Discovery, Synthesis, and Scale-up of Efficient Palladium Catalysts Useful for the Modification of Nucleosides and Heteroarenes
Abstract
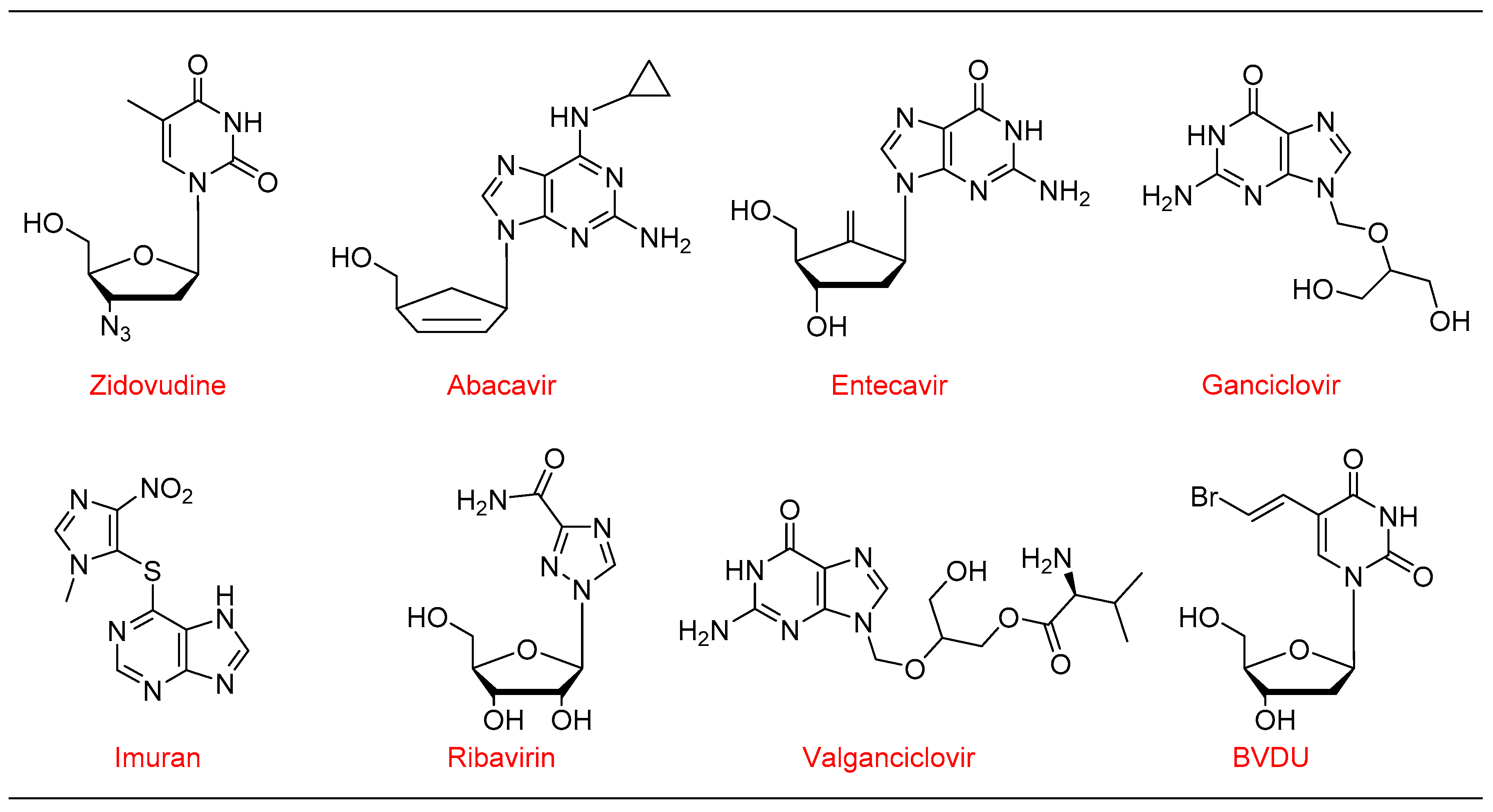
1. Introduction
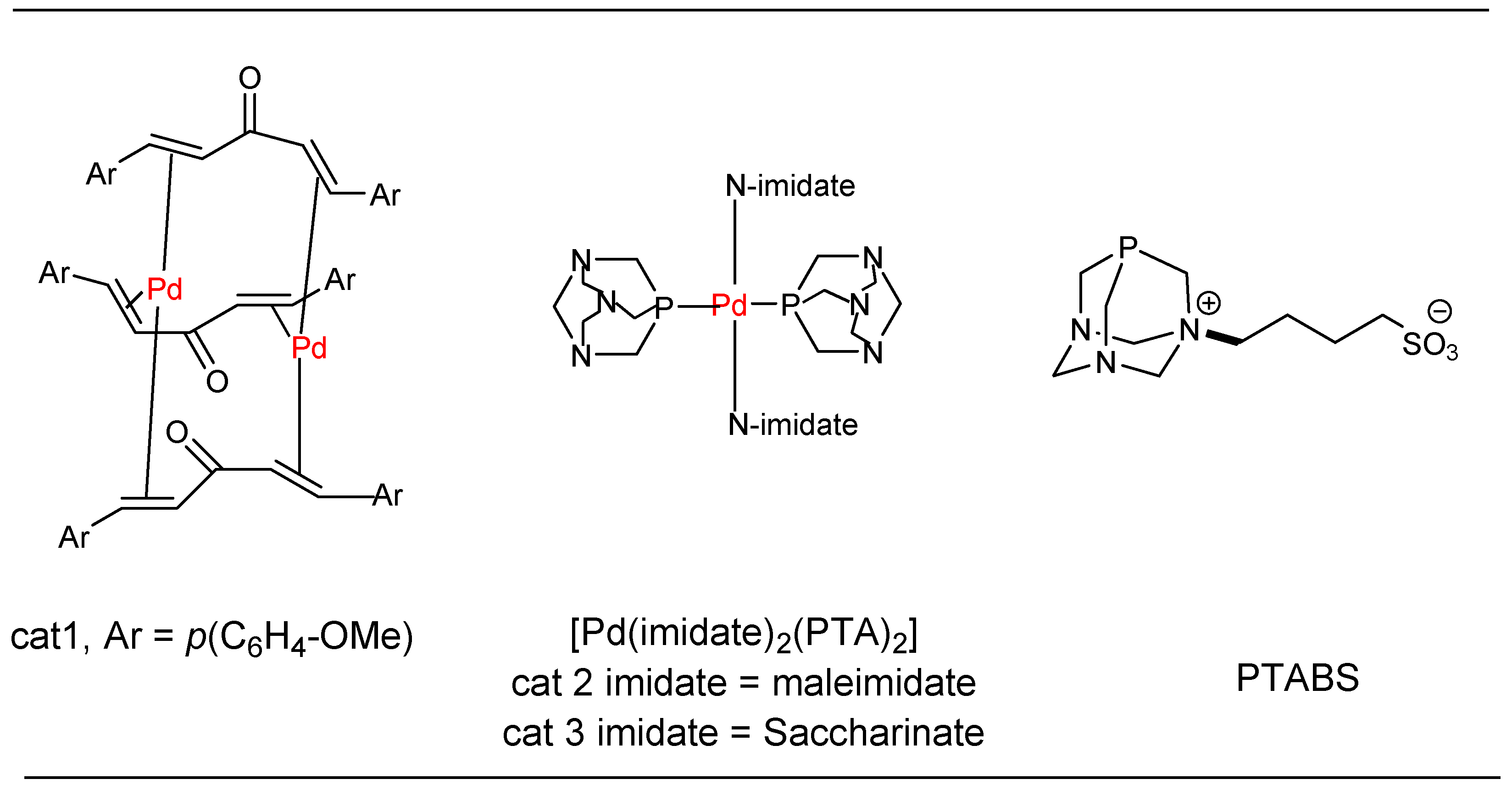
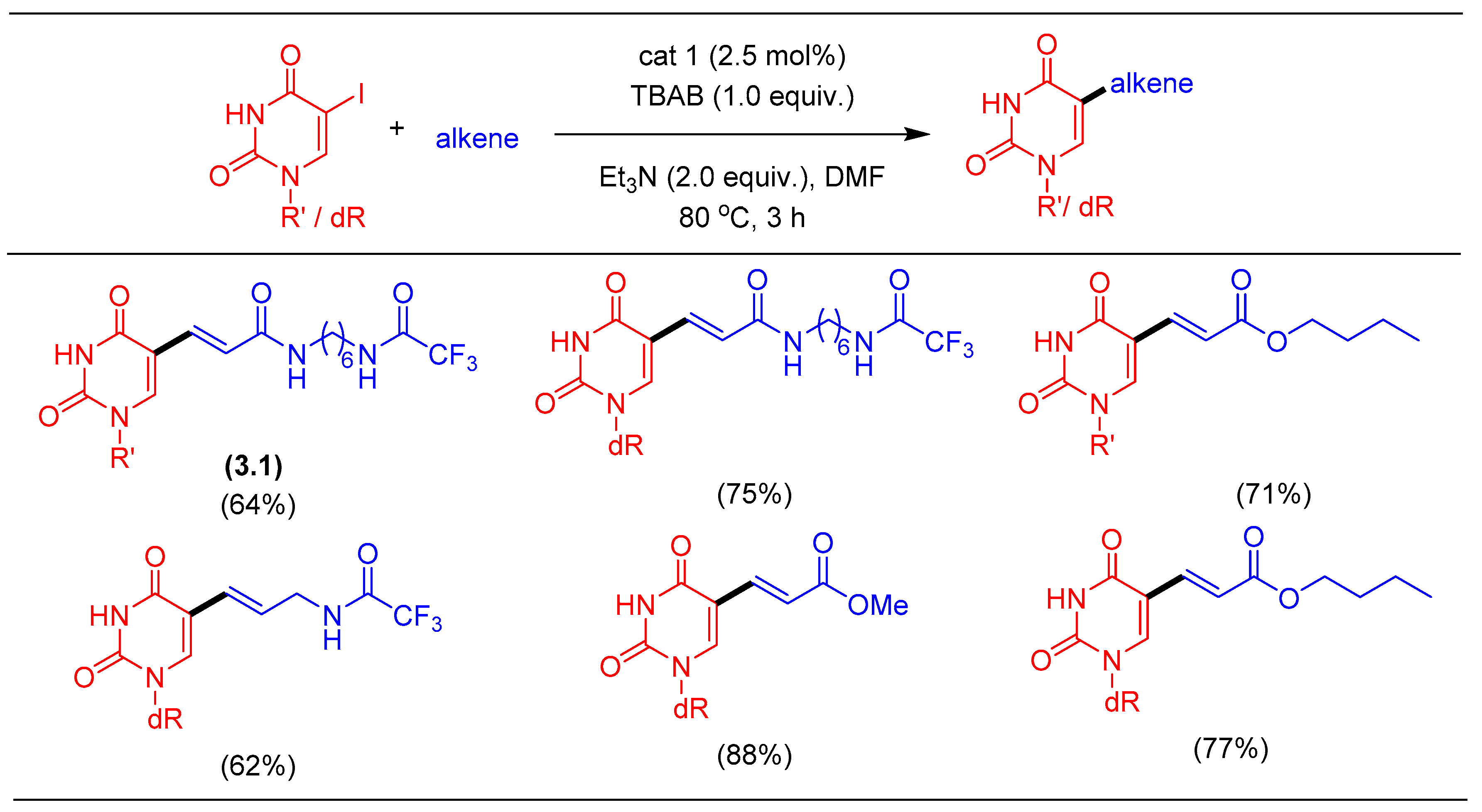
1.1. Heck Alkenylation Using cat1
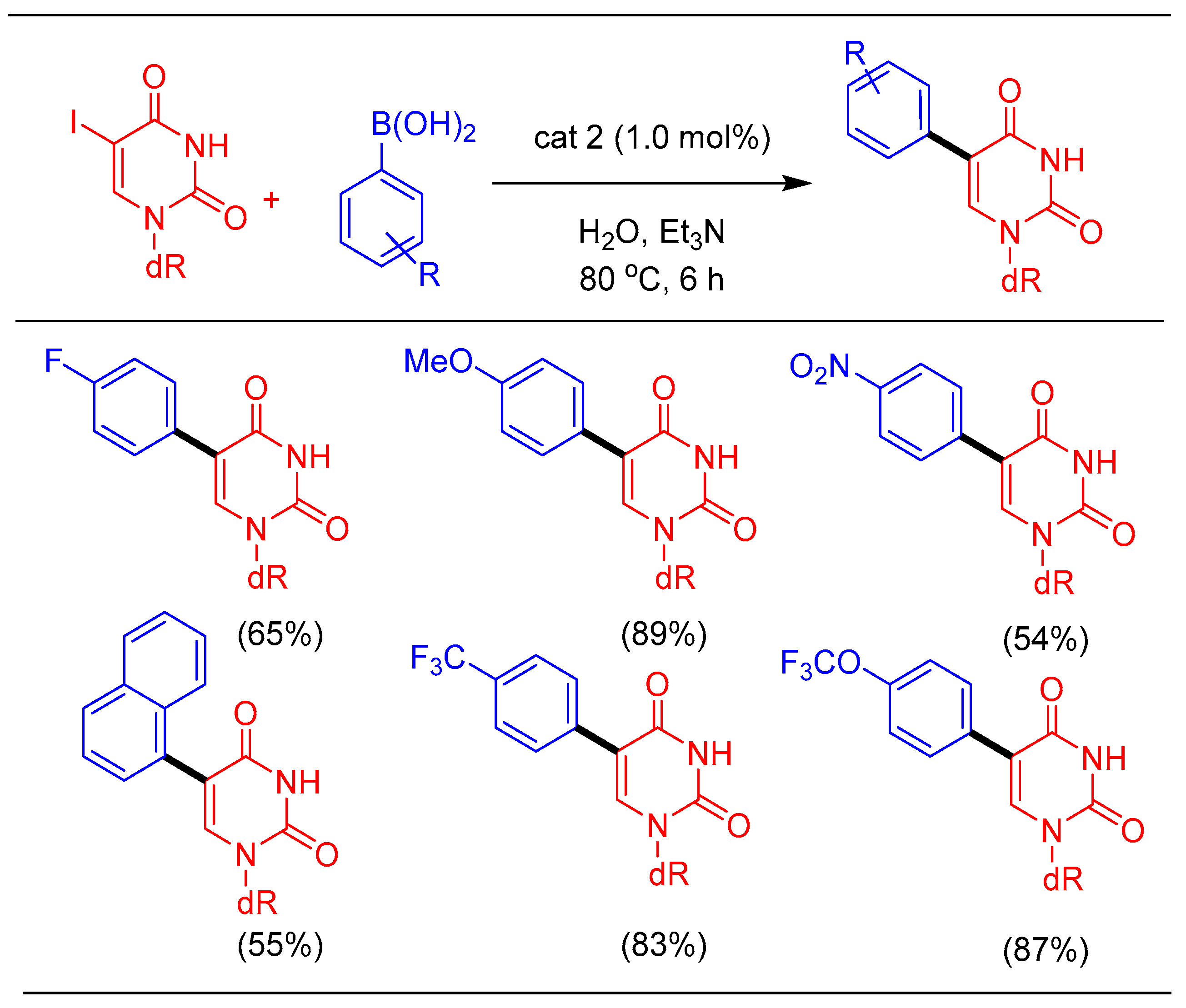
1.2. Suzuki-Miyaura Cross-Coupling Using cat 2
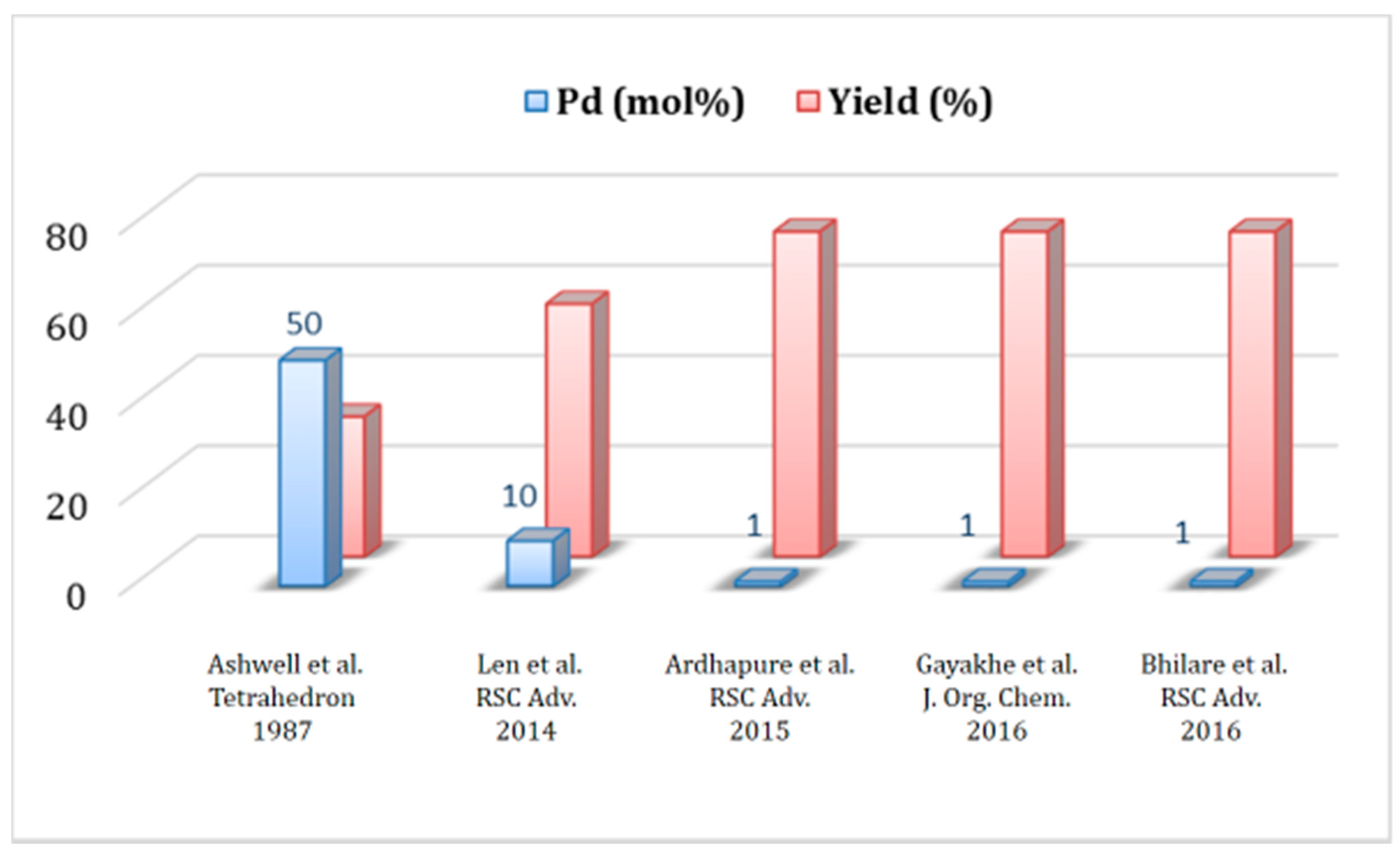
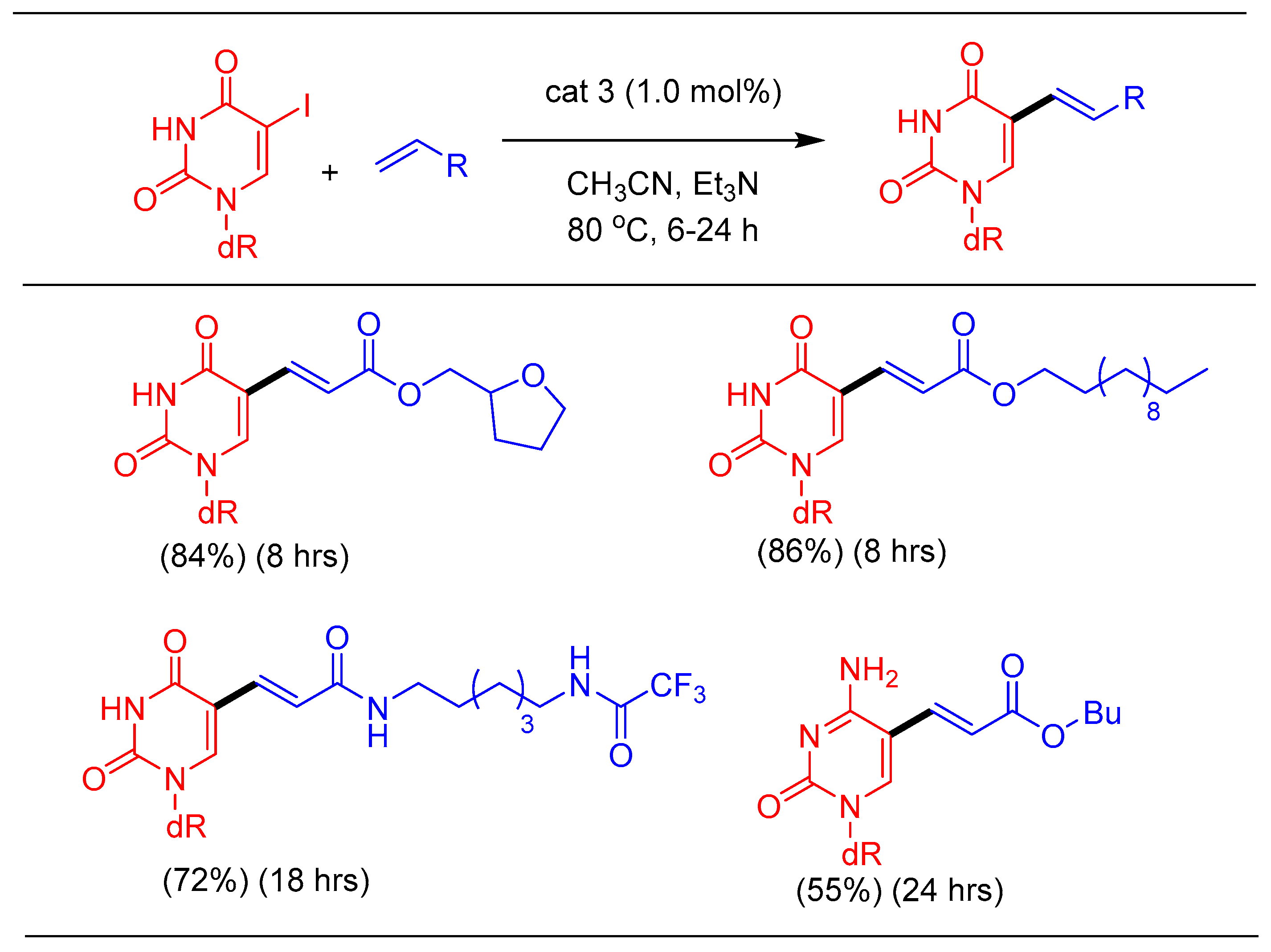
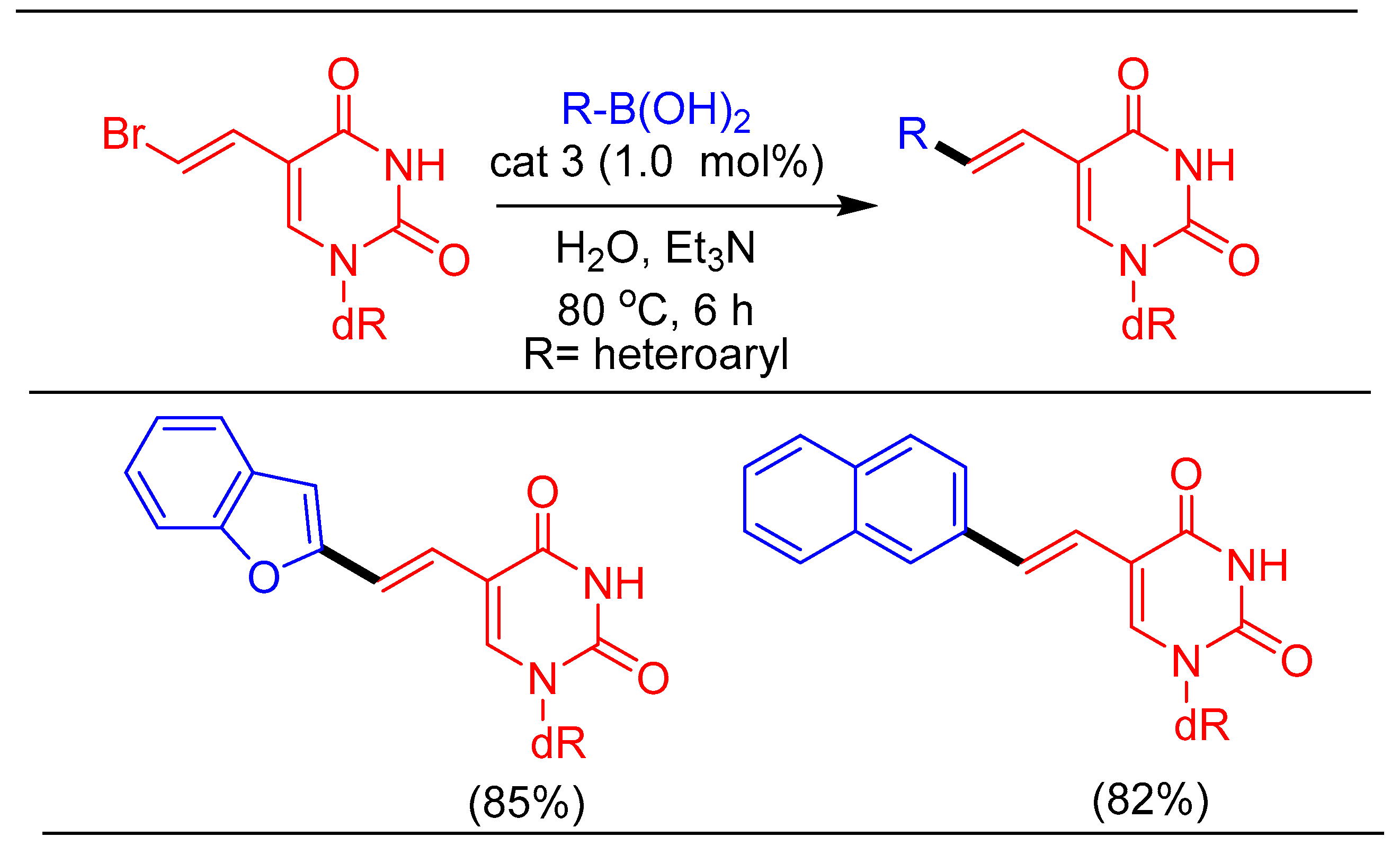
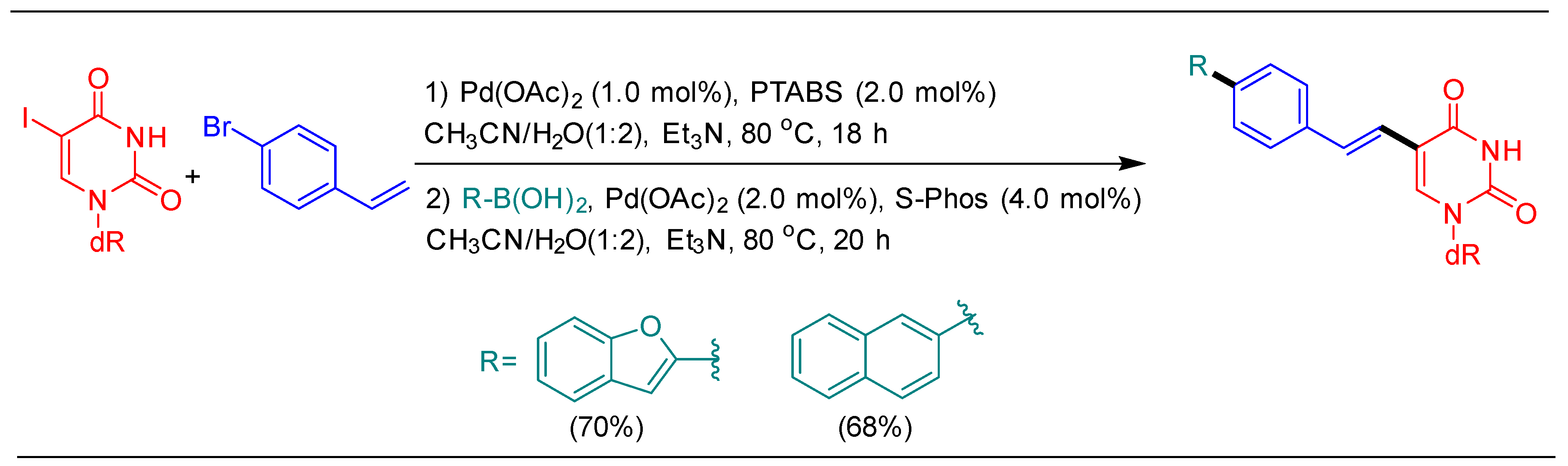
1.3. Heck Alkenylation Using cat 3
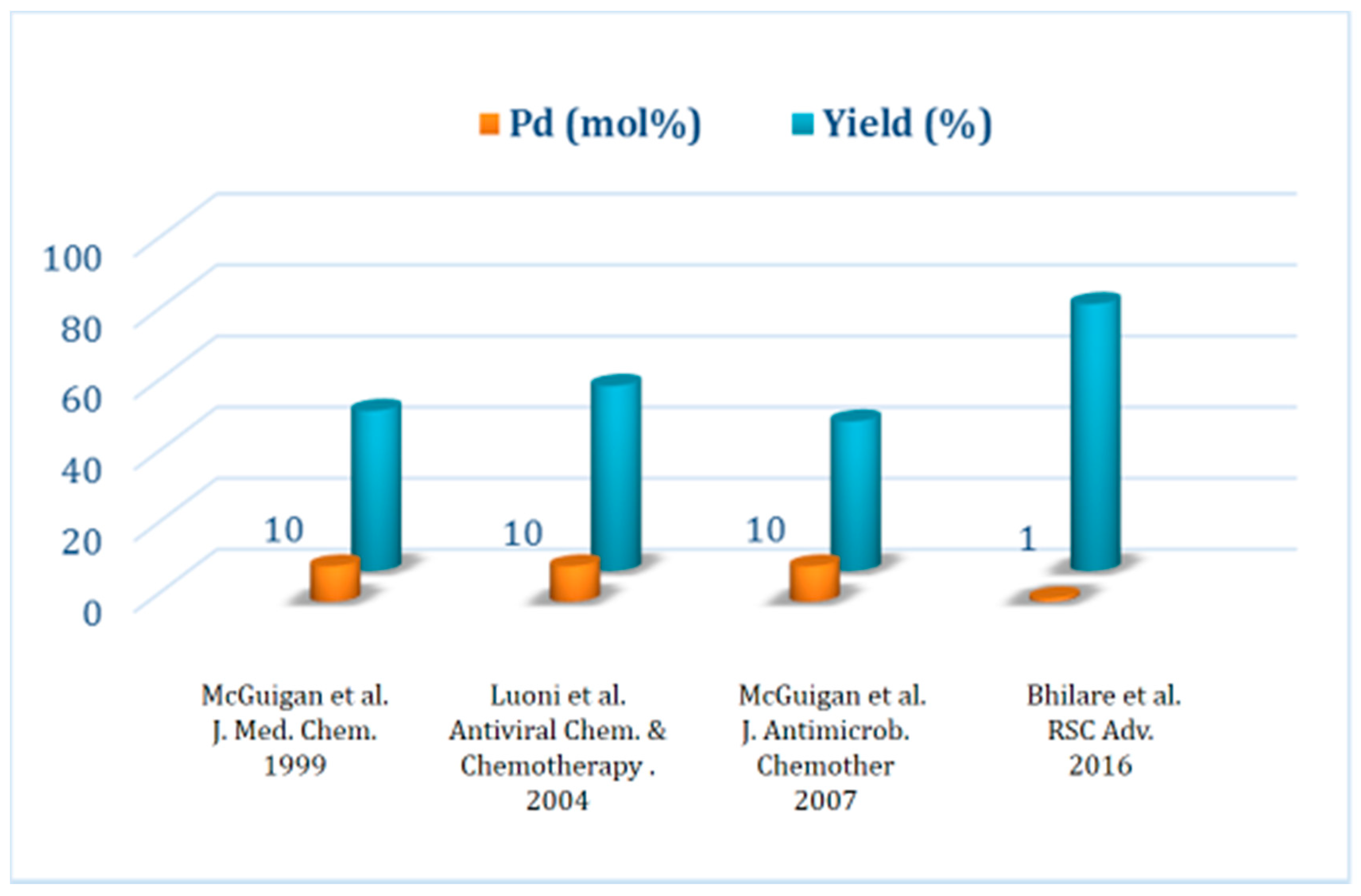
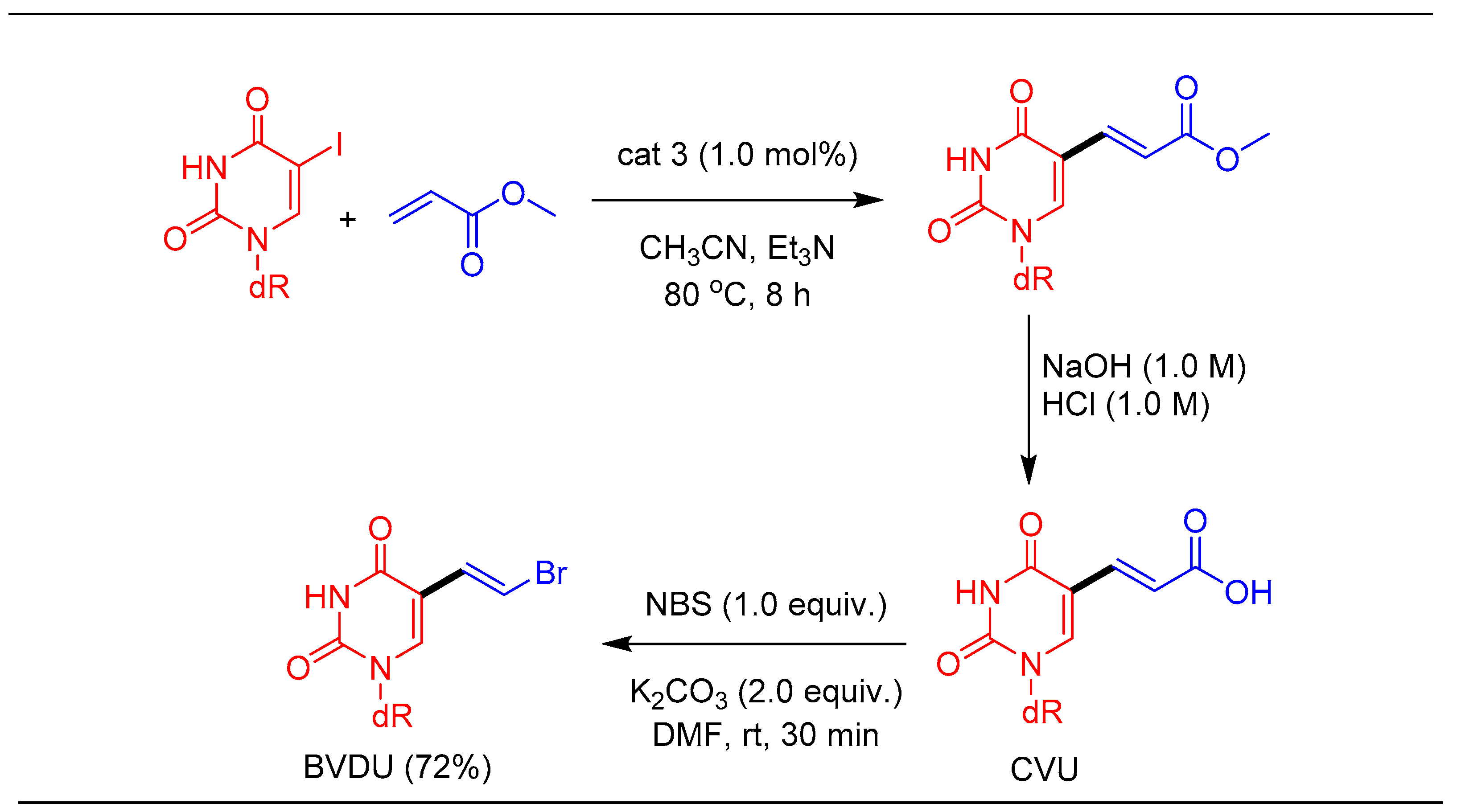
1.4. Synthesis of Antiviral Drug Brivudine (BVDU) Using cat 3
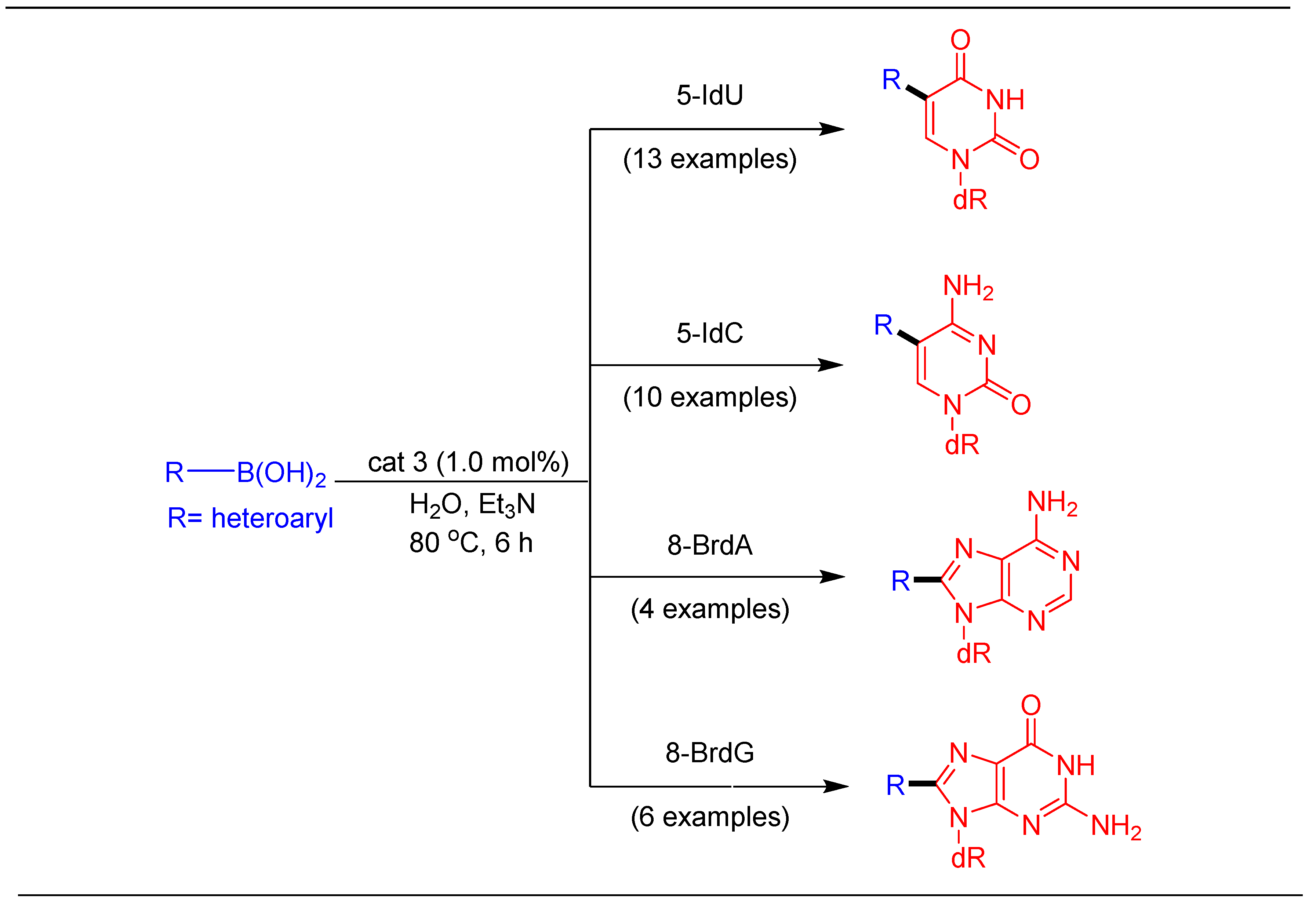
1.5. Suzuki–Miyaura cross Coupling on Four Natural Nucleosides Using cat 3
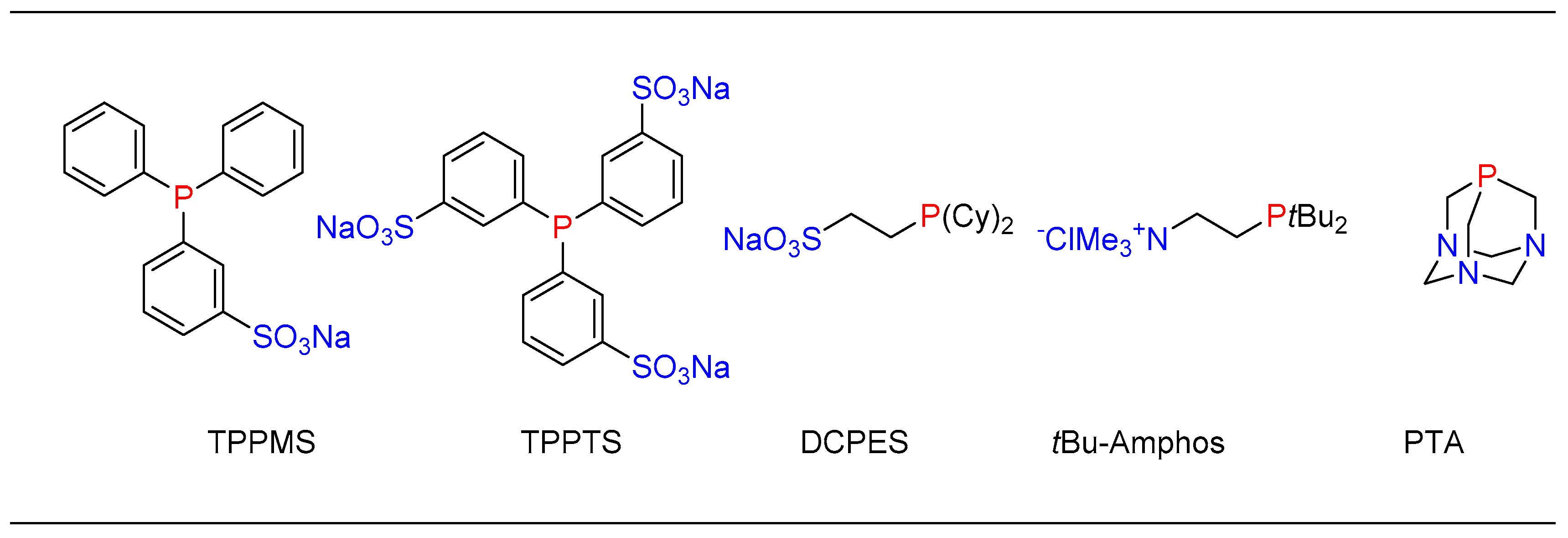
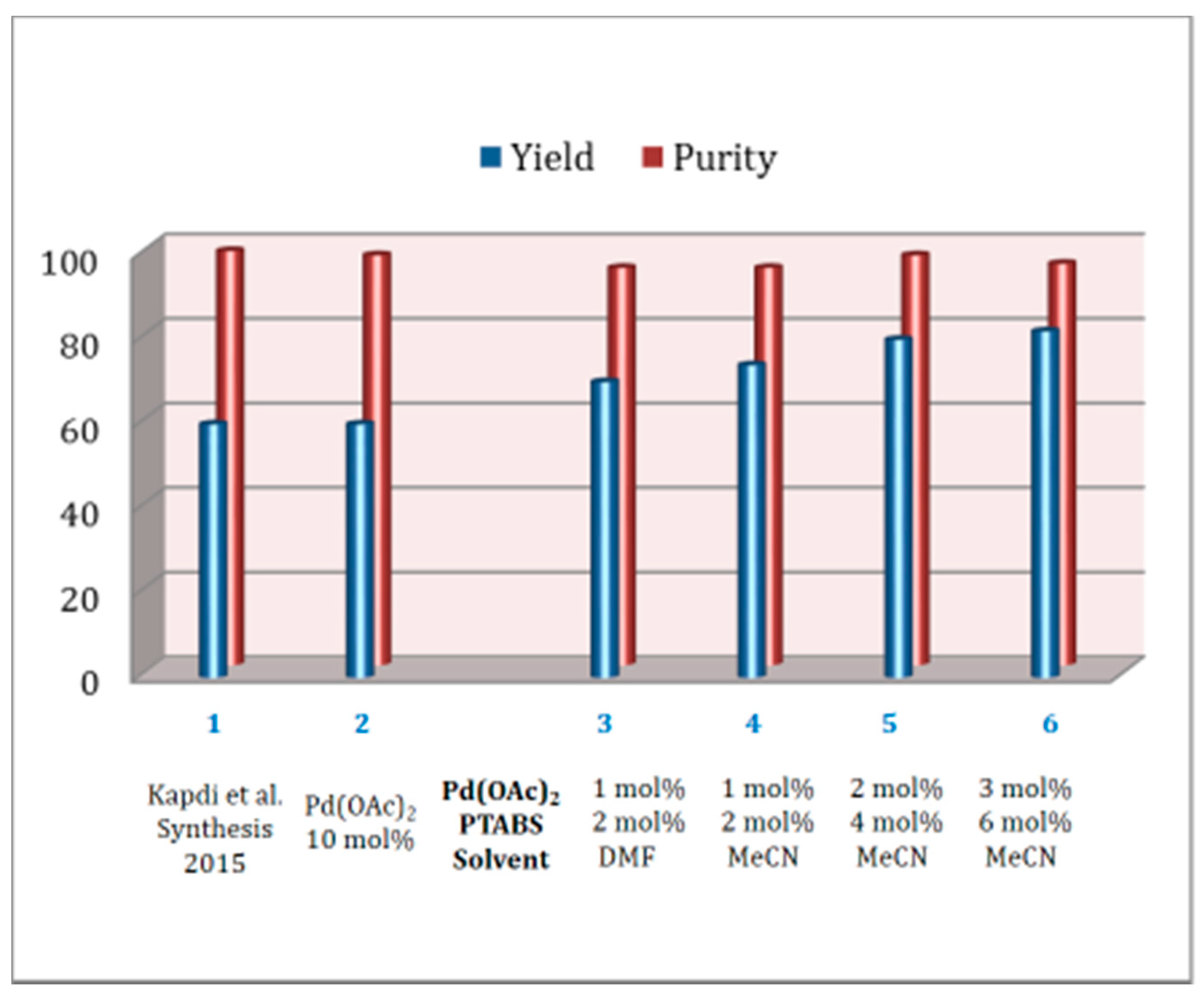
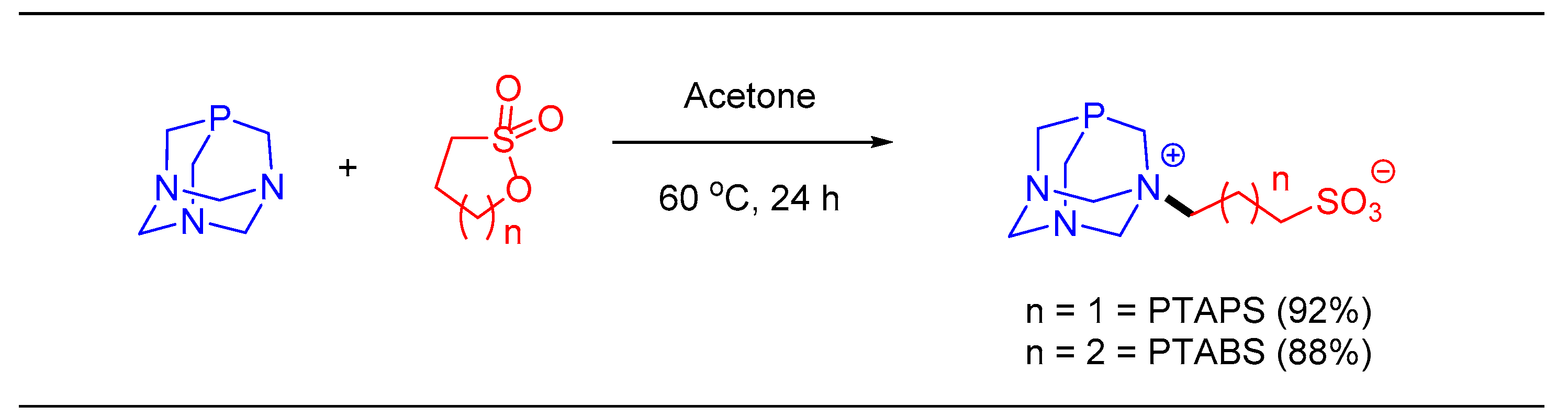
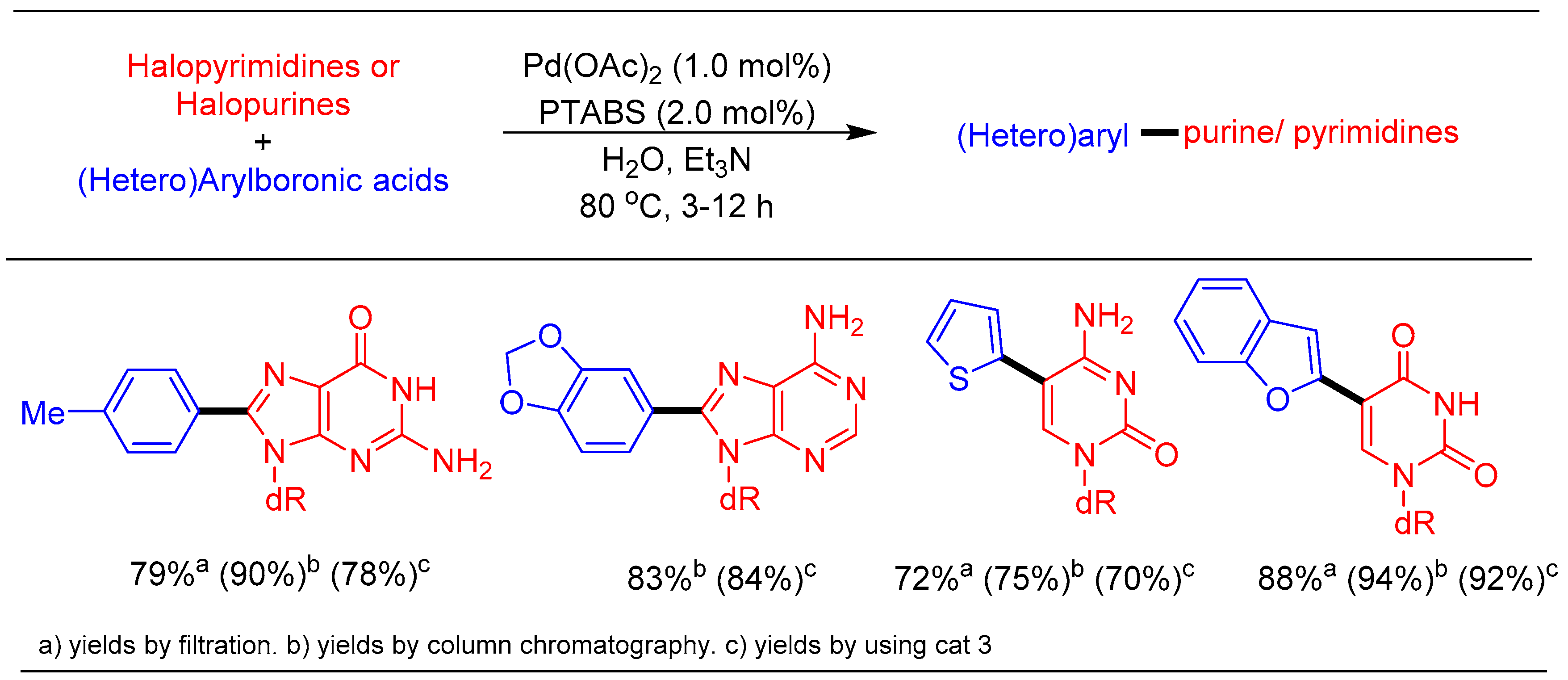
1.6. Suzuki–Miyaura cross Coupling Using Pd/PTABS
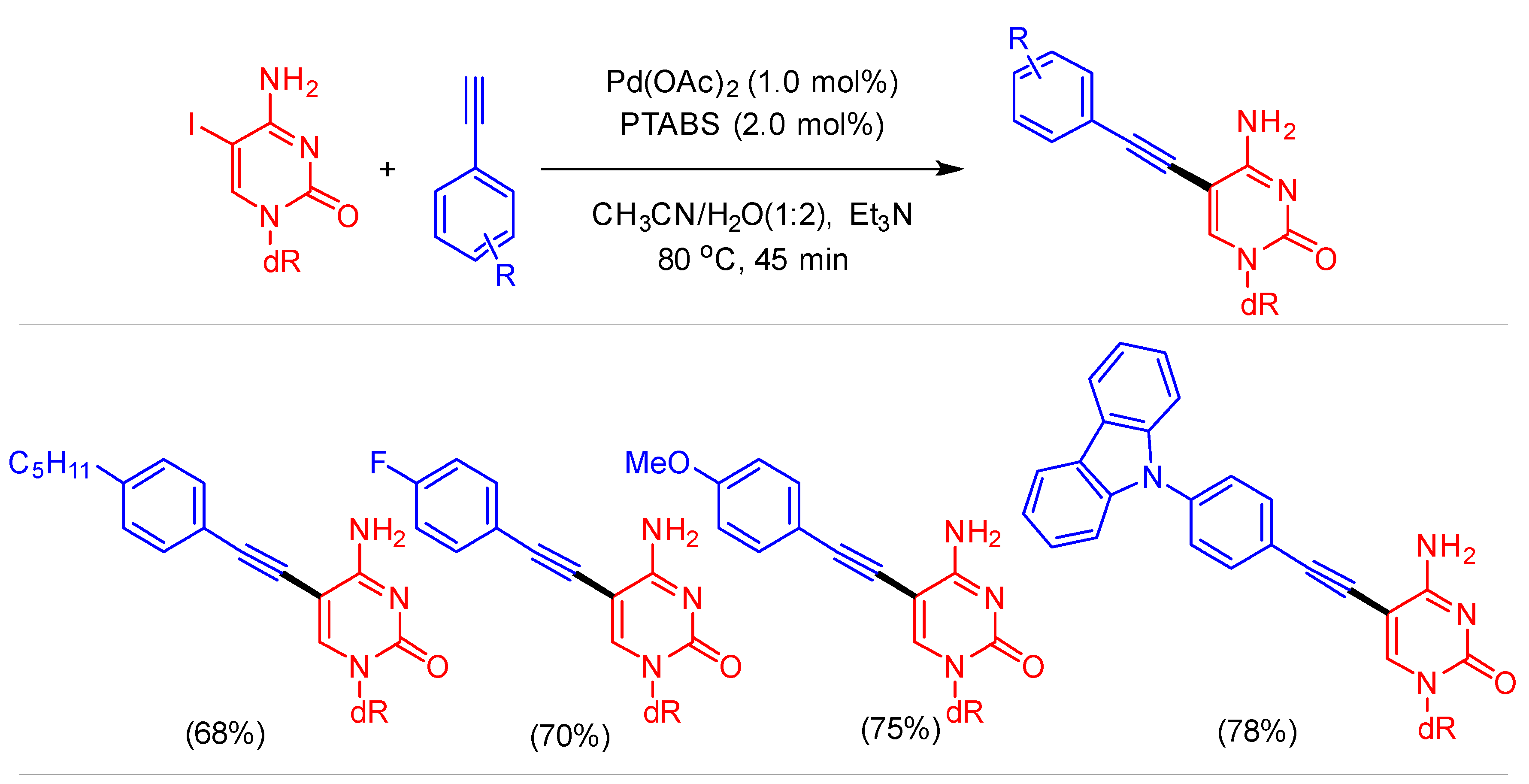
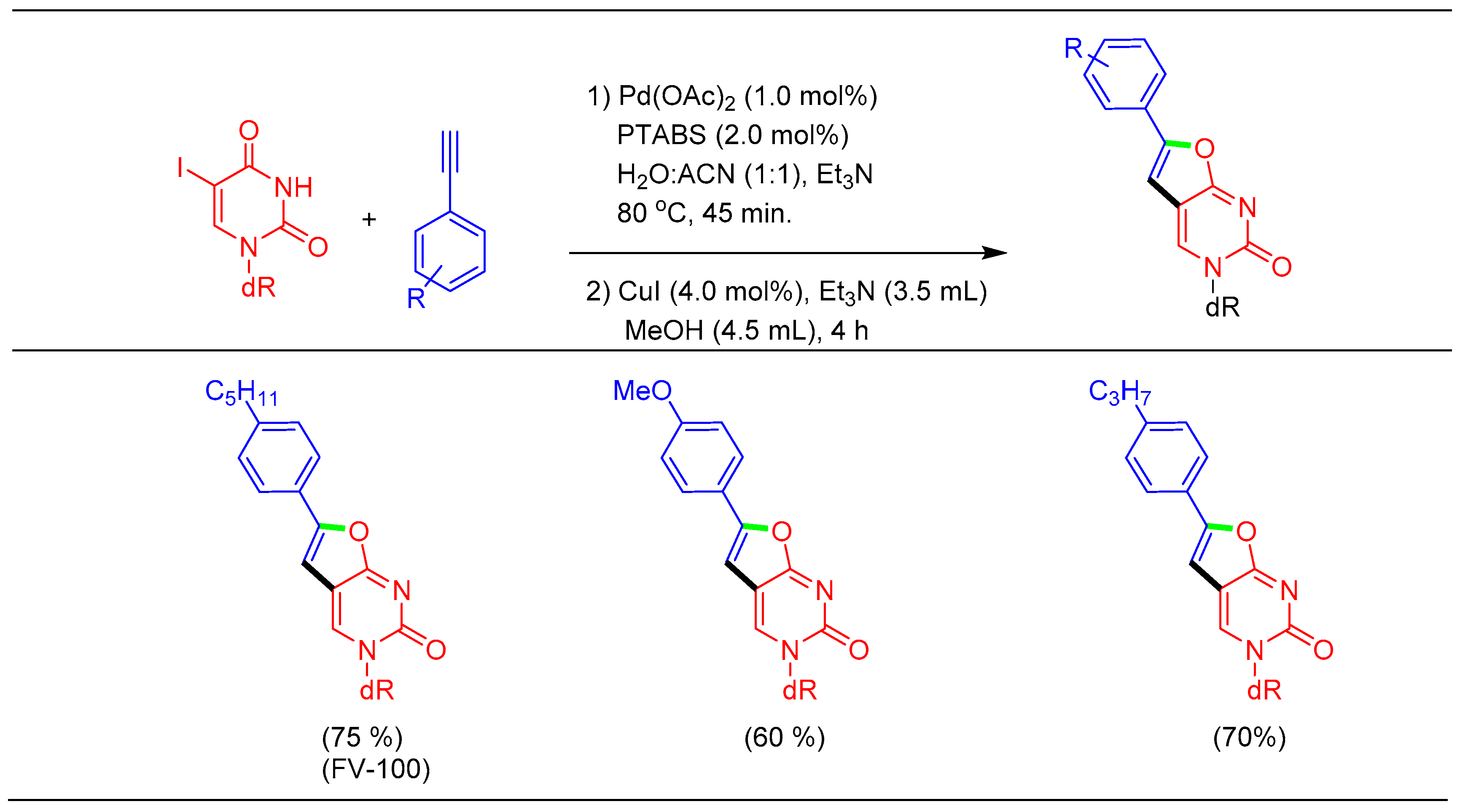
1.7. Sonogashira Cross-Coupling Using Pd/PTABS
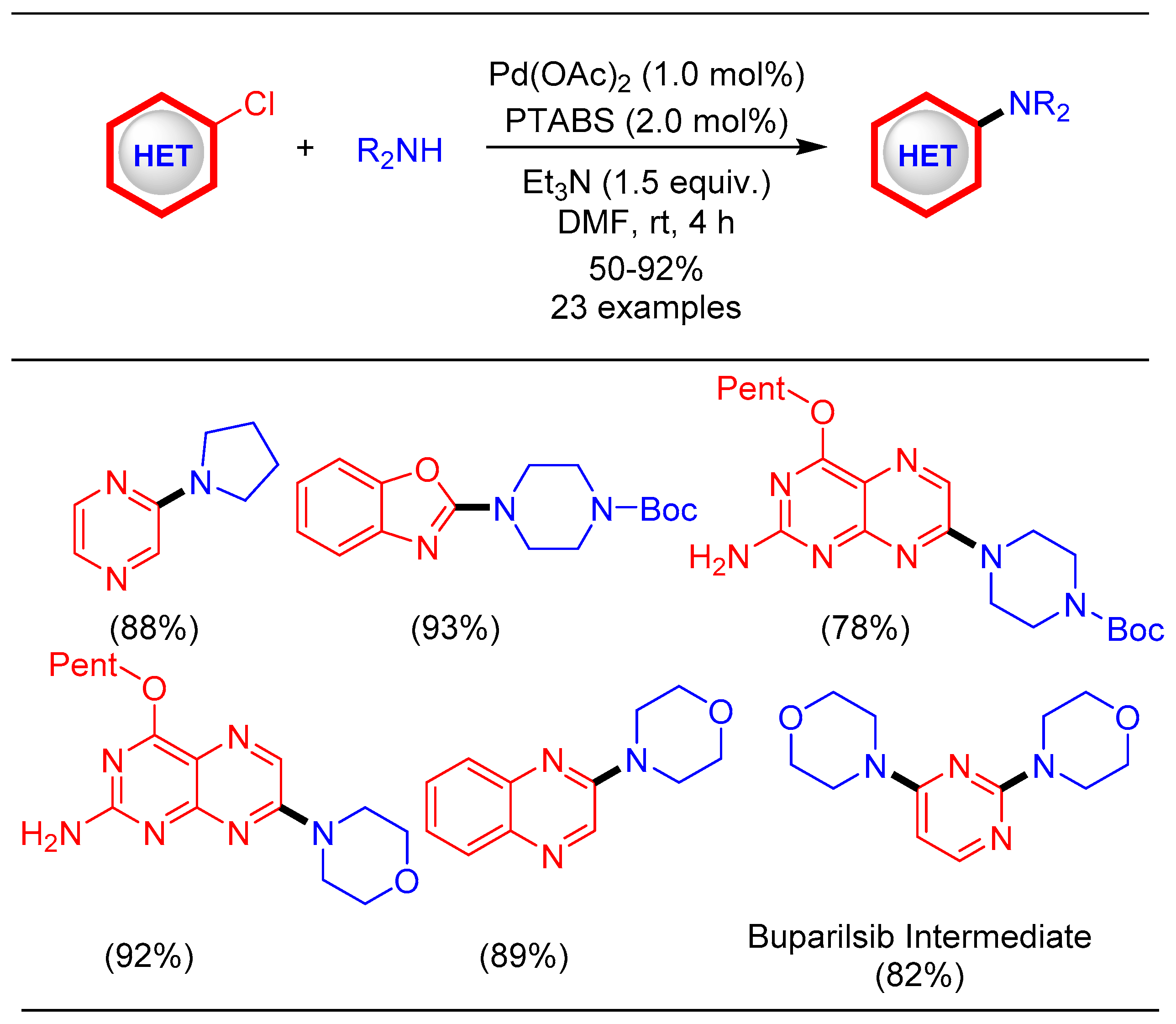
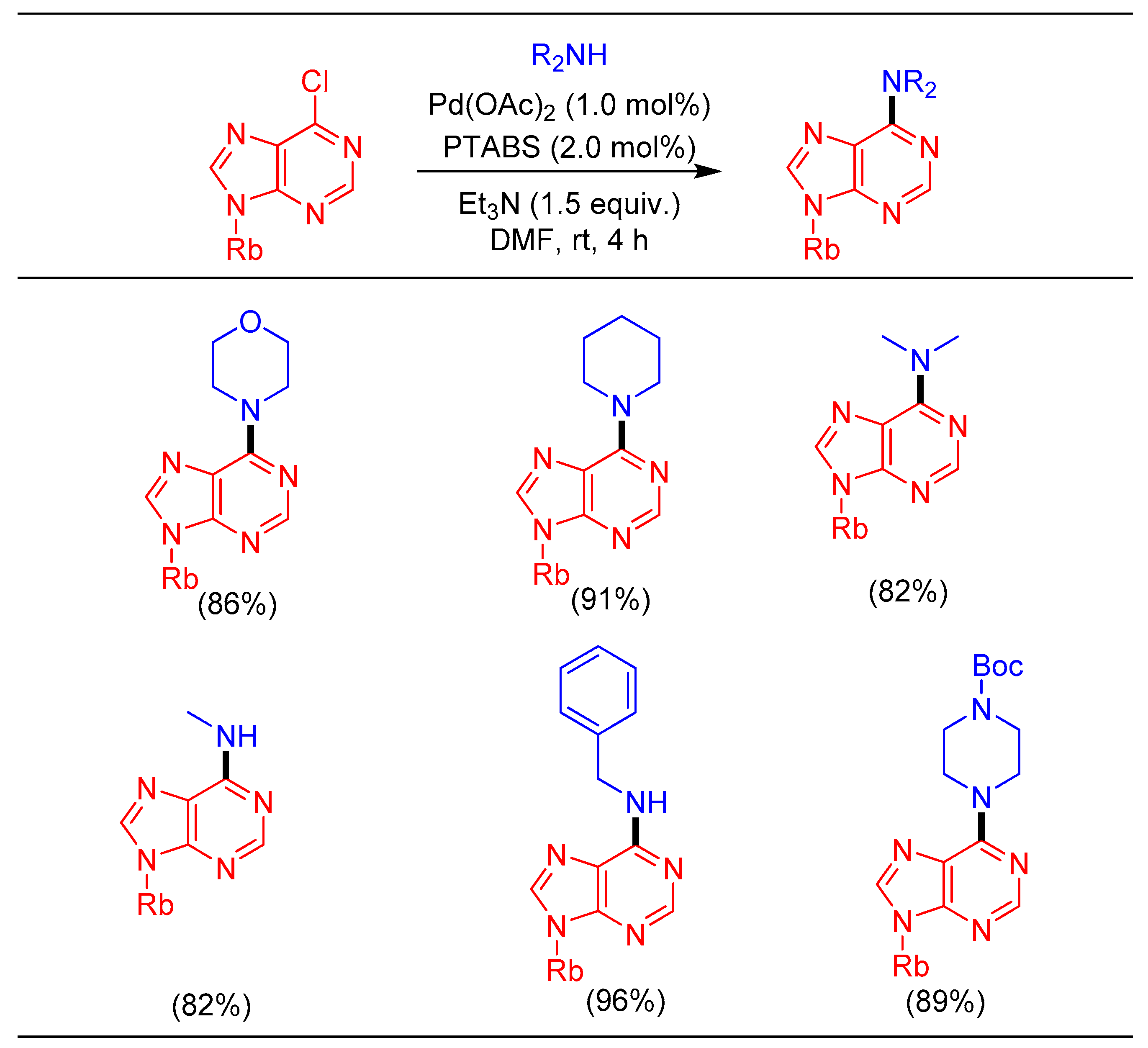
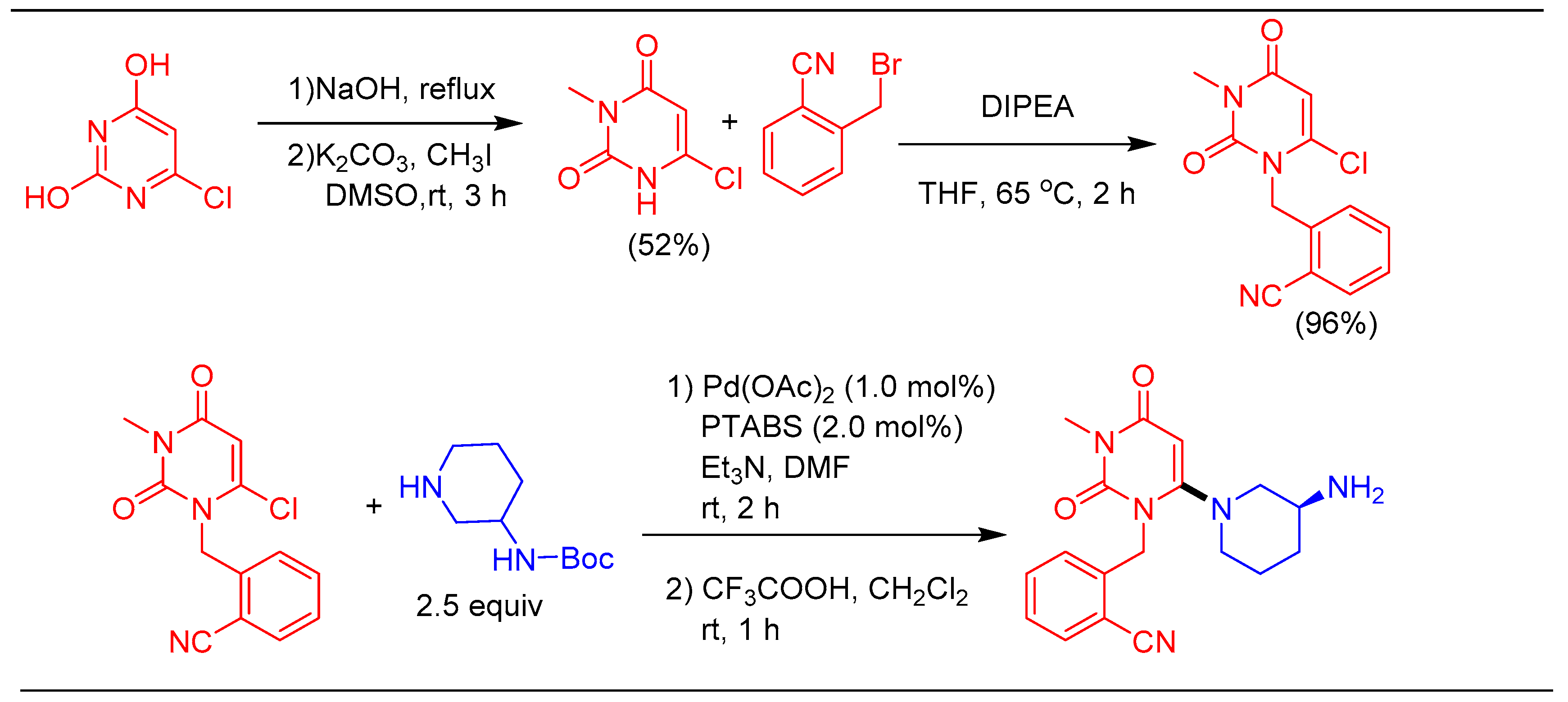
1.8. Amination Reaction Using Pd/PTABS
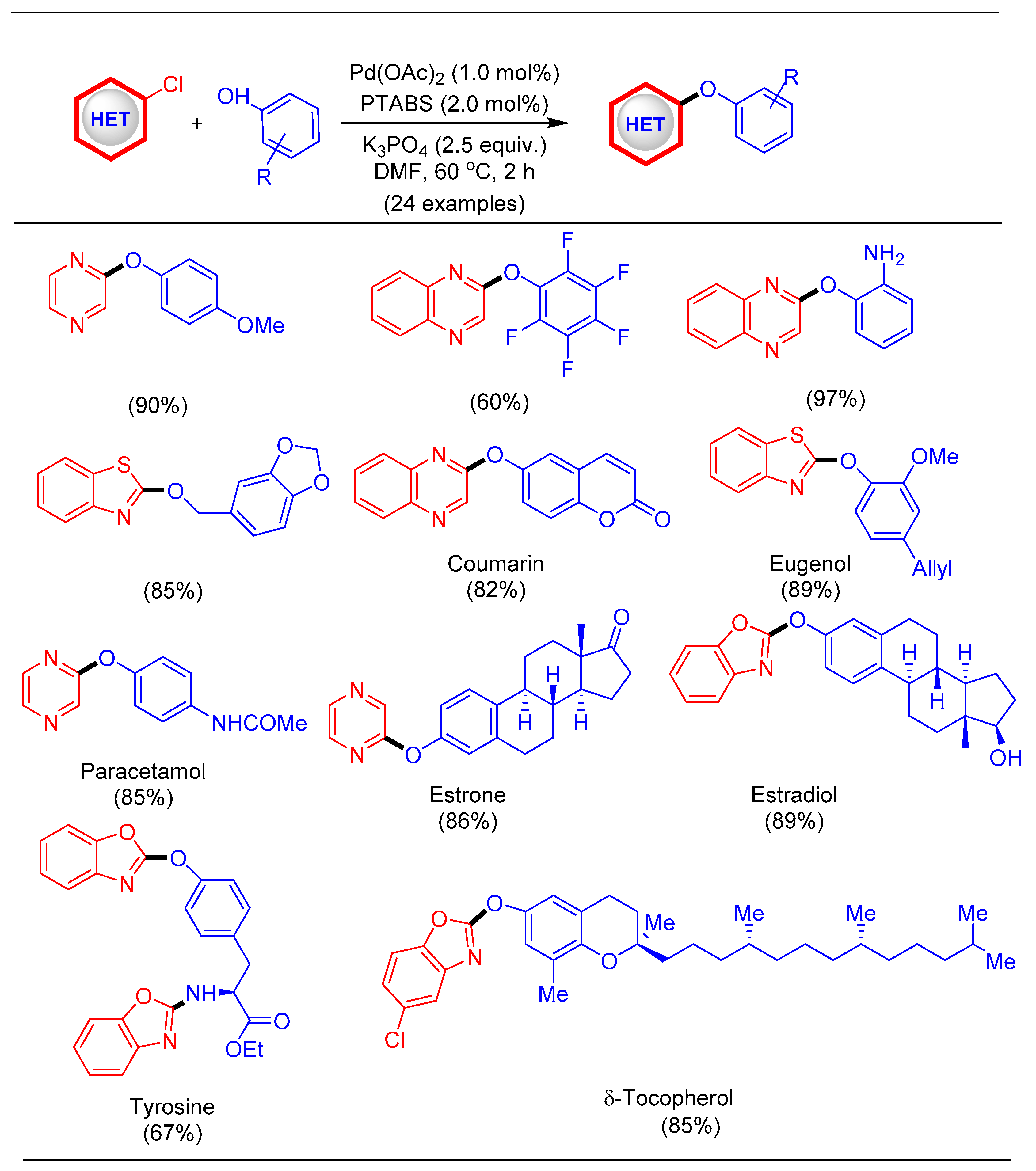
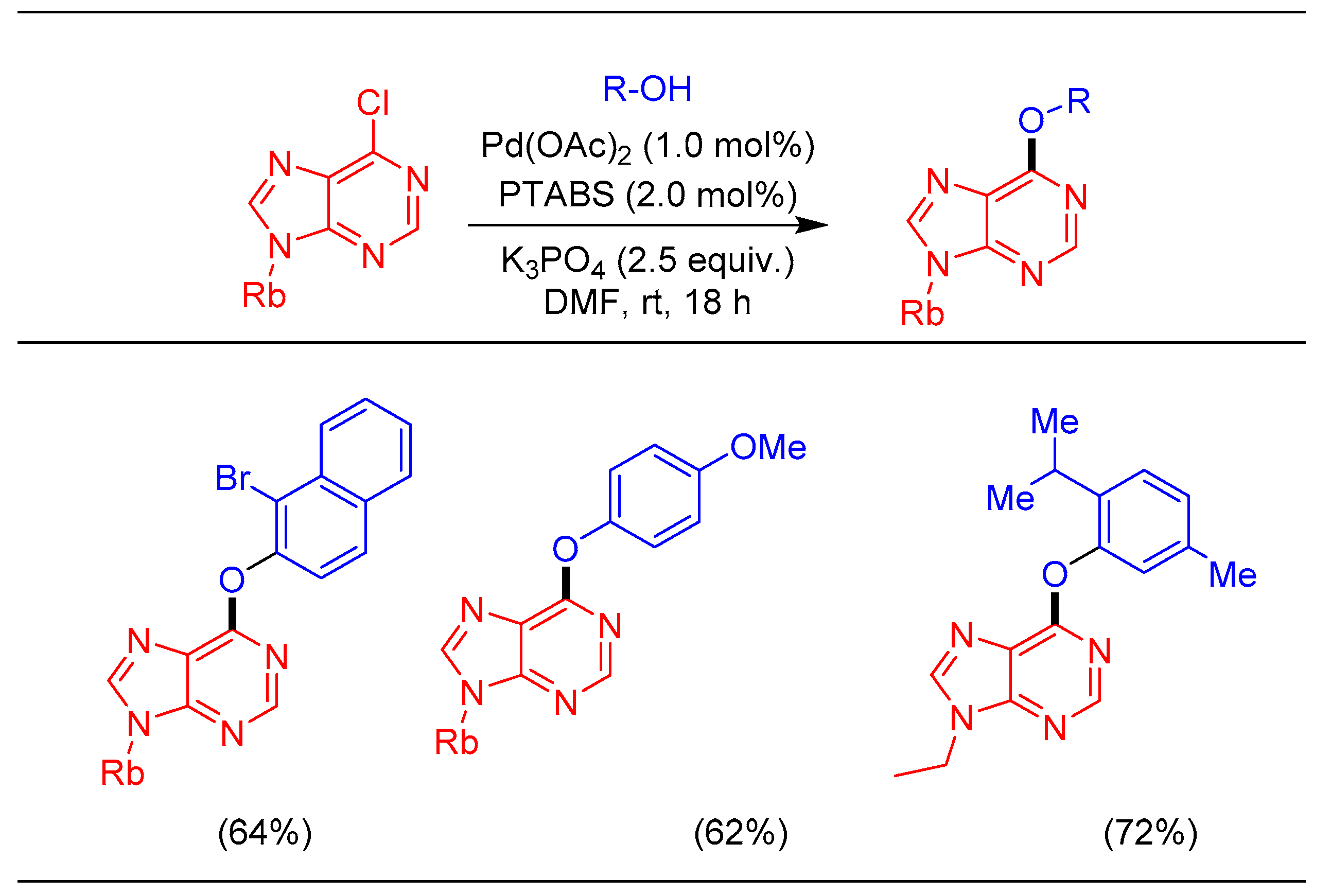
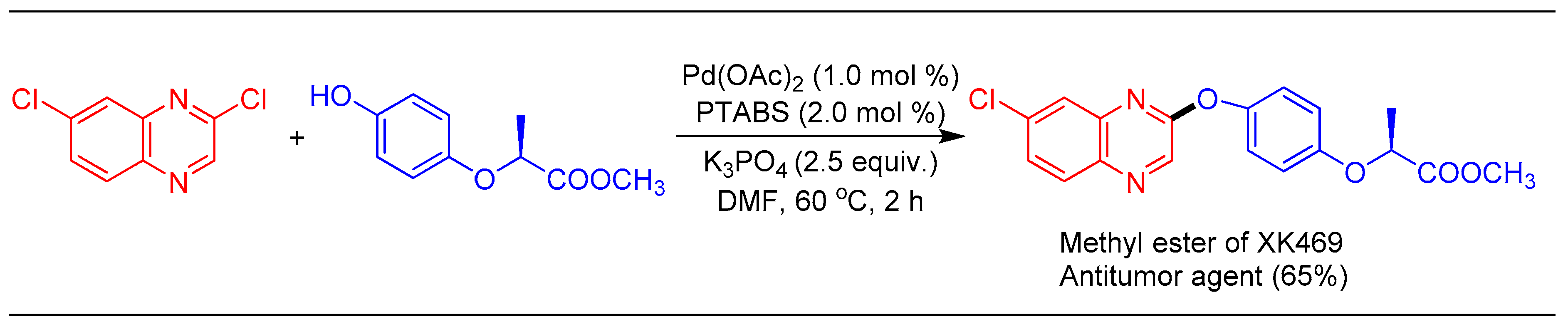
1.9. Etherification Reaction Using Pd/PTABS
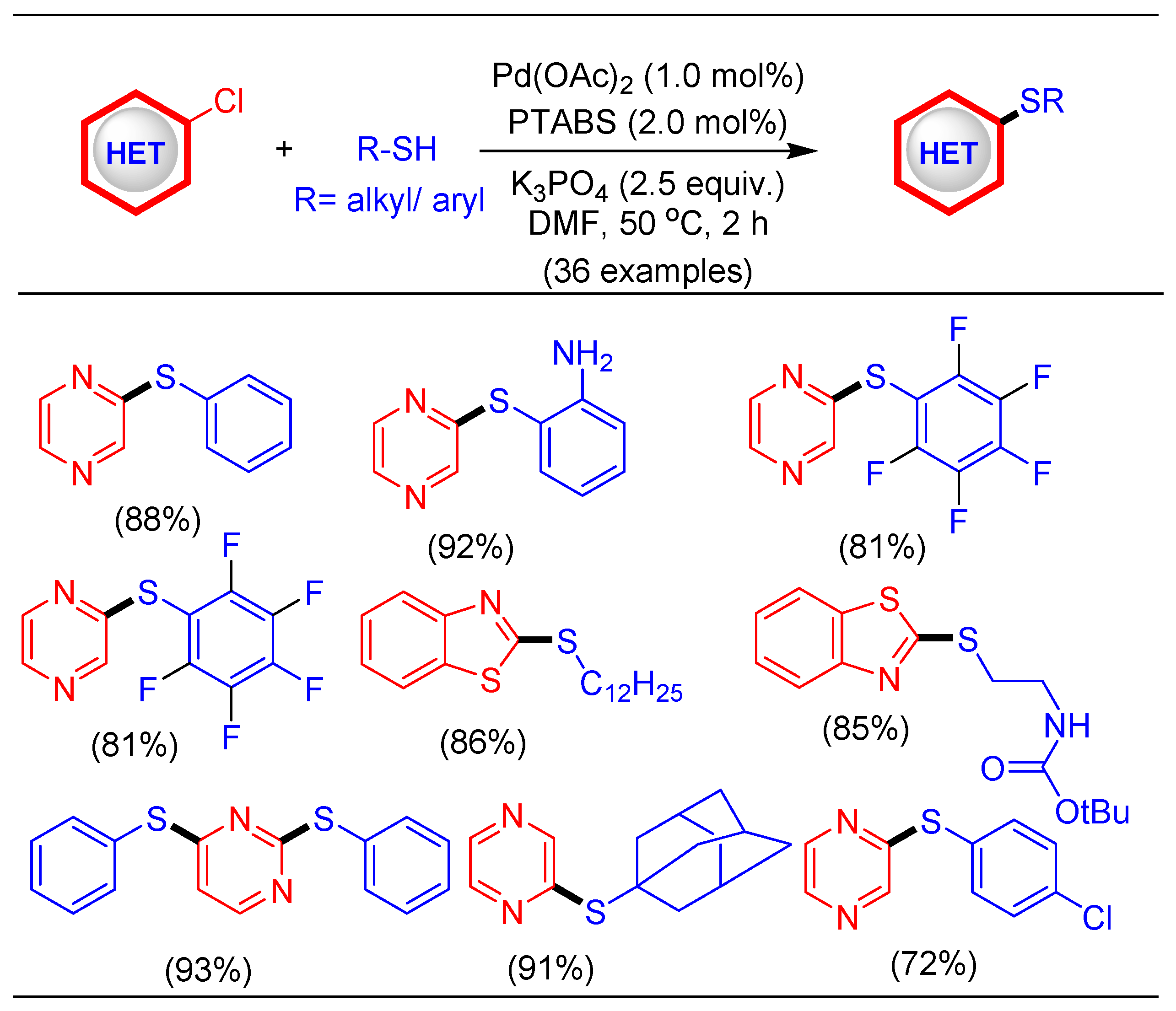
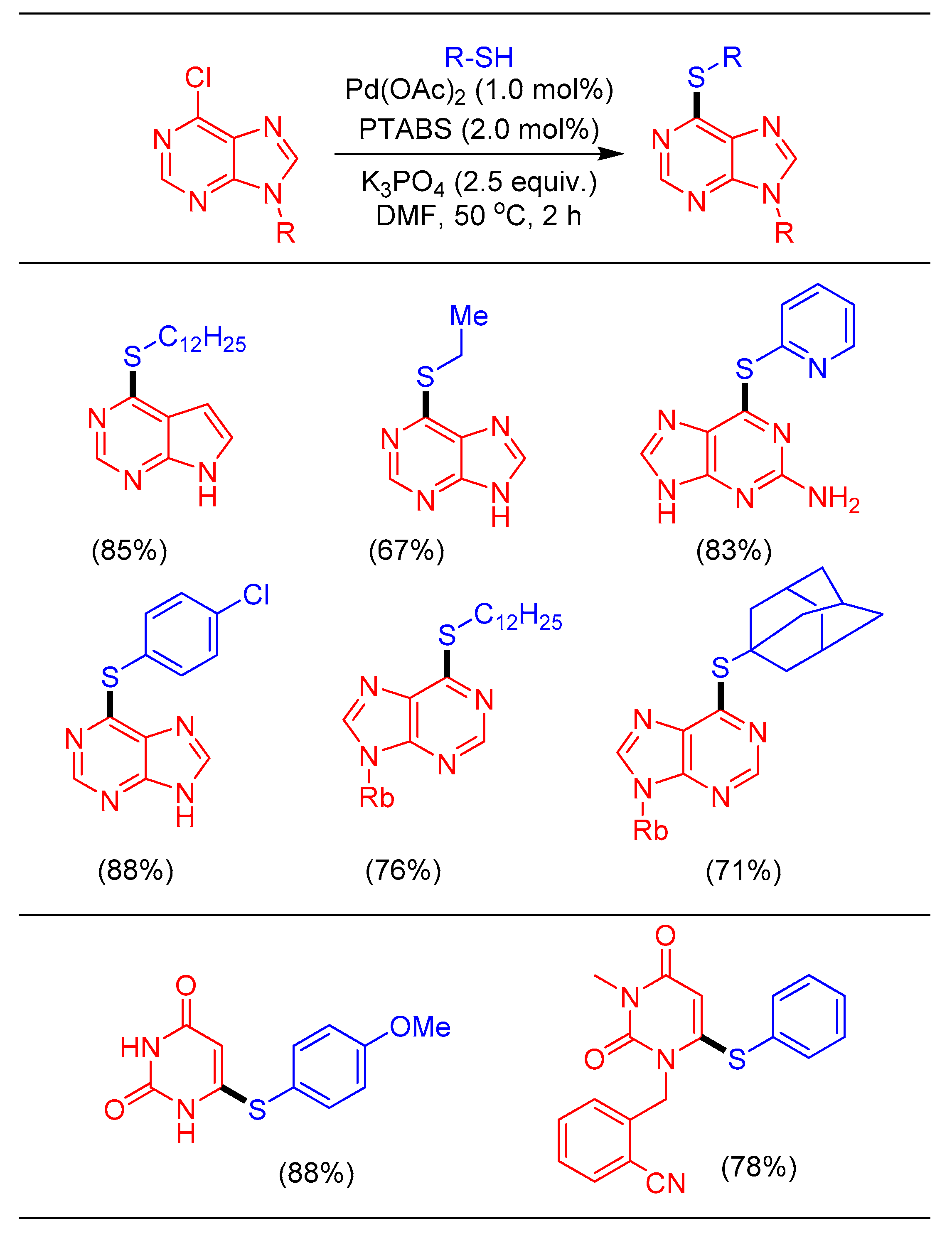
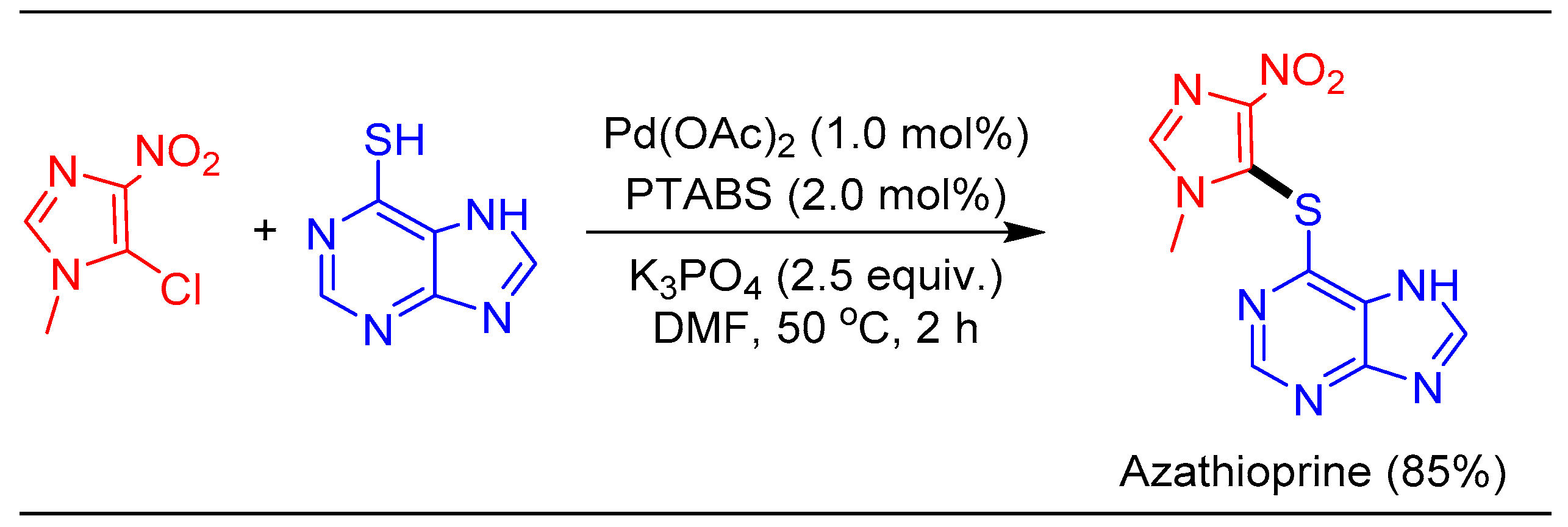
1.10. Thioetherification Reaction Using Pd/PTABS
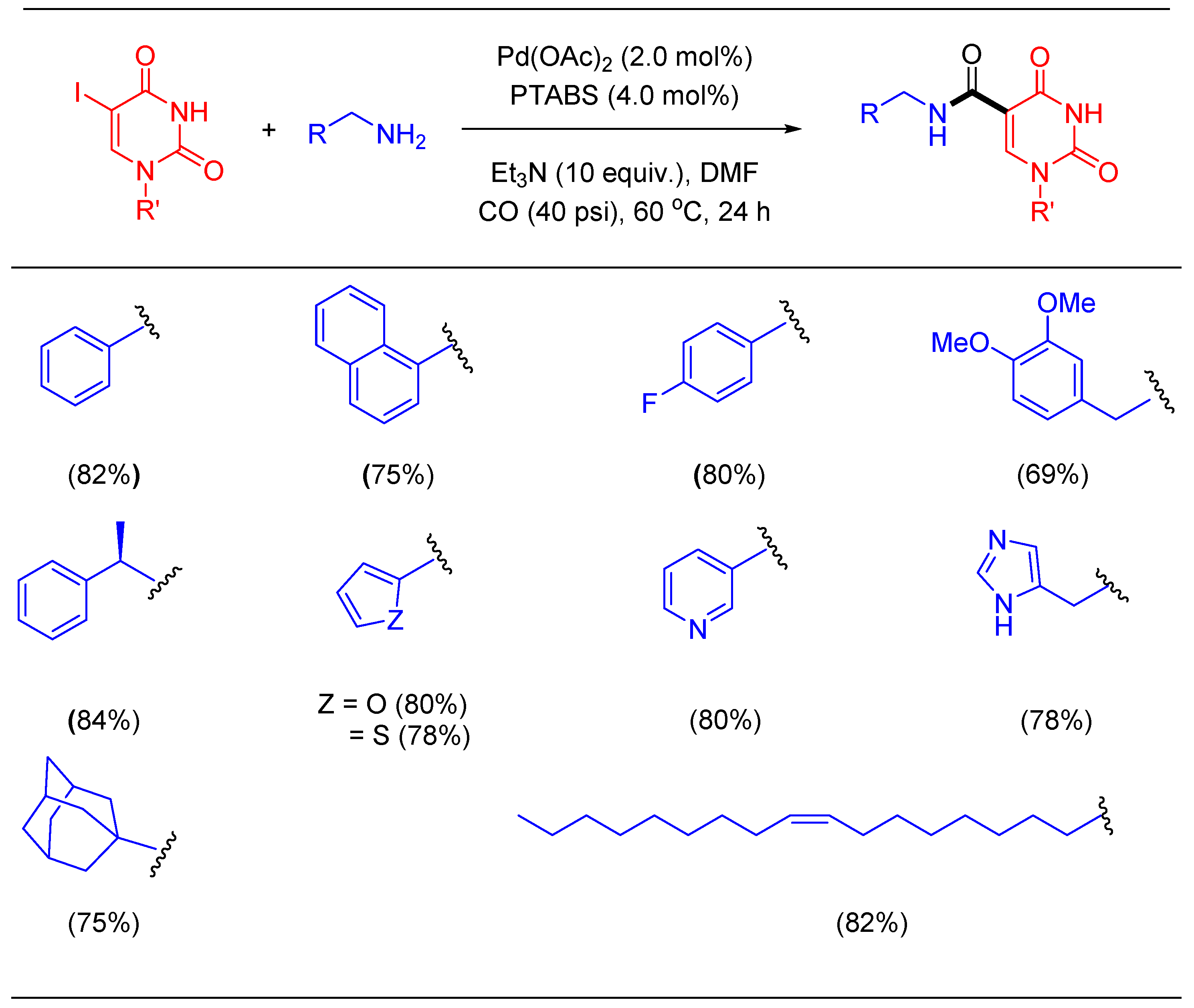
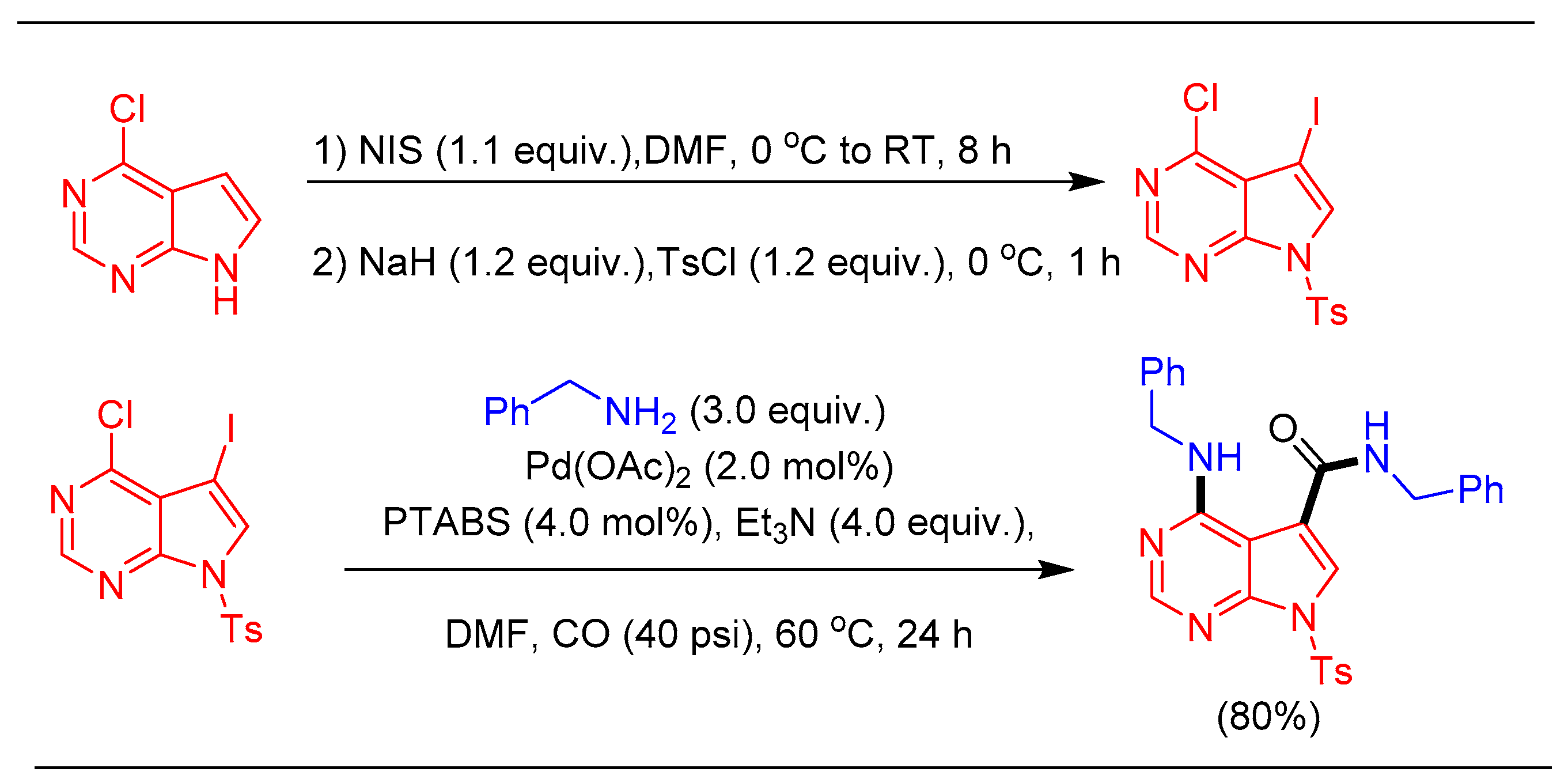
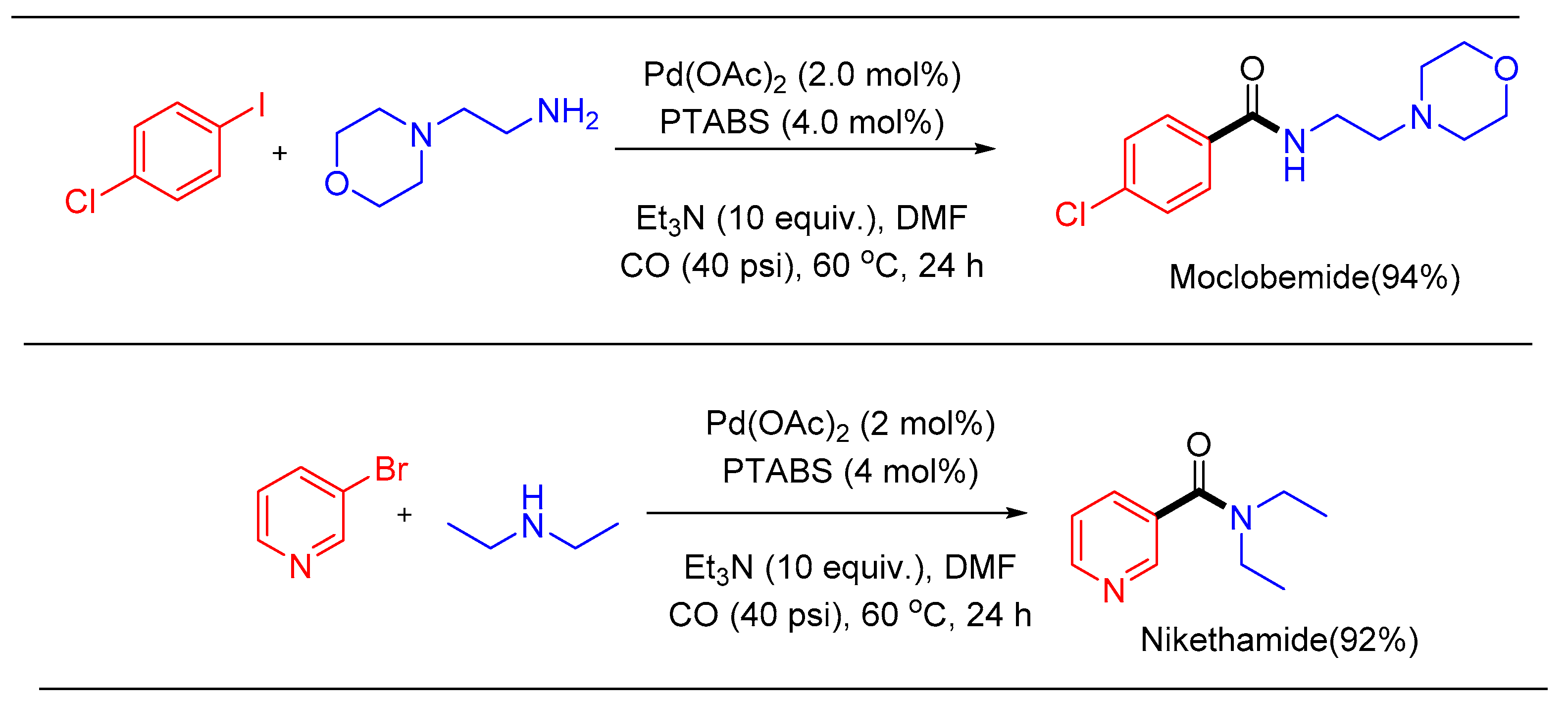
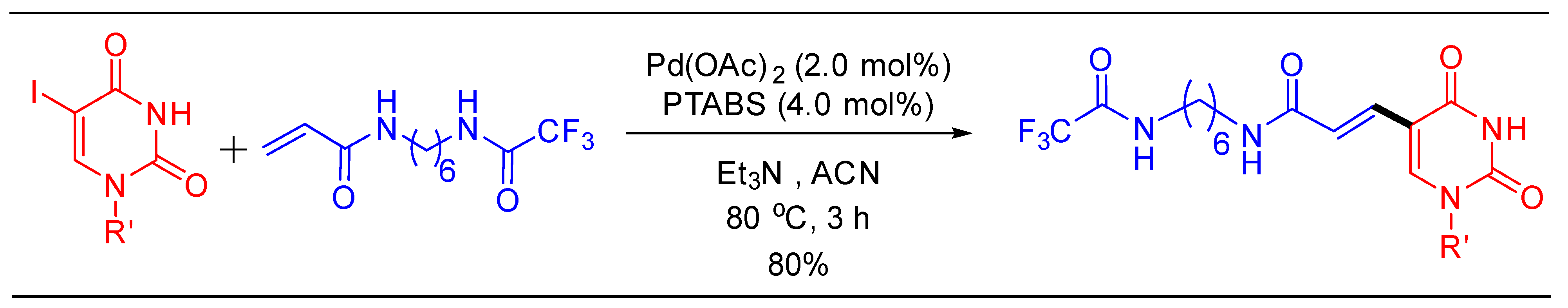
1.11. Aminocarbonylation Reaction Using Pd/PTABS
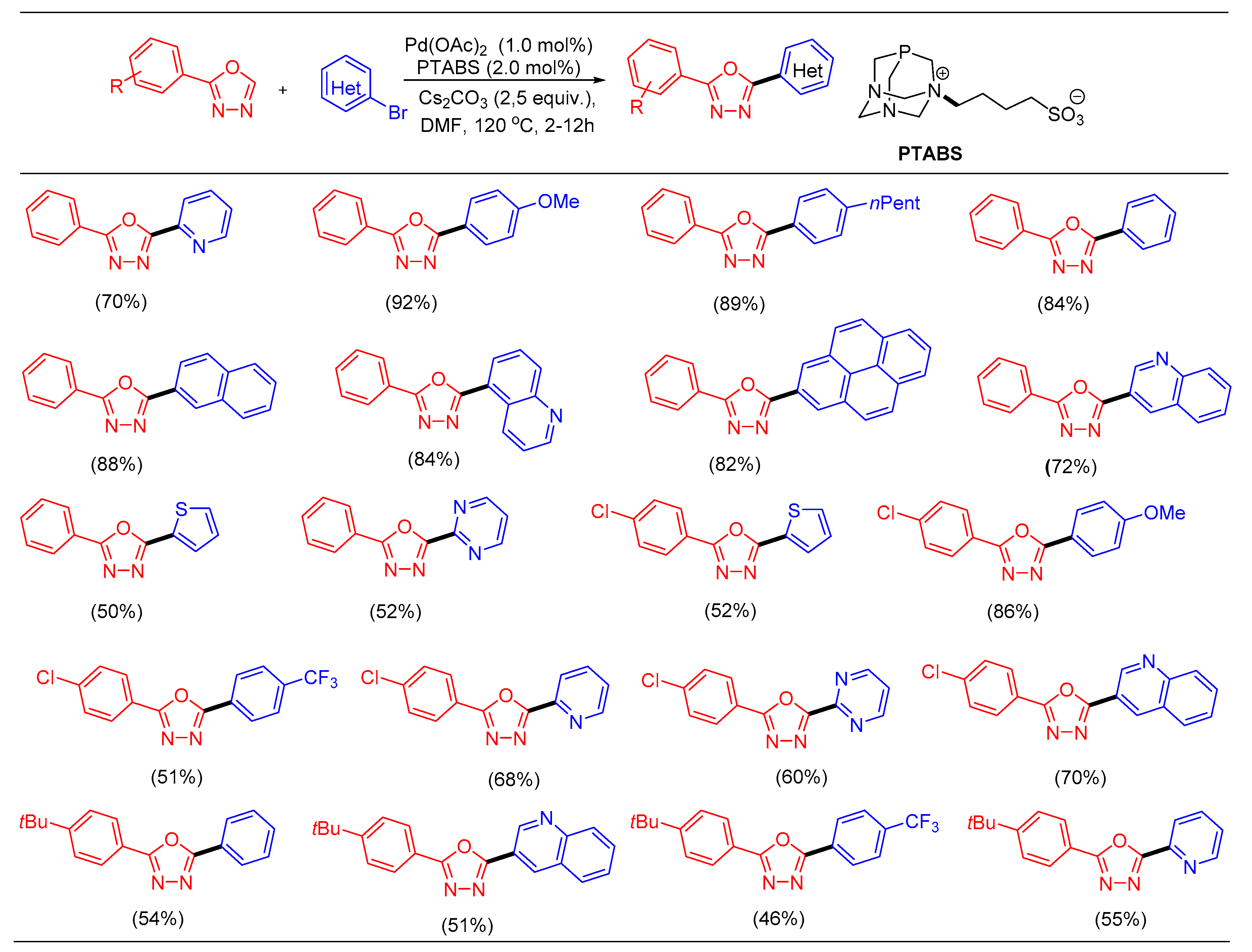
1.12. C─H Bond Functionalization of 1,3,4-oxadiazoles Using Pd/PTABS
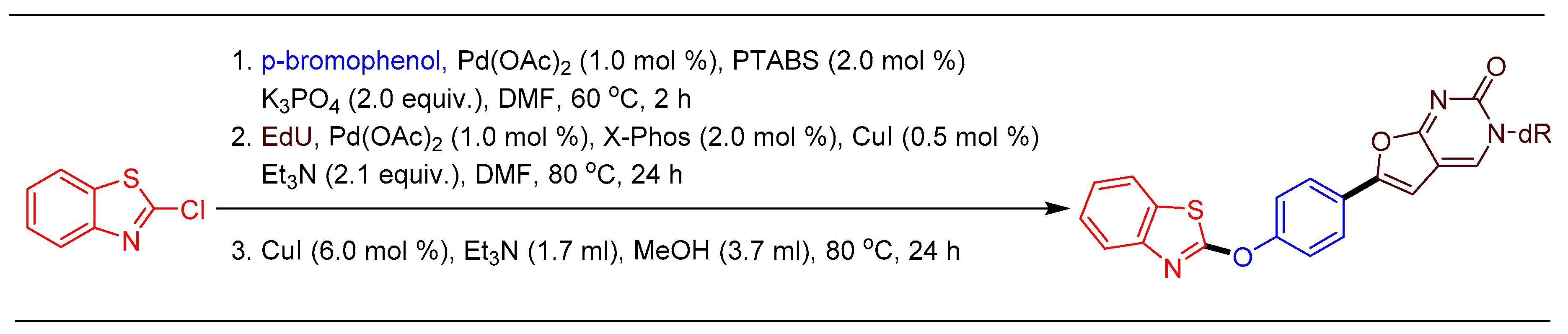
1.13. Scale-Up of Ruth Linker Using Pd/PTABS
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FDA | Food and Drug Administration |
| PTA | Triazaphosphaadamantane |
| PTABS | Triazaphosphaadamantane butane sulfonate |
| PTAPS | Triazaphosphaadamantane propane sulfonate |
| ICP-MS | Inductively Coupled Plasma Mass spectrometry |
| BVDU | Brivudine |
| CVU | Carboxyvinyl2’-deoxy uridine |
References
- Mikhailopulo, I.A.; Miroshnikov, A.I. Biologically Important Nucleosides: Modern Trends in Biotechnology and Application. Mendeleev Commun. 2011, 21, 57–68. [Google Scholar] [CrossRef]
- Girke, C.; Daumann, M.; Niopek-Witz, S.; Mohlmann, T. Nucleobase and Nucleoside Transport and Integration into Plant Metabolism. Front. Plant. Sci. 2014, 5, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Du, Y.; Tang, F.; Liu, J.; Zhao, H.; Chen, Q. Review of α-Nucleosides: From Discovery, Synthesis to Properties and Potential Applications. RSC Adv. 2019, 9, 14302–14320. [Google Scholar] [CrossRef]
- Merino, P. Chemical Synthesis of Nucleoside Analogues; John Wiley& Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Lehsten, D.M.; Baehr, D.N.; Lobl, T.J.; Vaino, A.R. An Improved Procedure for the Synthesis of Nucleoside Phosphoramidates. Org. Process. Res. Dev. 2002, 6, 819–822. [Google Scholar] [CrossRef]
