Excited-State Proton Transfer in 8-Azapurines I: A Kinetic Analysis of 8-Azaxanthine Fluorescence
Abstract
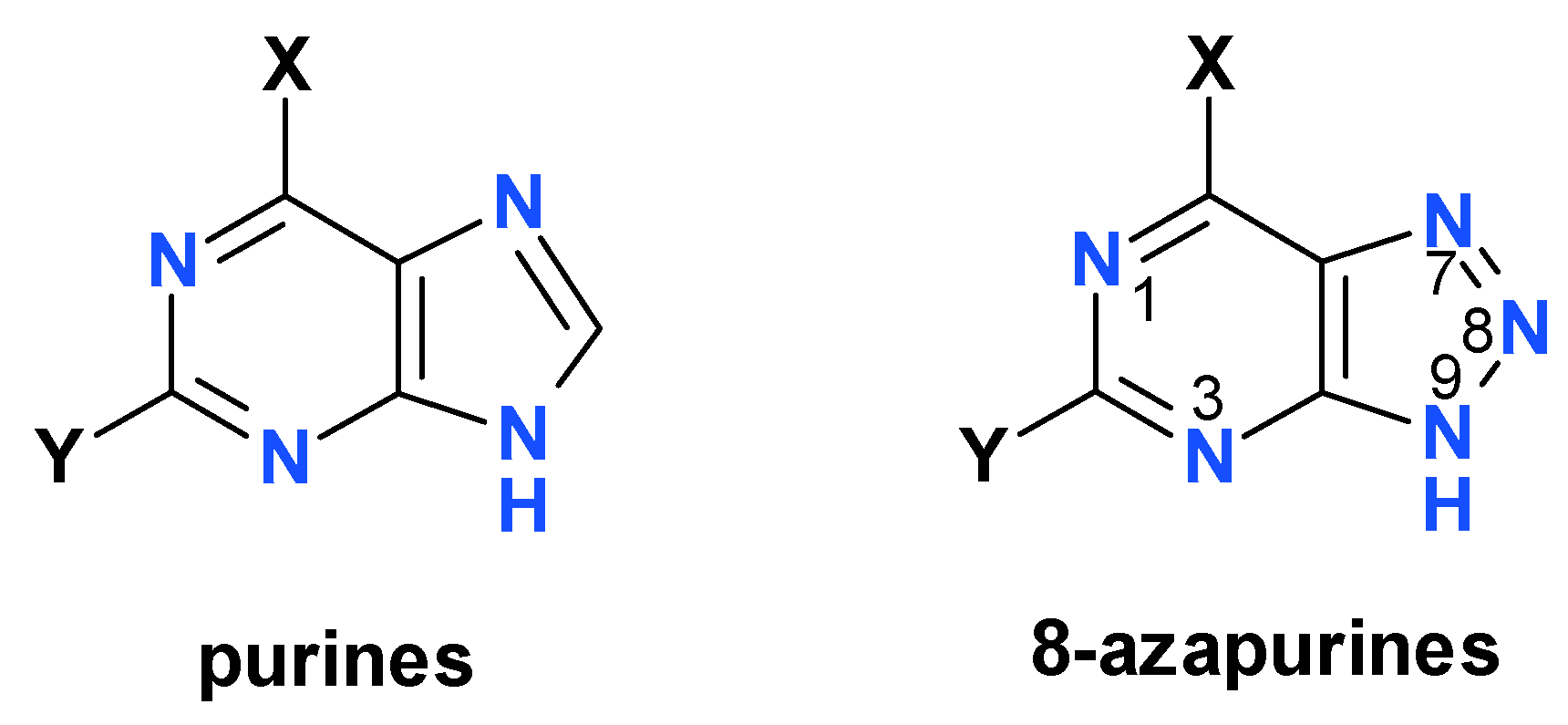
1. Introduction
2. Results
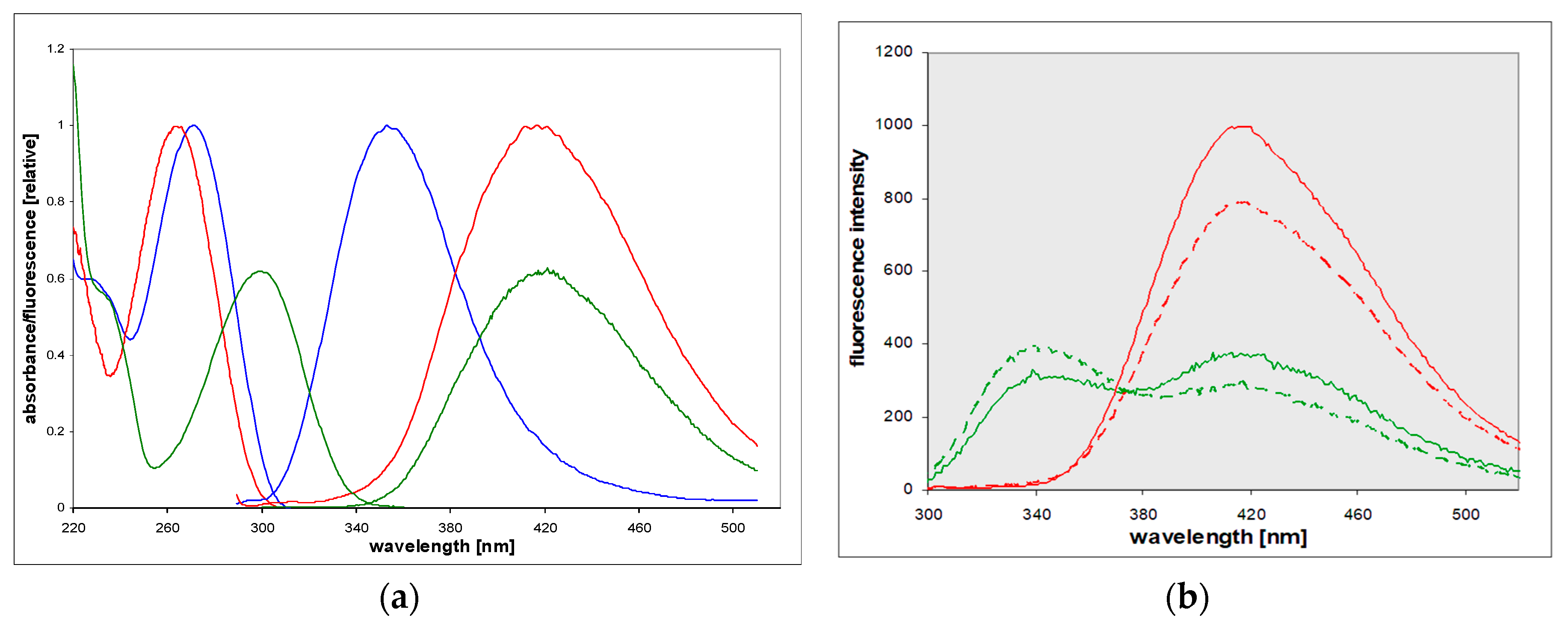
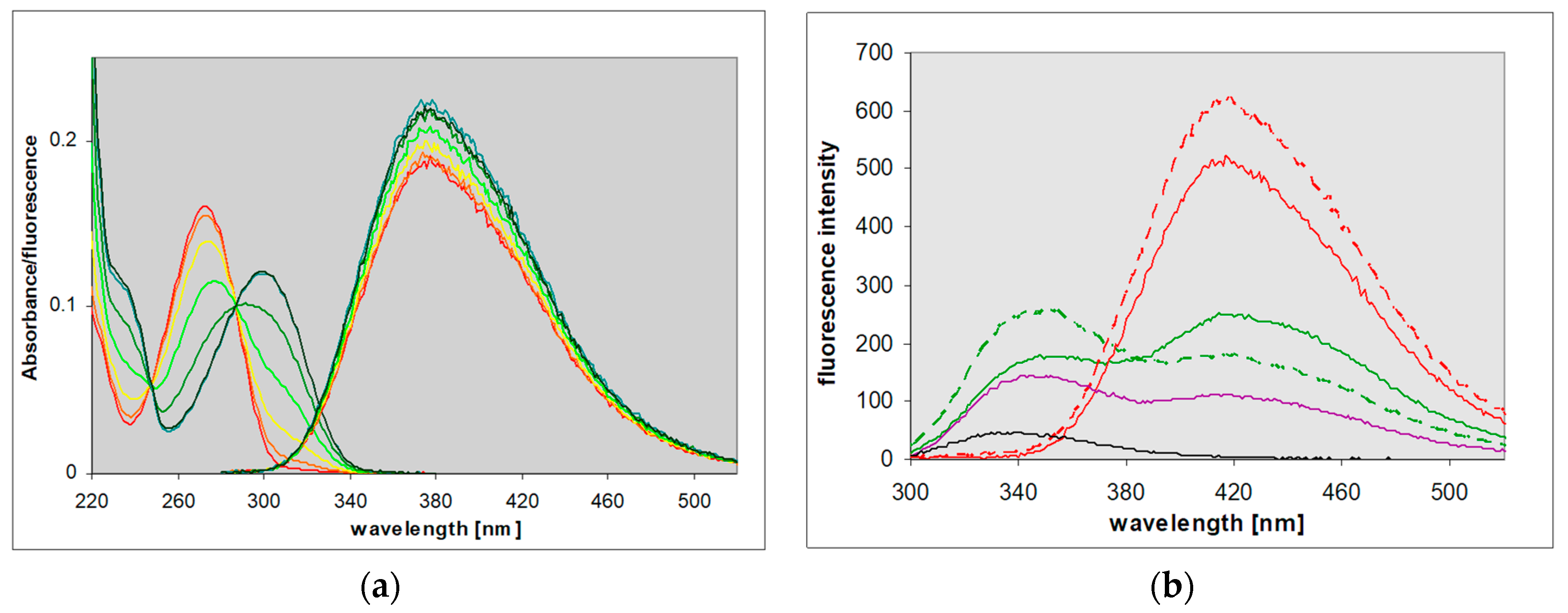
2.1. Steady-State Fluorescence of 8-Azaxanthine and Its Derivatives
2.2. Time-Resolved Spectroscopy of 8-Azaxanthine and Its N8-Methyl Derivative
3. Discussion
3.1. The Origins of Dual Emission and Calculations of pK*
3.2. Time-Resolved Spectra
3.3. Isotope Effects
3.4. Extension to Other Azapurine Derivatives
3.5. Perspectives
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sinkeldam, R.W.; Greco, N.J.; Tor, Y. Fluorescent analogs of biomolecular building blocks: Design, properties, and applications. Chem. Rev. 2010, 110, 2579–2619. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ke Min, C.; Kool, E.T. Fluorescent nucleobases as tools for studying DNA and RNA. Nat. Chem. 2017, 9, 1043–1055. [Google Scholar] [CrossRef]
- Albert, A. Chemistry of 8-azapurines. Adv. Heterocycl. Chem. 1986, 39, 117–178. [Google Scholar]
- Giorgi, I.; Scartoni, V. 8-Azapurine nucleus: A versatile scaffold for different targets. Mini Rev. Med. Chem. 2009, 9, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, J.; Antosiewicz, J.M.; Shugar, D. 8-Azapurines as isosteric purine fluorescent probes for nucleic acid and enzymatic research. Mol. BioSyst. 2014, 10, 2756–2774. [Google Scholar] [CrossRef]
- El Kouni, M.H. Potential chemotherapeutic targets in the purine metabolism of parasites. Pharmacol. Ther. 2003, 99, 283–309. [Google Scholar] [CrossRef]
