High Preventive Effect of G2-S16 Anionic Carbosilane Dendrimer against Sexually Transmitted HSV-2 Infection
Abstract
1. Introduction
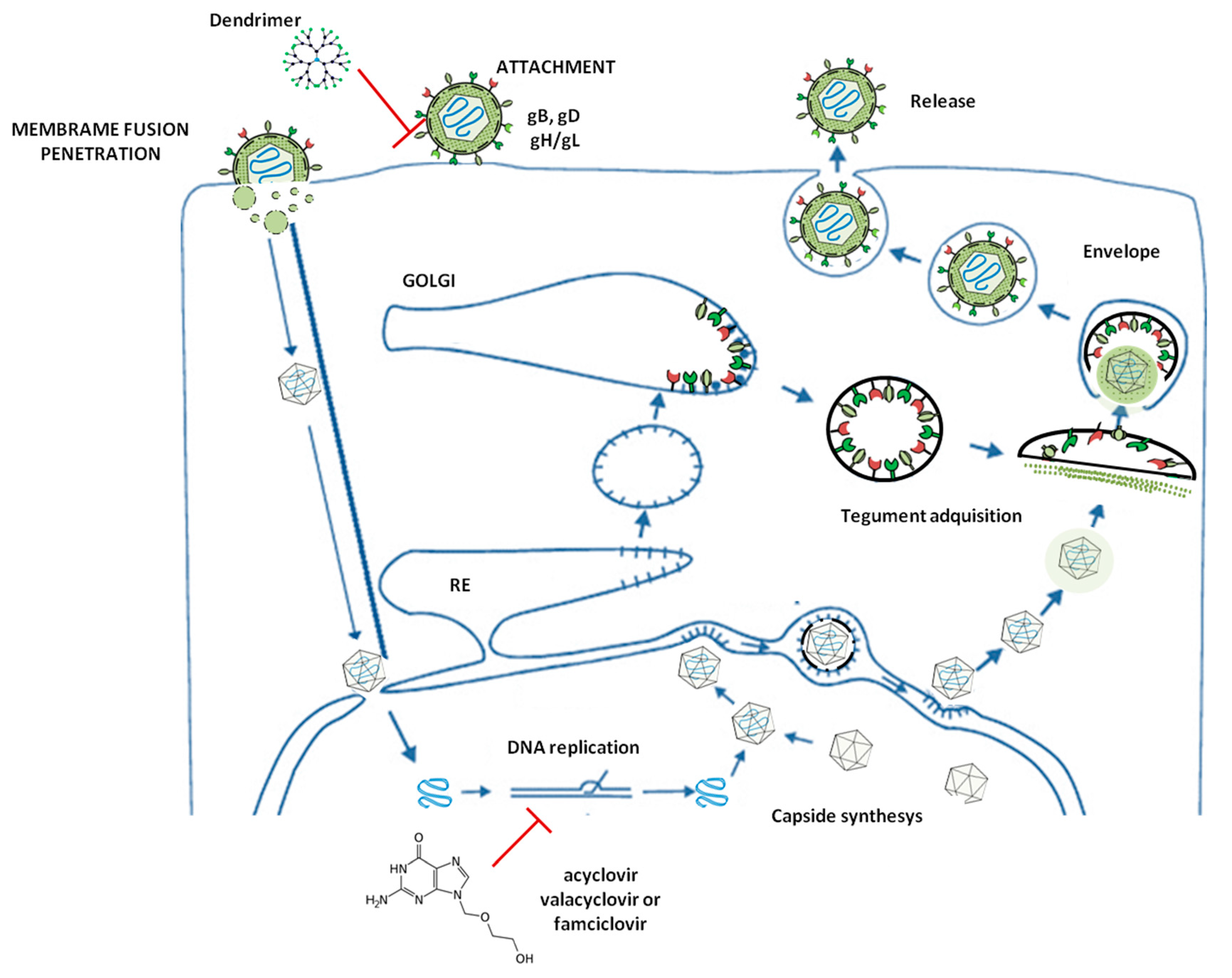
2. HSV-2 Infection Idiosyncrasy and HSV-2 Morphology and Structure
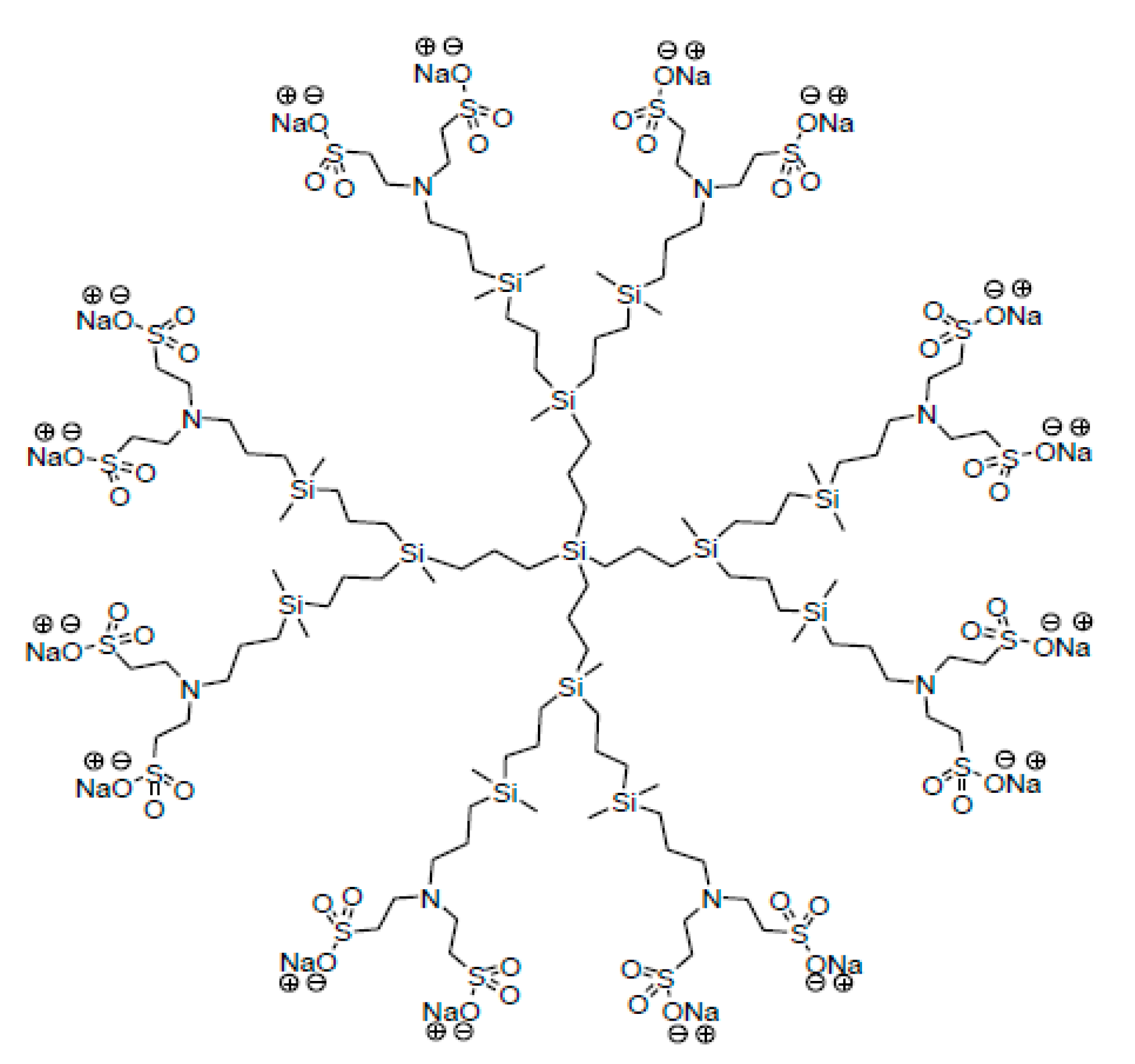
3. G2-S16 Anionic Carbosilane Dendrimer
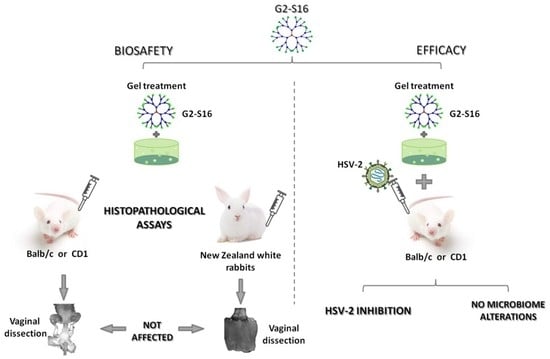
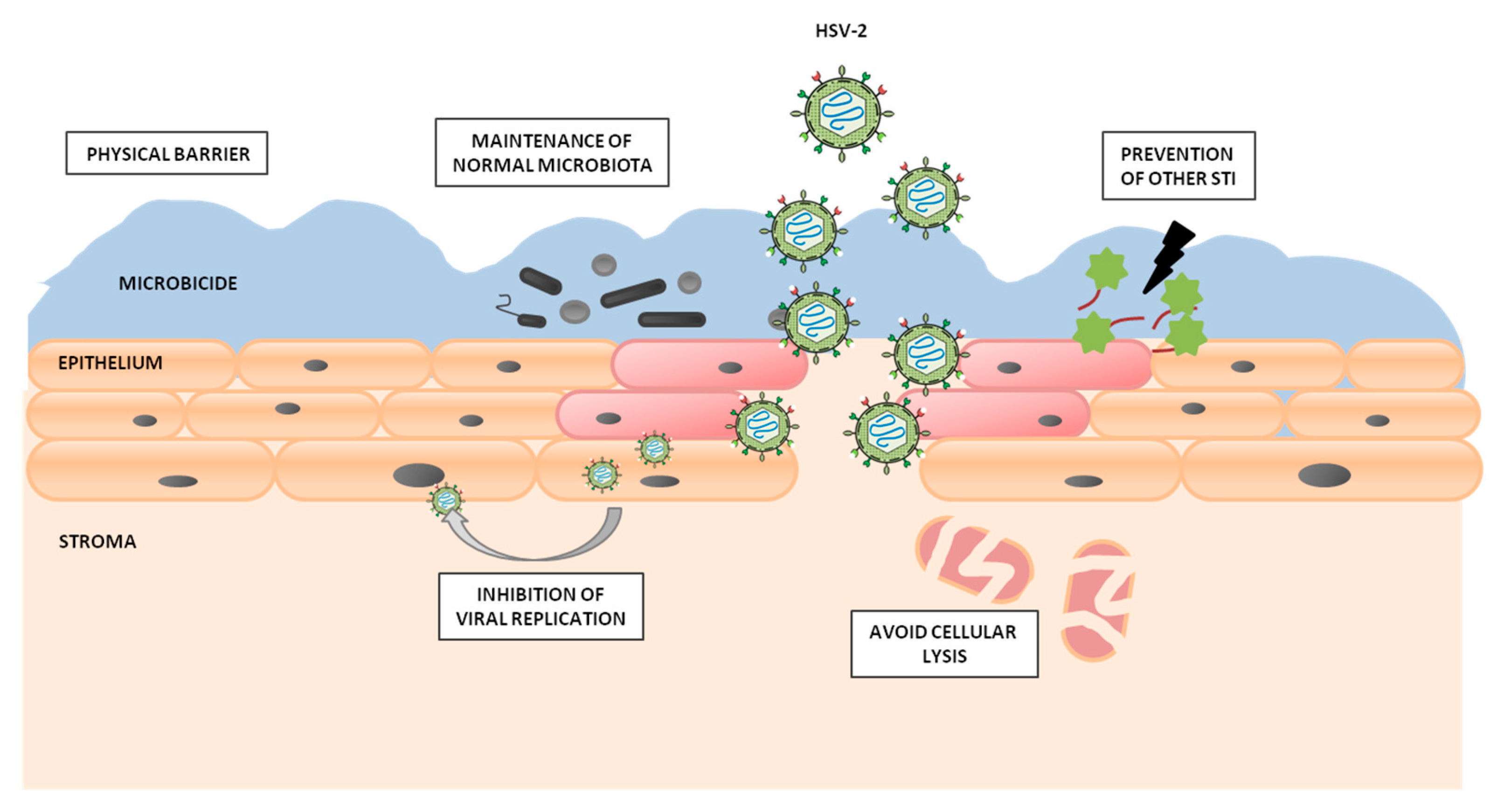
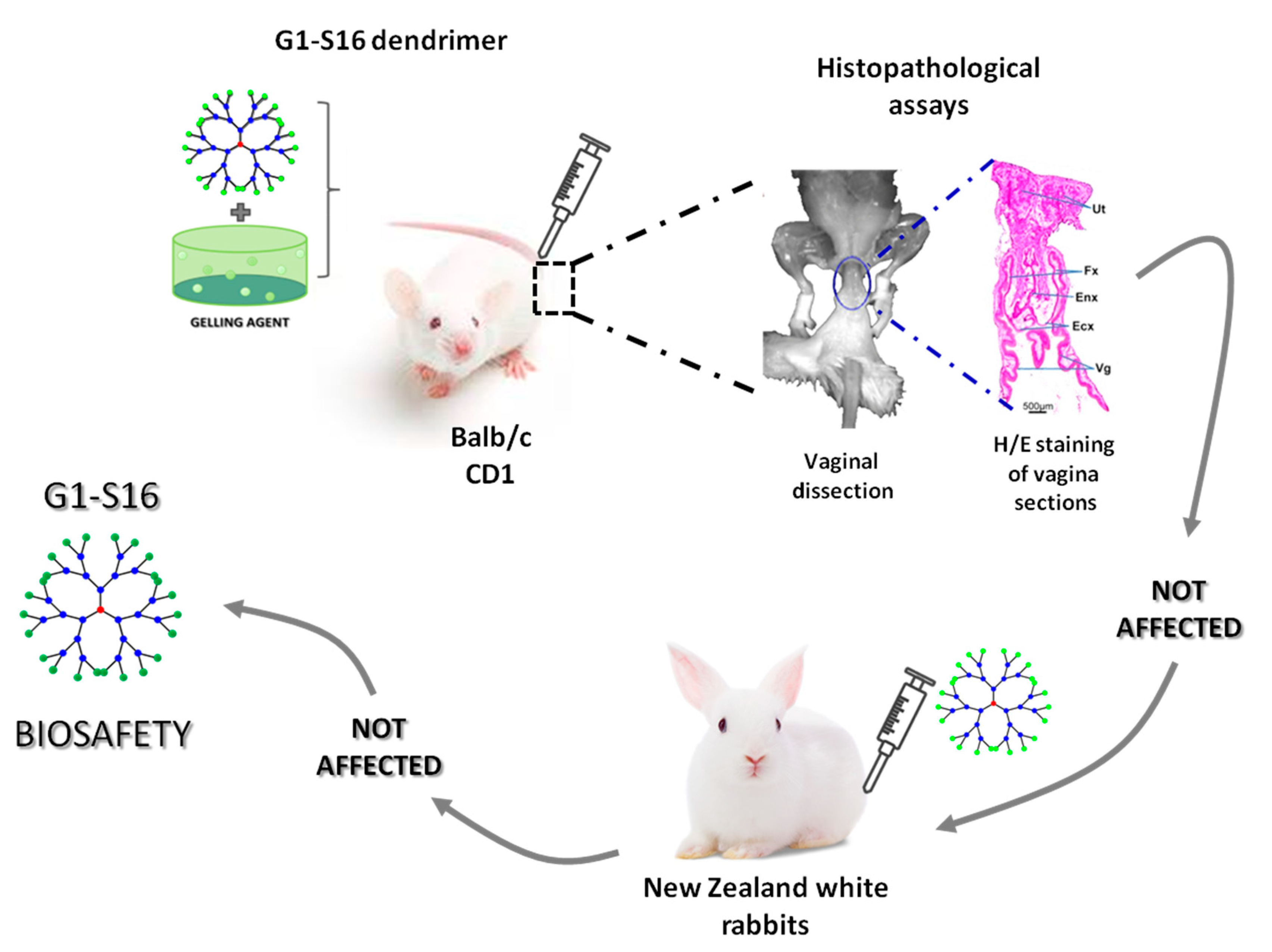
4. G2-S16 Anionic Carbosilane Dendrimer as an In Vivo Vaginal Microbicide
5. Prevention of Vaginal HSV-2 Infection in Presence of G2-S16 Anionic Carbosilane Dendrimer in Female Mice
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tomalia, D.A.; Fréchet, J.M. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Pol. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polim. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Tomalia, D.A. The dendritic state. Mater. Today 2005, 8, 34–46. [Google Scholar] [CrossRef]
- Tomalia, D.A. Dendrimer Molecules. Sci. Amer. 1995, 272, 62–66. [Google Scholar] [CrossRef]
- Tomalia, D.A. Starburst dendrimers—Nanoscopic supermolecules according to dendritic rules and principles. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 1996; Volume 101. [Google Scholar]
- Kaminskas, L.M.; Boyd, B.J.; Porter, C.J. Dendrimer pharmacokinetics: The effect of size, structure and surface characteristics on ADME properties. Nanomedicine 2011, 6, 1063–1084. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications--reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Int. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N. Dendrimer as nanocarrier for drug delivery. Prog. Poly. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Poly. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Amer. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Caminade, A.M.; Laurent, R.; Majoral, J.P. Characterization of dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2130–2146. [Google Scholar] [CrossRef] [PubMed]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Mecke, A.; Majoros, I.J.; Patri, A.K.; Baker, J.R., Jr.; Holl, M.M.; Orr, B.G. Lipid bilayer disruption by polycationic polymers: The roles of size and chemical functional group. Langmuir 2005, 21, 10348–10354. [Google Scholar] [CrossRef] [PubMed]
- Briz, V.; Serramia, M.J.; Madrid, R.; Hameau, A.; Caminade, A.M.; Majoral, J.P.; Munoz-Fernandez, M.A. Validation of a generation 4 phosphorus-containing polycationic dendrimer for gene delivery against HIV-1. Curr. Med. Chem. 2012, 19, 5044–5051. [Google Scholar] [CrossRef]
- Cena-Diez, R.; Vacas-Cordoba, E.; Garcia-Broncano, P.; de la Mata, F.J.; Gomez, R.; Maly, M.; Munoz-Fernandez, M.A. Prevention of vaginal and rectal herpes simplex virus type 2 transmission in mice: Mechanism of antiviral action. Int. J. Nanomed. 2016, 11, 2147–2162. [Google Scholar]
- Relano-Rodriguez, I.; Juarez-Sanchez, R.; Pavicic, C.; Munoz, E.; Munoz-Fernandez, M.A. Polyanionic carbosilane dendrimers as a new adjuvant in combination with latency reversal agents for HIV treatment. J. Nanobiotechnol. 2019, 17, 69. [Google Scholar] [CrossRef]
- Caminade, A.M. Phosphorus dendrimers for nanomedicine. Chem. Commun. (Camb.) 2017, 53, 9830–9838. [Google Scholar] [CrossRef]