- Sanjeev, A.; Mattaparthi, V.S.K.; Kaushik, S. Nucleic-Acid Structure Database; Ranganathan, S., Gribskov, M., Nakai, K., Schonbach, C., Eds.; Academic Press: Oxford, UK, 2019; pp. 567–574. [Google Scholar]
- Huang, R.M.; Chen, Y.N.; Zeng, Z.; Gao, C.H.; Su, X.; Peng, Y. Marine Nucleosides: Structure, Bioactivity, Synthesis and Biosynthesis. Mar. Drugs 2014, 12, 5817–5838. [Google Scholar] [CrossRef]
- Eyer, L.; Nencka, R.; de Clercq, E.; Seley-Radtke, K.; Ruzek, D. Nucleoside Analogs as a Rich Source of Antiviral Agents Active against Arthropod-Borne Flaviviruses. Antivir. Chem. Chemother. 2018, 26, 1–28. [Google Scholar] [CrossRef]
- Singh, J.; Ripp, A.; Haas, T.M.; Qiu, D.; Keller, M.; Wender, P.A.; Siegel, J.S.; Baldridge, K.K.; Jessen, H.J. Synthesis of Modified Nucleoside Oligophosphates Simplified: Fast, Pure, and Protecting Group Free. J. Am. Chem. Soc. 2019, 141, 15013–15017. [Google Scholar] [CrossRef]
- Allerson, C.R.; Chen, S.L.; Verdine, G.L. A Chemical Method for Site-Specific Modification of RNA: The Convertible Nucleoside Approach. J. Am. Chem. Soc. 1997, 119, 7423–7433. [Google Scholar] [CrossRef]
- Roy, B.; Depaix, A.; Perigaud, C.; Peyrottes, S. Recent Trends in Nucleotide Synthesis. Chem. Rev. 2016, 116, 7854–7897. [Google Scholar] [CrossRef]
- Matsuda, A.; Sasaki, T. Antitumor Activity of Sugar-Modified Cytosine Nucleosides. Cancer Sci. 2004, 95, 105–111. [Google Scholar] [CrossRef]
- Le, B.T.; Hornum, M.; Sharma, P.K.; Nielsen, P.; Veedu, R.N. Nucleobase-Modified Antisense Oligonucleotides Containing 5-(Phenyltriazol)-2′-Deoxyuridine Nucleotides Induce Exon-Skipping in Vitro. RSC Adv. 2017, 7, 54542–54545. [Google Scholar] [CrossRef]
- Loft, S.; Poulsen, H.E. Cancer Risk and Oxidative DNA Damage in Man. J. Mol. Med. 1996, 74, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Onidas, D.; Markovitsi, D.; Marguet, S.; Sharonov, A.; Gustavsson, T. Fluorescence Properties of DNA Nucleosides and Nucleotides: A Refined Steady-State and Femtosecond Investigation. J. Phys. Chem. B 2002, 106, 11367–11374. [Google Scholar] [CrossRef]
- De Clercq, E. C-Nucleosides To Be Revisited. J. Med. Chem. 2016, 59, 2301–2311. [Google Scholar] [CrossRef]
- Xu, W.; Chan, K.M.; Kool, E.T. Fluorescent Nucleobases as Tools for Studying DNA and RNA. Nat. Chem. 2017, 9, 1043–1055. [Google Scholar] [CrossRef]
- Agrofoglio, L.A.; Gillaizeau, I.; Saito, Y. Palladium-Assisted Routes to Nucleosides. Chem. Rev. 2003, 103, 1875–1916. [Google Scholar] [CrossRef]
- Sharapa, D.I.; Doronkin, D.E.; Studt, F.; Grunwaldt, J.D.; Behrens, S. Moving Frontiers in Transition Metal Catalysis: Synthesis, Characterization and Modeling. Adv. Mater. 2019, 31, 1807381–1807392. [Google Scholar] [CrossRef]
- Shin, K.; Kim, H.; Chang, S. Transition-Metal-Catalyzed C–N Bond Forming Reactions Using Organic Azides as the Nitrogen Source: A Journey for the Mild and Versatile C–H Amination. Acc. Chem. Res. 2015, 48, 1040–1052. [Google Scholar] [CrossRef]
- Balasubramanian, M. Chapter 14 Industrial Scale Palladium Chemistry. In Palladium in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; Volume 26, pp. 587–620. [Google Scholar]
- Lipshutz, B.H. Catalyst: Imagine Doing Chemistry at No Cost to the Environment! Chem 2018, 4, 2004–2007. [Google Scholar] [CrossRef]
