Effective Removal of Pb(II) Ions by Electrospun PAN/Sago Lignin-Based Activated Carbon Nanofibers
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Electrospun PAN Nanofibers
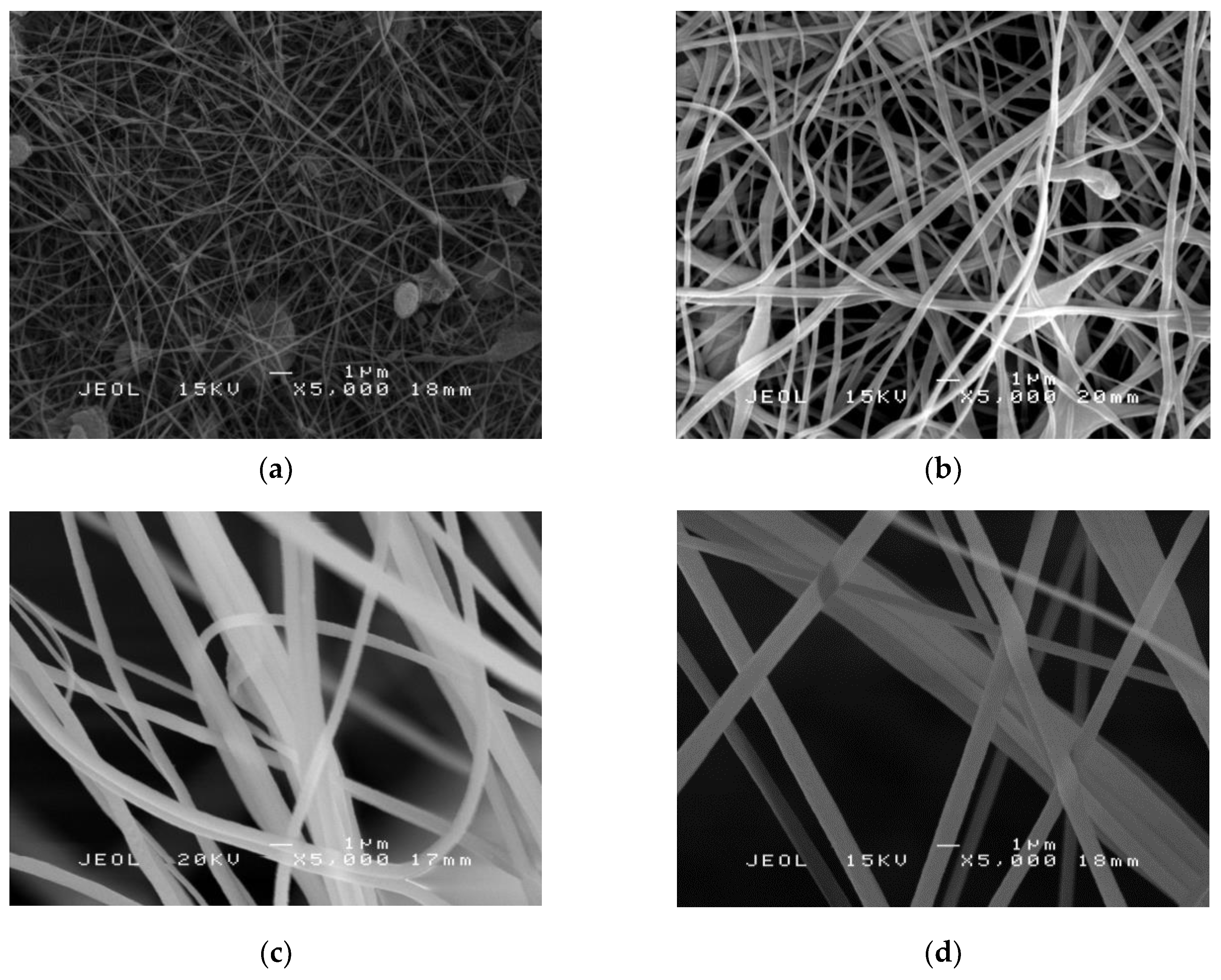
2.2. Electrospun PAN/SL Nanofibers
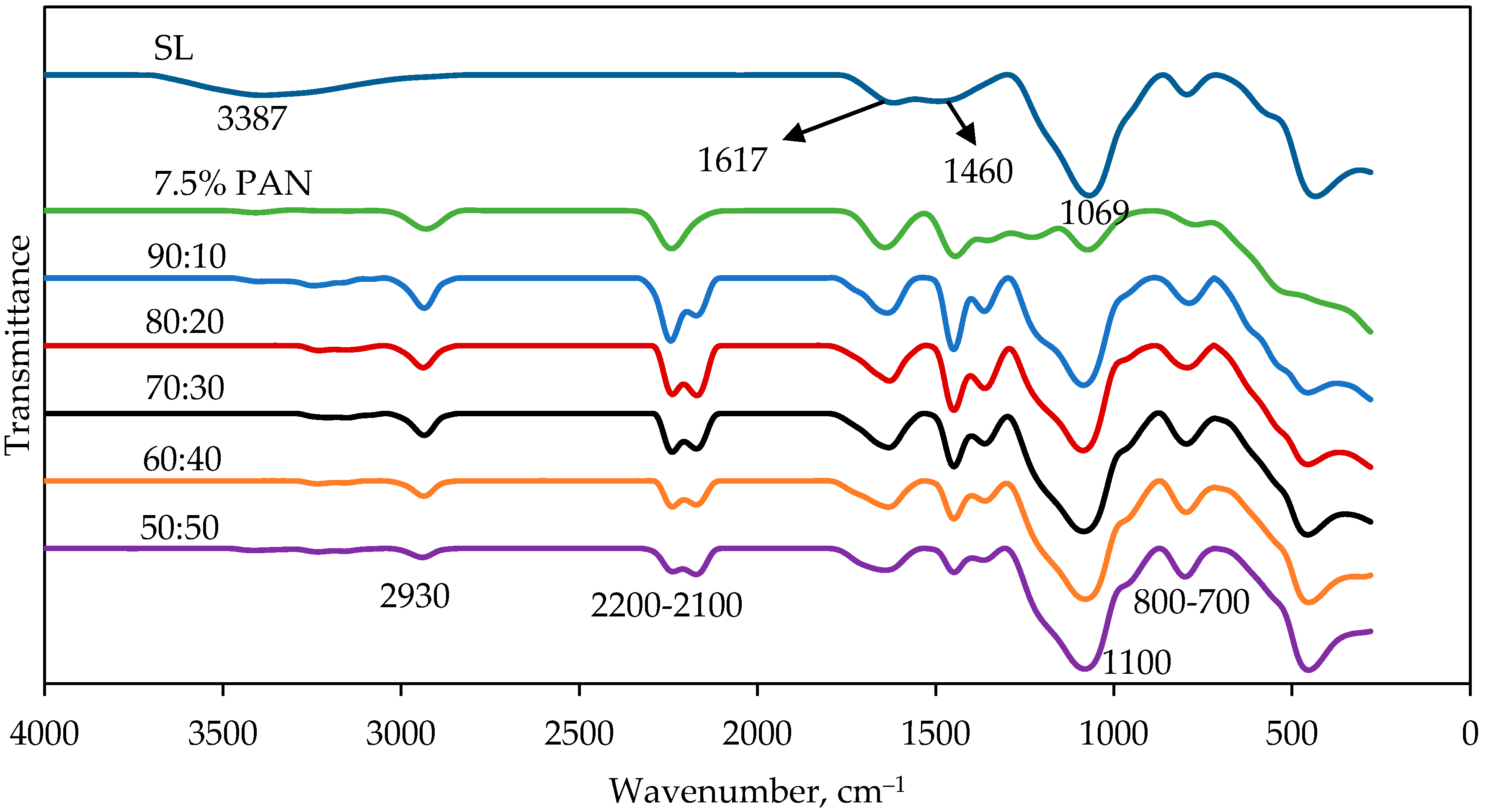
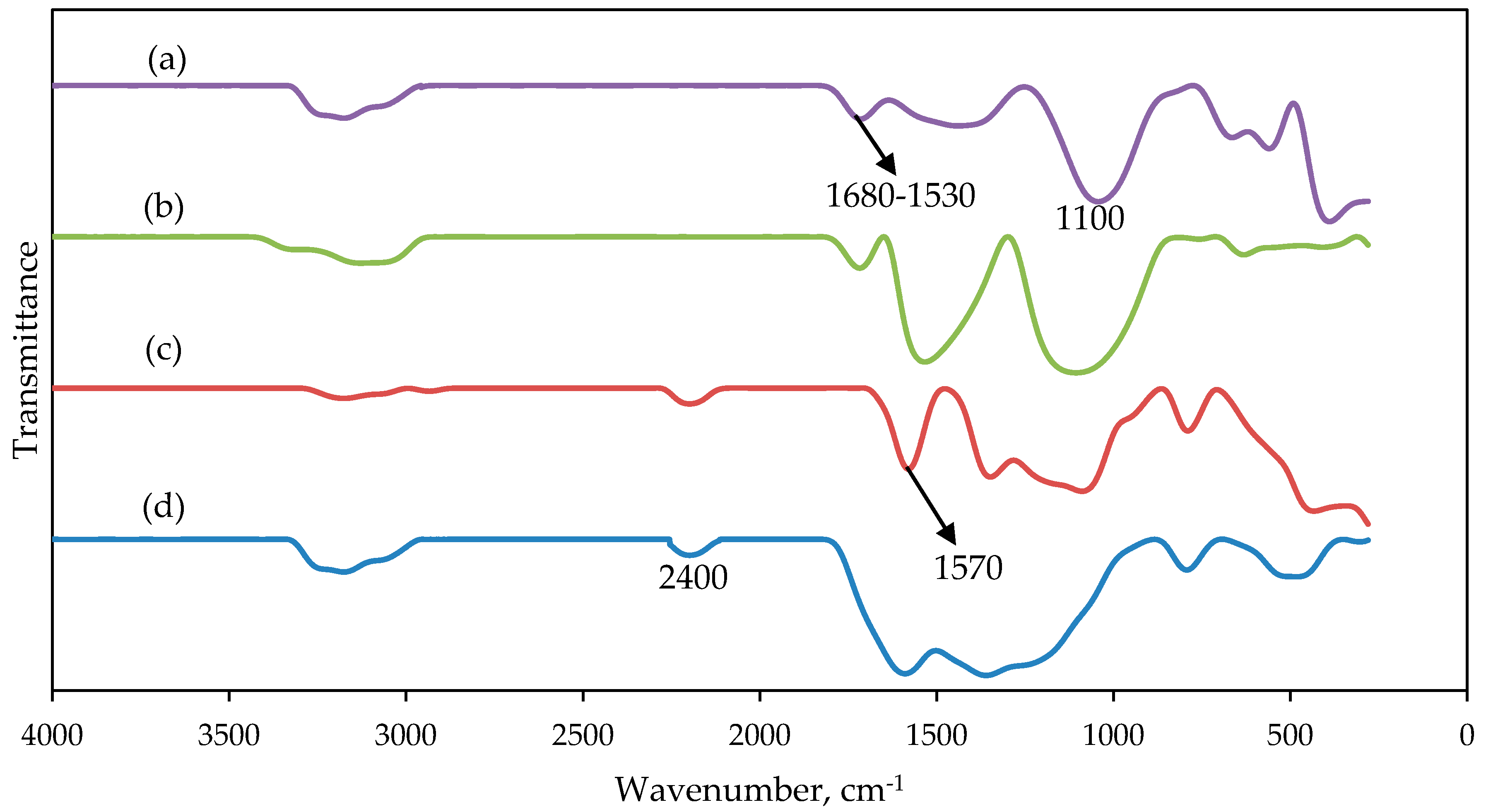
2.3. Spectroscopic Study of The Nanofibers After Thermal Treatment (Stabilization and Carbonization Process)
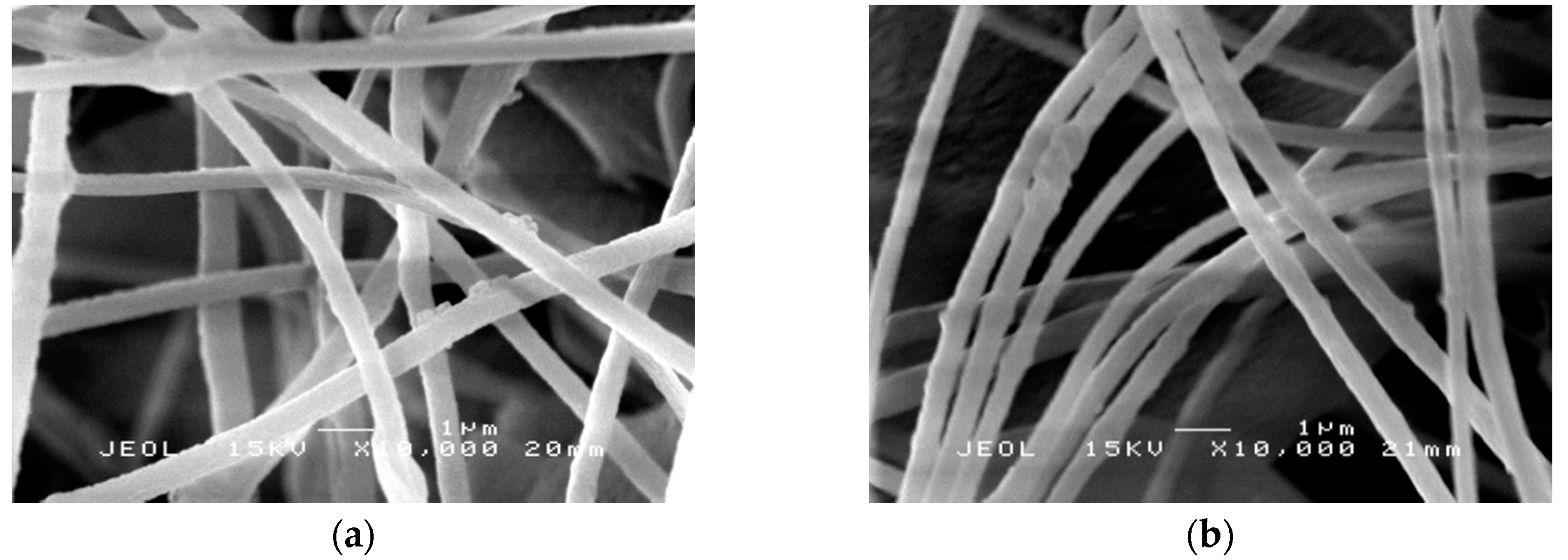
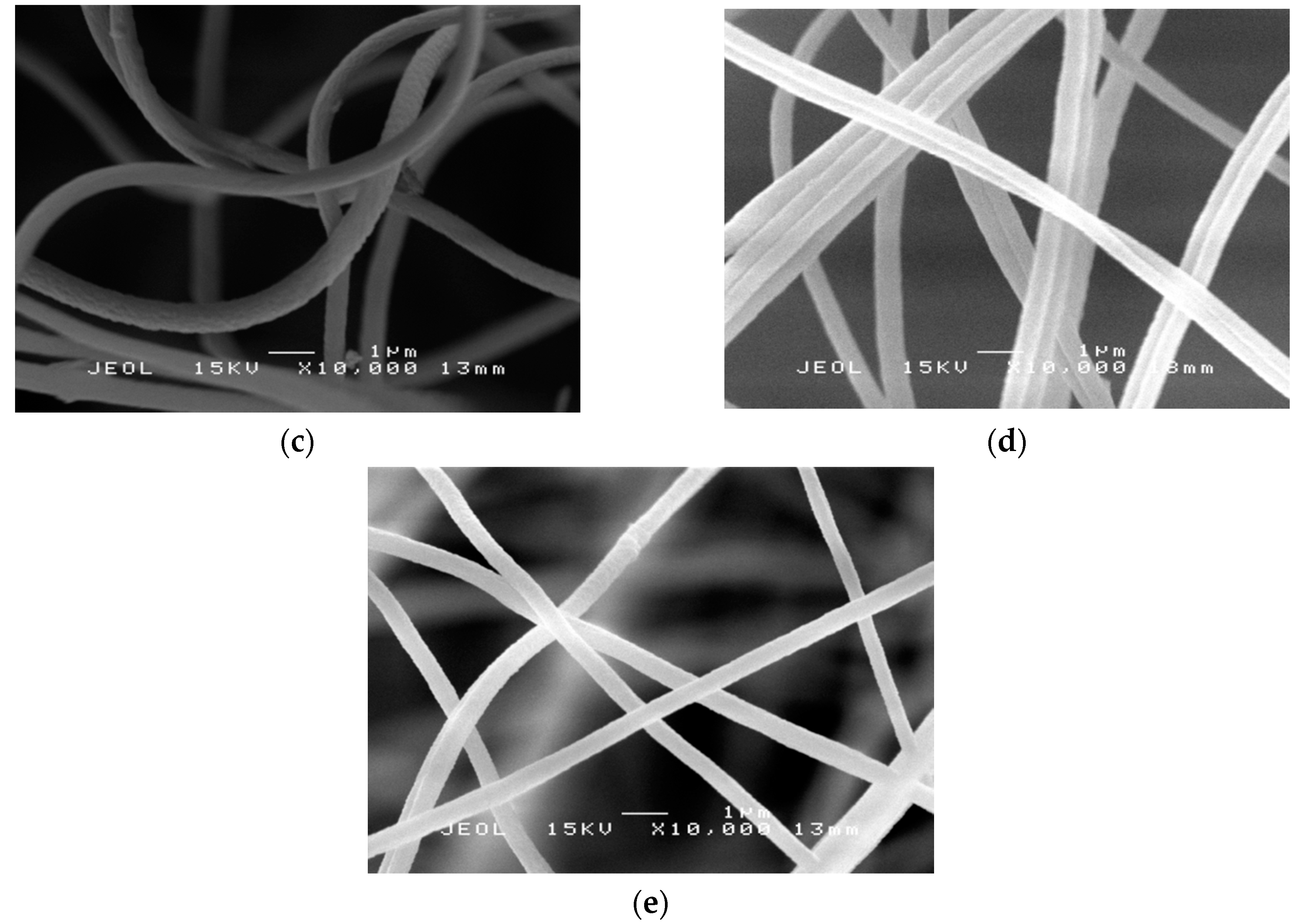
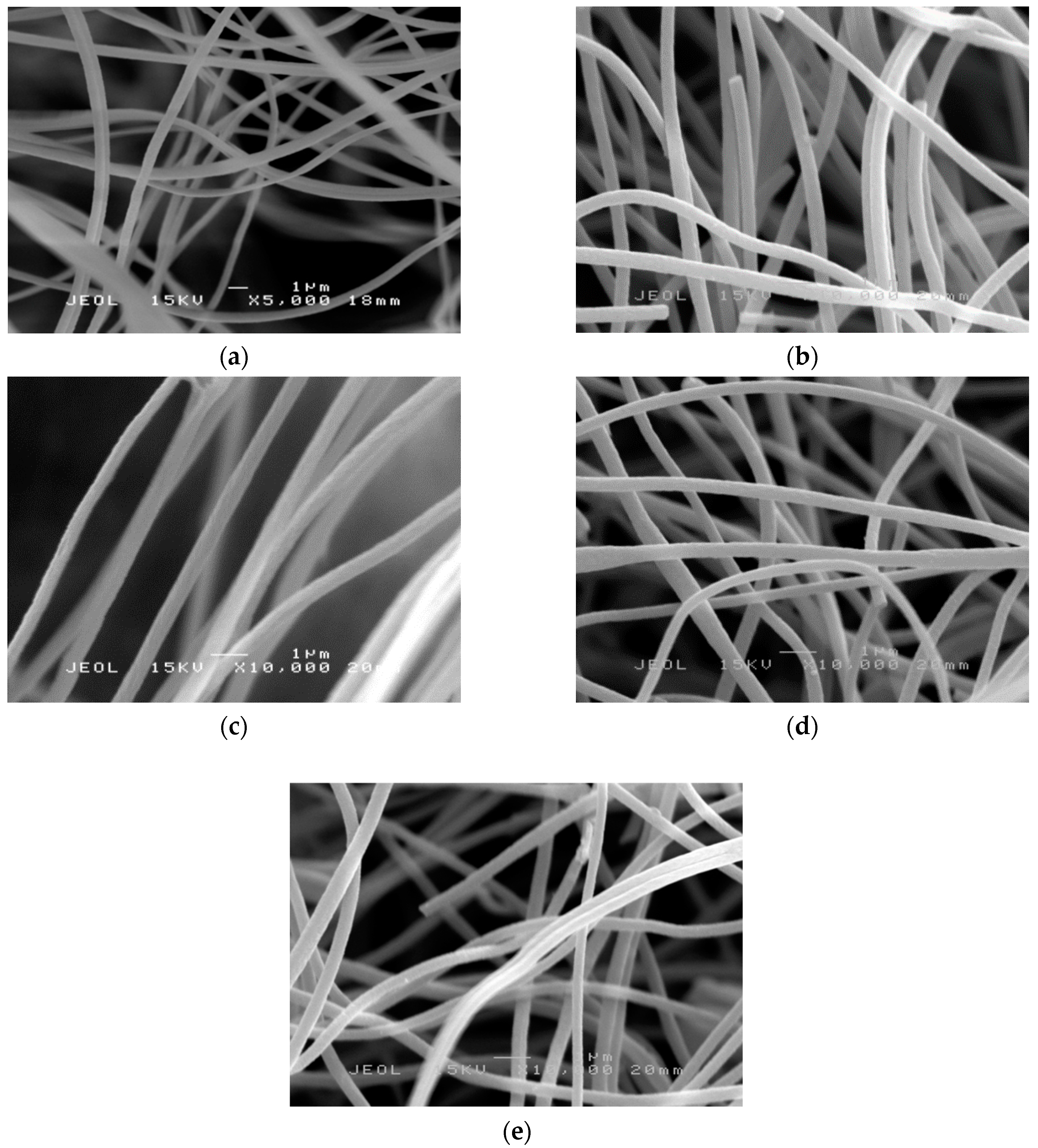
2.4. Morphology Study of PAN Nanofibers and PAN/SL Nanofibers After Stabilization and Carbonization Process
2.5. Carbon Yield
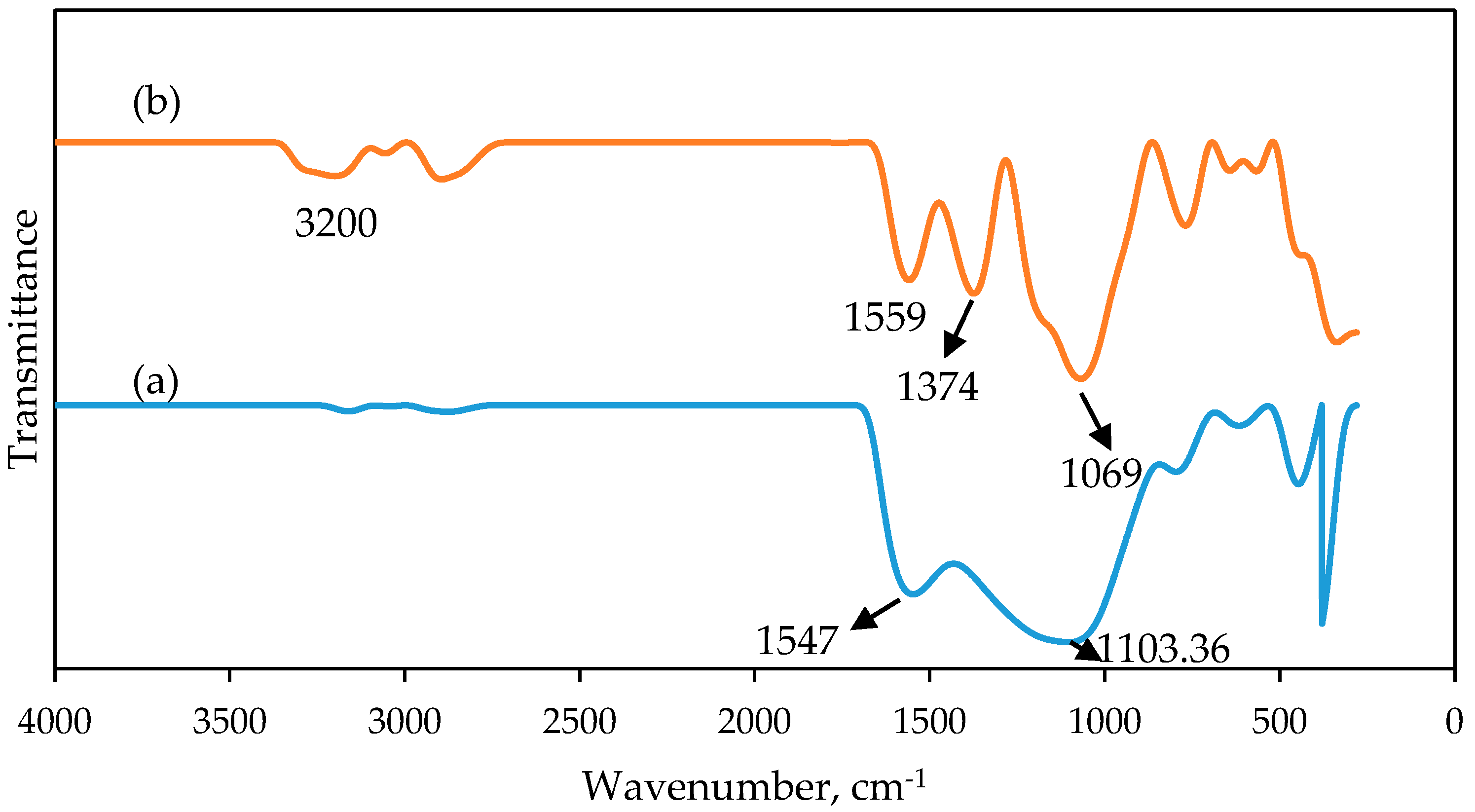
2.6. Spectroscopic Study of PAN/SL ACNFs
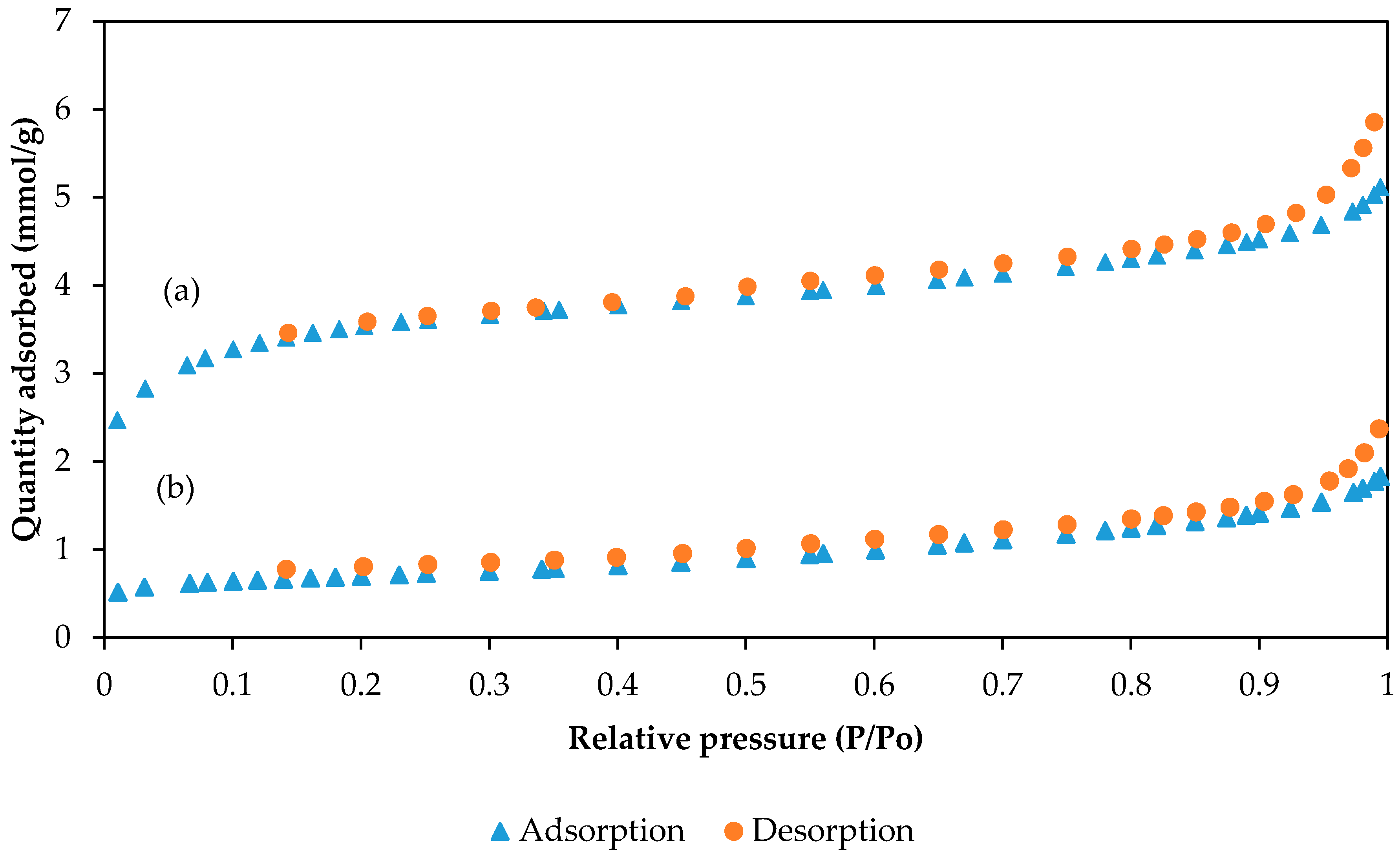
2.7. BET Analysis of PAN/SL CNFs and PAN/SL ACNFs
2.8. XPS Analysis of PAN/SL ACNFs
2.9. Adsorption of Pb(II) Ions
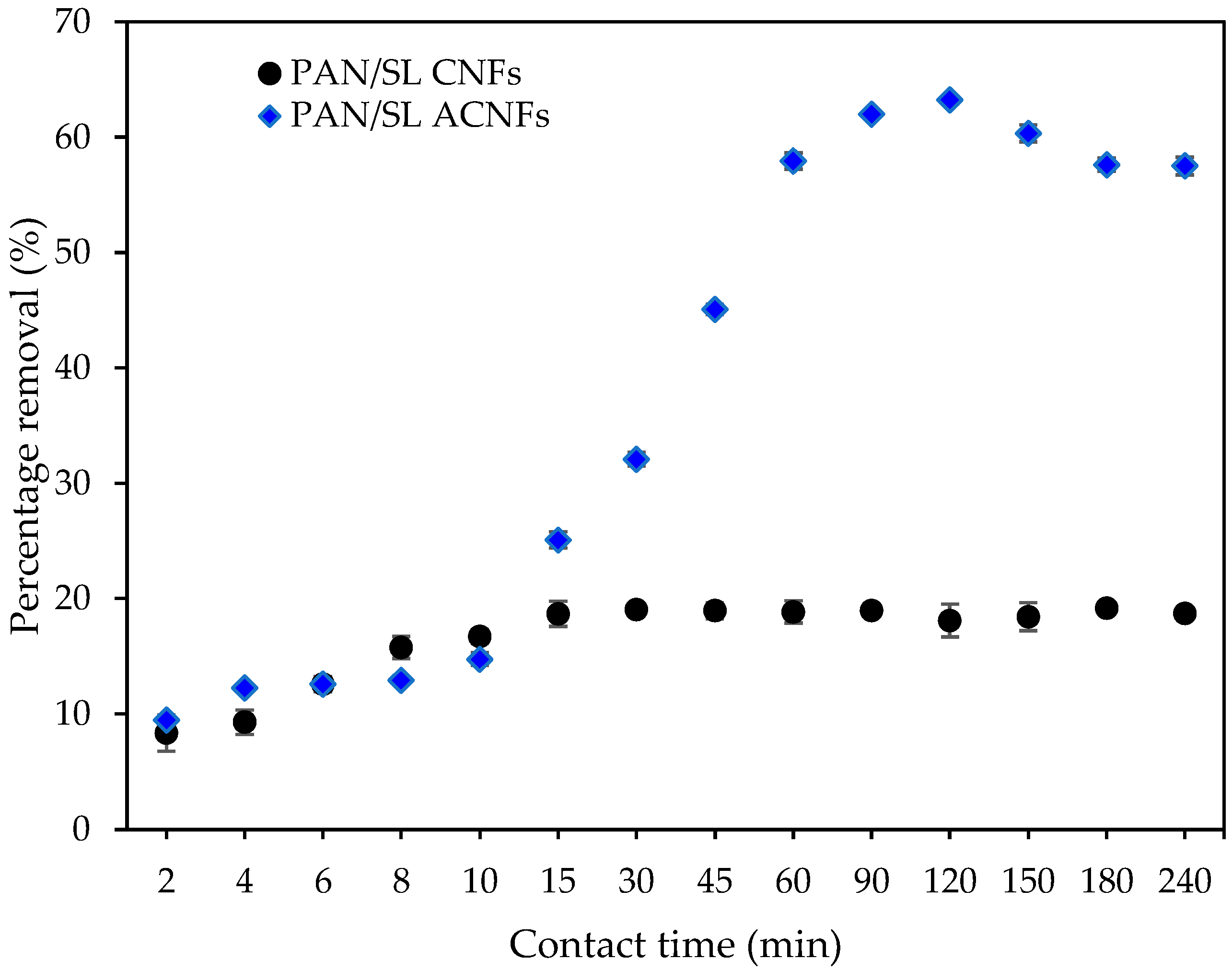
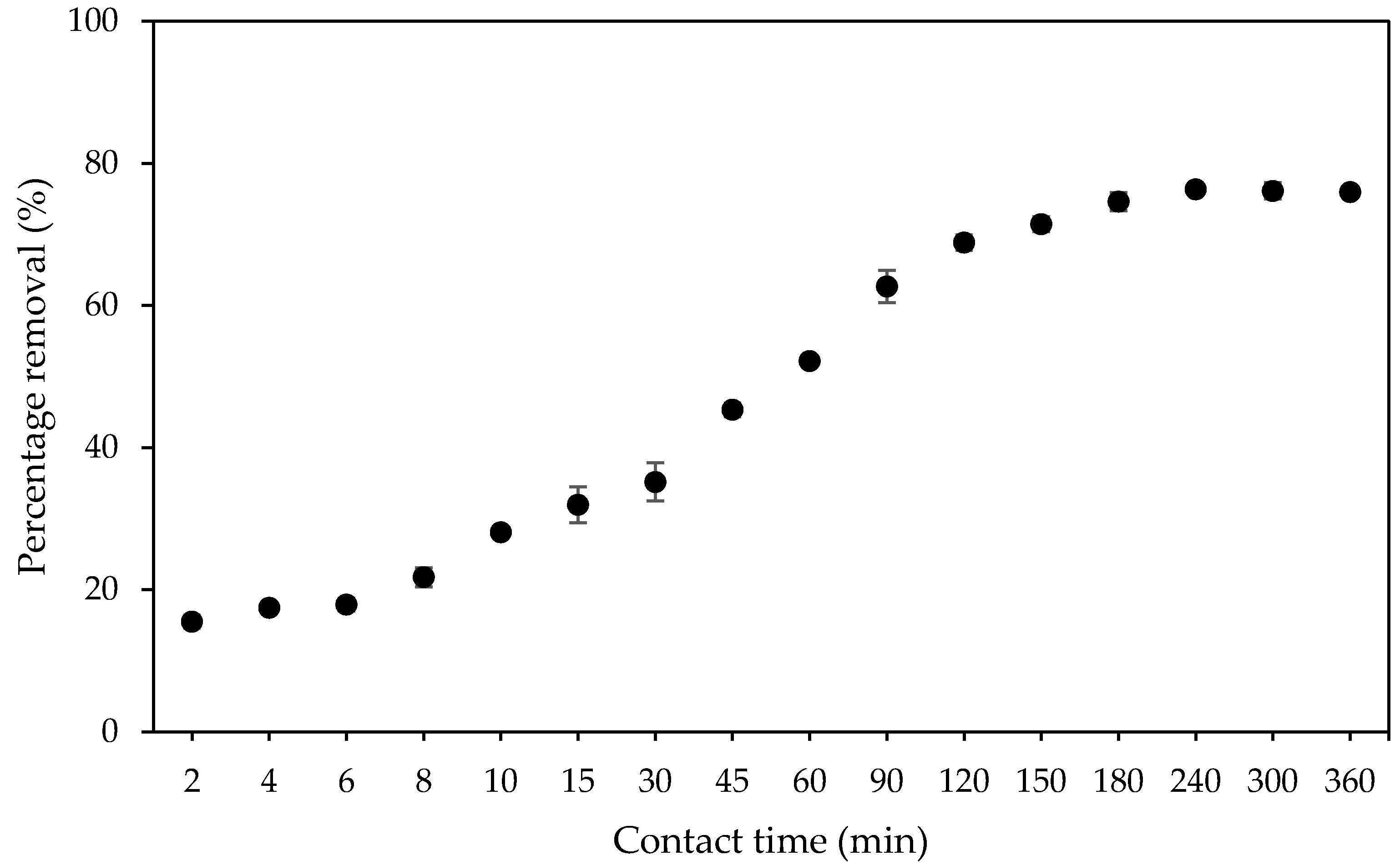
2.9.1. Effect of Contact Time
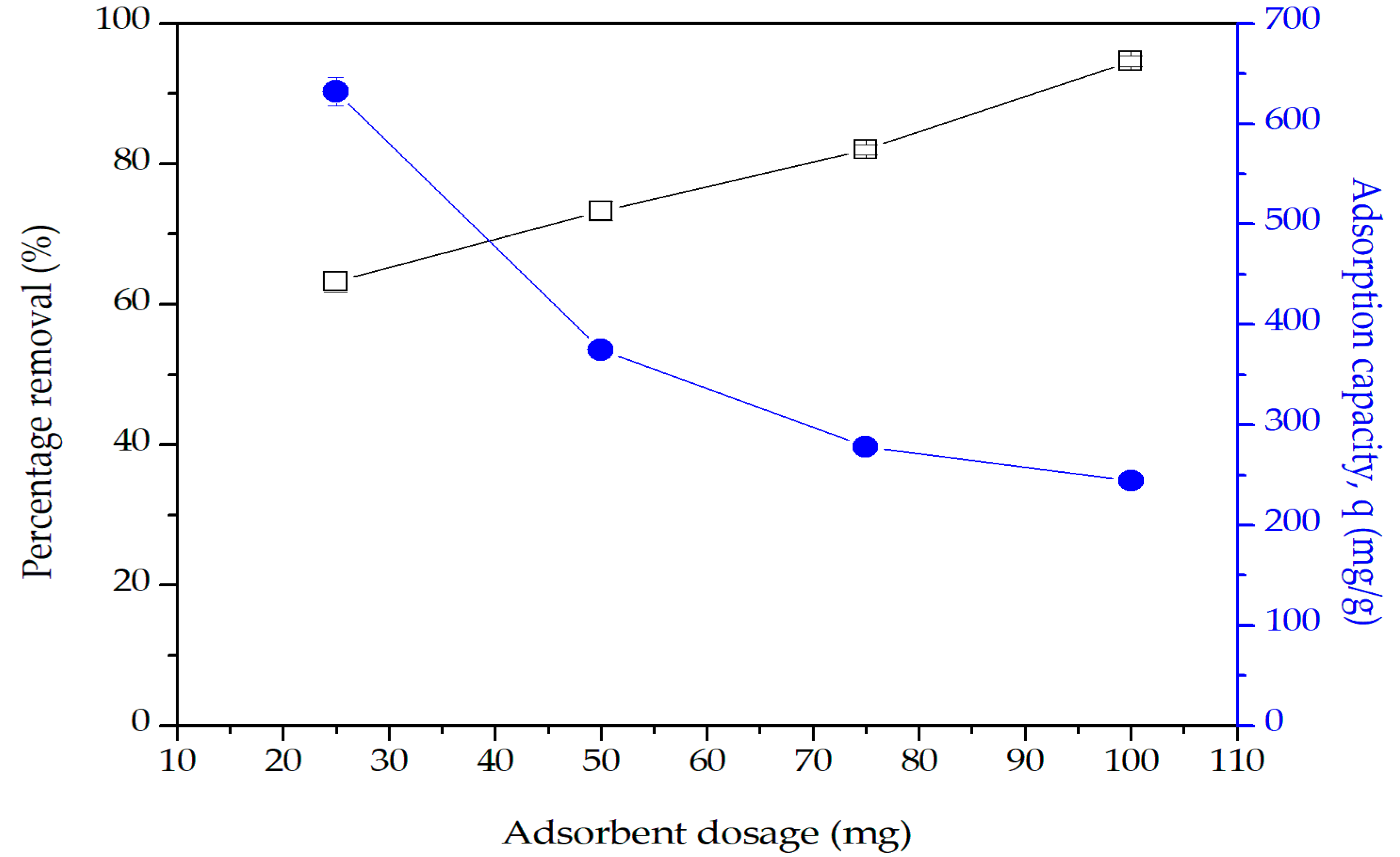
2.9.2. Effect of Adsorbent Dosage
2.9.3. Effect of Initial Concentration of Pb(II) Solutions
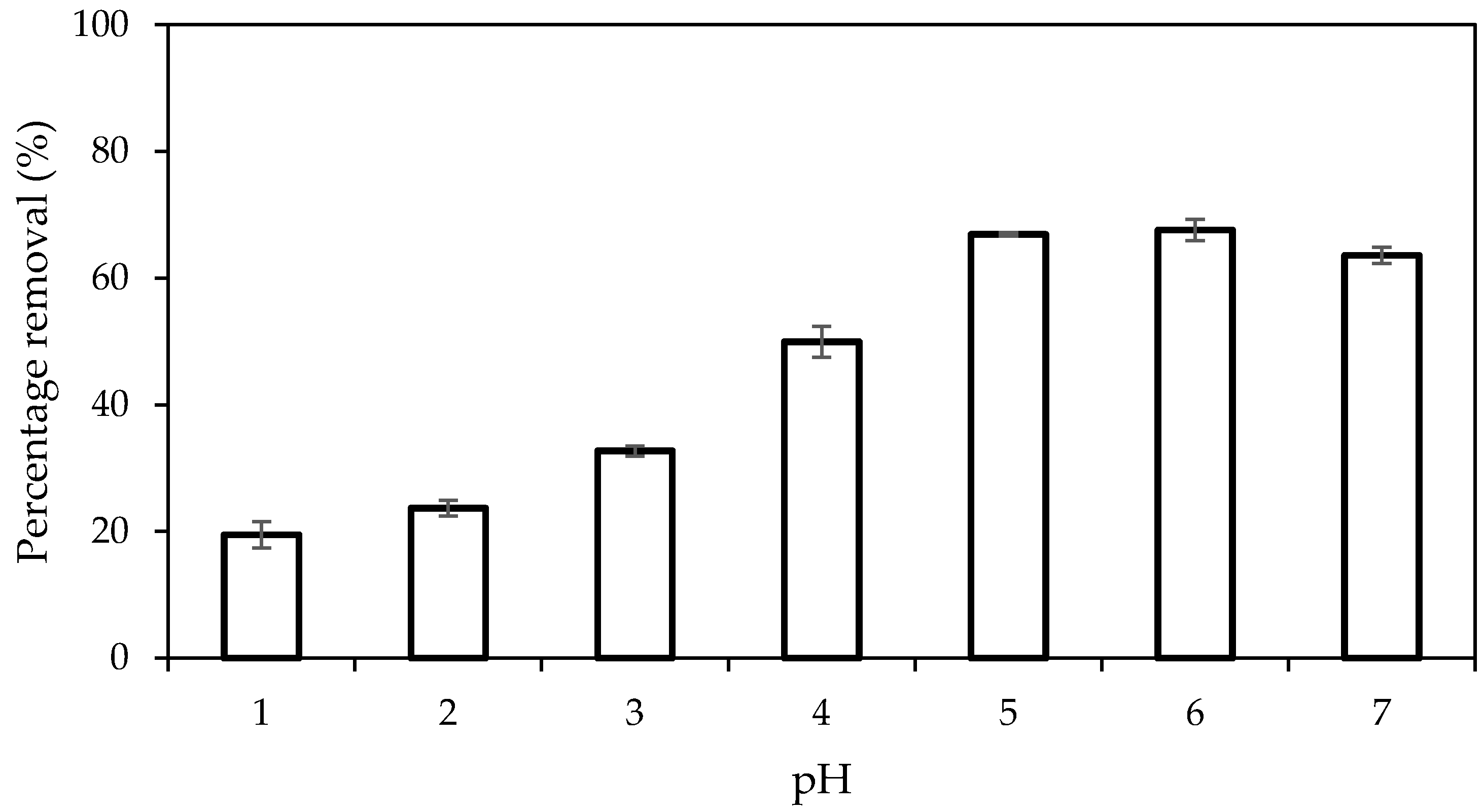
2.9.4. Effect of pH
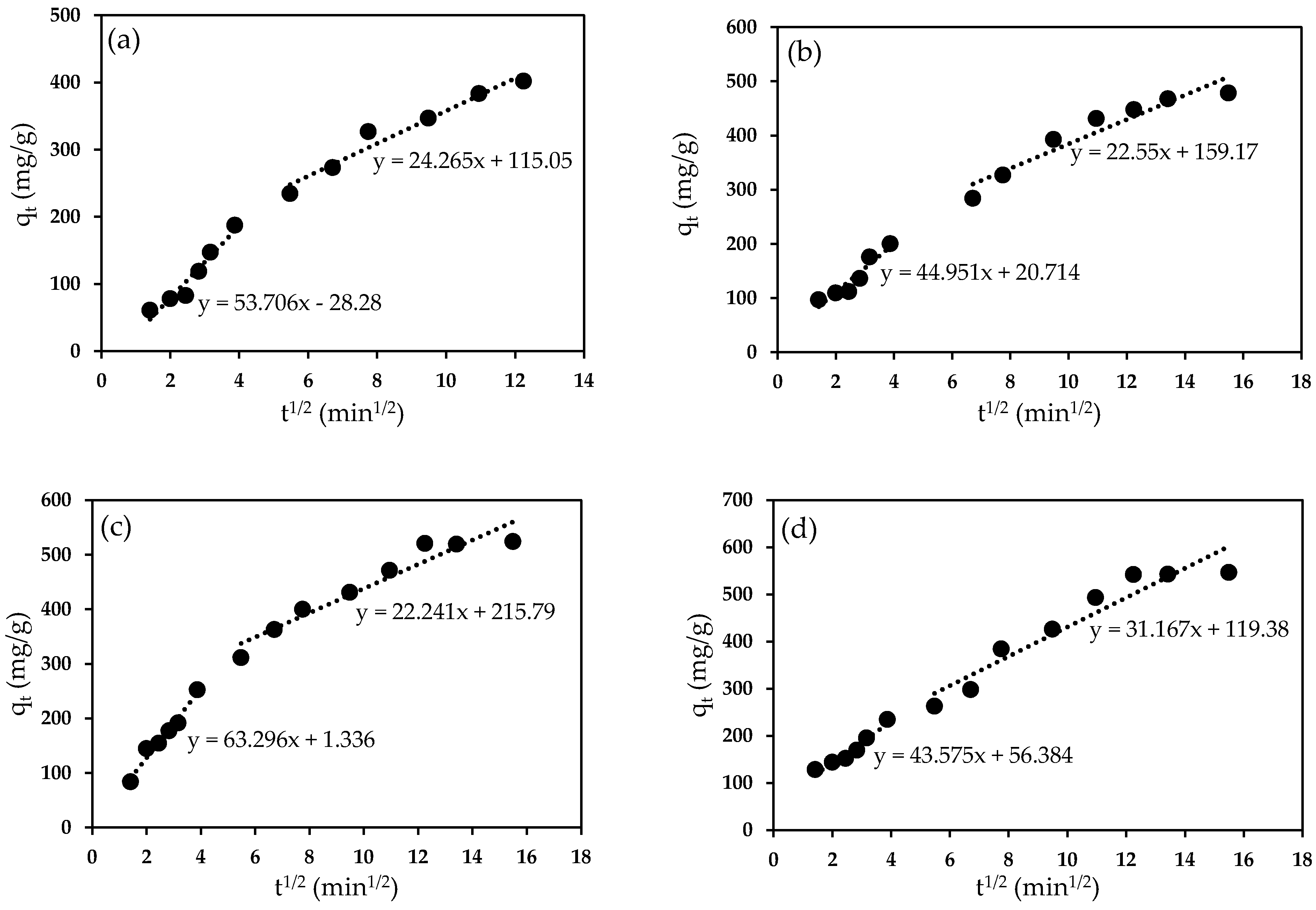
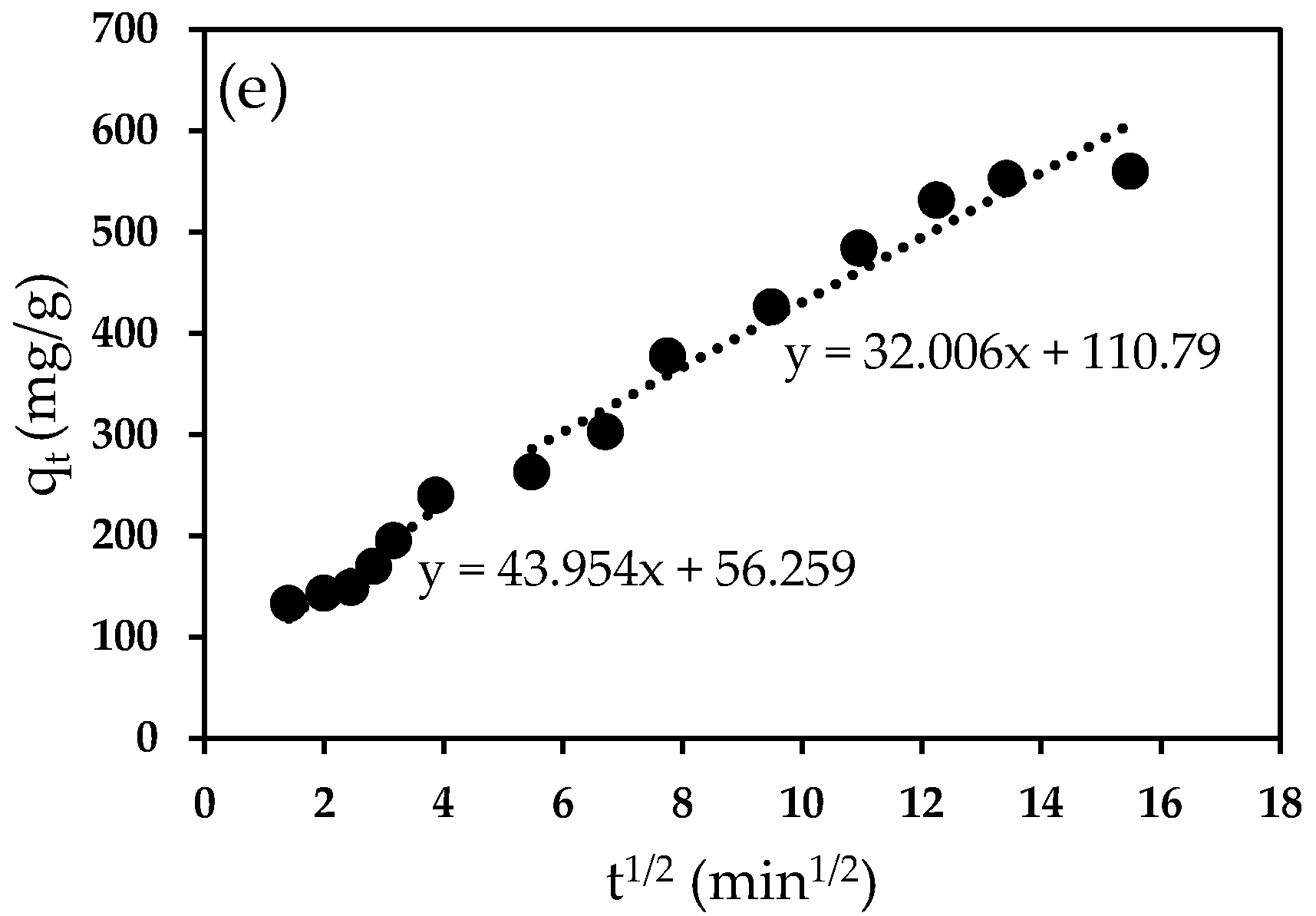
2.9.5. Adsorption Kinetics and Isotherm
3. Materials and Methods
3.1. Preparation of Electrospun PAN and PAN/SL Nanofibers
3.2. Preparation of Carbon Nanofibers
3.3. Characterizations of PAN/SL ACNFs
3.4. Batch Adsorption of Pb(II) Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desta, M.B. Batch Sorption Experiments: Langmuir and Freundlich Isotherm Studies for the Adsorption of Textile Metal Ions onto Teff Straw (Eragrostis tef) Agricultural Waste. J. Thermodyn. 2013, 6. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 3613–3677. [Google Scholar] [CrossRef]
