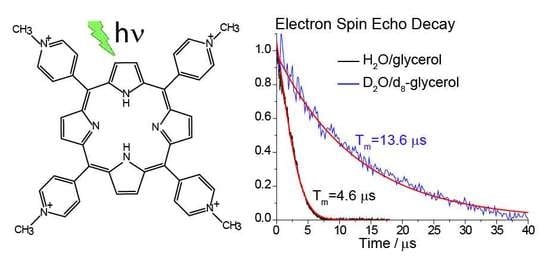
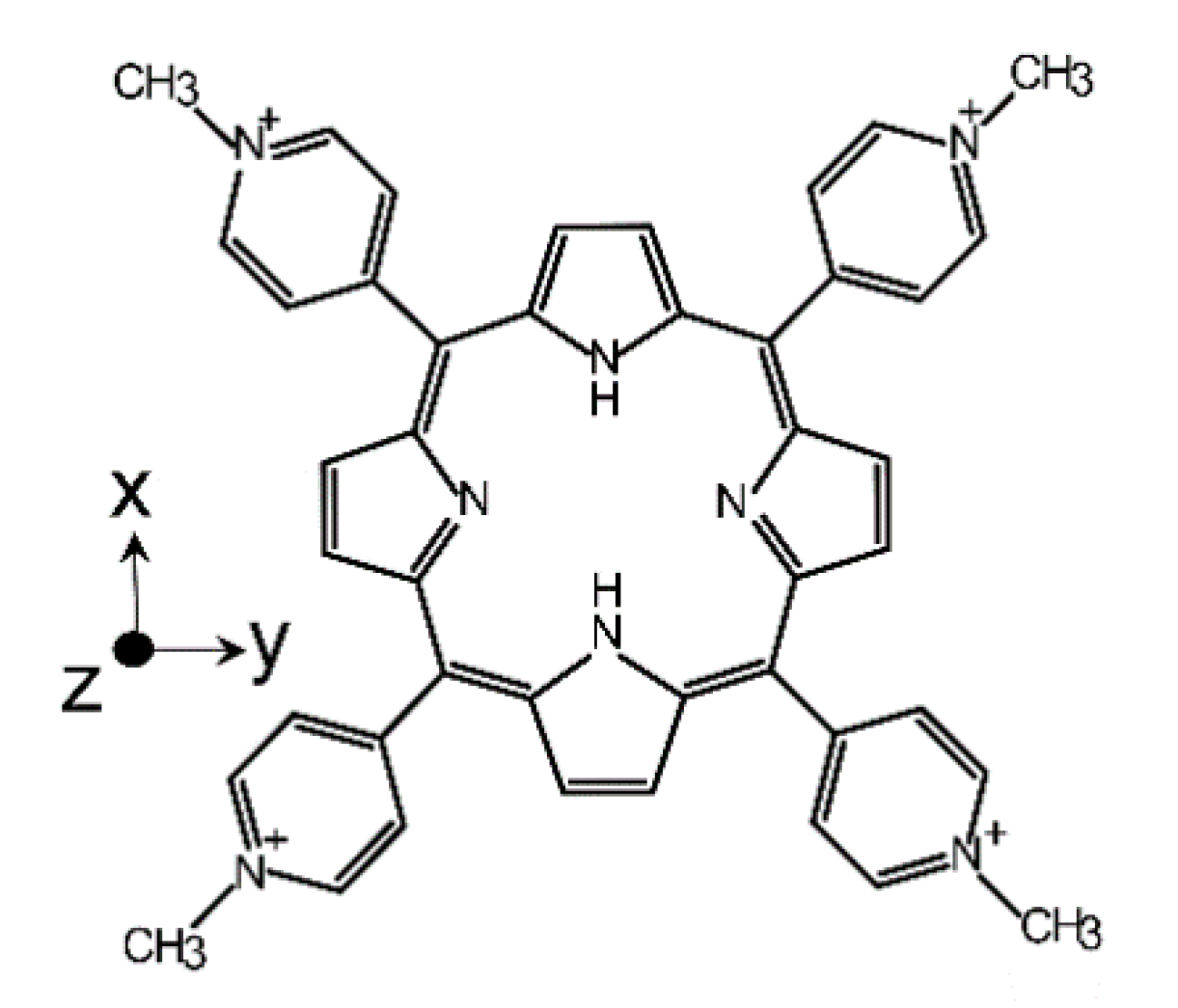
Electron Spin Relaxation of Photoexcited Porphyrin in Water—Glycerol Glass
Abstract
1. Introduction
2. Results and Discussion
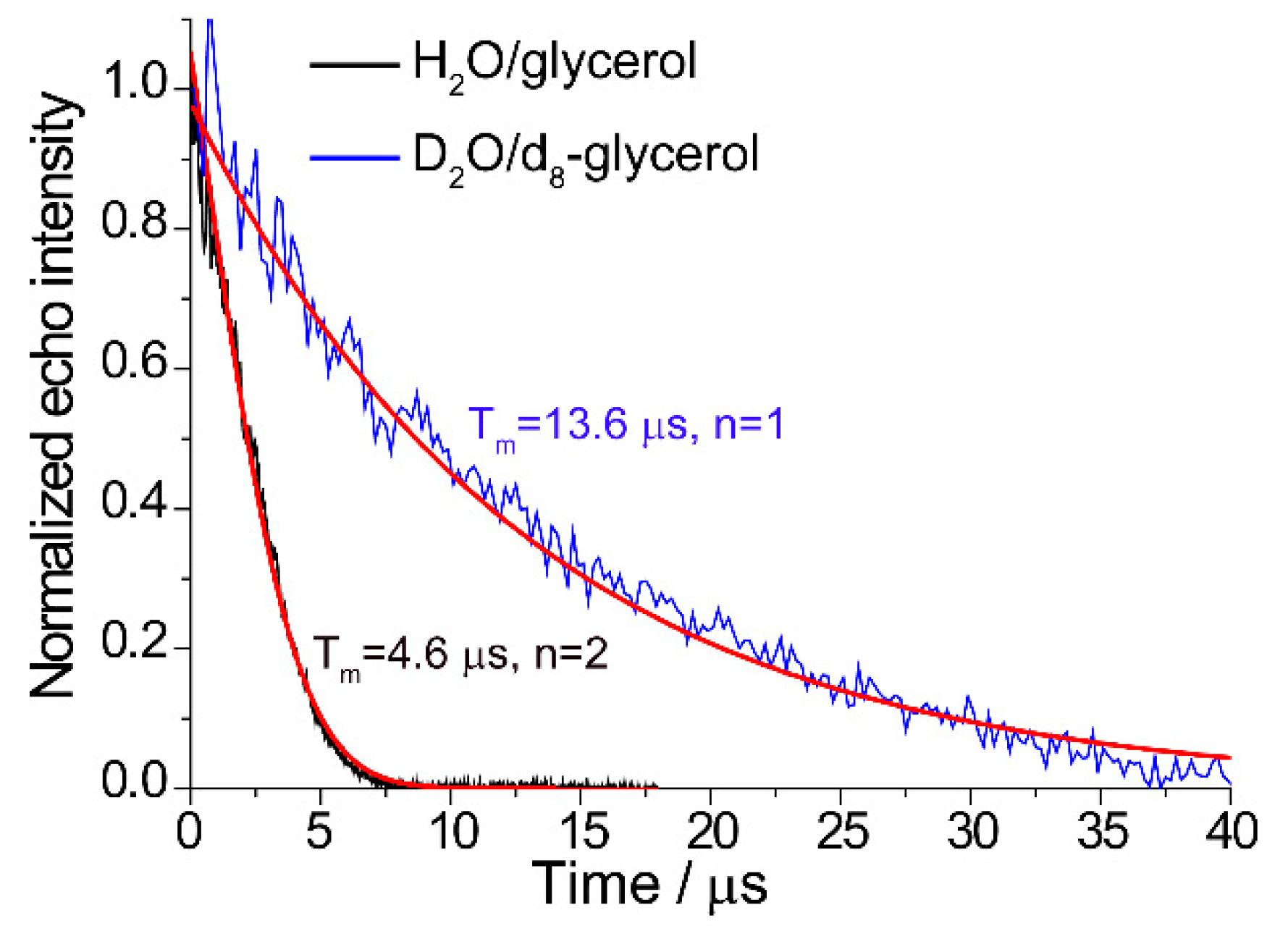
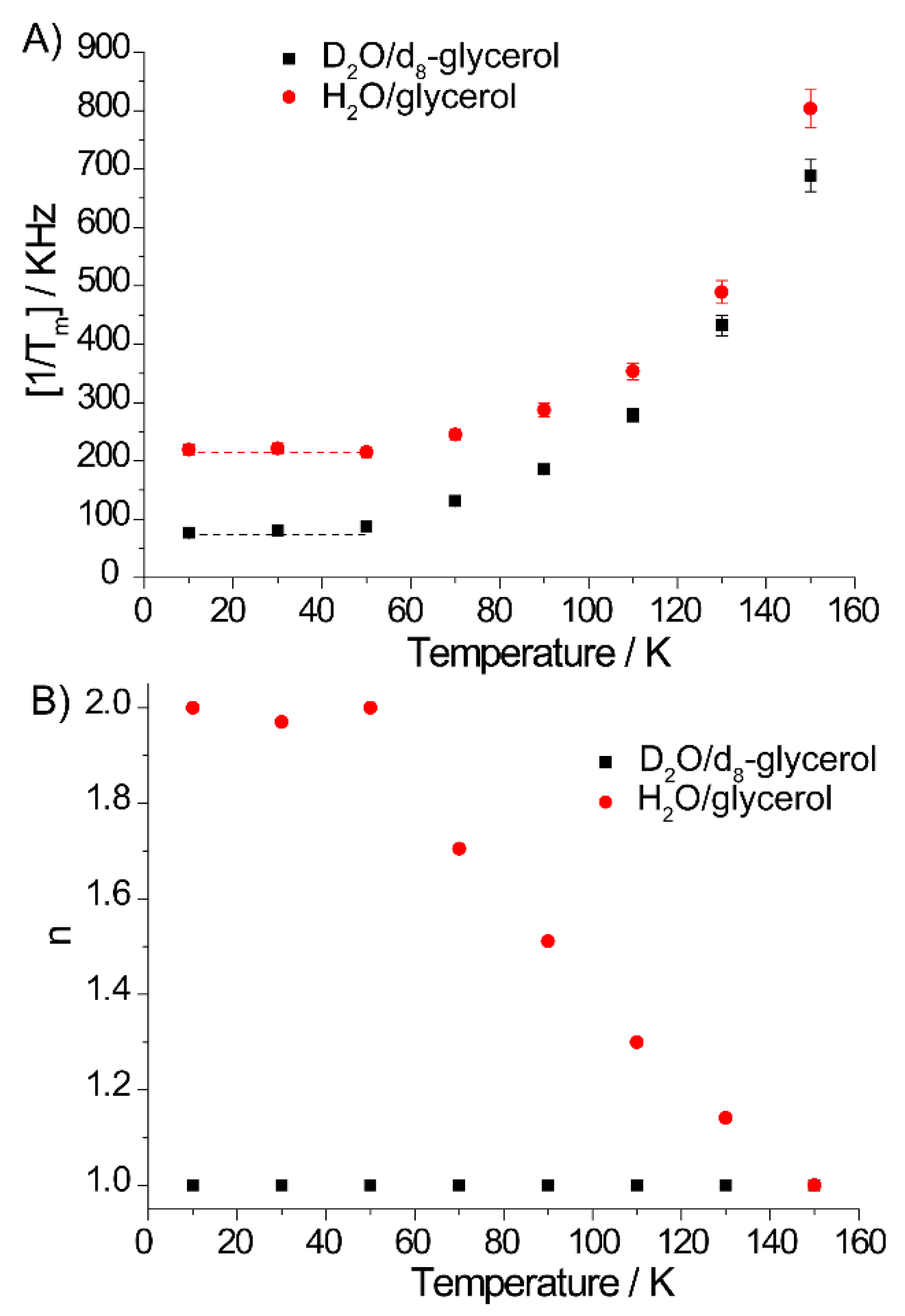
2.1. Tm in Photoexcited TMPyP4
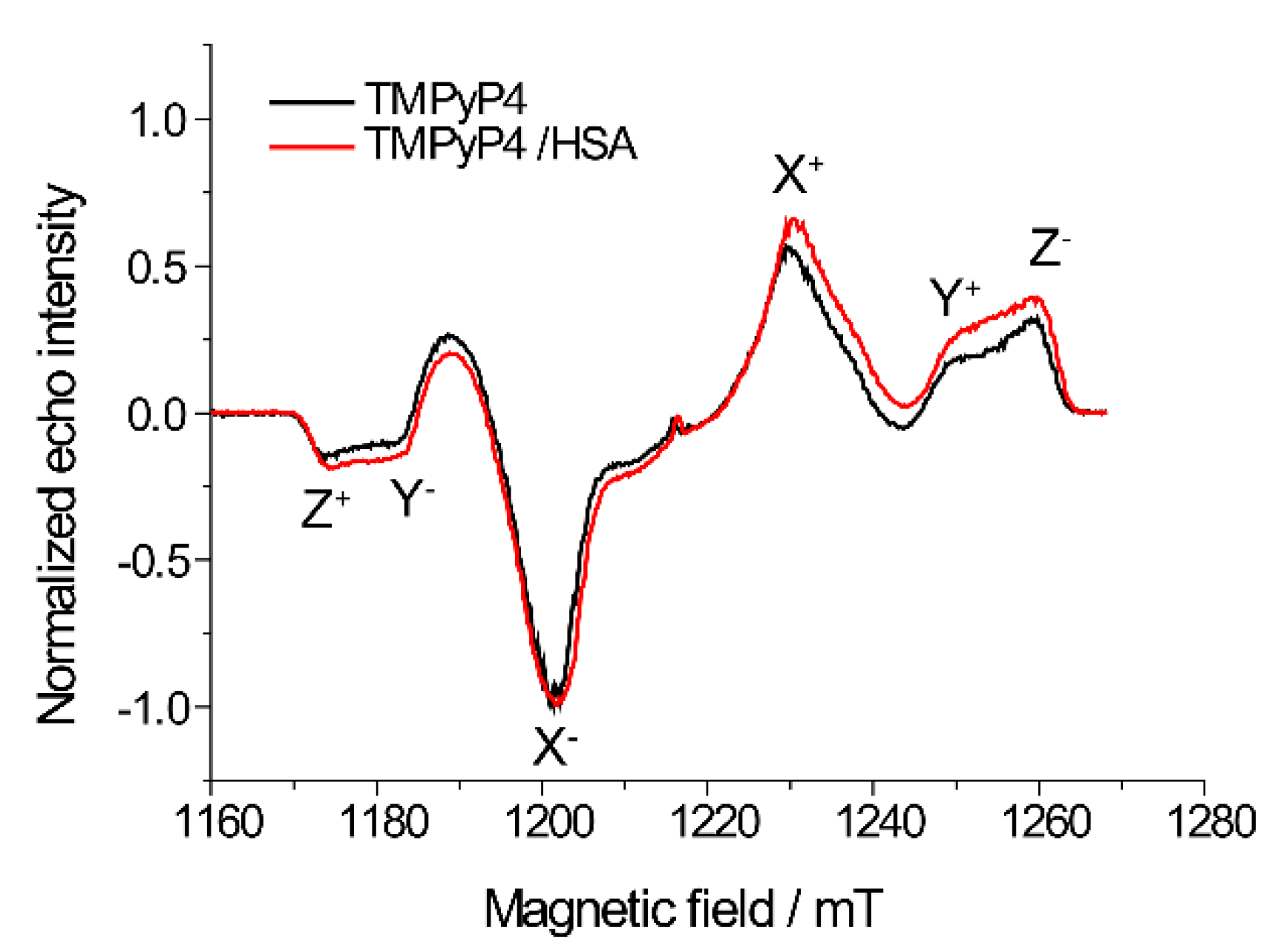
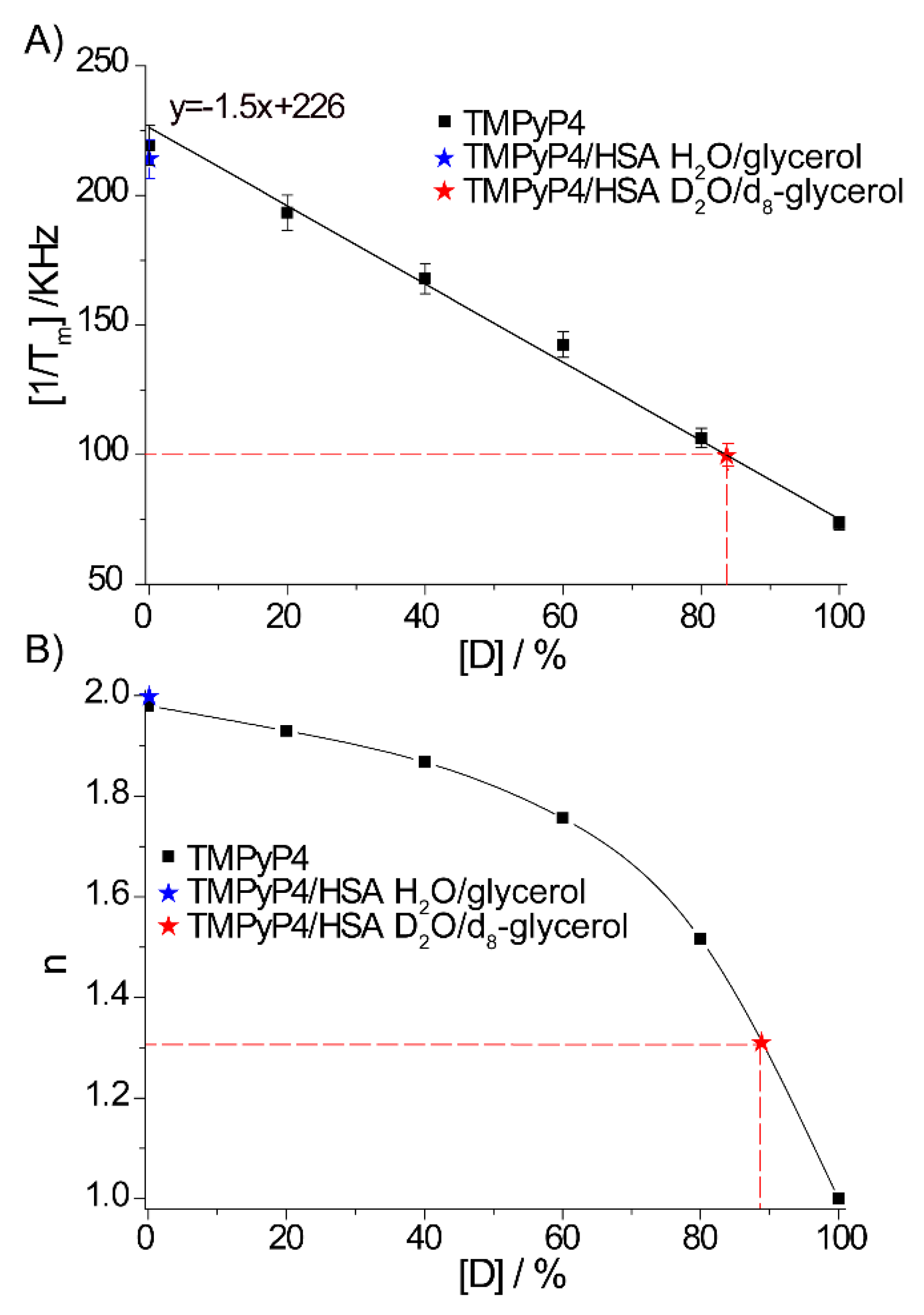
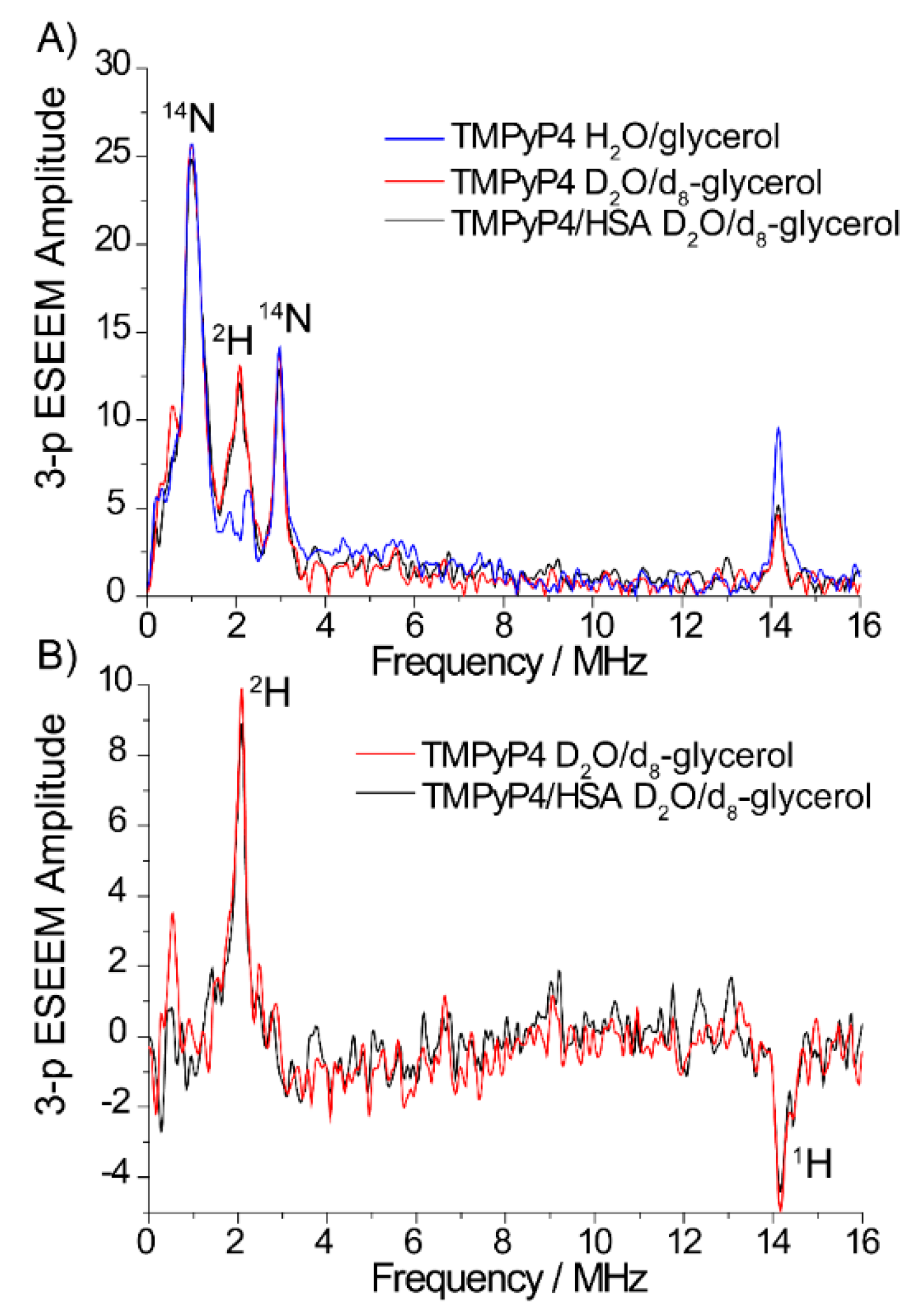
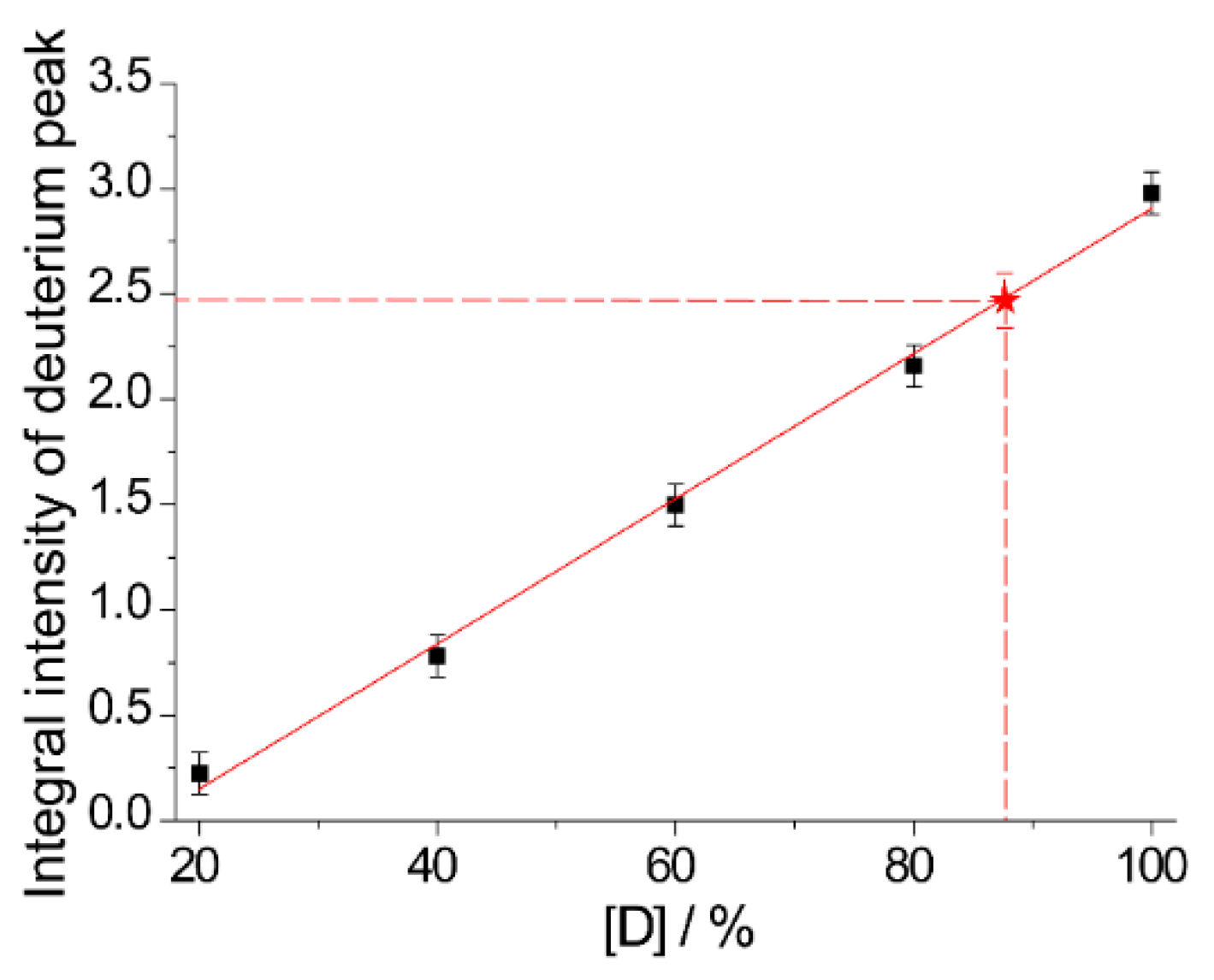
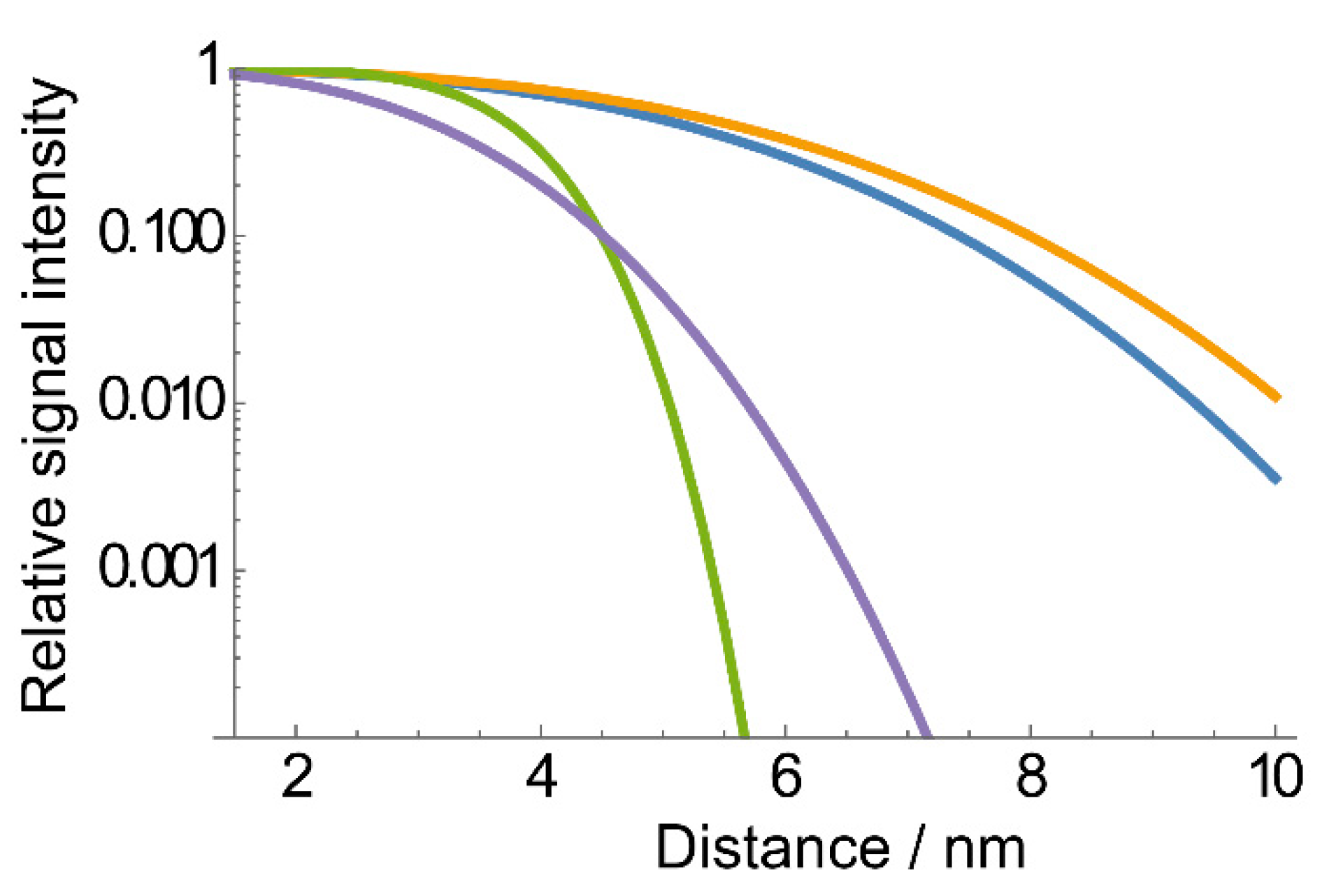
2.2. The Use of Tm to Determine Porphyrin Location in a Biopolymer
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duss, O.; Michel, E.; Yulikov, M.; Schubert, M.; Jeschke, G.; Allain, F.H.T. Structural basis of the non-coding RNA RsmZ acting as a protein sponge. Nature 2014, 509, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. DEER Distance Measurements on Proteins. Annu. Rev. Phys. Chem. 2012, 63, 419–446. [Google Scholar] [CrossRef]
- Schiemann, O.; Prisner, T.F. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q. Rev. Biophys. 2007, 40, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Malygin, A.A.; Krumkacheva, O.A.; Graifer, D.M.; Timofeev, I.O.; Ochkasova, A.S.; Meschaninova, M.I.; Venyaminova, A.G.; Fedin, M.V.; Bowman, M.; Karpova, G.G.; et al. Exploring the interactions of short RNAs with the human 40S ribosomal subunit near the mRNA entry site by EPR spectroscopy. Nucleic Acids Res. 2019, 47, 11850–11860. [Google Scholar] [CrossRef] [PubMed]