- Tu, S.; Yusuf, S.; Muehlfeld, M.; Bauman, R.; Vanchura, B. The Destiny of Palladium: Development of Efficient Palladium Analysis Techniques in Enhancing Palladium Recovery. Org. Process. Res. Dev. 2019, 23, 2175–2180. [Google Scholar] [CrossRef]
- Takale, B.S.; Thakore, R.R.; Mallarapu, R.; Gallou, F.; Lipshutz, B.H. A Sustainable 1-Pot, 3-Step Synthesis of Boscalid Using Part per Million Level Pd Catalysis in Water. Org. Process. Res. Dev. 2020, 24, 101–105. [Google Scholar] [CrossRef]
- Kapdi, A.R.; Sanghvi, Y.S. Chapter 1-The Future of Drug Discovery: The Importance of Modified Nucleosides, Nucleotides, and Oligonucleotides; Kapdi, A.R., Maiti, D., Sanghvi, Y.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–18. [Google Scholar]
- Hazari, N.; Melvin, P.R.; Beromi, M.M. Well-Defined Nickel and Palladium Precatalysts for Cross-Coupling. Nat. Rev. Chem. 2017, 1, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, H.K. Palladium-Catalyzed Modification of Unprotected Nucleosides, Nucleotides, and Oligonucleotides. Molecules 2015, 20, 9419–9454. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, D.E.; Ruth, J.L. Synthesis of C-5 Substituted Pyrimidine Nucleosides via Organopalladium Intermediates. J. Am. Chem. Soc. 1976, 98, 1587–1589. [Google Scholar] [CrossRef]
- Delbecq, F.; Len, C. Chapter 5-Application of Heck Alkenylation Reaction in Modified Nucleoside Synthesis; Kapdi, A.R., Maiti, D., Sanghvi, Y.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 147–166. [Google Scholar]
- Yang, Q.; Sane, N.; Klosowski, D.; Lee, M.; Rosenthal, T.; Wang, N.X.; Wiensch, E. Mizoroki–Heck Cross-Coupling of Bromobenzenes with Styrenes: Another Example of Pd-Catalyzed Cross-Coupling with Potential Safety Hazards. Org. Process. Res. Dev. 2019, 23, 2148–2156. [Google Scholar] [CrossRef]
- Yang, Q.; Canturk, B.; Gray, K.; McCusker, E.; Sheng, M.; Li, F. Evaluation of Potential Safety Hazards Associated with the Suzuki–Miyaura Cross-Coupling of Aryl Bromides with Vinylboron Species. Org. Process. Res. Dev. 2018, 22, 351–359. [Google Scholar] [CrossRef]
- Yang, Q.; Babji, N.R.; Good, S. Potential Safety Hazards Associated with Pd-Catalyzed Cross-Coupling Reactions. Org. Process Res. Dev. 2019, 23, 2608–2626. [Google Scholar] [CrossRef]
- Cho, J.H.; Prickett, C.D.; Shaughnessy, K.H. Efficient Sonogashira Coupling of Unprotected Halonucleosides in Aqueous Solvents Using Water-Soluble Palladium Catalysts. Eur. J. Org. Chem. 2010, 2010, 3678–3683. [Google Scholar] [CrossRef]
- Rohloff, J.C.; Fowler, C.; Ream, B.; Carter, J.D.; Wardle, G.; Fitzwater, T. Practical Synthesis of Cytidine-5-Carboxamide-Modified Nucleotide Reagents. Nucleosides Nucleotides Nucleic Acids 2015, 34, 180–198. [Google Scholar] [CrossRef]
- Kapdi, A.R.; Ardhapure, A.; Sanghvi, Y.S. Modulation of the Electronic Properties of Non-Innocent (E,E)-Dibenzylideneacetone for Palladium(0)-Mediated Heck Alkenylation of 5-Iodo-2′-Deoxyuridine and Scale-Up Studies. Synthesis 2015, 47, 1163–1169. [Google Scholar] [CrossRef][Green Version]
- Gayakhe, V.; Ardhapure, A.V.; Kapdi, A.R.; Sanghvi, Y.S.; Serrano, J.L.; Schulzke, C. C-C Bond Formation: Synthesis of C-5 Substituted Pyrimidine and C-8 Substituted Purine Nucleosides Using Water Soluble Pd-Imidate Complex. Curr. Protoc. Nucleic Acid Chem. 2016, 65, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Gayakhe, V.; Ardhapure, A.; Kapdi, A.R.; Sanghvi, Y.S.; Serrano, J.L.; Garcia, L.; Perez, J.; Garcia, J.; Sanchez, G.; Fischer, C. Water-Soluble Pd–Imidate Complexes: Broadly Applicable Catalysts for the Synthesis of Chemically Modified Nucleosides via Pd-Catalyzed Cross-Coupling. J. Org. Chem. 2016, 81, 2713–2729. [Google Scholar] [CrossRef] [PubMed]