- Stachelska-Wierzchowska, A.; Wierzchowski, J.; Wielgus-Kutrowska, B.; Bzowska, A.; Mikleuševic, G. Enzymatic synthesis of highly fluorescent 8-azapurine ribosides using purine-nucleoside phosphorylase reverse reaction: Variable ribosylation sites. Molecules 2013, 18, 12587–12598. [Google Scholar] [CrossRef]
- Wierzchowski, J.; Stachelska-Wierzchowska, A. Two fluorogenic substrates for purine-nucleoside phosphorylase, selective for mammalian and bacterial forms of the enzyme. Anal. Biochem. 2014. [Google Scholar] [CrossRef]
- Saito, Y.; Hudson, R.H.E. Base-modified fluorescent purine nucleosides and nucleotides for use in oligonucleotide probes. J. Photochem. Photobiol. C-Photochem. Revs 2018, 36, 48–73. [Google Scholar] [CrossRef]
- Liu, J.; Ingale, S.A.; Seela, F. Guanine and 8-Azaguanine in Anomeric DNA Hybrid Base Pairs: Stability, Fluorescence Sensing, and Efficient Mismatch Discrimination with alpha-D-Nucleosides. Bioconjugate Chem. 2018, 29, 2265–2277. [Google Scholar] [CrossRef]
- Da Costa, C.P.; Fedor, M.J.; Scott, L.G. 8-Azaguanine reporter of purine ionization states in structured RNAs. J. Am. Chem. Soc. 2007, 129, 3426–3432. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, J.W.; Scott, L.G.; Fedor, M.J. The pH dependence of hairpin ribozyme catalysis reflects ionization of an active site adenine. J. Biol. Chem. 2011, 286, 17658–17664. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, J.; Bzowska, A.; Stępniak, K.; Shugar, D. Interactions of calf spleen purine nucleoside phosphorylase with 8-Azaguanine, and a bisubstrate analogue inhibitor: Implications for the reaction mechanism. Z. Naturforsch. 2004, 59c, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, J.; Stępniak, K.; Bzowska, A.; Shugar, D. Spectroscopic and kinetic studies of interactions of calf spleen purine nucleoside phosphorylase with 8-azaguanine and its 9-(2-phosphonylmethoxyethyl) derivative. Nucleosides Nucleotides Nucl. Acids 2005, 24, 459–464. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, A.V.S.; Borin, A.C. Photochemical Relaxation Pathways of 9H-8-Azaguanine and 8H-8-Azaguanine. J. Phys. Chem. A 2019, 123, 3109–3120. [Google Scholar] [CrossRef]
- Wierzchowski, J. Excited-state proton transfer and phototautomerism in nucleobase and nucleoside analogs: A mini-review. Nucleosides Nucleotides Nucl. Acids 2014, 33, 626–644. [Google Scholar] [CrossRef]
- Wierzchowski, J.; Sepiol, J.; Sulikowski, D.; Kierdaszuk, B.; Shugar, D. Fluorescence emission properties of 8-azaxanthine and its N-alkyl derivatives: Excited-state proton transfer and potential applications in enzymology. J. Photochem. Photobiol. A 2006, 179, 276–282. [Google Scholar] [CrossRef]
- Wierzchowski, J.; Mędza, G.; Szabelski, M.; Stachelska-Wierzchowska, A. Properties of 2,6-diamino-8-azapurine, a highly fluorescent purine analog and its N-alkyl derivatives: Tautomerism and excited-state proton transfer reactions. J. Photochem. Photobiol. A 2013, 265, 49–57. [Google Scholar] [CrossRef]
- Wierzchowski, J.; Mędza, G.; Sepioł, J.; Szabelski, M.; Shugar, D. Fluorescence emission properties of 8-azaisoguanine and its N-methyl derivatives: Ground- and excited state tautomerism. J. Photochem. Photobiol. A 2012, 237, 64–70. [Google Scholar] [CrossRef]
- Ireland, J.F.; Wyatt, P.A.H. Acid-Base Properties of Electronically Excited States of Organic Molecules. Adv. Phys. Org. Chem. 1976, 12, 131–221. [Google Scholar]
- Schulman, S.; Fernando, Q. Excited State Prototropic Equilibria of Some Quinolinols. Tetrahedron 1968, 24, 1777–1783. [Google Scholar] [CrossRef]