- Zulkali, M.M.D.; Ahmad, A.L.; Norulakmal, N.H. Oryza sativa L. husk as heavy metal adsorbent: Optimization with lead as model solution. Bioresour. Technol. 2006, 97, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Jalali, R.; Ghafourian, H.; Asef, Y.; Davarpanah, S.J.; Sepehr, S. Removal and recovery of lead using nonliving biomass of marine algae. J. Hazard. Mater. 2002, 92, 2532–2562. [Google Scholar] [CrossRef]
- Volesky, B. Advances in biosorption of metals: Selection of biomass types. FEMS Microbiol. Rev. 1994, 14, 2913–3002. [Google Scholar] [CrossRef] [PubMed]
- Quintelas, C.; Rocha, Z.; Silva, B.; Froseca, B.; Figueiredo, H.; Tavares, T. Removal of Cd(II), Cr(VI), Fe(III) and Ni(II) from aqueous solutions by an E. coli biofilm supported on kaolin. Chem. Eng. J. 2009, 149, 3193–3224. [Google Scholar]
- Chen, T.; Zhao, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 2862–2893. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Du, M.; Zhu, H.; Bao, S.; Yang, T.; Zou, M. Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J. Hazard. Mater. 2015, 286, 5335–5344. [Google Scholar] [CrossRef]
- Jiang, M.-Q.; Jin, X.-Y.; Lu, X.-Q.; Chen, Z.-L. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 333–339. [Google Scholar]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 112–114. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 5745–5785. [Google Scholar]
- Wang, F.; Lu, X.; Li, X.-y. Selective removals of heavy metals (Pb2+, Cu2+, and Cd2+) from wastewater by gelation with alginate for effective metal recovery. J. Hazard. Mater. 2016, 308, 758–763. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Liu, R.; Li, Y.; Wang, D.; Tan, J.; Wu, R.; Huang, Y. Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment. Carbohydr. Polym. 2011, 83, 7437–7448. [Google Scholar] [CrossRef]