- Krumkacheva, O.A.; Shevelev, G.Y.; Lomzov, A.A.; Dyrkheeva, N.S.; Kuzhelev, A.A.; Koval, V.V.; Tormyshev, V.M.; Polienko, Y.F.; Fedin, M.V.; Pyshnyi, D.V.; et al. DNA complexes with human apurinic/apyrimidinic endonuclease 1: Structural insights revealed by pulsed dipolar EPR with orthogonal spin labeling. Nucleic Acids Res. 2019, 47, 7767–7780. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, Y.D.; Bowman, M.K.; Grishin, Y.A. Experimental Techniques BT—Pulsed Electron–Electron Double Resonance: Nanoscale Distance Measurement in the Biological, Materials and Chemical Sciences; Tsvetkov, Y.D., Bowman, M.K., Grishin, Y.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 37–65. ISBN 978-3-030-05372-7. [Google Scholar]
- Pannier, M.; Veit, S.; Godt, A.; Jeschke, G.; Spiess, H.W. Dead-time free measurement of dipole–dipole interactions between electron spins. J. Magn. Reson. 2000, 142, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Milov, A.; Salikohov, K.; Shirov, M. Application of ELDOR in Electron-Spin Echo for Paramagnetic Center Space Distribution in Solids. Fiz. Tverd. Tela 1981, 23, 975–982. [Google Scholar]
- Bagryanskaya, E.G.; Krumkacheva, O.A.; Fedin, M.V.; Marque, S.R.A. Development and Application of Spin Traps, Spin Probes, and Spin Labels. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2015; Volume 563, pp. 365–396. [Google Scholar]
- Weickert, S.; Cattani, J.; Drescher, M. Intrinsically disordered proteins (IDPs) studied by EPR and in-cell EPR. Electron. Paramagn. Reson. 2019, 26, 1–37. [Google Scholar]
- Jeschke, G. Dipolar spectroscopy-double-resonance methods. eMagRes 2016, 5, 1459–1476. [Google Scholar]
- Albertini, M.; Carbonera, D.; Gobbo, M.; Zurlo, E.; Di Valentin, M. Porphyrin Triplet State as a Potential Spin Label for Nanometer Distance Measurements by PELDOR Spectroscopy. J. Am. Chem. Soc. 2014, 136, 6582–6585. [Google Scholar]
