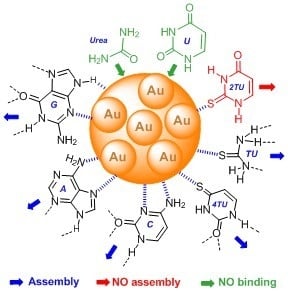
Gold Nanoparticles as Colorimetric Sensors for the Detection of DNA Bases and Related Compounds
Abstract
1. Introduction
2. Results and Discussion
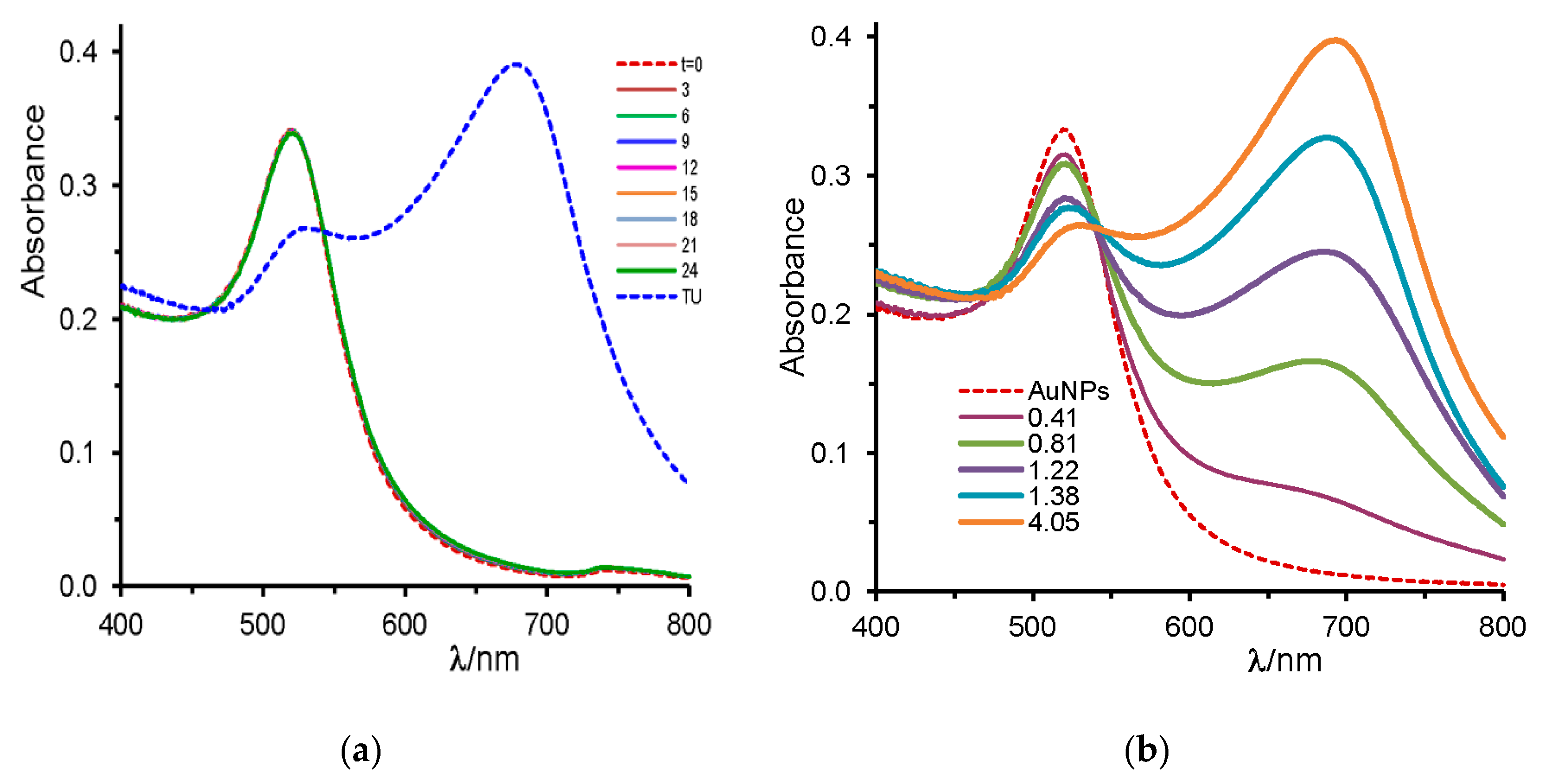
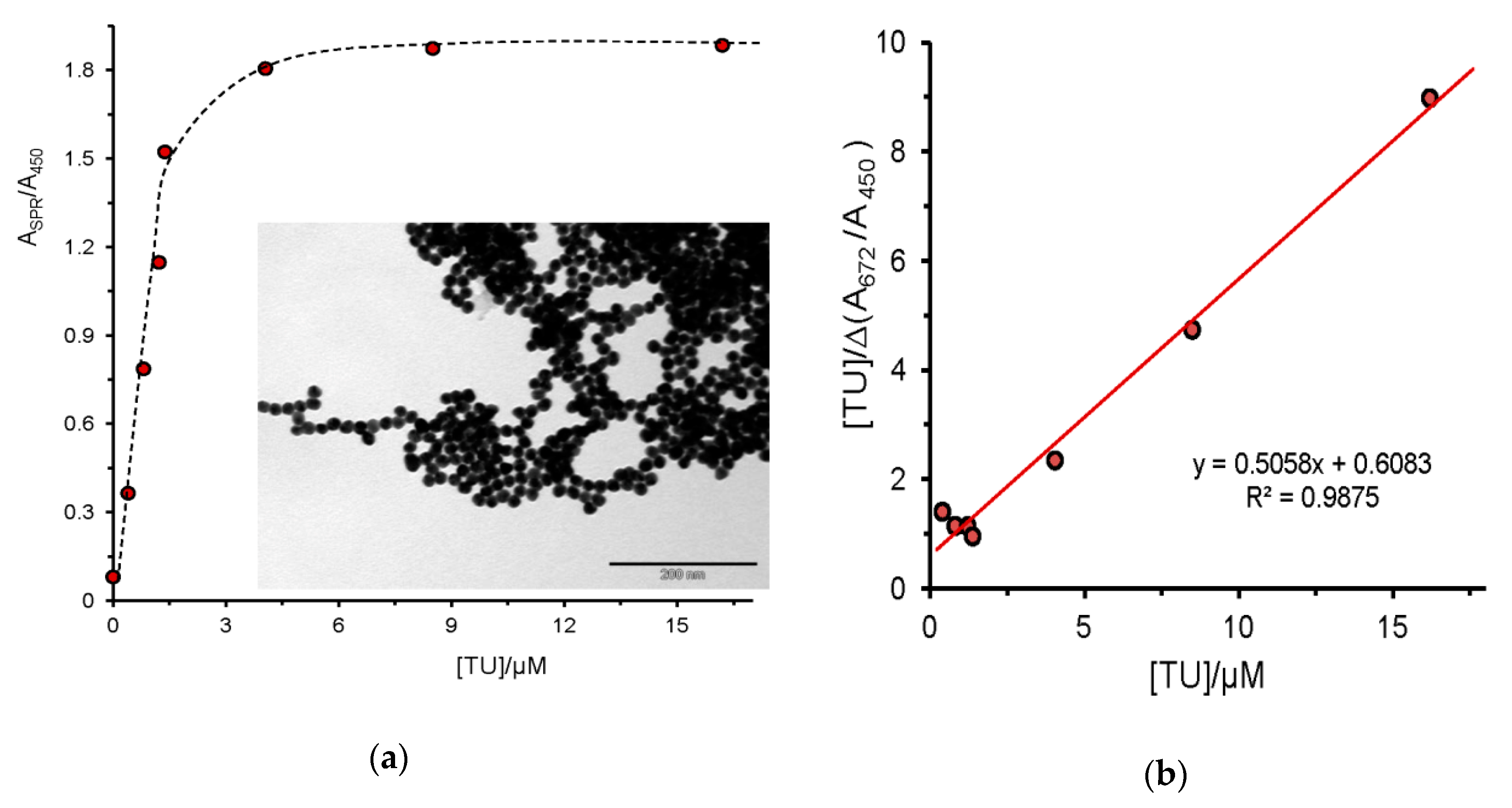
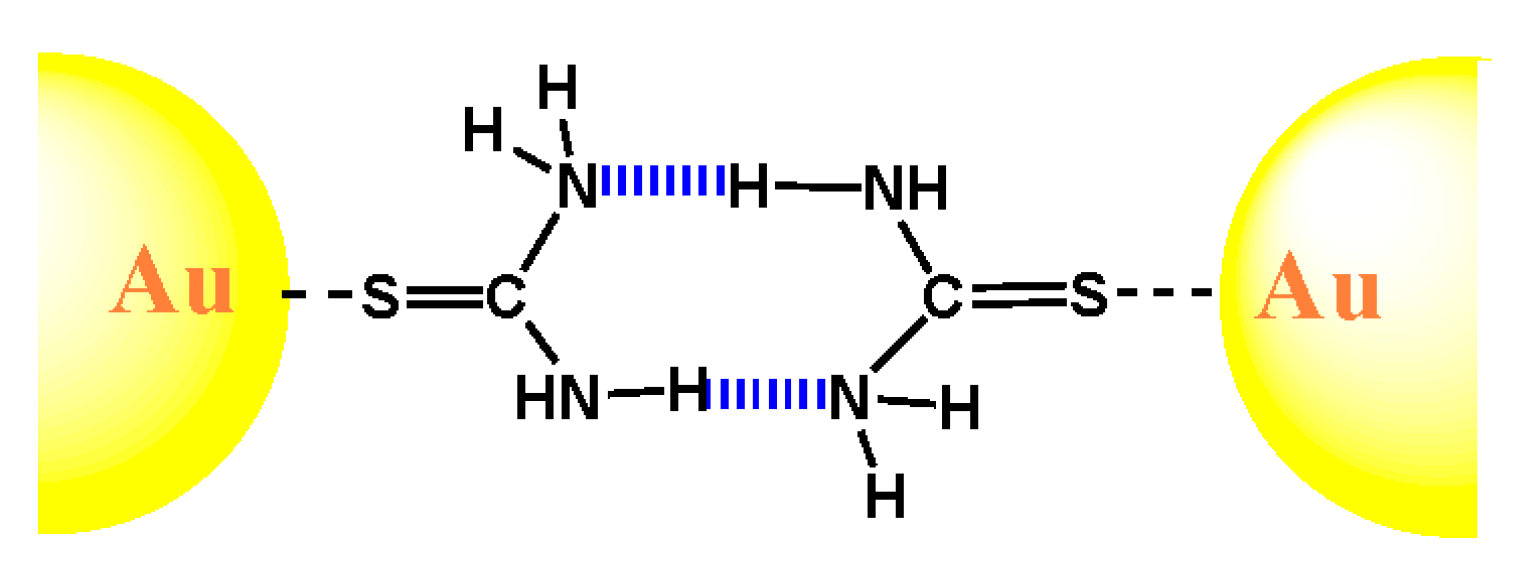
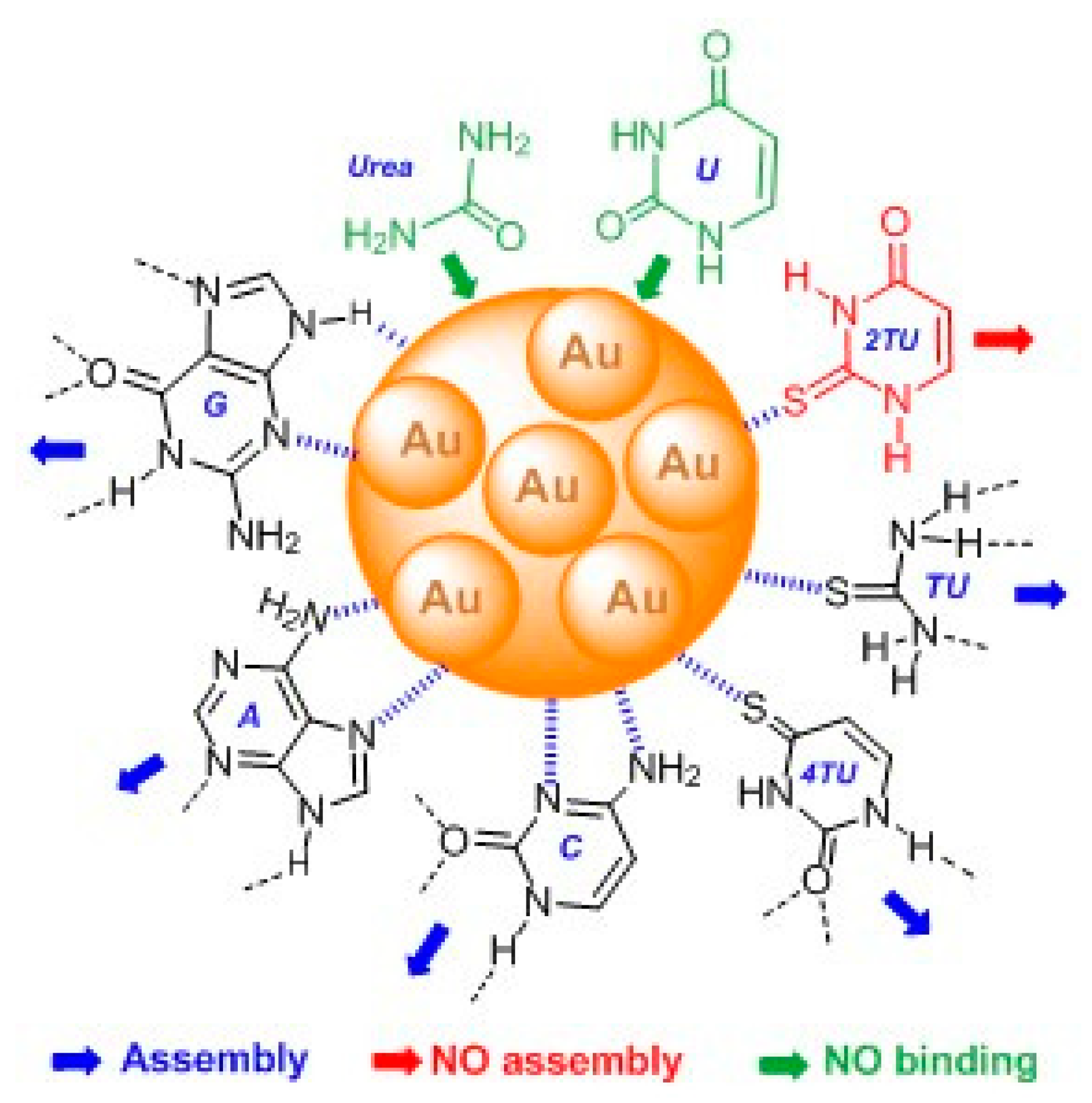
2.1. Urea and Thiourea
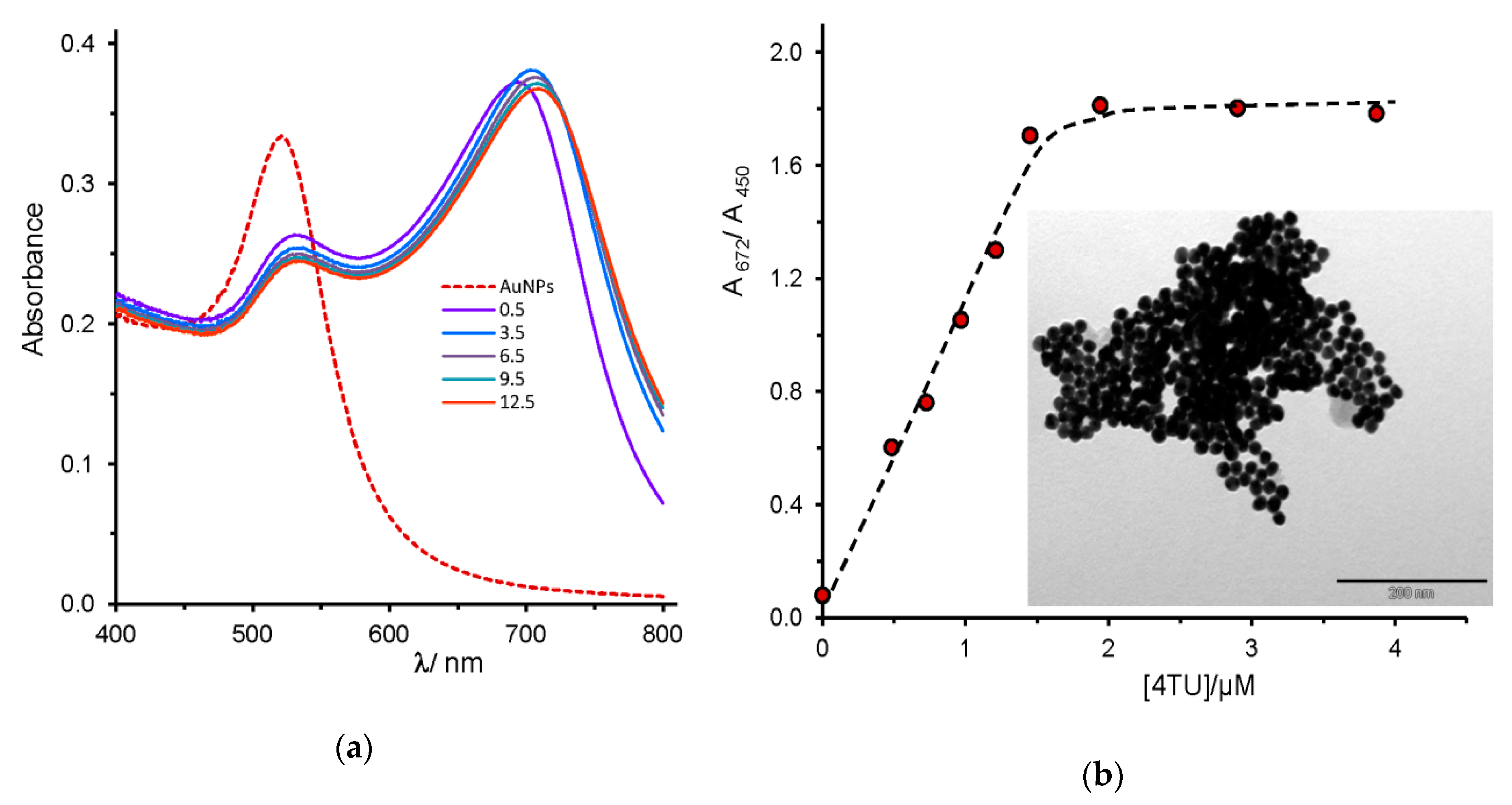
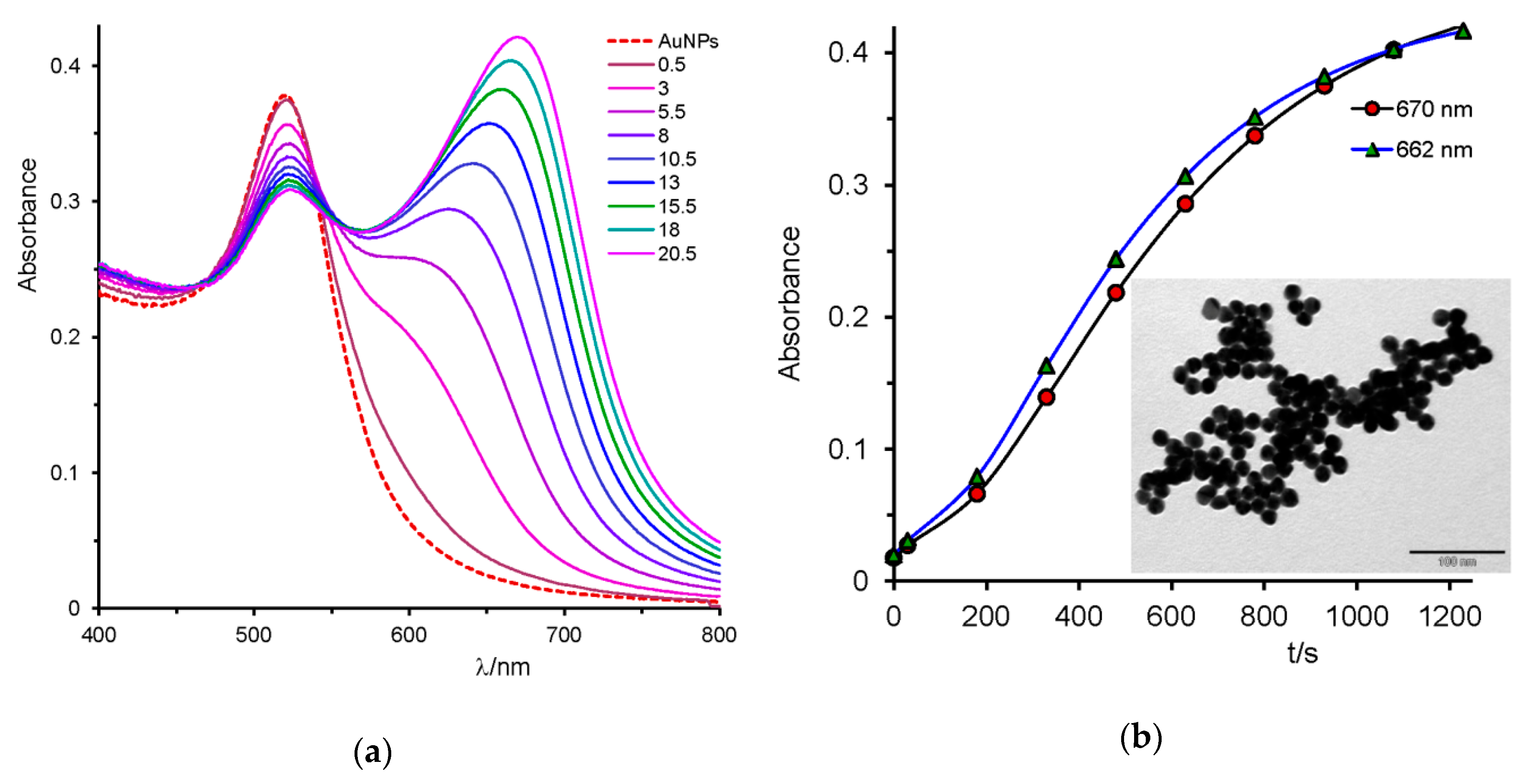
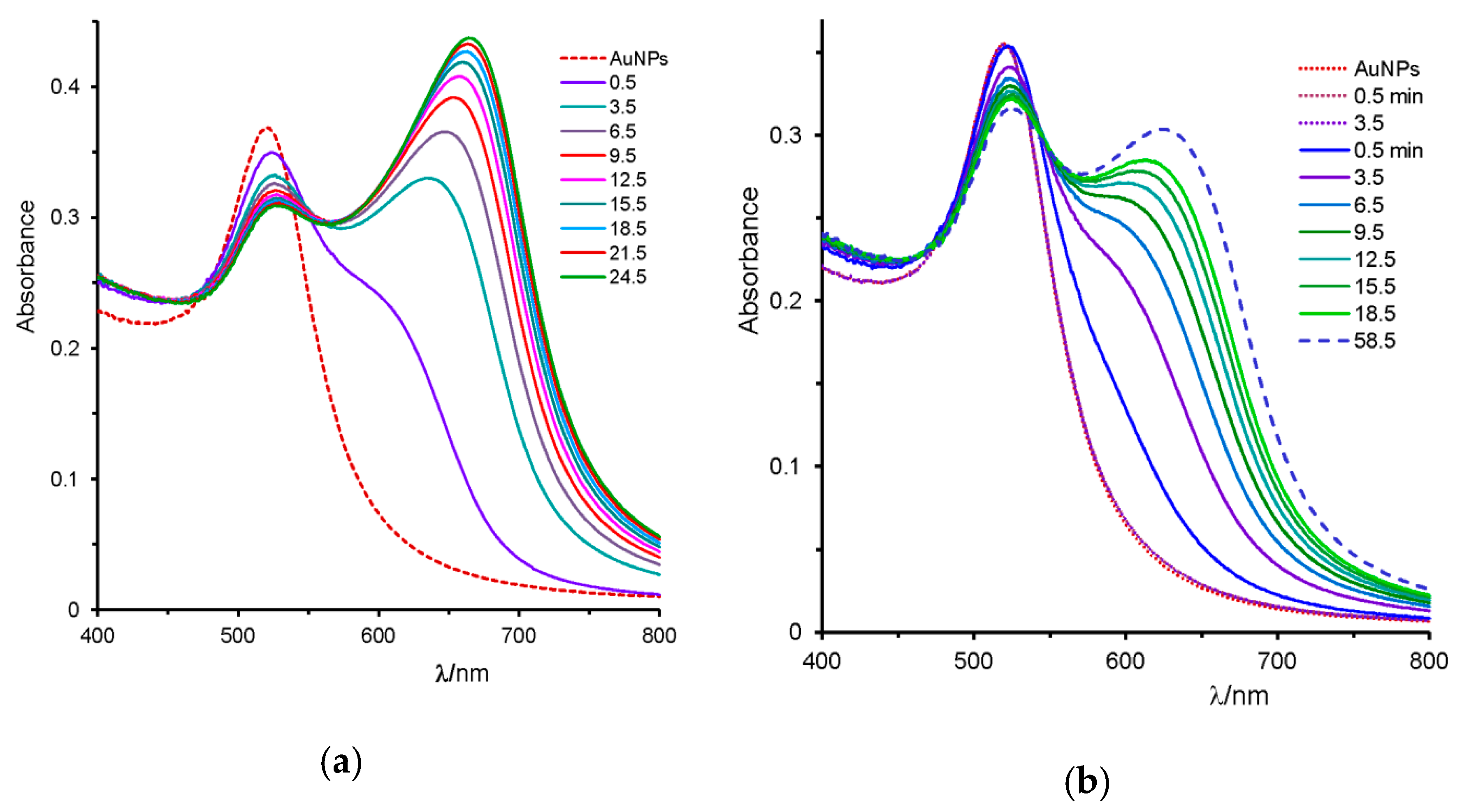
2.2. Uracil, 2-Thiouracil, and 4-Thiouracil
2.3. Cytosine, Adenine, and Guanine
3. Materials and Methods
3.1. Materials
3.2. Synthesis of AuNPs
3.3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Liu, J. Interface-driven hybrid materials based on DNA-functionalized gold nanoparticles. Matter 2019, 1, 825–847. [Google Scholar] [CrossRef]
- Carnerero, J.M.; Jimenez-Ruiz, A.; Castilli, P.M.; Prado-Gotor, R. Covalent and non-covalent DNA-gold nanoparticle interactions: New avenues of research. ChemPhysChem 2017, 18, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Montané, X.; Bajek, A.; Roszkowski, K.; Montornés, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for Cancer Therapy. Molecules 2020, 25, 1605. [Google Scholar] [CrossRef] [PubMed]