- Kapdi, A.; Gayakhe, V.; Sanghvi, Y.S.; Garcia, J.; Lozano, P.; da Silva, I.; Perez, J.; Serrano, J.L. New Water Soluble Pd-Imidate Complexes as Highly Efficient Catalysts for the Synthesis of C5-Arylated Pyrimidine Nucleosides. RSC Adv. 2014, 4, 17567–17572. [Google Scholar] [CrossRef]
- Bhilare, S.; Gayakhe, V.; Ardhapure, A.V.; Sanghvi, Y.S.; Schulzke, C.; Borozdina, Y.; Kapdi, A.R. Novel Water-Soluble Phosphatriazenes: Versatile Ligands for Suzuki–Miyaura, Sonogashira and Heck Reactions of Nucleosides. RSC Adv. 2016, 6, 83820–83830. [Google Scholar] [CrossRef]
- Bhilare, S.; Murthy Bandaru, S.S.; Shah, J.; Chrysochos, N.; Schulzke, C.; Sanghvi, Y.S.; Kapdi, A.R. Pd/PTABS: Low Temperature Etherification of Chloroheteroarenes. J. Org. Chem. 2018, 83, 13088–13102. [Google Scholar] [CrossRef]
- Bandaru, S.S.M.; Bhilare, S.; Cardozo, J.; Chrysochos, N.; Schulzke, C.; Sanghvi, Y.S.; Gunturu, K.C.; Kapdi, A.R. Pd/PTABS: Low-Temperature Thioetherification of Chloro(Hetero)Arenes. J. Org. Chem. 2019, 84, 8921–8940. [Google Scholar] [CrossRef] [PubMed]
- Murthy, B.S.S.; Bhilare, S.; Chrysochos, N.; Gayakhe, V.; Trentin, I.; Schulzke, C.; Kapdi, A.R. Pd/PTABS: Catalyst for Room Temperature Amination of Heteroarenes. Org. Lett. 2018, 20, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Bhilare, S.; Bandaru, S.S.M.; Kapdi, A.R.; Sanghvi, Y.S.; Schulzke, C. Pd/PTABS: An Efficient Water-Soluble Catalytic System for the Amination of 6-Chloropurine Ribonucleoside and Synthesis of Alogliptin. Curr. Protoc. Nucleic Acid Chem. 2018, 74, 1–10. [Google Scholar] [CrossRef]
- Singh, Y.; Murat, P.; Defrancq, E. Recent Developments in Oligonucleotide Conjugation. Chem. Soc. Rev. 2010, 39, 2054–2070. [Google Scholar] [CrossRef] [PubMed]
- Lyttle, M.H.; Walton, T.A.; Dick, D.J.; Carter, T.G.; Beckman, J.H.; Cook, R.M. New Reagents and Methods for the Synthesis of Internal and 3’-Labeled DNA. Bioconjug. Chem. 2002, 13, 1146–1154. [Google Scholar] [CrossRef]
- Fairlamb, I.J.S.; Kapdi, A.R.; Lee, A.F.; McGlacken, G.P.; Weissburger, F.; de Vries, A.H.M.; Vondervoort, L.S. Exploiting Noninnocent (E,E)-Dibenzylideneacetone (Dba) Effects in Palladium(0)-Mediated Cross-Coupling Reactions: Modulation of the Electronic Properties of Dba Affects Catalyst Activity and Stability in Ligand and Ligand-Free Reaction Systems. Chem. A Eur. J. 2006, 12, 8750–8761. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Biafora, A.; Doppiu, A.; Bongard, H.-J.; Kelm, H.; Gooßen, L.J. A Comparative Study of Dibenzylideneacetone Palladium Complexes in Catalysis. Org. Process. Res. Dev. 2019, 23, 1462–1470. [Google Scholar] [CrossRef]
- Lipshutz, B.H. Synthetic Chemistry in a Water World. New Rules Ripe for Discovery. Curr. Opin. Green Sustain. Chem. 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Li, C.J.; Chen, L. Organic Chemistry in Water. Chem. Soc. Rev. 2006, 35, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef] [PubMed]
