Characteristic Metabolic Changes of the Crust from Dry-Aged Beef Using 2D NMR Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
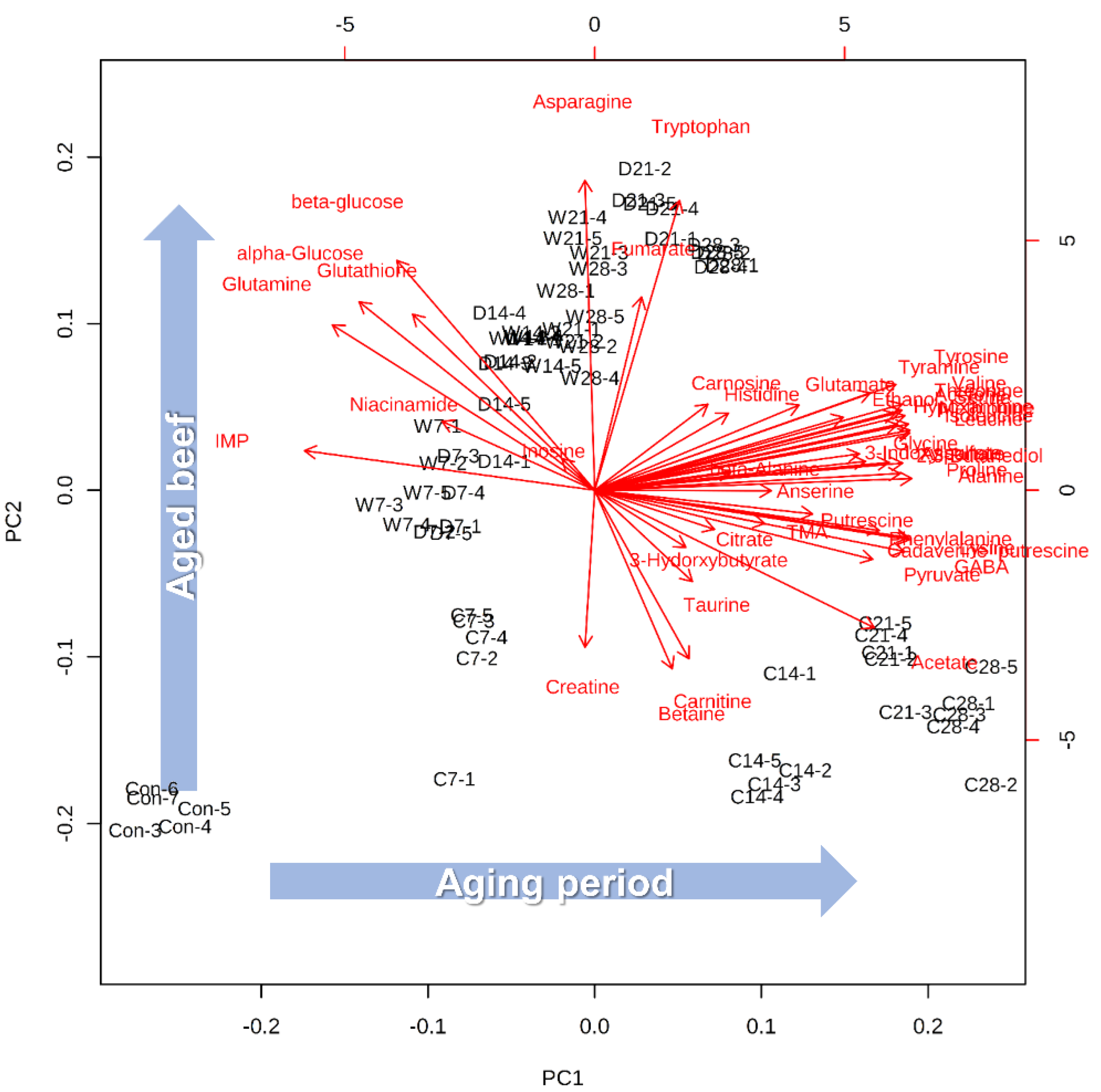
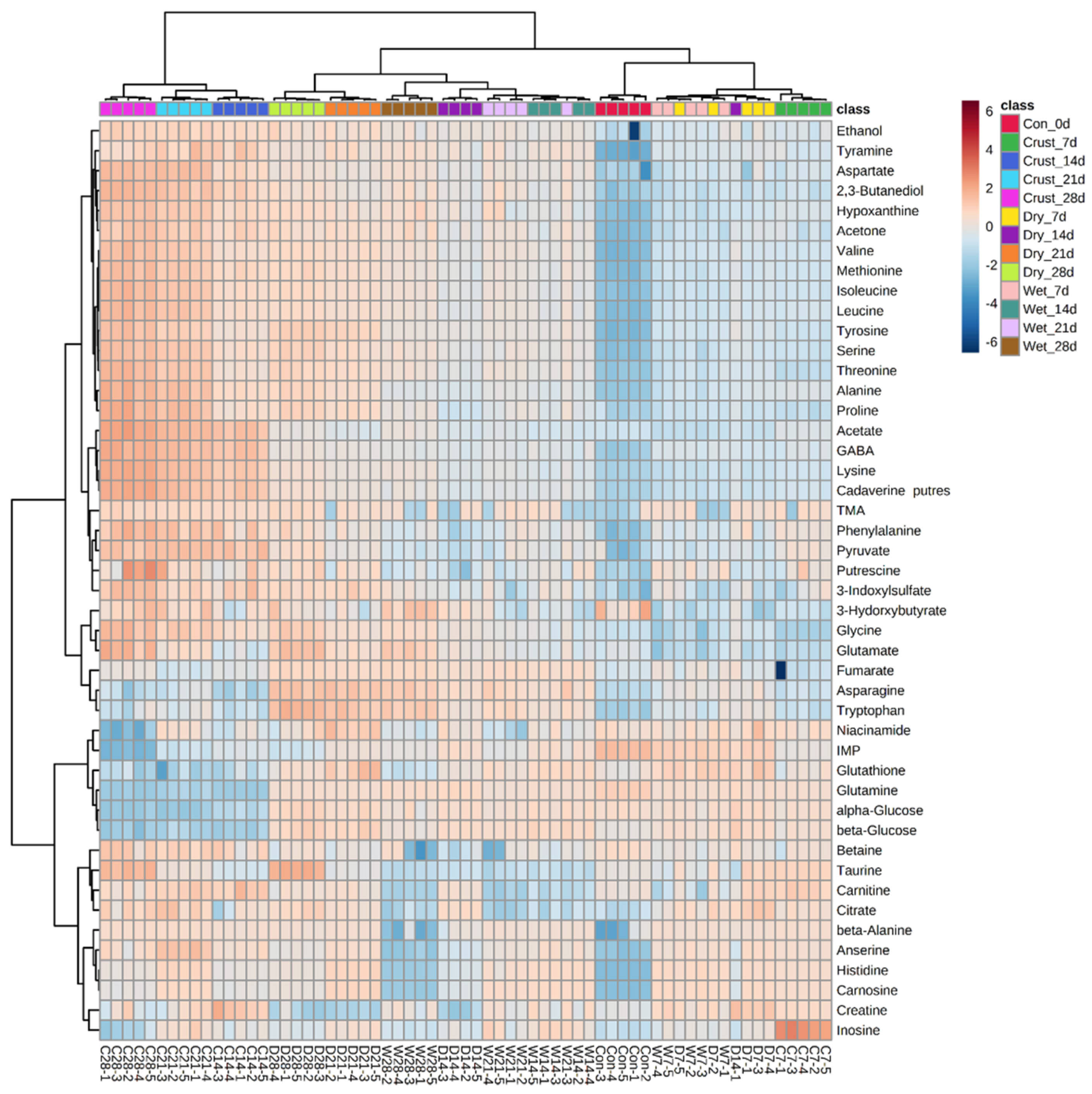
2.1. Multivariable Analyses
2.2. Metabolic Characteristics
2.2.1. Proteolysis
2.2.2. Bioactive Compounds
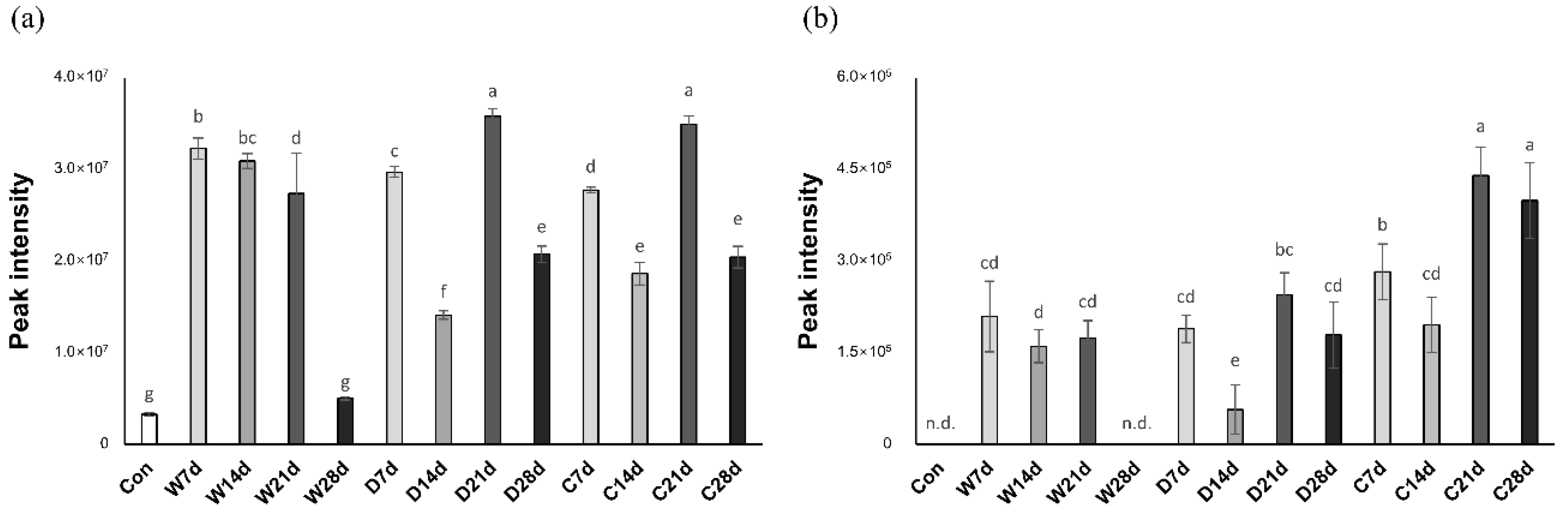
2.2.3. Nucleotides
2.3. Unique Metabolic Characteristics of the Crust during Aging
3. Materials and Methods
3.1. Sample Preparation and the Aging Process
3.2. Sample Extraction
3.3. NMR Experiments
3.4. Multivariable Analysis
3.5. Quantification of Metabolites
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koohmaraie, M. Muscle proteinases and meat aging. Meat Sci. 1994, 36, 93–104. [Google Scholar] [CrossRef]
- Dashdorj, D.; Tripathi, V.K.; Kim, J.-H.; Kim, Y.; Hwang, I. Dry aging of beef; Review. J. Anim. Sci. Technol. 2016, 58, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choe, J.; Kim, M.; Kim, H.C.; Yoon, J.W.; Oh, S.W.; Jo, C. Role of moisture evaporation in the taste attributes of dry- and wet-aged beef determined by chemical and electronic tongue analyses. Meat Sci. 2019, 151, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Nicholson, K.; Nicholson, J.; Harris, K.; Miller, R.; Griffin, D.; Savell, J.W. Dry versus wet aging of beef: Retail cutting yields and consumer palatability evaluations of steaks from US Choice and US Select short loins. Meat Sci. 2008, 79, 631–639. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.J.; Kim, H.C.; Kim, H.-J.; Yun, Y.G.; Kim, K.T.; Choi, Y.I.; Jo, C. The effects of dry or wet aging on the quality of the longissimus muscle from 4-year-old Hanwoo cows and 28-month-old Hanwoo steers. Anim. Prod. Sci. 2018, 58, 2344. [Google Scholar] [CrossRef]
- Perry, N. Dry aging beef. Int. J. Gastron. Food Sci. 2012, 1, 78–80. [Google Scholar] [CrossRef]
- Dikeman, M.E.; Obuz, E.; Gök, V.; Akkaya, L.; Stroda, S. Effects of dry, vacuum, and special bag aging; USDA quality grade; and end-point temperature on yields and eating quality of beef Longissimus lumborum steaks. Meat Sci. 2013, 94, 228–233. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, J.W.; Kim, M.; Oh, H.; Yoon, Y.; Jo, C. Changes in microbial composition on the crust by different air flow velocities and their effect on sensory properties of dry-aged beef. Meat Sci. 2019, 153, 152–158. [Google Scholar] [CrossRef]