- Galan, M.; Sanchez Rodriguez, J.; Jimenez, J.L.; Relloso, M.; Maly, M.; de la Mata, F.J.; Munoz-Fernandez, M.A.; Gomez, R. Synthesis of new anionic carbosilane dendrimers via thiol-ene chemistry and their antiviral behaviour. Organ. Biomol. Chem. 2014, 12, 3222–3237. [Google Scholar] [CrossRef]
- Caminade, A.M.; Zibarov, A.; Cueto Diaz, E.; Hameau, A.; Klausen, M.; Moineau-Chane Ching, K.; Majoral, J.P.; Verlhac, J.B.; Mongin, O.; Blanchard-Desce, M. Fluorescent phosphorus dendrimers excited by two photons: Synthesis, two-photon absorption properties and biological uses. Beilstein. J. Org. Chem. 2019, 15, 2287–2303. [Google Scholar] [CrossRef] [PubMed]
- Rasines, B.; Sanchez-Nieves, J.; Maiolo, M.; Maly, M.; Chonco, L.; Jimenez, J.L.; Munoz-Fernandez, M.A.; de la Mata, F.J.; Gomez, R. Synthesis, structure and molecular modelling of anionic carbosilane dendrimers. Dalton Trans. 2012, 41, 12733–12748. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, E.V.; Pion, M.; Rasines, B.; Filippini, D.; Komber, H.; Ionov, M.; Bryszewska, M.; Appelhans, D.; Munoz-Fernandez, M.A. Glycodendrimers as new tools in the search for effective anti-HIV DC-based immunotherapies. Nanomedicine 2013, 9, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Vacas-Cordoba, E.; Climent, N.; De La Mata, F.J.; Plana, M.; Gomez, R.; Pion, M.; Garcia, F.; Munoz-Fernandez, M.A. Dendrimers as nonviral vectors in dendritic cell-based immunotherapies against human immunodeficiency virus: Steps toward their clinical evaluation. Nanomedicine 2014, 9, 2683–2702. [Google Scholar] [CrossRef] [PubMed]
- Vacas-Cordoba, E.; Bastida, H.; Pion, M.; Hameau, A.; Ionov, M.; Bryszewska, M.; Caminade, A.M.; Majoral, J.P.; Munoz-Fernandez, M.A. HIV-antigens charged on phosphorus dendrimers as tools for tolerogenic dendritic cells-based immunotherapy. Curr. Med. Chem. 2014, 21, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Ciepluch, K.; Moreno, B.R.; Appelhans, D.; Sanchez-Nieves, J.; Gomez, R.; de la Mata, F.J.; Munoz-Fernandez, M.A.; Bryszewska, M. Biophysical characterization of glycodendrimers as nano-carriers for HIV peptides. Curr. Med. Chem. 2013, 20, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Ciepluch, K.; Klajnert, B.; Glinska, S.; Gomez-Ramirez, R.; de la Mata, F.J.; Munoz-Fernandez, M.A.; Bryszewska, M. Complexation of HIV derived peptides with carbosilane dendrimers. Colloids Surfaces B Biointerfaces 2013, 101, 236–242. [Google Scholar] [CrossRef]
- Tambe, V.; Thakkar, S.; Raval, N.; Sharma, D.; Kalia, K.; Tekade, R.K. Surface Engineered Dendrimers in siRNA Delivery and Gene Silencing. Curr. Pharm. Design 2017, 23, 2952–2975. [Google Scholar] [CrossRef]
- Klementieva, O.; Benseny-Cases, N.; Gella, A.; Appelhans, D.; Voit, B.; Cladera, J. Dense shell glycodendrimers as potential nontoxic anti-amyloidogenic agents in Alzheimer’s disease. Amyloid-dendrimer aggregates morphology and cell toxicity. Biomacromolecules 2011, 12, 3903–3909. [Google Scholar] [CrossRef]
- de Las Cuevas, N.; Garcia-Gallego, S.; Rasines, B.; de la Mata, F.J.; Guijarro, L.G.; Munoz-Fernandez, M.A.; Gomez, R. In vitro studies of water-stable cationic carbosilane dendrimers as delivery vehicles for gene therapy against HIV and hepatocarcinoma. Curr. Med. Chem. 2012, 19, 5052–5061. [Google Scholar] [CrossRef]