- Jans, H.; Huo, Q. Gold nanoparticle-enabled biological and chemical detection analysis. Chem. Soc. Rev. 2012, 41, 2849–2866. [Google Scholar] [CrossRef] [PubMed]
- Saka, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar]
- Liu, J. Adsorption of DNA onto gold nanoparticles and grapheme oxide: Surface science and applications. Phys. Chem. Chem. Phys. 2012, 14, 10485–10496. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Borse, S.; Joshi, S.; Khan, A. Enhanced in vitro cytotoxicity and cellular uptake of DNA bases functionalized gold nanoparticles in HeLa cell lines. RSC Adv. 2015, 5, 13402–13410. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kinling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.; Coronado, E.; Zhao, L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.-Y.; McLellan, J.; Siekkinen, A.R.; Xia, Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef] [PubMed]
- Lim, I.-I.S.; Ip, W.; Crew, E.; Njoki, P.M.; Mott, D.; Zhong, C.-J.; Pan, Y.; Zhou, Z. Homocysteine-mediated reactivity and assembly of gold nanoparticles. Langmuir 2007, 23, 826–833. [Google Scholar] [CrossRef]
- Zhang, F.X.; Han, I.; Israel, I.B.; Daras, J.G.; Maye, M.M.; Ly, N.K.; Zhong, C.-J. Colorimetric detection of thiol-containing amino acids using gold nanoparticles. Analyst 2002, 127, 462–465. [Google Scholar] [CrossRef]
- Li, L.; Li, B. Sensitive colorimetric detection of cysteine using gold nanoparticles as colorimetric probes. Analyst 2009, 134, 1361–1365. [Google Scholar] [CrossRef]
- Hu, B.; Cao, X.; Zhang, P. Selective colorimetric detection of glutathione based on quasi-stable gold nanoparticles assembly. New J. Chem. 2013, 37, 3853–3856. [Google Scholar] [CrossRef]
- Basu, S.; Panigrahi, S.; Praharaj, S.; Ghosh, S.K.; Pande, S.; Jana, S.; Pal, T. Dipole–dipole plasmon interactions in self-assembly of gold organosol induced by glutathione. New J. Chem. 2006, 30, 1333–1339. [Google Scholar] [CrossRef]
- Filbrun, S.L.; Driskell, J.D. A fluorescence-based method to directly quantify antibodies immobilized on gold nanoparticles. Analyst 2016, 141, 3851–3857. [Google Scholar] [CrossRef]