- Ryu, S.; Park, M.R.; Maburutse, B.E.; Lee, W.J.; Park, D.-J.; Cho, S.; Hwang, I.; Oh, S.; Kim, Y. Diversity and characteristics of the meat microbiological community on dry aged beef. J. Microbiol. Biotechnol. 2018, 28, 105–108. [Google Scholar] [CrossRef]
- Khan, M.I.; Jung, S.; Nam, K.C.; Jo, C. Postmortem Aging of Beef with a Special Reference to the Dry Aging. Food Sci. Anim. Resour. 2016, 36, 159–169. [Google Scholar] [CrossRef]
- Koutsidis, G.; Elmore, J.S.; Concha, M.J.O.; Campo, M.M.; Wood, J.; Mottram, D.S. Water-soluble precursors of beef flavour. Part II: Effect of post-mortem conditioning. Meat Sci. 2008, 79, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Napolitano, J.G.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Universal quantitative NMR analysis of complex natural samples. Curr. Opin. Biotechnol. 2013, 25, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Ko, Y.-J.; Kim, M.; Choe, J.; Yong, H.I.; Jo, C. Optimization of 1D 1H Quantitative NMR (Nuclear Magnetic Resonance) Conditions for Polar Metabolites in Meat. Food Sci. Anim. Resour. 2019, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Castejón, D.; Garcia-Segura, J.M.; Escudero, R.; Herrera, A.; Cambero, M. Metabolomics of meat exudate: Its potential to evaluate beef meat conservation and aging. Anal. Chim. Acta 2015, 901, 1–11. [Google Scholar] [CrossRef]
- Graham, S.F.; Farrell, D.; Kennedy, T.; Gordon, A.; Farmer, L.; Elliott, C.; Moss, B. Comparing GC–MS, HPLC and 1H NMR analysis of beef longissimus dorsi tissue extracts to determine the effect of suspension technique and ageing. Food Chem. 2012, 134, 1633–1639. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2012, 1, 92–107. [Google Scholar] [CrossRef]
- Ergon, R. Informative PLS score-loading plots for process understanding and monitoring. J. Process. Control. 2004, 14, 889–897. [Google Scholar] [CrossRef]
- Fu, Y.; Young, J.F.; Therkildsen, M. Bioactive peptides in beef: Endogenous generation through postmortem aging. Meat Sci. 2017, 123, 134–142. [Google Scholar] [CrossRef]