- Dal Farra, M.G.; Ciuti, S.; Gobbo, M.; Carbonera, D.; Di Valentin, M. Triplet-state spin labels for highly sensitive pulsed dipolar spectroscopy. Mol. Phys. 2019, 117, 2673–2687. [Google Scholar] [CrossRef]
- Carbonera, D.; Dal Farra, M.G.; Albertini, M.; Zurlo, E.; Polimeno, A.; Di Valentin, M.; Orian, L.; Gobbo, M. Light-Induced Porphyrin-Based Spectroscopic Ruler for Nanometer Distance Measurements. Chem. Eur. J. 2016, 22, 17204–17214. [Google Scholar]
- Hintze, C.; Bücker, D.; Domingo Köhler, S.; Jeschke, G.; Drescher, M. Laser-Induced Magnetic Dipole Spectroscopy. J. Phys. Chem. Lett. 2016, 7, 2204–2209. [Google Scholar] [CrossRef]
- Krumkacheva, O.A.; Timofeev, I.O.; Politanskaya, L.V.; Polienko, Y.F.; Tretyakov, E.V.; Rogozhnikova, O.Y.; Trukhin, D.V.; Tormyshev, V.M.; Chubarov, A.S.; Bagryanskaya, E.G.; et al. Triplet Fullerenes as Prospective Spin Labels for Nanoscale Distance Measurements by Pulsed Dipolar EPR Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 13271–13275. [Google Scholar] [CrossRef]
- Gonen, O.; Levanon, H. Energy transfer and fine structure axes determination in a hybrid porphyrin dimer oriented in a liquid crystal. Time resolved triplet EPR spectroscopy. J. Chem. Phys. 1986, 84, 4132–4141. [Google Scholar] [CrossRef]
- Levanon, H.; Vega, S. Analysis of the transient EPR signals in the photoexcited triplet state. Application to porphyrin molecules. J. Chem. Phys. 1974, 61, 2265–2274. [Google Scholar] [CrossRef]
- Wili, N.; Hintz, H.; Vanas, A.; Godt, A.; Jeschke, G. Distance measurement between trityl radicals by pulse dressed electron paramagnetic resonance with phase modulation. Magn. Reson. 2020, 1, 75–87. [Google Scholar] [CrossRef]