- Adebisi, G.A.; Chowdhury, Z.Z.; Alaba, P.A. Equilibrium, kinetic, and thermodynamic studies of lead ion and zinc ion adsorption from aqueous solution onto activated carbon prepared from palm oil mill effluent. J. Clean. Prod. 2017, 148, 9589–9668. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Ge, Y. Removal of lead ion and oil droplet from aqueous solution by lignin-grafted carbon nanotubes. Chem. Eng. J. 2017, 308, 8098–8117. [Google Scholar] [CrossRef]
- Alizadeh, B.; Ghorbani, M.; Salehi, M.A. Application of polyrhodanine modified multi-walled carbon nanotubes for high efficiency removal of Pb(II) from aqueous solution. J. Mol. Liq. 2016, 220, 1421–1449. [Google Scholar] [CrossRef]
- Pyrzyńska, K.; Bystrzejewski, M. Comparative study of heavy metal ions sorption onto activated carbon, carbon nanotubes, and carbon-encapsulated magnetic nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 362, 1021–1109. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Removal of heavy metals from their aqueous solutions through adsorption onto natural polymers. Carbohydr. Polym. 2011, 84, 4544–4558. [Google Scholar] [CrossRef]
- Lai, J.C.; Rahman, W.A.W.A.; Toh, W.Y. Characterisation of sago pith waste and its composites. Ind. Crops Prod. 2013, 45, 3193–3226. [Google Scholar] [CrossRef]
- Igura, M.; Okazaki, M. Cadmium sorption characteristics of phosphorylated sago starch-extraction residue. J. Hazard. Mater. 2010, 178, 6866–6892. [Google Scholar] [CrossRef] [PubMed]
- Mohd Kanafi, N.; Abdul Rahman, N.; Rosdi, H.N.; Bahruji, H.; Maarof, H. Hydrogel Nanofibers from Carboxymethyl Sago Pulp and Its Controlled Release Studies as a Methylene Blue Drug Carrier. Fibers 2019, 7, 56. [Google Scholar] [CrossRef]
- Mamun, A.A.; Ahmed, Y.M.; Muyibi, S.A.; Al-Khatib, M.F.R.; Jameel, A.T.; AlSaadi, M.A. Synthesis of carbon nanofibers on impregnated powdered activated carbon as cheap substrate. Arab. J. Chem. 2016, 9, 5325–5336. [Google Scholar] [CrossRef]