- Galgano, F.; Favati, F.; Bonadio, M.; Lorusso, V.; Romano, P. Role of biogenic amines as index of freshness in beef meat packed with different biopolymeric materials. Food Res. Int. 2009, 42, 1147–1152. [Google Scholar] [CrossRef]
- Shumilina, E.; Slizyte, R.; Mozuraityte, R.; Dykyy, A.; Stein, T.A.; Dikiy, A. Quality changes of salmon by-products during storage: Assessment and quantification by NMR. Food Chem. 2016, 211, 803–811. [Google Scholar] [CrossRef]
- Byun, J.-S.; Min, J.S.; Kim, I.S.; Kim, J.-W.; Chung, M.S.; Lee, M. Comparison of indicators of microbial quality of meat during aerobic cold storage. J. Food Prot. 2003, 66, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Prieto, B.; Eriksson, L.; Trygg, J. Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS). J. Chemom. 2014, 28, 623–632. [Google Scholar] [CrossRef]
- Škuta, C.; Bartunek, P.; Svozil, D. InCHlib—Interactive cluster heatmap for web applications. J. Cheminform. 2014, 6, 645. [Google Scholar] [CrossRef]
- Casal, M.; Paiva, S.; Queirós, O.; Soares-Silva, I.; Silva, I.J.S. Transport of carboxylic acids in yeasts. FEMS Microbiol. Rev. 2008, 32, 974–994. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jung, S.; Kim, H.-J.; Yong, H.I.; Nam, K.C.; Jo, C. Taste-active compound levels in Korean native chicken meat: The effects of bird age and the cooking process. Poult. Sci. 2015, 94, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Mottram, N.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Vermeulen, N.P.; Gänzle, M.G.; Vogel, R.F. Glutamine deamidation by cereal-associated lactic acid bacteria. J. Appl. Microbiol. 2007, 103, 1197–1205. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Stouthamer, A.; Bettenhaussen, C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. Biochim. Biophys. Acta Bioenerg. 1973, 301, 53–70. [Google Scholar] [CrossRef]
- Mei, L.; Cromwell, G.; Crum, A.; Decker, E.A. Influence of dietary β-alanine and histidine on the oxidative stability of pork. Meat Sci. 1998, 49, 55–64. [Google Scholar] [CrossRef]