- Perise-Barrios, A.J.; Gomez, R.; Corbi, A.L.; de la Mata, J.; Dominguez-Soto, A.; Munoz-Fernandez, M.A. Use of carbosilane dendrimer to switch macrophage polarization for the acquisition of antitumor functions. Nanoscale 2015, 7, 3857–3866. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, B.; Franiak-Pietryga, I.; Pion, M.; Appelhans, D.; Munoz-Fernandez, M.A.; Voit, B.; Bryszewska, M.; Klajnert-Maculewicz, B. Toxicity and proapoptotic activity of poly(propylene imine) glycodendrimers in vitro: Considering their contrary potential as biocompatible entity and drug molecule in cancer. Int. J. Pharm. 2014, 461, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Deeksha; Tewari, D.; Gupta, G.; Awasthi, R.; Singh, H.; Pandey, P.; Chellappan, D.K.; Wadhwa, R.; Collet, T.; et al. Oligonucleotide therapy: An emerging focus area for drug delivery in chronic inflammatory respiratory diseases. Chem.-Biol. Inter. 2019, 308, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Riyaz, B.; Patil, A.; Kaur, A.; Kapoor, B.; Mishra, V. Novel drug delivery systems for NSAIDs in management of rheumatoid arthritis: An overview. Biomed. Pharm. 2018, 106, 1011–1023. [Google Scholar] [CrossRef]
- Mhlwatika, Z.; Aderibigbe, B.A. Application of Dendrimers for the Treatment of Infectious Diseases. Molecules 2018, 23, 2205. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomedicine 2007, 3, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The ’right’ size in nanobiotechnology. Nat. Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef]
- Svenson, S. The dendrimer paradox--high medical expectations but poor clinical translation. Chem. Soc. Rev. 2015, 44, 4131–4144. [Google Scholar] [CrossRef]
- Briz, V.; Sepulveda-Crespo, D.; Diniz, A.R.; Borrego, P.; Rodes, B.; de la Mata, F.J.; Gomez, R.; Taveira, N.; Munoz-Fernandez, M.A. Development of water-soluble polyanionic carbosilane dendrimers as novel and highly potent topical anti-HIV-2 microbicides. Nanoscale 2015, 7, 14669–14683. [Google Scholar] [CrossRef]
- Cena-Diez, R.; Martin-Moreno, A.; de la Mata, F.J.; Gomez-Ramirez, R.; Munoz, E.; Ardoy, M.; Munoz-Fernandez, M.A. G1-S4 or G2-S16 carbosilane dendrimer in combination with Platycodin D as a promising vaginal microbicide candidate with contraceptive activity. Int. J. Nanomed. 2019, 14, 2371–2381. [Google Scholar] [CrossRef]
- Guerrero-Beltran, C.; Rodriguez-Izquierdo, I.; Serramia, M.J.; Araya-Duran, I.; Marquez-Miranda, V.; Gomez, R.; de la Mata, F.J.; Leal, M.; Gonzalez-Nilo, F.; Munoz-Fernandez, M.A. Anionic Carbosilane Dendrimers Destabilize the GP120-CD4 Complex Blocking HIV-1 Entry and Cell to Cell Fusion. Bioconjug. Chem. 2018, 29, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Izquierdo, I.; Serramia, M.J.; Gomez, R.; De La Mata, F.J.; Bullido, M.J.; Muñoz-Fernández, M.A. Gold Nanoparticles Crossing Blood-Brain Barrier Prevent HSV-1 Infection and Reduce Herpes Associated Amyloid-betasecretion. J. Clin. Med. 2020, 9, 155. [Google Scholar]