- Abdul Rahman, N.; Gisdavic-Nikolaidis, M.; Ray, S.; Easteal, A.J.; Travas-Sejdic, J. Functional electrospun nanofibres of poly(lactic acid) blends with polyaniline or poly(aniline-co-benzoic acid). Synth. Met. 2010, 160, 20152–21022. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, J.; Kim, H. Preparation of poly(vinylidene fluoride)/phosphotungstic acid composite nanofiber membranes by electrospinning for proton conductivity. React. Funct. Polym. 2010, 70, 697–704. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Li, K.; Wu, Y.; Liu, H. Optimization of stabilization conditions for electrospun polyacrylonitrile nanofibers. Polym. Degrad. Stabil. 2012, 97, 15111–15519. [Google Scholar] [CrossRef]
- Patel, S.; Hota, G. Synthesis of novel surface functionalized electrospun PAN nanofibers matrix for efficient adsorption of anionic CR dye from water. J. Environ. Chem. Eng. 2018, 6, 53015–53310. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, Q.; Ye, J.; Yao, Q.; Zhao, C. Study on the thermal degradation behaviors and kinetics of alkali lignin for production of phenolic-rich bio-oil using TGA–FTIR and Py–GC/MS. J. Anal. Appl. Pyrolysis. 2016, 117, 1161–1224. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stabil. 2014, 108, 1331–1338. [Google Scholar] [CrossRef]
- Lai, C.; Zhao, Z.; Zhang, L.; Wang, X.; Zhou, Q.; Zhao, Y.; Wang, Y.; Wu, X.-F.; Zhu, Z.; Fong, H. Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J. Power Sources 2014, 247, 1341–1441. [Google Scholar] [CrossRef]
- Beck, R.J.; Zhao, Y.; Fong, H.; Menkhaus, T.J. Electrospun lignin carbon nanofiber membranes with large pores for highly efficient adsorptive water treatment applications. J. Water Process Eng. 2017, 16, 2402–2448. [Google Scholar] [CrossRef]
- Arshad, S.N.; Naraghi, M.; Chasiotis, I. Strong carbon nanofibers from electrospun polyacrylonitrile. Carbon 2011, 49, 17101–17719. [Google Scholar] [CrossRef]
- Rajzer, I.; Kwiatkowski, R.; Piekarczyk, W.; Biniaś, W.; Janicki, J. Carbon nanofibers produced from modified electrospun PAN/hydroxyapatite precursors as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2012, 32, 25622–26569. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon Nanofibers Prepared via Electrospinning. Adv. Mater. 2012, 24, 25472–25566. [Google Scholar] [CrossRef]