- Zecevic, A.; Eaton, G.R.; Eaton, S.S.; Lindgren, M. Dephasing of electron spin echoes for nitroxyl radicals in glassy solvents by non-methyl and methyl protons. Mol. Phys. 1998, 95, 1255–1263. [Google Scholar] [CrossRef]
- Mims, W.B. Electron spin echoes. In Electron Paramagnetic Resonance; Geschwind, S., Ed.; Plenum Press: New York, NY, USA, 1972; pp. 263–351. [Google Scholar]
- Salikhov, K.M.; Tsvetkov, Y.D. Electron spin-echo studies of spin-spin interactions in solids. In Time Domain Electron Spin Resonance; Kevan, L., Schwartz, R.N., Eds.; Wiley: New York, NY, USA, 1979; pp. 231–277. [Google Scholar]
- Brown, I.M. Electron spin echo studies of relaxation processes in molecular solids. In Time Domain Electron Spin Resonance; Kevan, L., Schwartz, R.N., Eds.; Wiley: New York, NY, USA, 1979; pp. 195–229. [Google Scholar]
- Dal Farra, M.G.; Richert, S.; Martin, C.; Larminie, C.; Gobbo, M.; Bergantino, E.; Timmel, C.R.; Bowen, A.M.; Di Valentin, M. Light-Induced Pulsed EPR Dipolar Spectroscopy on a Paradigmatic Hemeprotein. ChemPhysChem 2019, 20, 931–935. [Google Scholar] [CrossRef]
- Bieber, A.; Bücker, D.; Drescher, M. Light-induced dipolar spectroscopy—A quantitative comparison between LiDEER and LaserIMD. J. Magn. Reson. 2018, 296, 29–35. [Google Scholar] [CrossRef]
- Ward, R.; Bowman, A.; Sozudogru, E.; El-Mkami, H.; Owen-Hughes, T.; Norman, D.G. EPR distance measurements in deuterated proteins. J. Magn. Reson. 2010, 207, 164–167. [Google Scholar] [CrossRef]
- El Mkami, H.; Ward, R.; Bowman, A.; Owen-Hughes, T.; Norman, D.G. The spatial effect of protein deuteration on nitroxide spin-label relaxation: Implications for EPR distance measurement. J. Magn. Reson. 2014, 248, 36–41. [Google Scholar] [CrossRef]