- Hulankova, R.; Kameník, J.; Saláková, A.; Závodský, D.; Borilova, G. The effect of dry aging on instrumental, chemical and microbiological parameters of organic beef loin muscle. LWT-Food Sci. Technol. 2018, 89, 559–565. [Google Scholar] [CrossRef]
- Chan, K.M.; Decker, E.A.; Feustman, C. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994, 34, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Bae, Y.S.; Kim, H.-J.; Jayasena, D.D.; Lee, J.-H.; Park, H.-B.; Heo, K.N.; Jo, C. Carnosine, anserine, creatine, and inosine 5′-monophosphate contents in breast and thigh meats from 5 lines of Korean native chicken. Poult. Sci. 2013, 92, 3275–3282. [Google Scholar] [CrossRef]
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: An overview. Eur. Food Res. Technol. 2015, 241, 157–171. [Google Scholar] [CrossRef]
- Rebouche, C.J. Carnitine function and requirements during the life cycle. FASEB J. 1992, 6, 3379–3386. [Google Scholar] [CrossRef]
- Jung, S.; Bae, Y.S.; Yong, H.I.; Lee, H.J.; Seo, D.W.; Park, H.B.; Lee, J.-H.; Jo, C. Proximate composition, and L-carnitine and betaine contents in meat from Korean indigenous chicken. Asian-Australas. J. Anim. Sci. 2015, 28, 1760–1766. [Google Scholar] [CrossRef]
- Falony, G.; Vieira-Silva, S.; Raes, J. Microbiology meets big fata: The case of gut microbiota–derived trimethylamine. Annu. Rev. Microbiol. 2015, 69, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Jung, S.; Kim, S.H.; Kim, H.-J.; Alahakoon, A.U.; Lee, J.-H.; Jo, C. Endogenous functional compounds in Korean native chicken meat are dependent on sex, thermal processing and meat cut. J. Sci. Food Agric. 2014, 95, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Lowe, J.; Warnes, K.; Orme, C. The concentration of creatine in meat, offal and commercial dog food. Res. Vet. Sci. 1997, 62, 58–62. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Kemp, R.; Samuelsson, L.M. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016, 111, 168–176. [Google Scholar] [CrossRef]
- Yamaguchi, S. The synergistic taste effect of monosodium glutamate and disodium 5′-inosinate. J. Food Sci. 1967, 32, 473–478. [Google Scholar] [CrossRef]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, H.J.; Yoon, J.W.; Choe, J.; Jo, C. Electrical resistance and mold distribution on beef surface as indicators of dry aging. J. Food Process Eng. 2019, 42, e13122. [Google Scholar] [CrossRef]