- Ji, L.; Yao, Y.; Toprakci, O.; Lin, Z.; Liang, Y.; Shi, Q.; Medford, A.J.; Millns, C.R.; Zhang, X. Fabrication of carbon nanofiber-driven electrodes from electrospun polyacrylonitrile/polypyrrole bicomponents for high-performance rechargeable lithium-ion batteries. J. Power Sources 2010, 195, 20502–21056. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Hamshary, H.; Al-Deyab, S.S.; El-Newehy, M.H. Functionalized electrospun carbon nanofibers for removal of cationic dye. Arab. J. Chem. 2019, 12, 7477–7559. [Google Scholar] [CrossRef]
- Thunga, M.; Chen, K.; Grewell, D.; Kessler, M.R. Bio-renewable precursor fibers from lignin/polylactide blends for conversion to carbon fibers. Carbon 2014, 68, 1591–1666. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, G.; Xu, X.; Shen, D.; Jin, B. Study on carbonization of lignin by TG-FTIR and high-temperature carbonization reactor. Fuel Process. Technol. 2013, 106, 414–417. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Sui, Z.-J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 7857–7896. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 3523–3559. [Google Scholar] [CrossRef]
- Yagmur, E.; Inal, I.I.G.; Gokce, Y.; Ulusoy Ghobadi, T.G.; Aktar, T.; Aktas, Z. Examination of gas and solid products during the preparation of activated carbon using phosphoric acid. J. Environ. Manag. 2018, 228, 3283–3335. [Google Scholar] [CrossRef]
- Keyser, M.M.; Prinsloo, F.F. Loading of Cobalt on Carbon Nanofibers. In Studies in Surface Science and Catalysis; Davis, B.H., Occelli, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 457–463. [Google Scholar]