- Schmidt, T.; Wälti, M.A.; Baber, J.L.; Hustedt, E.J.; Clore, G.M. Long Distance Measurements up to 160 Åin the GroEL Tetradecamer Using Q-Band DEER EPR Spectroscopy. Angew. Chem. Int. Ed. 2016, 55, 15905–15909. [Google Scholar] [CrossRef]
- Salikhov, K.M.; Semenov, A.G.; Tsvetkov, Y.D. Electron Spin Echo and Its Applications; Nauka: Novosibirsk, Russia, 1976. [Google Scholar]
- Halpern, H.J.; Quine, R.W.; Yong, L.; Barth, E.; Eaton, G.R.; Rinard, G.A.; Eaton, S.S.; Mailer, C.; Harbridge, J. Electron Spin Relaxation of Triarylmethyl Radicals in Fluid Solution. J. Magn. Reson. 2002, 152, 156–161. [Google Scholar]
- Eaton, S.S.; Eaton, G.R. Relaxation Times of Organic Radicals and Transition Metal Ions. In Distance Measurements in Biological Systems by EPR; Springer: Boston, MA, USA, 2002; pp. 29–154. [Google Scholar]
- Dzuba, S.A. Libration motion of guest spin probe molecules in organic glasses: CW EPR and electron spin echo study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 227–234. [Google Scholar] [CrossRef]
- Kirilina, E.P.; Dzuba, S.A.; Maryasov, A.G.; Tsvetkov, Y.D. Librational Dynamics of Nitroxide Molecules in a Molecular Glass Studied by Echo - Detected EPR. Appl. Magn. Reson. 2001, 21, 203–221. [Google Scholar] [CrossRef]
- Golysheva, E.A.; Shevelev, G.Y.; Dzuba, S.A. Dynamical transition in molecular glasses and proteins observed by spin relaxation of nitroxide spin probes and labels. J. Chem. Phys. 2017, 147, 064501. [Google Scholar] [CrossRef]
- Kay, C.W.M.; Elger, G.; Möbius, K. The photoexcited triplet state of free-base porphycene: A time-resolved EPR and electron spin echo investigation. Phys. Chem. Chem. Phys. 1999, 1, 3999–4002. [Google Scholar] [CrossRef]