- Ardhapure, A.V.; Sanghvi, Y.S.; Kapdi, A.R.; García, J.; Sanchez, G.; Lozano, P.; Serrano, J.L. Pd–Imidate Complexes as Recyclable Catalysts for the Synthesis of C5-Alkenylated Pyrimidine Nucleosides via Heck Cross-Coupling Reaction. RSC Adv. 2015, 5, 24558–24563. [Google Scholar] [CrossRef]
- Hervé, G.; Len, C. First Ligand-Free, Microwave-Assisted, Heck Cross-Coupling Reaction in Pure Water on a Nucleoside–Application to the Synthesis of Antiviral BVDU. RSC Adv. 2014, 4, 46926–46929. [Google Scholar] [CrossRef]
- Tan, L.; Liu, Y.; Yang, Q.; Li, X.; Wu, X.Y.; Gong, B.; Shen, Y.M.; Shao, Z. Design and Synthesis of Fluorescence-Labeled Nucleotide with a Cleavable Azo Linker for DNA Sequencing. Chem. Commun. 2016, 52, 954–957. [Google Scholar] [CrossRef]
- Jantke, D.; Cokoja, M.; Pothig, A.; Herrmann, W.A.; Kuhn, F.E. Synthesis and Characterization of Highly Water Soluble Ruthenium(II) and Osmium(II) Complexes Bearing Chelating Sulfonated N-Heterocyclic Carbene Ligands. Organometallics 2013, 32, 741–744. [Google Scholar] [CrossRef]
- Rajaraman, A.; Sahoo, A.R.; Hild, F.; Fischmeister, C.; Achard, M.; Bruneau, C. Ruthenium(II) and Iridium(III) Complexes Featuring NHC–Sulfonate Chelate. Dalton. Trans. 2015, 44, 17467–17472. [Google Scholar] [CrossRef]
- Bangde, P.S.; Prajapati, D.S.; Dandekar, P.P.; Kapdi, A.R. New Water-Soluble N-Heterocyclic Carbene-Palladium Complexes as Promising Anti-Tumor Agents: Investigating DNA and Protein Interactions. ChemistrySelect 2018, 3, 5709–5716. [Google Scholar] [CrossRef]
- Hervé, G.; Len, C. Heck and Sonogashira Couplings in Aqueous Media–Application to Unprotected Nucleosides and Nucleotides. Sustain. Chem. Process. 2015, 3, 1–23. [Google Scholar] [CrossRef]
- McGuigan, C.; Yarnold, C.J.; Jones, G.; Velázquez, S.; Barucki, H.; Brancale, A.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. Potent and Selective Inhibition of Varicella-Zoster Virus (VZV) by Nucleoside Analogues with an Unusual Bicyclic Base. J. Med. Chem. 1999, 42, 4479–4484. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Vorholt, A.J.; Ostrowski, K.A.; Seidensticker, T. Towards Resource Efficient Chemistry: Tandem Reactions with Renewables. Green Chem. 2014, 16, 982–1006. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Luoni, G.M.; McGuigan, C.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. Bicyclic Nucleoside Inhibitors of Varicella-Zoster Virus Modified on the Sugar Moiety: 3’ and 5’ Derivatives. Antivir. Chem. Chemother. 2004, 15, 333–341. [Google Scholar] [CrossRef][Green Version]
- McGuigan, C.; Pathirana, R.N.; Migliore, M.; Adak, R.; Luoni, G.; Jones, A.T.; Diez-Torrubia, A.; Camarasa, M.J.; Velazquez, S.; Henson, G. Preclinical Development of Bicyclic Nucleoside Analogues as Potent and Selective Inhibitors of Varicella Zoster Virus. J. Antimicrob. Chemother. 2007, 60, 1316–1330. [Google Scholar] [CrossRef]
- Ashwell, M.; Jones, A.S.; Kumar, A.; Sayers, J.R.; Walker, R.T.; Sakuma, T.; De Clercq, E. The Synthesis and Antiviral Properties of (E)-5-(2-Bromovinyl)-2’-Deoxyuridine-Related Compounds. Tetrahedron 1987, 43, 4601–4608. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Q.X.; Long, C.Y.; Tan, Y.; Qu, Y.X.; Su, M.H.; Huang, S.J.; Tan, W.; Wang, X.Q. Anticancer-Active N-Heteroaryl Amines Syntheses: Nucleophilic Amination of N-Heteroaryl Alkyl Ethers with Amines. Org. Lett. 2019, 21, 5111–5115. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, M.M.; Huffman, M.A.; McNamara, J.M. Pd-Catalyzed N-Arylation of Heteroarylamines. Org. Lett. 2002, 4, 3481–3484. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Viale, G.; Curigliano, G.; Goldhirsch, A. Profile of Buparlisib and Its Potential in the Treatment of Breast Cancer: Evidence to Date. Breast Cancer 2018, 10, 23–29. [Google Scholar] [PubMed]