- Kumpulainen, T.; Lang, B.; Rosspeintner, A.; Vauthey, E. Ultrafast Elementary Photochemical Processes of Organic Molecules in Liquid Solution. Chem. Rev. 2017, 117, 10826–10939. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, L.M.; Solntsev, K.M. Excited-State Proton Transfer: From constrained systems to “Super” photoacids to superfast proton transfer. Acc. Chem. Res. 2002, 35, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mędza, G.; Wierzchowski, J.; Kierdaszuk, B.; Shugar, D. Fluorescence emission properties of 8-aza analogues of caffeine, theophylline, and related N-alkyl xanthines. Bioorg. Med. Chem. 2009, 17, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Maroncelli, M.; Fleming, G.R. Picosecond solvation dynamics of coumarin 153: The importance of molecular aspects of solvation. J. Chem. Phys. 1987, 86, 6221–6239. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer Science & Busines Media: New York, NY, USA, 2006; Chapter 7; pp. 237–276. [Google Scholar]
- Nubel, G.; Pfleiderer, W.; Purine, V. Über die Synthese und Struktur von 8-Aza-xanthin (5.7-Dioxo- tetrahydro-ν-triazolo[4.5-d]pyrimidin) und seinen N-Methyl-Derivaten. Chem. Berichte 1965, 98, 1060–1072. [Google Scholar] [CrossRef]
- Huppert, D.; Tolbert, L.M.; Linares-Samaniego, S. Ultrafast Excited-State Proton Transfer from Cyano-Substituted 2-Naphthols. J. Phys. Chem. A 1997, 101, 4602–4605. [Google Scholar] [CrossRef]
- Popov, A.V.; Gould, E.A.; Salvitti, M.A.; Hernandez, R.; Solntsev, K.M. Diffusional Effects on the Reversible Excited-State Proton Transfer. From Experiments to Brownian Dynamics Simulations. Phys. Chem. Chem. Phys. 2011, 13, 14914–14927. [Google Scholar] [CrossRef]