- Choma, J.; Burakiewicz-Mortka, W.; Jaroniec, M.; Li, Z.; Klinik, J. Monitoring Changes in Surface and Structural Properties of Porous Carbons Modified by Different Oxidizing Agents. J. Colloid Interface Sci. 1999, 214, 4384–4446. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon. Carbon 2003, 41, 7137–7222. [Google Scholar] [CrossRef]
- Song, X.; Liu, H.; Cheng, L.; Qu, Y. Surface modification of coconut-based activated carbon by liquid-phase oxidation and its effects on lead ion adsorption. Desalination 2010, 255, 788–793. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodríguez-Mirasol, J.; Cordero, T. The production of submicron diameter carbon fibers by the electrospinning of lignin. Carbon 2010, 48, 6967–7005. [Google Scholar] [CrossRef]
- Wang, Z.; Qie, L.; Yuan, L.; Zhang, W.; Hu, X.; Huang, Y. Functionalized N-doped interconnected carbon nanofibers as an anode material for sodium-ion storage with excellent performance. Carbon 2013, 55, 3283–3334. [Google Scholar] [CrossRef]
- Bouhamed, F.; Elouear, Z.; Bouzid, J. Adsorptive removal of copper(II) from aqueous solutions on activated carbon prepared from Tunisian date stones: Equilibrium, kinetics and thermodynamics. J. Taiwan Inst. Chem. Eng. 2012, 43, 7417–7449. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.W.P.K.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 2993–3009. [Google Scholar] [CrossRef]
- Arshadi, M.; Amiri, M.J.; Mousavi, S. Kinetic, equilibrium and thermodynamic investigations of Ni(II), Cd(II), Cu(II) and Co(II) adsorption on barley straw ash. Water Resour. Ind. 2014, 6, 11–17. [Google Scholar] [CrossRef]
- Abdelwahab, O.; Amin, N.K.; El-Ashtoukhy, E.S.Z. Removal of zinc ions from aqueous solution using a cation exchange resin. Chem. Eng. Res. Des. 2013, 91, 1651–1673. [Google Scholar] [CrossRef]
- Gao, J.-J.; Qin, Y.-B.; Zhou, T.; Cao, D.-D.; Xu, P.; Hochstetter, D.; Wang, Y.-F. Adsorption of methylene blue onto activated carbon produced from tea (Camellia sinensis L.) seed shells: Kinetics, equilibrium, and thermodynamics studies. J. Zhejiang Univ. Sci. B 2013, 14, 6506–6558. [Google Scholar]