- Xu, S.; Hao, Q.; Li, H.; Liu, Z.; Zhou, W. Synthesis of Trelagliptin Succinate. Org. Process. Res. Dev. 2017, 21, 585–589. [Google Scholar] [CrossRef]
- Lian, Y.; Coffey, S.B.; Li, Q.; Londregan, A.T. Preparation of Heteroaryl Ethers from Azine N-Oxides and Alcohols. Org. Lett. 2016, 18, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Transition Metal Catalyzed Synthesis of Arylamines and Aryl Ethers from Aryl Halides and Triflates: Scope and Mechanism. Angew. Chem. Int. Ed. 1998, 37, 2046–2067. [Google Scholar] [CrossRef]
- Gowrisankar, S.; Sergeev, A.G.; Anbarasan, P.; Spannenberg, A.; Neumann, H.; Beller, M. A General and Efficient Catalyst for Palladium-Catalyzed C−O Coupling Reactions of Aryl Halides with Primary Alcohols. J. Am. Chem. Soc. 2010, 132, 11592–11598. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.E.; Arris, C.E.; Bentley, J.; Boyle, F.T.; Curtin, N.J.; Davies, T.G.; Endicott, J.A.; Golding, B.T.; Grant, S.; Griffin, R.J.; et al. Probing the ATP Ribose-Binding Domain of Cyclin-Dependent Kinases 1 and 2 with O6-Substituted Guanine Derivatives. J. Med. Chem. 2002, 45, 3381–3393. [Google Scholar] [CrossRef]
- Guo, H.M.; Xin, P.Y.; Niu, H.Y.; Wang, D.C.; Jiang, Y.; Qu, G.R. Microwave Irradiated C6-Functionalization of 6-Chloropurine Nucleosides with Various Mild Nucleophiles under Solvent-Free Conditions. Green Chem. 2010, 12, 2131–2134. [Google Scholar] [CrossRef]
- Balzarini, J.; McGuigan, C. Bicyclic Pyrimidine Nucleoside Analogues (BCNAs) as Highly Selective and Potent Inhibitors of Varicella-Zoster Virus Replication. J. Antimicrob. Chemother. 2002, 50, 5–9. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, J.Y.; Wei, W.Z.; Baker, V.V.; Wu, G.S. Induction of Apoptosis by the New Anticancer Drug XK469 in Human Ovarian Cancer Cell Lines. Oncogene 2002, 21, 4530–4538. [Google Scholar] [CrossRef]
- Hazeldine, S.T.; Polin, L.; Kushner, J.; White, K.; Corbett, T.H.; Horwitz, J.P. Synthetic Modification of the 2-Oxypropionic Acid Moiety in 2-{4-[(7-Chloro-2-Quinoxalinyl)Oxy]Phenoxy}propionic Acid (XK469), and Consequent Antitumor Effects. Part 4. Bioorg. Med. Chem. 2005, 13, 3910–3920. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.; Fan, J.; Zhang, Q.; Wang, Y.; Zhao, Y.; Huang, M.; Ding, M.; Zhang, Y. Semisynthesis and Insecticidal Activity of Some Novel Fraxinellone-Based Thioethers Containing 1,3,4-Oxadiazole Moiety. R. Soc. Open Sci. 2017, 4, 171053–171063. [Google Scholar] [CrossRef] [PubMed]
- Dishington, A.; Fillery, S.; Finlay, M.R.V. A One-Pot Sulfide to Sulfone Oxidation with m-Chloroperoxybenzoic Acid and Sodium Permanganate. Tetrahedron Lett. 2010, 51, 4211–4213. [Google Scholar] [CrossRef]
- Li, L.; Ding, Y. Recent Advances in the Synthesis of Thioether. Mini-Rev. Org. Chem. 2017, 14, 407–431. [Google Scholar] [CrossRef]
- Norris, T.; Leeman, K. Development of a New Variant of the Migita Reaction for Carbon−Sulfur Bond Formation Used in the Manufacture of Tetrahydro-4-[3-[4-(2-Methyl-1H-Imidazol-1-Yl)Phenyl]Thio]Phenyl-2H-Pyran-4-Carboxamide. Org. Process. Res. Dev. 2008, 12, 869–876. [Google Scholar] [CrossRef]