- Angiolillo, P.J.; Vanderkooi, J.M. Electron paramagnetic resonance of the excited triplet state of metal-free and metal-substituted cytochrome c. Biophys. J. 1995, 68, 2505–2518. [Google Scholar] [CrossRef][Green Version]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Lang, K.; Mosinger, J.; Wagnerová, D.M. Photophysical properties of porphyrinoid sensitizers non-covalently bound to host molecules; models for photodynamic therapy. Coord. Chem. Rev. 2004, 248, 321–350. [Google Scholar] [CrossRef]
- Zheng, X.H.; Nie, X.; Liu, H.Y.; Fang, Y.M.; Zhao, Y.; Xia, L.X. TMPyP4 promotes cancer cell migration at low doses, but induces cell death at high doses. Sci. Rep. 2016, 6, 26592. [Google Scholar] [CrossRef]
- Ouyang, D.; Inoue, S.; Okazaki, S.; Hirakawa, K. Tetrakis(N-methyl-p-pyridinio)porphyrin and its zinc complex can photosensitize damage of human serum albumin through electron transfer and singlet oxygen generation. J. Porphyr. Phthalocyanines 2016, 20, 813–821. [Google Scholar] [CrossRef]
- Groh, S.E.; Nagahisa, A.; Tan, S.L.; Orme-Johnson, W.H. Electron Spin Echo Modulation Demonstrates P-450scc Complexation. J. Am. Chem. Soc. 1983, 105, 7445–7446. [Google Scholar] [CrossRef]
- Mims, W.B.; Dams, J.L.; Peisach, J. The exchange of hydrogen ions and of water molecules near the active site of cytochrome c. J. Magn. Reson. 1990, 86, 273–292. [Google Scholar] [CrossRef]
- Erilov, D.A.; Bartucci, R.; Guzzi, R.; Shubin, A.A.; Maryasov, A.G.; Marsh, D.; Dzuba, S.A.; Sportelli, L. Water concentration profiles in membranes measured by ESEEM of spin-labeled lipids. J. Phys. Chem. B 2005, 109, 12003–12013. [Google Scholar] [CrossRef]