- Abdelnaeim, M.Y.; El Sherif, I.Y.; Attia, A.A.; Fathy, N.A.; El-Shahat, M.F. Impact of chemical activation on the adsorption performance of common reed towards Cu(II) and Cd(II). Int. J. Miner. Process. 2016, 157, 808–818. [Google Scholar] [CrossRef]
- Abdullah, N.; Tajuddin, M.H.; Yusof, N.; Jaafar, J.; Aziz, F.; Misdan, N. Removal of Lead(Ii) from Aqueous Solution Using Polyacrylonitrile/Zinc Oxide Activated Carbon Nanofibers. Malays. J. Anal. Sci. 2017, 21, 619–626. [Google Scholar]
- Schwanninger, M.; Hinterstoisser, B. Klason Lignin: Modifications to Improve the Precision of the Standardized Determination. Holzforschung 2002, 161. [Google Scholar] [CrossRef]
- Al-Khaldi, F.A.; Abusharkh, B.; Khaled, M.; Atieh, M.A.; Nasser, M.S.; Iaoui, T.; Saleh, T.A.; Agarwal, S.; Tyagi, I.; Gupta, V.K.; et al. Adsorptive removal of cadmium(II) ions from liquid phase using acid modified carbon-based adsorbents. J. Mol. Liq. 2015, 204, 2552–2563. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 192–196. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Shen, M.; Tan, X.; Ding, Y.; Jiang, Z.; Wang, C. Preparation of phosphorylated polyacrylonitrile-based nanofiber mat and its application for heavy metal ion removal. Chem. Eng. J. 2015, 268, 2902–2999. [Google Scholar] [CrossRef]
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 1251–1336. [Google Scholar] [CrossRef]
- Faghihian, H.; Kooravand, M.; Atarodi, H. Synthesis of a novel carbon nanofiber structure for removal of lead. Korean J. Chem. Eng. 2012, 30, 3573–3663. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Nanofibers | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | ||
|---|---|---|---|---|---|
| a Total | b Micropore | c Total | b Micropore | ||
| PAN/SL CNFs | 292.24 | 168.27 | 0.163 | 0.0683 | 3.18 |
| PAN/SL ACNFs | 57.37 | 28.25 | 0.0534 | 0.0116 | 4.51 |
| C0 (mg/L) | qe, expt. (mg/g) | Pseudo 1st Order | Pseudo 2nd Order | ||||