- Moreno, S.; Sepulveda-Crespo, D.; de la Mata, F.J.; Gomez, R.; Munoz-Fernandez, M.A. New anionic carbosilane dendrons functionalized with a DO3A ligand at the focal point for the prevention of HIV-1 infection. Antiviral Res. 2017, 146, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Crespo, D.; Jimenez, J.L.; Gomez, R.; De La Mata, F.J.; Majano, P.L.; Munoz-Fernandez, M.A.; Gastaminza, P. Polyanionic carbosilane dendrimers prevent hepatitis C virus infection in cell culture. Nanomedicine 2017, 13, 49–58. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Lorente, R.; Leal, M.; Gomez, R.; De la Mata, F.J.; Jimenez, J.L.; Munoz-Fernandez, M.A. Synergistic activity profile of carbosilane dendrimer G2-STE16 in combination with other dendrimers and antiretrovirals as topical anti-HIV-1 microbicide. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 609–618. [Google Scholar] [CrossRef]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Archiv. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef]
- Marchi, S.; Trombetta, C.M.; Gasparini, R.; Temperton, N.; Montomoli, E. Epidemiology of herpes simplex virus type 1 and 2 in Italy: A seroprevalence study from 2000 to 2014. J. Prevent. Med. Hygiene 2017, 58, E27–E33. [Google Scholar]
- Pickering, J.M.; Whitworth, J.A.; Hughes, P.; Kasse, M.; Morgan, D.; Mayanja, B.; Van der Paal, L.; Mayaud, P. Aetiology of sexually transmitted infections and response to syndromic treatment in southwest Uganda. Sexual. Trans. Infect. 2005, 81, 488–493. [Google Scholar] [CrossRef][Green Version]
- Gupta, R.; Warren, T.; Wald, A. Genital herpes. Lancet 2007, 370, 2127–2137. [Google Scholar] [CrossRef]
- Shukla, D.; Spear, P.G. Herpesviruses and heparan sulfate: An intimate relationship in aid of viral entry. J. Clin. Invest. 2001, 108, 503–510. [Google Scholar] [CrossRef]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Schiffer, J.T.; Corey, L. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat. Med. 2013, 19, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Pinninti, S.G.; Kimberlin, D.W. Neonatal herpes simplex virus infections. Pediatric Clin. North Amer. 2013, 60, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Thellman, N.M.; Triezenberg, S.J. Herpes Simplex Virus Establishment, Maintenance, and Reactivation: In vitro Modeling of Latency. Pathogens 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Wald, A.; Patel, R.; Sacks, S.L.; Tyring, S.K.; Warren, T.; Douglas, J.M., Jr.; Paavonen, J.; Morrow, R.A.; Beutner, K.R.; et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. New Eng. J. Med. 2004, 350, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Warren, T.; Hamed, K.; Fife, K.; Wald, A. Famciclovir reduces viral mucosal shedding in HSV-seropositive persons. Sexual. Trans. Diseases 2007, 34, 900–907. [Google Scholar] [CrossRef]
- Polansky, H.; Javaherian, A.; Itzkovitz, E. Clinical study in genital herpes: Natural Gene-Eden-VIR/Novirin versus acyclovir, valacyclovir, and famciclovir. Drug Design Develop. Therapy 2016, 10, 2713–2722. [Google Scholar] [CrossRef][Green Version]
- Wald, A.; Timmler, B.; Magaret, A.; Warren, T.; Tyring, S.; Johnston, C.; Fife, K.; Selke, S.; Huang, M.L.; Stobernack, H.P.; et al. Effect of Pritelivir Compared With Valacyclovir on Genital HSV-2 Shedding in Patients With Frequent Recurrences: A Randomized Clinical Trial. Jama 2016, 316, 2495–2503. [Google Scholar] [CrossRef]