- Zakaria, H.M.; Shah, A.; Konieczny, M.; Hoffmann, J.A.; Nijdam, A.J.; Reeves, M.E. Small molecule- and amino acid-induced aggregation of gold nanoparticles. Langmuir 2013, 29, 7661–7673. [Google Scholar] [CrossRef]
- Iglesias, E.; Prado-Gotor, R. Interaction of gold nanoparticles mediated by captopril and S-nitrosocaptopril: The effect of manganese ions in mild acid medium. Phys. Chem. Chem. Phys. 2015, 17, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Cobley, C.M.; Chen, J.; Cho, E.C.; Wang, L.V.; Xia, Y. Gold nanostructures: A class of multifunctional materials for biomedical applications. Chem. Soc. Rev. 2011, 40, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Izatt, R.M.; Christensen, J.J.; Rytting, J.H. Sizes and thermodynamic quantities associated with proton and metal ions interaction with ribonucleic acid, deoxyribonucleic acid, and their constituent bases, nucleosides, and nucleotides. Chem. Rev. 1971, 71, 439–481. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R. Strengths or neutral organic bases. In The Proton: Applications to Organic Chemistry; Academic Press: Orlando, FL, USA, 1985; pp. 87–145. [Google Scholar]
- Shugar, D.; Fox, J.J. Absoption spectra and structures of 2-thiouracil derivatives as a function of pH. Bull. Soc. Chim. Belg. 1952, 61, 293–309. [Google Scholar] [CrossRef]
- Verdolino, V.; Cammi, R.; Munk, B.H.; Schlegel, H.B. Calculation of pKa values of nucleobases and the guanine oxidation products guanidinohydantoin and spiroiminodihydantoin using density functional theory and a polarizable continuum model. J. Phys. Chem. B 2008, 112, 16860–16873. [Google Scholar] [CrossRef]
- Jang, Y.H.; Goddard, W.A.; Noyes, K.T.; Sowers, L.C.; Hwang, S.; Chung, D.S. pKa Values of guanine in water: Density functional theory calculations combined with Poisson-Boltzmann continuum solvation model. J. Phys. Chem. B 2003, 107, 344–357. [Google Scholar] [CrossRef]
- Close, D.M. Calculated pKa’s of DNA base radical ions. J. Phys. Chem. A 2013, 117, 473–480. [Google Scholar] [CrossRef]
- Les, A.; Adamowicz, L. Tautomerism of 2- and 4-thiouracil. Ab initio theoretical study. J. Am. Chem. Soc. 1990, 112, 1504–1509. [Google Scholar] [CrossRef]
- Psoda, A.; Kazimierczuk, Z.; Shugar, D. Structure and tautomerism of the neutral and monoanionic forms of 4-thiouracil derivatives. J. Am. Chem. Soc. 1974, 96, 6832–6839. [Google Scholar] [CrossRef]