- Adib, M.; Sadeghi, V.; Veisi, H. CuI Catalyzed-Novel One-Pot Synthesis of Aryl Alkenyl Thioethers through Ullmann-Type Coupling Reactions Using Carbon Disulfide as a Sulfur Surrogate in the Presence of Nitroalkanes and Aryl Iodides. Tetrahedron Lett. 2018, 59, 1928–1931. [Google Scholar] [CrossRef]
- Schmink, J.R.; Dockrey, S.A.B.; Zhang, T.; Chebet, N.; van Venrooy, A.; Sexton, M.; Lew, S.I.; Chou, S.; Okazaki, A. Palladium-Catalyzed Synthesis of Aryl Vinyl Sulfides via 1,3-Oxathiolanes As Vinyl Sulfide Surrogates. Org. Lett. 2016, 18, 6360–6363. [Google Scholar] [CrossRef]
- Wise, H. Mechanisms of Catalyst Poisoning by Sulfur Species. In Catalyst Deactivation 1991; Elsevier: Amsterdam, The Netherlands, 1991; pp. 497–504. [Google Scholar]
- Kolpin, A.; Jones, G.; Jones, S.; Zheng, W.; Cookson, J.; York, A.P.E.; Collier, P.J.; Tsang, S.C.E. Quantitative Differences in Sulfur Poisoning Phenomena over Ruthenium and Palladium: An Attempt To Deconvolute Geometric and Electronic Poisoning Effects Using Model Catalysts. ACS Catal. 2017, 7, 592–605. [Google Scholar] [CrossRef]
- Guilbaud, J.; Labonde, M.; Selmi, A.; Kammoun, M.; Cattey, H.; Pirio, N.; Roger, J.; Hierso, J.-C. Palladium-Catalyzed Heteroaryl Thioethers Synthesis Overcoming Palladium Dithiolate Resting States Inertness: Practical Road to Sulfones and NH-Sulfoximines. Catal. Commun. 2018, 111, 52–58. [Google Scholar] [CrossRef]
- Lazar, A.; Tomalik-Scharte, D.; Fuhr, U. Chapter 13-Applications of Genotyping and Phenotyping for Clinically-Relevant Polymorphisms of Drug Metabolizing Enzymes and Drug Transporters. In Drug Monitoring and Clinical Chemistry; Hempel, G., Ed.; Elsevier Science: Amsterdam The Netherlands, 2004; pp. 321–353. [Google Scholar]
- Warner, B.; Johnston, E.; Arenas-Hernandez, M.; Marinaki, A.; Irving, P.; Sanderson, J. A Practical Guide to Thiopurine Prescribing and Monitoring in IBD. Frontline Gastroenterol. 2018, 9, 10–15. [Google Scholar] [CrossRef]
- Patel, A.A.; Swerlick, R.A.; McCall, C.O. Azathioprine in Dermatology: The Past, the Present, and the Future. J. Am. Acad. Dermatol. 2006, 55, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Donald, J.A. Subchapter 103B-Carbon Monoxide; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 603–606. [Google Scholar]
- Barnard, C.F.J. Palladium-Catalyzed Carbonylation—A Reaction Come of Age. Organometallics 2008, 27, 5402–5422. [Google Scholar] [CrossRef]
- Gadge, S.T.; Bhanage, B.M. Recent Developments in Palladium Catalysed Carbonylation Reactions. RSC Adv. 2014, 4, 10367–10389. [Google Scholar] [CrossRef]
- Martinelli, J.R.; Watson, D.A.; Freckmann, D.M.M.; Barder, T.E.; Buchwald, S.L. Palladium-Catalyzed Carbonylation Reactions of Aryl Bromides at Atmospheric Pressure: A General System Based on Xantphos. J. Org. Chem. 2008, 73, 7102–7107. [Google Scholar] [CrossRef] [PubMed]
- Brennführer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chem. Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef]
- Czernecki, S.; Viswanadham, G.; Valery, J.M. Synthesis of Amide Linked Nucleosides at the 6 Position of Deoxy Inosine and Their Application to DNA Synthesis, Hybridization Studies. Nucleosides Nucleotides 1998, 17, 2087–2091. [Google Scholar] [CrossRef]