- Agmon, N. Elementary Steps in Excited-State Proton Transfer. J. Phys. Chem. A 2005, 109, 13–35. [Google Scholar] [CrossRef]
- Barroso, M.; Arnault, L.G.; Formosinho, S. Intersecting-state model calculations on fast and ultrafast excited-state proton transfers in naphthols and substituted naphthols. J. Photochem. Photobiol. A 2002, 154, 13–21. [Google Scholar] [CrossRef]
- Franchetti, P.; Messini, L.; Cappellacci, L.; Grifantini, M.; Lucacchini, A.; Martini, C.; Senatore, G. 8-Azaxanthine derivatives as antagonists of adenosine receptors. J. Medicinal Chem. 1994, 37, 2970–2975. [Google Scholar] [CrossRef]
- Colloch, N.; ElHajji, M.; Bachet, B.; LHermite, G.; Schiltz, M.; Prange, T.; Castro, B.; Mornon, J.P. Crystal Structure of the protein drug urate oxidase-inhibitor complex at 2.05 angstrom resolution. Nat. Struct. Biol. 1997, 4, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, F.; Ungar-Waron, H.; Kwietny-Govrin, H. Action of 8-azaguanine + 8-azaxanthine on pseudomonas aeruginosa. Biochem. J. 1964, 91, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Domcke, W.; Ehrmaier, J.; Sobolewski, A.L. Solar energy harvesting with carbon nitrides and N-heterocyclic frameworks: Do we understand the mechanism? ChemPhotoCem 2019, 3, 10–23. [Google Scholar] [CrossRef]
- Kasparek, A.; Smyk, B. A new approach to the old problem: Inner filter effect type I and II in fluorescence. Spectrochim. Acta A-M 2018, 198, 297–303. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (2), (3) and (4) are available from the authors. |




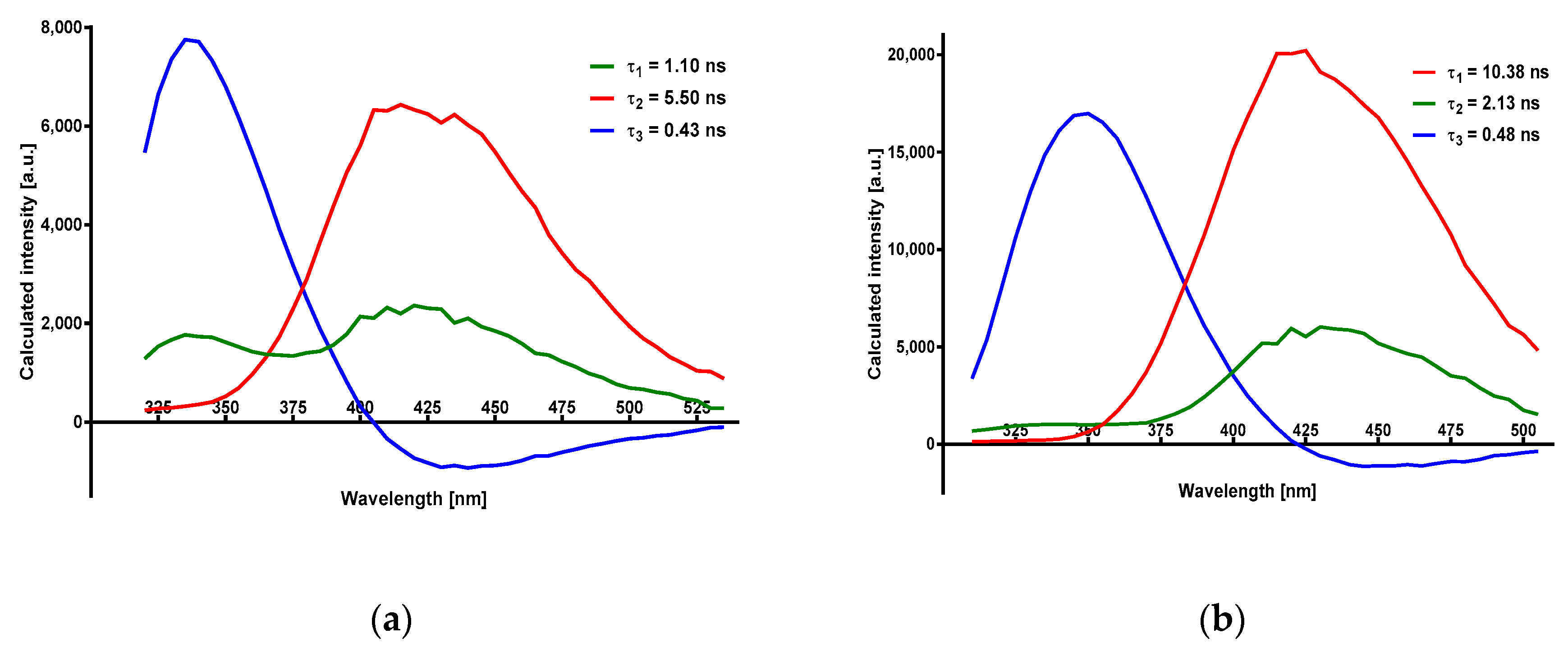
| Compound/Medium | λobs (nm) a | Rise Time (ns) | Decay: τ1 (ns) | Decay: τ2 (ns) | χ2 (Global) |