- Kumar, S.; Punekar, N.S. The metabolism of 4-aminobutyrate (GABA) in fungi. Mycol. Res. 1997, 101, 403–409. [Google Scholar] [CrossRef]
- Inoue, K.; Shirai, T.; Ochiai, H.; Kasao, M.; Hayakawa, K.; Kimura, M.; Sansawa, H. Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 2003, 57, 490–495. [Google Scholar] [CrossRef]
- Lee, H.J.; Choe, J.-H.; Kim, K.T.; Oh, J.; Lee, D.G.; Kwon, K.M.; Choi, Y.I.; Jo, C. Analysis of low-marbled Hanwoo cow meat aged with different dry-aging methods. Asian-Australas. J. Anim. Sci. 2017, 30, 1733–1738. [Google Scholar] [CrossRef]
- Peromingo, B.; Sulyok, M.; Lemmens, M.; Rodríguez, A.; Rodríguez, M. Diffusion of mycotoxins and secondary metabolites in dry-cured meat products. Food Control 2019, 101, 144–150. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Contents | Aging Method | Aging Period | SEM 1 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | |||
| Alanine | Crust | 15.95 e | 27.26 d,x | 37.91 c,x | 47.19 b,x | 52.95 a,x | 0.852 |
| Dry | 15.95 d | 26.25 c,x | 26.52 c,y | 36.35 b,y | 39.93 a,y | 0.264 | |
| Wet | 15.95 d | 23.30 c,y | 27.35 b,y | 27.92 b,z | 29.42 a,z | 0.312 | |
| SEM 2 | 0.359 | 0.339 | 0.517 | 0.980 | |||
| Asparagine | Crust | 3.79 b | 4.69 ab,y | 3.30 b,y | 5.49 a,z | 3.95 b,z | 0.363 |
| Dry | 3.79 d | 7.07 c,x | 8.63 b,x | 13.23 a,x | 13.59 a,x | 0.388 | |
| Wet | 3.79 d | 6.94 c,x | 9.30 b,x | 11.02 a,y | 11.96 a,y | 0.481 | |
| SEM 2 | 0.448 | 0.445 | 0.518 | 0.428 | |||
| Aspartic acid | Crust | 1.39 d | 4.11 c | 13.37 b,x | 25.22 a,x | 25.15 a,x | 0.699 |
| Dry | 1.39 d | 3.77 c | 5.71 b,y | 7.87 a,y | 9.14 a,y | 0.585 | |
| Wet | 1.39 d | 3.54 c | 7.80 b,y | 8.66 ab,y | 9.82 a,y | 0.462 | |
| SEM 2 | 0.654 | 0.589 | 0.630 | 0.625 | |||
| Glutamic acid | Crust | 6.74 e | 27.25 d,x | 67.31 a,x | 62.59 b,x | 56.80 c,x | 0.844 |
| Dry | 6.74 e | 12.88 d,y | 18.53 c,y | 23.25 b,y | 30.44 a,z | 0.489 | |
| Wet | 6.74 e | 11.95 d,y | 17.84 c,y | 24.92 b,y | 35.83 a,y | 0.855 | |
| SEM 2 | 0.369 | 0.934 | 1.013 | 0.865 | |||
| Glutamine | Crust | 49.09 a | 34.14 b | 6.10 d,z | 8.12 c,z | 6.07 d,z | 0.553 |