- Jang, Y.H.; Sowers, L.C.; Çagin, T.; Goddard, W.A. First principles calculations of pKa values for 5-substituted uracils. J. Phys. Chem. A 2001, 105, 274–280. [Google Scholar] [CrossRef]
- Connors, K.A. Binding constants. In Binding Constants. The Measurement of Molecular Complex Stability; John Wiley & Sons: Hoboken, NJ, USA, 1987; pp. 21–101. [Google Scholar]
- Bera, R.K.; Anoop, A.; Raj, C.R. Enzyme-free colorimetric assay of serum uric acid. Chem. Commun. 2011, 47, 11498–11500. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Frankel, D.J.; Richardson, N.V. Self-assembly of adenide on Cu(110) surfaces. Langmuir 2002, 18, 3219–3225. [Google Scholar] [CrossRef]
- Giese, B.; McNaughton, D. Surface-enhanced raman spectroscopic and density functional theory study of adenine adsorption to silver surfaces. J. Phys. Chem. B 2002, 106, 101–112. [Google Scholar] [CrossRef]
- Jang, N.H. The coordination chemistry of DNA nucleosides on gold nanoparticles as a probe by SERS. Bull. Koream Chem. Soc. 2002, 23, 1790–1800. [Google Scholar]
- Camafeita, L.E.; Sánchez-Cortés, S.; García-Ramos, J.V. SERS of guanine and its alkyl derivatives on gold sols. J. Raman Spectrosc. 1996, 27, 533–537. [Google Scholar] [CrossRef]
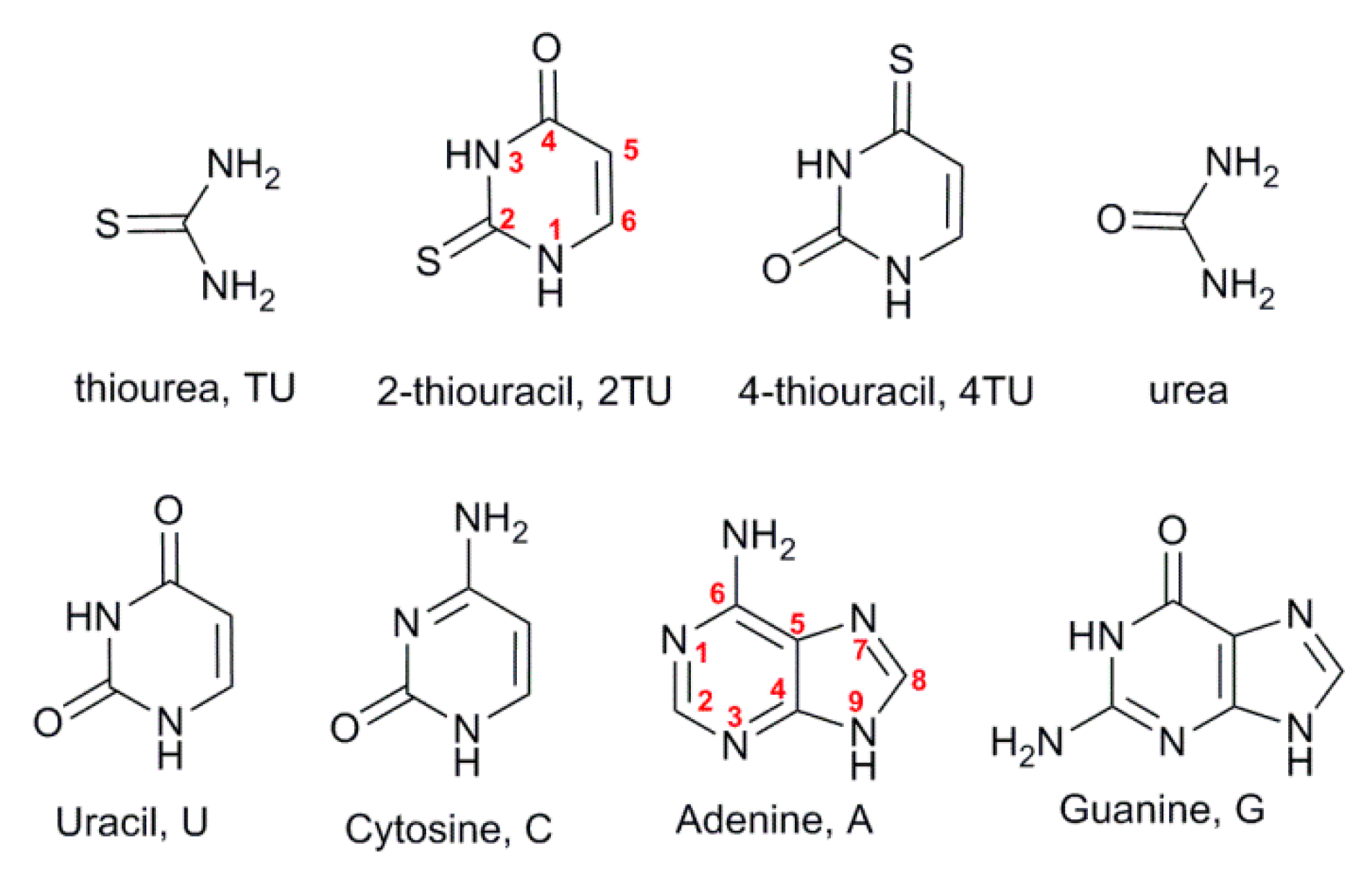

| Base (a) | λ1/nm | Ɛ1/mol−1dm3s−1 | λ2/nm | Ɛ2/mol−1dm3s−1 | pKa1 |
|---|---|---|---|---|---|
| urea | --- | --- | --- | --- | 0.05 |
| thiourea (TU) | --- | --- | --- | --- | −0.90 |
| uracil (U) | 259 | 8150 | --- | --- | <0.5 |
| 2-thiouracil (2TU) | 212 | 17,000 | 270 | 14,000 | --- |
| 4-thiouracil (4TU) | 242 | 4100 | 328 | 19,000 | --- |
| cytosine (C) | 268 (neutral) | 7050 | 275 (acid) (b) | 9750 | 4.4 |
| adenine (A) | 261 | 13,200 | 263 (acid) (c) | 12,900 | 4.1 |
| guanine (G) | 246 | 6300 | 276 | 4600 | 3.3 |
| Base 1 (c/μM) | Base 2 (c/μM) | λmax (SPR band)/nm | Color | Effect |
|---|---|---|---|---|
| urea (2500) | --- | 520 | red | − |
| thiourea, TU (0.4 to 8.1) | --- | 670 | blue | + |
| urea (2500) | thiourea, TU (8.1) | 670 | blue | + |
| uracil, U (196) | --- | 520 | red | − |
| 2-thiouracil, 2TU (9.34) | --- | 523 | red | functionalized |
| 4-thiouracil, 4TU (25.3) | --- | 672 | blue | + |