- Robins, M.J.; Doboszewski, B.; Nilsson, B.L.; Peterson, M.A. Synthesis of Amide-Linked [(3′)CH2CO-NH(5′)] Nucleoside Analogues of Small Oligonucleotides. Nucleos. Nucleot. Nucl. 2000, 19, 69–86. [Google Scholar] [CrossRef]
- Vaught, J.D.; Bock, C.; Carter, J.; Fitzwater, T.; Otis, M.; Schneider, D.; Rolando, J.; Waugh, S.; Wilcox, S.K.; Eaton, B.E. Expanding the Chemistry of DNA for in Vitro Selection. J. Am. Chem. Soc. 2010, 132, 4141–4151. [Google Scholar] [CrossRef]
- Vaught, J.D.; Dewey, T.; Eaton, B.E. T7 RNA Polymerase Transcription with 5-Position Modified UTP Derivatives. J. Am. Chem. Soc. 2004, 126, 11231–11237. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. SELEX—A (r)evolutionary Method to Generate High-Affinity Nucleic Acid Ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Y.; Jiang, F.; Zhou, J.; Li, Y.; Liang, C.; Dang, L.; Lu, A.; Zhang, G. Development of Cell-SELEX Technology and Its Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2016, 17, 2079. [Google Scholar] [CrossRef] [PubMed]
- Bhilare, S.; Shah, J.; Gaikwad, V.; Gupta, G.; Sanghvi, Y.S.; Bhanage, B.M.; Kapdi, A.R. Pd/PTABS: An Efficient Catalytic System for the Aminocarbonylation of a Sugar-Protected Nucleoside. Synthesis 2019, 51, 4239–4248. [Google Scholar] [CrossRef]
- Bastea, L.I.; Hollant, L.M.A.; Döppler, H.R.; Reid, E.M.; Storz, P. Sangivamycin and Its Derivatives Inhibit Haspin-Histone H3-Survivin Signaling and Induce Pancreatic Cancer Cell Death. Sci. Rep. 2019, 9, 16588–16597. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, U. Moclobemide: Therapeutic Use and Clinical Studies. CNS Drug Rev. 2003, 9, 97–140. [Google Scholar] [CrossRef] [PubMed]
- Macarthur, J.G. The Effect of Nikethamide by Mouth in Man. Br. Med. J. 1953, 1, 547. [Google Scholar] [CrossRef]
- Bhujabal, Y.B.; Vadgaonkar, K.S.; Kapdi, A.R. Pd/PTABS: Catalyst for Efficient C-H (Hetero)Arylation of 1,3,4-Oxadiazoles Using Bromo(Hetero)Arenes. Asian J. Org. Chem. 2019, 8, 289–295. [Google Scholar] [CrossRef]
- SapalaOrganic Private Limited, Home Page. Available online: http://www.sapalaorganics.com/#/SapalaOrganic (accessed on 29 February 2020).
- Kapdi, A.R.; Bhilare, S.; Shah, J. 3,5-Diaza-1-azonia-7-phosphatricyclo[3.3.1.13,7]decane, 1-(4-Sulfobutyl)-, Inner Salt. Encycl. Reag. Org. Synth. 2020, 1–6. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhilare, S.; Shet, H.; Sanghvi, Y.S.; Kapdi, A.R. Discovery, Synthesis, and Scale-up of Efficient Palladium Catalysts Useful for the Modification of Nucleosides and Heteroarenes. Molecules 2020, 25, 1645. https://doi.org/10.3390/molecules25071645
Bhilare S, Shet H, Sanghvi YS, Kapdi AR. Discovery, Synthesis, and Scale-up of Efficient Palladium Catalysts Useful for the Modification of Nucleosides and Heteroarenes. Molecules. 2020; 25(7):1645. https://doi.org/10.3390/molecules25071645
Chicago/Turabian StyleBhilare, Shatrughn, Harshita Shet, Yogesh S. Sanghvi, and Anant R. Kapdi. 2020. "Discovery, Synthesis, and Scale-up of Efficient Palladium Catalysts Useful for the Modification of Nucleosides and Heteroarenes" Molecules 25, no. 7: 1645. https://doi.org/10.3390/molecules25071645
APA StyleBhilare, S., Shet, H., Sanghvi, Y. S., & Kapdi, A. R. (2020). Discovery, Synthesis, and Scale-up of Efficient Palladium Catalysts Useful for the Modification of Nucleosides and Heteroarenes. Molecules, 25(7), 1645. https://doi.org/10.3390/molecules25071645