- Milov, A.D.; Samoilova, R.I.; Shubin, A.A.; Grishin, Y.A.; Dzuba, S.A. ESEEM measurements of local water concentration in D 2O- containing spin-labeled systems. Appl. Magn. Reson. 2008, 35, 73–94. [Google Scholar] [CrossRef]
- Syryamina, V.N.; De Zotti, M.; Peggion, C.; Formaggio, F.; Toniolo, C.; Raap, J.; Dzuba, S.A. A molecular view on the role of cholesterol upon membrane insertion, aggregation, and water accessibility of the antibiotic lipopeptide trichogin GA IV as revealed by EPR. J. Phys. Chem. B 2012, 116, 5653–5660. [Google Scholar] [CrossRef]
- Carmieli, R.; Papo, N.; Zimmermann, H.; Potapov, A.; Shai, Y.; Goldfarb, D. Utilizing ESEEM spectroscopy to locate the position of specific regions of membrane-active peptides within model membranes. Biophys. J. 2006, 90, 492–505. [Google Scholar] [CrossRef]
- Liu, L.; Mayo, D.J.; Sahu, I.D.; Zhou, A.; Zhang, R.; McCarrick, R.M.; Lorigan, G.A. Determining the Secondary Structure of Membrane Proteins and Peptides Via Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2015; Volume 564, pp. 289–313. [Google Scholar]
- Cieslak, J.A.; Focia, P.J.; Gross, A. Electron spin-echo envelope modulation (ESEEM) reveals water and phosphate interactions with the KcsA potassium channel. Biochemistry 2010, 49, 1486–1494. [Google Scholar] [CrossRef][Green Version]
- Ionita, G.; Florent, M.; Goldfarb, D.; Chechik, V. Studying supramolecular assemblies by ESEEM spectroscopy: Inclusion complexes of cyclodextrins. J. Phys. Chem. B 2009, 113, 5781–5787. [Google Scholar] [CrossRef]
- Krumkacheva, O.A.; Fedin, M.V.; Polovyanenko, D.N.; Jicsinszky, L.; Marque, S.R.A.; Bagryanskaya, E.G. Structural equilibrium in new nitroxide-capped cyclodextrins: CW and pulse EPR study. J. Phys. Chem. B 2013, 117, 8223–8231. [Google Scholar] [CrossRef]