|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe, calc. (mg/g) | R2 | k2 (g/mg/min) | qe, calc. (mg/g) | R2 | ||
| 75 | 441.34 | 0.0258 | 427.3 | 0.8747 | 8.51 × 10−5 | 476.19 | 0.9986 |
| 100 | 478.44 | 0.0178 | 375.67 | 0.9257 | 8.75 × 10−5 | 500.00 | 0.9951 |
| 125 | 523.91 | 0.0256 | 475.61 | 0.9513 | 9.73 × 10−5 | 555.56 | 0.9952 |
| 150 | 546.66 | 0.0260 | 554.13 | 0.9502 | 7.30 × 10−5 | 588.24 | 0.9811 |
| 175 | 574.75 | 0.0197 | 506.44 | 0.9425 | 7.10 × 10−5 | 588.24 | 0.98 |
| Ci (mg/L) | 1st Step | 2nd | ||
|---|---|---|---|---|
| Kid (mg/g.min1/2) | R2 | kid (mg/g.min1/2) | R2 | |
| 75 | 53.706 | 0.9391 | 24.265 | 0.9573 |
| 100 | 44.951 | 0.9002 | 22.55 | 0.9124 |
| 125 | 63.296 | 0.9697 | 22.241 | 0.9244 |
| 150 | 43.575 | 0.9443 | 31.167 | 0.9089 |
| 175 | 43.954 | 0.9091 | 32.006 | 0.9432 |
| PAN/SL ACNFs | Langmuir Isotherm | Freundlich Isotherm | ||||
| qm (mg/g) | KL (L/mg) | R2 | KF (mg/g) | 1/n | R2 | |
| 588.24 | 0.2537 | 0.999 | 339.94 | 0.1132 | 0.9902 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordin, N.A.; Abdul Rahman, N.; Abdullah, A.H. Effective Removal of Pb(II) Ions by Electrospun PAN/Sago Lignin-Based Activated Carbon Nanofibers. Molecules 2020, 25, 3081. https://doi.org/10.3390/molecules25133081
Nordin NA, Abdul Rahman N, Abdullah AH. Effective Removal of Pb(II) Ions by Electrospun PAN/Sago Lignin-Based Activated Carbon Nanofibers. Molecules. 2020; 25(13):3081. https://doi.org/10.3390/molecules25133081
Chicago/Turabian StyleNordin, Nurul Aida, Norizah Abdul Rahman, and Abdul Halim Abdullah. 2020. "Effective Removal of Pb(II) Ions by Electrospun PAN/Sago Lignin-Based Activated Carbon Nanofibers" Molecules 25, no. 13: 3081. https://doi.org/10.3390/molecules25133081
APA StyleNordin, N. A., Abdul Rahman, N., & Abdullah, A. H. (2020). Effective Removal of Pb(II) Ions by Electrospun PAN/Sago Lignin-Based Activated Carbon Nanofibers. Molecules, 25(13), 3081. https://doi.org/10.3390/molecules25133081