| Dry | 49.09 a | 36.12 c | 42.98 b,x | 34.71 c,x | 35.46 c,y | 0.688 | |
| Wet | 49.09 a | 35.05 c | 35.21 c,y | 32.16 d,y | 39.22 b,x | 0.781 | |
| SEM 2 | 0.828 | 0.327 | 0.381 | 0.567 | |||
| Glycine | Crust | 25.09 d | 21.16 e,y | 35.55 c,x | 40.28 b,x | 44.72 a,x | 0.761 |
| Dry | 25.09 d | 25.83 d,x | 31.83 c,y | 34.15 b,y | 39.66 a,y | 0.450 | |
| Wet | 25.09 c | 21.65 d,y | 28.46 b,z | 30.40 b,z | 36.09 a,z | 0.702 | |
| SEM 2 | 0.832 | 0.702 | 0.664 | 0.700 | |||
| Isoleucine | Crust | 3.77 e | 10.08 d,x | 21.79 c,x | 30.62 b,x | 37.25 a,x | 0.616 |
| Dry | 3.77 e | 10.69 d,x | 13.58 c,y | 20.80 b,y | 23.60 a,y | 0.188 | |
| Wet | 3.77 e | 9.07 d,y | 13.93 c,y | 16.78 b,z | 18.32 a,z | 0.290 | |
| SEM 2 | 0.228 | 0.396 | 0.253 | 0.736 | |||
| Leucine | Crust | 7.21 e | 17.79 d,x | 31.11 c,x | 43.72 b,x | 50.38 a,x | 0.821 |
| Dry | 7.21 e | 17.76 d,x | 20.58 c,z | 30.07 b,y | 32.42 a,y | 0.301 | |
| Wet | 7.21 d | 15.83 c,y | 22.63 b,y | 25.70 a,z | 26.83 a,z | 0.519 | |
| SEM 2 | 0.257 | 0.569 | 0.580 | 0.989 | |||
| Methionine | Crust | 4.43 e | 12.34 d,y | 18.68 c,x | 23.50 b,x | 27.08 a,x | 0.408 |
| Dry | 4.43 c | 13.77 b,x | 14.56 b,z | 21.61 a,y | 21.67 a,y | 0.344 | |
| Wet | 4.43 d | 12.41 c,y | 16.51 b,y | 18.48 a,z | 16.75 b,z | 0.283 | |
| SEM 2 | 0.285 | 0.364 | 0.430 | 0.416 | |||
| Proline | Crust | 8.56 d | 9.89 d,y | 16.97 c,x | 23.66 b,x | 27.18 a,x | 0.627 |
| Dry | 8.56 d | 10.72 c,x | 11.23 c,z | 17.98 b,y | 19.30 a,y | 0.375 | |
| Wet | 8.56 d | 10.58 c,x | 12.89 b,y | 14.11 ab,z | 14.65 a,z | 0.435 | |
| SEM 2 | 0.188 | 0.353 | 0.491 | 0.683 | |||
| Serine | Crust | 6.61 e | 9.92 d,y | 18.18 c,x | 24.04 b,x | 27.00 a,x | 0.481 |
| Dry | 6.61 e | 11.73 d,x | 14.58 c,y | 19.93 b,y | 22.07 a,y | 0.155 | |
| Wet | 6.61 e | 10.19 d,y | 14.55 c,y | 16.32 b,z | 17.92 a,z | 0.322 | |
| SEM 2 | 0.358 | 0.321 | 0.309 | 0.511 | |||
| Taurine | Crust | 45.68 d | 56.38 b,x | 39.30 e,x | 48.02 c,x | 67.42 a,y | 0.620 |
| Dry | 45.68 c | 55.03 b,x | 36.44 e,y | 43.50 d,y | 71.26 a,x | 0.692 | |
| Wet | 45.68 a | 41.56 b,y | 34.27 c,y | 34.26 c,z | 33.98 c,z | 0.883 | |
| SEM 2 | 0.698 | 0.911 | 0.689 | 0.930 | |||