- Tait, C.E.; Neuhaus, P.; Anderson, H.L.; Timmel, C.R.; Carbonera, D.; Di Valentin, M. HYSCORE on Photoexcited Triplet States. Appl. Magn. Reson. 2015, 46, 389–409. [Google Scholar] [CrossRef]
- Kruk, N.N.; Dzhagarov, B.M.; Galievsky, V.A.; Chirvony, V.S.; Turpin, P.Y. Photophysics of the cationic 5,10,15,20-tetrakis(4-N-methylpyridyl) porphyrin bound to DNA, [poly(dA-dT)]2 and [poly(dG-dC)]2: Interaction with molecular oxygen studied by porphyrin triplet-triplet absorption and singlet oxygen luminescence. J. Photochem. Photobiol. B Biol. 1998, 42, 181–190. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sannikova, N.; Timofeev, I.; Bagryanskaya, E.; Bowman, M.; Fedin, M.; Krumkacheva, O. Electron Spin Relaxation of Photoexcited Porphyrin in Water—Glycerol Glass. Molecules 2020, 25, 2677. https://doi.org/10.3390/molecules25112677
Sannikova N, Timofeev I, Bagryanskaya E, Bowman M, Fedin M, Krumkacheva O. Electron Spin Relaxation of Photoexcited Porphyrin in Water—Glycerol Glass. Molecules. 2020; 25(11):2677. https://doi.org/10.3390/molecules25112677
Chicago/Turabian StyleSannikova, Natalya, Ivan Timofeev, Elena Bagryanskaya, Michael Bowman, Matvey Fedin, and Olesya Krumkacheva. 2020. "Electron Spin Relaxation of Photoexcited Porphyrin in Water—Glycerol Glass" Molecules 25, no. 11: 2677. https://doi.org/10.3390/molecules25112677
APA StyleSannikova, N., Timofeev, I., Bagryanskaya, E., Bowman, M., Fedin, M., & Krumkacheva, O. (2020). Electron Spin Relaxation of Photoexcited Porphyrin in Water—Glycerol Glass. Molecules, 25(11), 2677. https://doi.org/10.3390/molecules25112677