| 2TU (94) | 4TU (94) | 523 | red | − |
| 2TU (94) + 4TU (94) | AuNPs addition | 523 | red | functionalized |
| cytosine, C (11.3) | --- | 670 | blue | + |
| 2TU (63) | C (34) | 523 | red | − |
| C (34) | 2TU (63) | red-shift | -- | stopped |
| adenine, A (1.55) | --- | 663 | blue | + |
| guanine, G (8.4) | --- | 620 | blue | + |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, E. Gold Nanoparticles as Colorimetric Sensors for the Detection of DNA Bases and Related Compounds. Molecules 2020, 25, 2890. https://doi.org/10.3390/molecules25122890
Iglesias E. Gold Nanoparticles as Colorimetric Sensors for the Detection of DNA Bases and Related Compounds. Molecules. 2020; 25(12):2890. https://doi.org/10.3390/molecules25122890
Chicago/Turabian StyleIglesias, Emilia. 2020. "Gold Nanoparticles as Colorimetric Sensors for the Detection of DNA Bases and Related Compounds" Molecules 25, no. 12: 2890. https://doi.org/10.3390/molecules25122890
APA StyleIglesias, E. (2020). Gold Nanoparticles as Colorimetric Sensors for the Detection of DNA Bases and Related Compounds. Molecules, 25(12), 2890. https://doi.org/10.3390/molecules25122890