- Bartlett, B.L.; Tyring, S.K.; Fife, K.; Gnann, J.W., Jr.; Hadala, J.T.; Kianifard, F.; Berber, E. Famciclovir treatment options for patients with frequent outbreaks of recurrent genital herpes: The RELIEF trial. J. Clin. Virol. Offic. Pub. Pan Amer. Soc. Clin. Virol. 2008, 43, 190–195. [Google Scholar] [CrossRef]
- Romanowski, B.; Marina, R.B.; Roberts, J.N. Patients’ preference of valacyclovir once-daily suppressive therapy versus twice-daily episodic therapy for recurrent genital herpes: A randomized study. Sexual. Trans. Diseases 2003, 30, 226–231. [Google Scholar] [CrossRef]
- Wald, A.; Carrell, D.; Remington, M.; Kexel, E.; Zeh, J.; Corey, L. Two-day regimen of acyclovir for treatment of recurrent genital herpes simplex virus type 2 infection. Clin. Infectious Diseases Off. Pub. Infect. Diseases Soc. Amer. 2002, 34, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Sudomova, M.; Masarcikova, R. Herpes simplex virus infection: An overview of the problem, pharmacologic therapy and dietary measures. Ceska Slov. Farm. 2017, 66, 95–102. [Google Scholar]
- Mitterreiter, J.G.; Titulaer, M.J.; van Nierop, G.P.; van Kampen, J.J.; Aron, G.I.; Osterhaus, A.D.; Verjans, G.M.; Ouwendijk, W.J. Prevalence of Intrathecal Acyclovir Resistant Virus in Herpes Simplex Encephalitis Patients. PLoS ONE 2016, 11, e0155531. [Google Scholar] [CrossRef] [PubMed]
- Fife, K.H.; Crumpacker, C.S.; Mertz, G.J.; Hill, E.L.; Boone, G.S. Recurrence and resistance patterns of herpes simplex virus following cessation of > or = 6 years of chronic suppression with acyclovir. Acyclovir Study Group. J. Infect. Diseases 1994, 169, 1338–1341. [Google Scholar] [CrossRef]
- Perkins, N.; Nisbet, M.; Thomas, M. Topical imiquimod treatment of aciclovir-resistant herpes simplex disease: Case series and literature review. Sexual. Trans. Infect. 2011, 87, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Gupta, N. Clinical use of vaginal or rectally applied microbicides in patients suffering from HIV/AIDS. HIV/Aids 2013, 5, 295–307. [Google Scholar]
- Johnston, C.; Saracino, M.; Kuntz, S.; Magaret, A.; Selke, S.; Huang, M.L.; Schiffer, J.T.; Koelle, D.M.; Corey, L.; Wald, A. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: Three randomised, open-label, cross-over trials. Lancet 2012, 379, 641–647. [Google Scholar] [CrossRef]
- Johnston, C.; Zhu, J.; Jing, L.; Laing, K.J.; McClurkan, C.M.; Klock, A.; Diem, K.; Jin, L.; Stanaway, J.; Tronstein, E.; et al. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J. Virol. 2014, 88, 4921–4931. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef]
- Cortini, R.; Wilkie, N.M. Physical maps for HSV type 2 DNA with five restriction endonucleases. J. General Virol. 1978, 39, 259–280. [Google Scholar] [CrossRef]
- Roizman, B. The structure and isomerization of herpes simplex virus genomes. Cell 1979, 16, 481–494. [Google Scholar] [CrossRef]