| Tryptophan | Crust | 3.91 c | 5.26 b,y | 5.29 b,y | 6.78 a,z | 5.76 b,z | 0.343 |
| Dry | 3.91 d | 6.86 c,x | 7.71 c,x | 10.92 b,x | 12.39 a,x | 0.303 | |
| Wet | 3.91 e | 6.45 d,x | 8.04 c,x | 9.32 b,y | 10.54 a,y | 0.232 | |
| SEM 2 | 0.347 | 0.220 | 0.274 | 0.414 | |||
| Valine | Crust | 3.18 e | 9.56 d,x | 17.94 c,x | 26.14 b,x | 30.92 a,x | 0.582 |
| Dry | 3.17 e | 9.88 d,x | 12.51 c,y | 19.48 b,y | 20.76 a,y | 0.256 | |
| Wet | 3.37 e | 8.99 d,y | 13.34 c,y | 16.06 b,z | 16.90 a,z | 0.263 | |
| SEM2 | 0.109 | 0.388 | 0.348 | 0.702 | |||
| Total free amino acids | Crust | 200.89 e | 280.30 d,x | 386.52 c,x | 478.71 b,x | 533.85 a,x | 6.282 |
| Dry | 200.89 e | 279.48 d,x | 301.81 c,y | 383.80 b,y | 446.23 a,y | 2.958 | |
| Wet | 200.89 e | 246.77 d,y | 300.74 c,y | 330.32 b,z | 360.88 a,z | 3.759 | |
| SEM 2 | 3.884 | 4.278 | 4.084 | 7.102 | |||
| Contents | Aging Method | Aging Period (Day) | SEM 1 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | |||
| Anserine | Crust | 19.93 c | 90.26 b,y | 95.08 b,x | 147.98 a,x | 96.89 b,x | 0.852 |
| Dry | 19.93 d | 100.21 a,x | 53.12 c,y | 98.30 a,y | 72.07 b,y | 0.264 | |
| Wet | 19.93 c | 92.04 a,y | 91.40 a,x | 78.30 b,z | 22.72 c,z | 0.312 | |
| SEM 2 | 2.438 | 3.932 | 3.193 | 5.267 | |||
| Betaine | Crust | 15.36 ab | 14.78 b | 16.34 ab,x | 17.15 ab,x | 17.67 a,x | 0.363 |
| Dry | 15.36 a | 14.76 ab | 13.58 b,y | 14.19 ab,y | 14.98 ab,xy | 0.388 | |
| Wet | 15.36 | 14.15 | 14.18 y | 13.08 y | 12.93 y | 0.481 | |
| SEM 2 | 0.302 | 0.637 | 0.546 | 0.873 | |||
| Carnitine | Crust | 39.61 c | 44.95 ab,x | 46.01 a,x | 45.35 ab,x | 42.70 b,x | 0.699 |
| Dry | 39.61 b | 43.41 a,x | 41.44 ab,y | 41.76 ab,y | 41.24 ab,x | 0.585 | |
| Wet | 39.61 a | 34.97 b,y | 34.87 b,z | 32.52 b,z | 32.47 b,y | 0.462 | |
| SEM 2 | 0.866 | 0.642 | 0.451 | 0.650 | |||
| Carnosine | Crust | 33.97 d | 418.83 b,z | 256.24 c,y | 498.79 a,y | 260.74 c,y | 0.844 |
| Dry | 33.97 e | 471.15 b,y | 205.82 d,z | 546.28 a,x | 297.89 c,x | 0.489 | |
| Wet | 33.97 e | 499.34 a,x | 476.09 b,x | 455.32 c,z | 53.02 d,z | 0.855 | |
| SEM 2 | 5.908 | 6.005 | 5.221 | 5.655 | |||
| Creatine/Phosphocreatine | Crust | 182.13 c | 215.28 b,y | 239.46 a,x | 205.85 bc,x | 192.74 b,c | 0.553 |