|---|---|---|---|---|---|
| 8-azaXan/H2O a | 350 ÷ 530 | - | 5.11 ± 0.05 | - | 1.110 |
| 8-azaXan/D2O a | 350 ÷ 530 | - | 8.076 ± 0.003 | - | 1.145 |
| 8-azaXan/MeOH | 320 ÷ 530 | 0.426 ± 0.004 | 5.50 ± 0.13 | 1.102 ± 0.026 | 1.167 |
| 8-azaXan/MeOD | 295 ÷ 500 | 0.510 ± 0.001 | 6.36 ± 0.04 | 1.407 ± 0.004 | 1.133 |
| 8-methyl-8azaXan/H2O | 350 ÷ 520 | - | 11.494 ± 0.004 | - | 1.104 |
| 8-methyl-8azaXan/D2O | 350 ÷ 520 | - | 14.55 ± 0.06 | - | 1.167 |
| 8-methyl-8azaXan/MeOH | 310 ÷ 505 | 0.484 ± 0.002 | 10.38 ± 0.08 | 2.134 ± 0.037 | 1.155 |
| 8-methyl-8azaXan/MeOD | 320 ÷ 505 | 0.498 ± 0.002 | 8.32 ± 0.02 | 0.865 ± 0.009 | 1.139 |
| 8-methyl-8azaXan/iprOH | 350 ÷ 500 | 0.454 ± 0.004 | 9.60 ± 0.30 | 1.401 ± 0.025 | 1.303 |
| 8-methyl-8azaXan/dioxane | 310 ÷ 370 | - | 0.13 ± 0.02 b | - | 1.279 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzchowski, J.; Smyk, B. Excited-State Proton Transfer in 8-Azapurines I: A Kinetic Analysis of 8-Azaxanthine Fluorescence. Molecules 2020, 25, 2740. https://doi.org/10.3390/molecules25122740
Wierzchowski J, Smyk B. Excited-State Proton Transfer in 8-Azapurines I: A Kinetic Analysis of 8-Azaxanthine Fluorescence. Molecules. 2020; 25(12):2740. https://doi.org/10.3390/molecules25122740
Chicago/Turabian StyleWierzchowski, Jacek, and Bogdan Smyk. 2020. "Excited-State Proton Transfer in 8-Azapurines I: A Kinetic Analysis of 8-Azaxanthine Fluorescence" Molecules 25, no. 12: 2740. https://doi.org/10.3390/molecules25122740
APA StyleWierzchowski, J., & Smyk, B. (2020). Excited-State Proton Transfer in 8-Azapurines I: A Kinetic Analysis of 8-Azaxanthine Fluorescence. Molecules, 25(12), 2740. https://doi.org/10.3390/molecules25122740