- Davison, A.J.; Wilkie, N.M. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J. General Virol. 1981, 55, 315–331. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, D.J.; Rixon, F.J.; Davison, A.J. Topics in herpesvirus genomics and evolution. Virus Res. 2006, 117, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.; Park, P.J.; Zanotti, B.; Maus, E.; Volin, M.V.; Shukla, D.; Tiwari, V. Susceptibility of human iris stromal cells to herpes simplex virus 1 entry. J. Virol. 2013, 87, 4091–4096. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, E.; Forghieri, C.; Campadelli-Fiume, G. Cross talk among the glycoproteins involved in herpes simplex virus entry and fusion: The interaction between gB and gH/gL does not necessarily require gD. J. Virol. 2009, 83, 10752–10760. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.S.; Heldwein, E.E. Herpesvirus gB: A Finely Tuned Fusion Machine. Viruses 2015, 7, 6552–6569. [Google Scholar] [CrossRef]
- Heldwein, E.E. gH/gL supercomplexes at early stages of herpesvirus entry. Curr. Opin. Virol. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Krummenacher, C.; Baribaud, F.; Ponce de Leon, M.; Baribaud, I.; Whitbeck, J.C.; Xu, R.; Cohen, G.H.; Eisenberg, R.J. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology 2004, 322, 286–299. [Google Scholar] [CrossRef]
- Lazear, E.; Whitbeck, J.C.; Zuo, Y.; Carfi, A.; Cohen, G.H.; Eisenberg, R.J.; Krummenacher, C. Induction of conformational changes at the N-terminus of herpes simplex virus glycoprotein D upon binding to HVEM and nectin-1. Virology 2014, 448, 185–195. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Khanna, S.N. A Systematic Framework and Nanoperiodic Concept for Unifying Nanoscience: Hard/Soft Nanoelements, Superatoms, Meta-Atoms, New Emerging Properties, Periodic Property Patterns, and Predictive Mendeleev-like Nanoperiodic Tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef]
- Sanchez-Nieves, J.; Frutos, L.M.; Royo, P.; Castano, O.; Herdtweck, E.; Mosquera, M.E. Trapping unstable terminal M-O multiple bonds of monocyclopentadienyl niobium and tantalum complexes with Lewis acids. Inorgan. Chem. 2010, 49, 10642–10648. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Mourtas, S. Recent advances on anti-HIV vaginal delivery systems development. Adv. Drug Deliv. Rev. 2015, 92, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Heffron, R.; Kidoguchi, L.; Mugo, N.R.; Katabira, E.; Bukusi, E.A.; Asiimwe, S.; Haberer, J.E.; Morton, J.; Ngure, K.; et al. Integrated Delivery of Antiretroviral Treatment and Pre-exposure Prophylaxis to HIV-1-Serodiscordant Couples: A Prospective Implementation Study in Kenya and Uganda. PLoS Med. 2016, 13, e1002099. [Google Scholar] [CrossRef] [PubMed]
- Chonco, L.; Pion, M.; Vacas, E.; Rasines, B.; Maly, M.; Serramia, M.J.; Lopez-Fernandez, L.; De la Mata, J.; Alvarez, S.; Gomez, R.; et al. Carbosilane dendrimer nanotechnology outlines of the broad HIV blocker profile. J. Control. Release Off. J. Control. Release Soc. 2012, 161, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Vacas Cordoba, E.; Arnaiz, E.; Relloso, M.; Sanchez-Torres, C.; Garcia, F.; Perez-Alvarez, L.; Gomez, R.; de la Mata, F.J.; Pion, M.; Munoz-Fernandez, M.A. Development of sulphated and naphthylsulphonated carbosilane dendrimers as topical microbicides to prevent HIV-1 sexual transmission. Aids 2013, 27, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, K.; Aka-Any-Grah, A.; Dereuddre-Bosquet, N.; Martin, L.; Lievin-Le-Moal, V.; Le Grand, R.; Nicolas, V.; Gibellini, D.; Lembo, D.; Pous, C.; et al. Thermosensitive and mucoadhesive pluronic-hydroxypropylmethylcellulose hydrogel containing the mini-CD4 M48U1 is a promising efficient barrier against HIV diffusion through macaque cervicovaginal mucus. Antimicro. Agents Chemother. 