| Dry | 182.13 b | 227.47 a,x | 167.30 b,y | 150.83 b,y | 157.55 b,y | 0.688 | |
| Wet | 182.13 b | 211.49 a,y | 200.98 ab,xy | 202.34 ab,x | 197.01 ab,x | 0.781 | |
| SEM 2 | 3.806 | 12.983 | 6.496 | 9.142 | |||
| Contents | Aging Method | Aging Period (Day) | SEM 1 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | |||
| IMP | Crust | 135.21 a | 57.42 b,y | 46.52 c,y | 36.54 d,z | 10.22 e,z | 1.104 |
| Dry | 135.21 a | 99.78 b,x | 80.49 c,x | 66.71 d,x | 38.29 e,y | 1.291 | |
| Wet | 135.21 a | 102.60 b,x | 75.64 c,x | 56.68 d,y | 53.05 d,x | 1.330 | |
| SEM 2 | 1.154 | 1.612 | 1.094 | 0.953 | |||
| Inosine | Crust | 16.04 d | 27.48 a,x | 18.58 c,y | 20.48 b | 14.93 d,y | 0.476 |
| Dry | 16.04 d | 18.79 b,y | 17.59 c,y | 20.17 a | 17.74 c,x | 0.287 | |
| Wet | 16.04 c | 18.50 b,y | 20.24 a,x | 20.03 a | 16.81 c,x | 0.476 | |
| SEM 2 | 0.408 | 0.423 | 0.589 | 0.400 | |||
| Hypoxanthine | Crust | 12.54 e | 25.45 d,x | 37.67 c,x | 43.53 b,x | 46.64 a,x | 0.540 |
| Dry | 12.54 e | 22.72 d.y | 27.64 c,y | 33.51 b,y | 37.36 a,y | 0.880 | |
| Wet | 12.54 d | 21.15 c,z | 26.90 b,y | 32.18 a,y | 36.04 a,y | 1.415 | |
| SEM 2 | 0.502 | 1.006 | 0.253 | 0.765 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.C.; Baek, K.H.; Ko, Y.-J.; Lee, H.J.; Yim, D.-G.; Jo, C. Characteristic Metabolic Changes of the Crust from Dry-Aged Beef Using 2D NMR Spectroscopy. Molecules 2020, 25, 3087. https://doi.org/10.3390/molecules25133087
Kim HC, Baek KH, Ko Y-J, Lee HJ, Yim D-G, Jo C. Characteristic Metabolic Changes of the Crust from Dry-Aged Beef Using 2D NMR Spectroscopy. Molecules. 2020; 25(13):3087. https://doi.org/10.3390/molecules25133087
Chicago/Turabian StyleKim, Hyun Cheol, Ki Ho Baek, Yoon-Joo Ko, Hyun Jung Lee, Dong-Gyun Yim, and Cheorun Jo. 2020. "Characteristic Metabolic Changes of the Crust from Dry-Aged Beef Using 2D NMR Spectroscopy" Molecules 25, no. 13: 3087. https://doi.org/10.3390/molecules25133087
APA StyleKim, H. C., Baek, K. H., Ko, Y.-J., Lee, H. J., Yim, D.-G., & Jo, C. (2020). Characteristic Metabolic Changes of the Crust from Dry-Aged Beef Using 2D NMR Spectroscopy. Molecules, 25(13), 3087. https://doi.org/10.3390/molecules25133087






