p3 | Zr3d |
|---|---|---|---|---|---|
| E-CZZ | 56.9 | 0.00 | 17.3 | 11.7 | 14.1 |
| E-CZZred | 55.6 | 0.00 | 17.4 | 12.3 | 14.7 |
| E-CNZ | 52.7 | 17.8 | 19.6 | 0.0 | 9.9 |
| E-CNZred | 52.5 | 15.4 | 19.4 | 0.0 | 12.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borovinskaya, E.S.; Oswald, S.; Reschetilowski, W. Effects of Promoter on Structural and Surface Properties of Zirconium Oxide-Based Catalyst Materials. Molecules 2020, 25, 2619. https://doi.org/10.3390/molecules25112619
Borovinskaya ES, Oswald S, Reschetilowski W. Effects of Promoter on Structural and Surface Properties of Zirconium Oxide-Based Catalyst Materials. Molecules. 2020; 25(11):2619. https://doi.org/10.3390/molecules25112619
Chicago/Turabian StyleBorovinskaya, Ekaterina S., Steffen Oswald, and Wladimir Reschetilowski. 2020. "Effects of Promoter on Structural and Surface Properties of Zirconium Oxide-Based Catalyst Materials" Molecules 25, no. 11: 2619. https://doi.org/10.3390/molecules25112619
APA StyleBorovinskaya, E. S., Oswald, S., & Reschetilowski, W. (2020). Effects of Promoter on Structural and Surface Properties of Zirconium Oxide-Based Catalyst Materials. Molecules, 25(11), 2619. https://doi.org/10.3390/molecules25112619