2015, 59, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tian, B.; Agy, M.B.; Saifuddin, M.; Tsai, C.C. Macaca fascicularis are highly susceptible to an RT-SHIV following intravaginal inoculation: A new model for microbicide evaluation. J. Med. Primatol. 2009, 38, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J.; Desjardins, D.; Dereuddre-Bosquet, N.; Brochard, P.; Perrot, L.; Pruvost, A.; Le Grand, R.; Lagatie, O.; Vanhooren, L.; Feyaerts, M.; et al. Pre-clinical development of a combination microbicide vaginal ring containing dapivirine and darunavir. J. Antimicrob. Chemother. 2014, 69, 2477–2488. [Google Scholar] [CrossRef]
- McGowan, I.; Gomez, K.; Bruder, K.; Febo, I.; Chen, B.A.; Richardson, B.A.; Husnik, M.; Livant, E.; Price, C.; Jacobson, C. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). Aids 2011, 25, 1057–1064. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Serramia, M.J.; Tager, A.M.; Vrbanac, V.; Gomez, R.; De La Mata, F.J.; Jimenez, J.L.; Munoz-Fernandez, M.A. Prevention vaginally of HIV-1 transmission in humanized BLT mice and mode of antiviral action of polyanionic carbosilane dendrimer G2-S16. Nanomedicine 2015, 11, 1299–1308. [Google Scholar] [CrossRef]
- Bunge, K.E.; Levy, L.; Szydlo, D.W.; Zhang, J.; Gaur, A.H.; Reirden, D.; Mayer, K.H.; Futterman, D.; Hoesley, C.; Hillier, S.L.; et al. Brief Report: Phase IIa Safety Study of a Vaginal Ring Containing Dapivirine in Adolescent Young Women. J. Acquir. Immune. Defic. Syndr. 2020, 83, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, K.; Iannelli, F.; Cuppone, A.M.; Desjardins, D.; Caldwell, A.; Dereuddre-Bosquet, N.; Scala, C.; Smith, K.A.; Mukhopadya, I.; Frank, B.; et al. In vivo Modulation of Cervicovaginal Drug Transporters and Tissue Distribution by Film-Released Tenofovir and Darunavir for Topical Prevention of HIV-1. Mol. Pharm. 2020, 17, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, R.K.; Boyd, P.J.; McCoy, C.F.; Murphy, D.J. Microbicide vaginal rings: Technological challenges and clinical development. Adv. Drug Deliv. Rev. 2016, 103, 33–56. [Google Scholar] [CrossRef]
- Nunes, R.; Sarmento, B.; das Neves, J. Formulation and delivery of anti-HIV rectal microbicides: Advances and challenges. J. Control. Release Off. J. Control. Release Soc. 2014, 194, 278–294. [Google Scholar] [CrossRef]
- Raymond, E.; Alvarado, G.; Ledesma, L.; Diaz, S.; Bassol, S.; Morales, E.; Fernandez, V.; Carlos, G. Acceptability of two spermicides in five countries. Contraception 1999, 60, 45–50. [Google Scholar] [CrossRef]
- Rodriguez-Izquierdo, I.; Natalia, C.; Garcia, F.; Los Angeles Munoz-Fernandez, M. G2-S16 sulfonate dendrimer as new therapy for treatment failure in HIV-1 entry inhibitors. Nanomedicine 2019, 14, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Cena-Diez, R.; Garcia-Broncano, P.; de la Mata, F.J.; Gomez, R.; Munoz-Fernandez, M.A. Efficacy of HIV antiviral polyanionic carbosilane dendrimer G2-S16 in the presence of semen. Int. J. Nanomed. 2016, 11, 2443–2450. [Google Scholar]
- Cena-Diez, R.; Garcia-Broncano, P.; Javier de la Mata, F.; Gomez, R.; Resino, S.; Munoz-Fernandez, M. G2-S16 dendrimer as a candidate for a microbicide to prevent HIV-1 infection in women. Nanoscale 2017, 9, 9732–9742. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Cena-Diez, R.; Jimenez, J.L.; Angeles Munoz-Fernandez, M. Mechanistic Studies of Viral Entry: An Overview of Dendrimer-Based Microbicides As Entry Inhibitors Against Both HIV and HSV-2 Overlapped Infections. Med. Res. Rev. 2017, 37, 149–179. [Google Scholar] [CrossRef]
- McGowan, I.; Cranston, R.D.; Duffill, K.; Siegel, A.; Engstrom, J.C.; Nikiforov, A.; Jacobson, C.; Rehman, K.K.; Elliott, J.; Khanukhova, E.; et al. A Phase 1 Randomized, Open Label, Rectal Safety, Acceptability, Pharmacokinetic, and Pharmacodynamic Study of Three Formulations of Tenofovir 1% Gel (the CHARM-01 Study). PLoS ONE 2015, 10, e0125363. [Google Scholar] [CrossRef]
- Winceslaus, S.J. Dapivirine Vaginal Ring for HIV-1 Prevention. New Eng. J. Med. 2017, 376, 994–995. [Google Scholar] [PubMed]
- Reddy, K.; Kelly, C.; Brown, E.R.; Jeenarain, N.; Naidoo, L.; Siva, S.; Bekker, L.G.; Nair, G.; Makanani, B.; Chinula, L.; et al. Use of the dapivirine vaginal ring and effect on cervical cytology abnormalities. Aids 2020, 34, 559–567. [Google Scholar] [CrossRef]
- Katz, A.W.K.; Naidoo, K.; Reddy, K.; Chitukuta, M.; Nabukeera, J.; Siva, S.; Zimba, C.; Montgomery, E.T. The Power of the Shared Experience: MTN-020/ASPIRE Trial Participants’ Descriptions of Peer Influence on Acceptability of and Adherence to the Dapivirine Vaginal Ring for HIV Prevention. AIDS Behav. 2020. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltran, C.; Prieto, A.; Leal, M.; Jimenez, J.L.; Munoz-Fernandez, M.A. Combination of G2-S16 dendrimer/dapivirine antiretroviral as a new HIV-1 microbicide. Future Med. Chem. 2019, 11, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Cheu, R.; Birse, K.; Zevin, A.S.; Perner, M.; Noel-Romas, L.; Grobler, A.; Westmacott, G.; Xie, I.Y.; Butler, J.; et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017, 356, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Velloza, J.; Heffron, R. The Vaginal Microbiome and its Potential to Impact Efficacy of HIV Pre-exposure Prophylaxis for Women. Curr. HIV/AIDS Rep. 2017, 14, 153–160. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Izquierdo, I.; Gasco, S.; Muñoz-Fernández, M.A. High Preventive Effect of G2-S16 Anionic Carbosilane Dendrimer against Sexually Transmitted HSV-2 Infection. Molecules 2020, 25, 2965. https://doi.org/10.3390/molecules25132965
Rodriguez-Izquierdo I, Gasco S, Muñoz-Fernández MA. High Preventive Effect of G2-S16 Anionic Carbosilane Dendrimer against Sexually Transmitted HSV-2 Infection. Molecules. 2020; 25(13):2965. https://doi.org/10.3390/molecules25132965
Chicago/Turabian StyleRodriguez-Izquierdo, Ignacio, Samanta Gasco, and Maria Angeles Muñoz-Fernández. 2020. "High Preventive Effect of G2-S16 Anionic Carbosilane Dendrimer against Sexually Transmitted HSV-2 Infection" Molecules 25, no. 13: 2965. https://doi.org/10.3390/molecules25132965
APA StyleRodriguez-Izquierdo, I., Gasco, S., & Muñoz-Fernández, M. A. (2020). High Preventive Effect of G2-S16 Anionic Carbosilane Dendrimer against Sexually Transmitted HSV-2 Infection. Molecules, 25(13), 2965. https://doi.org/10.3390/molecules25132965