Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model
Abstract
1. Introduction
2. Results
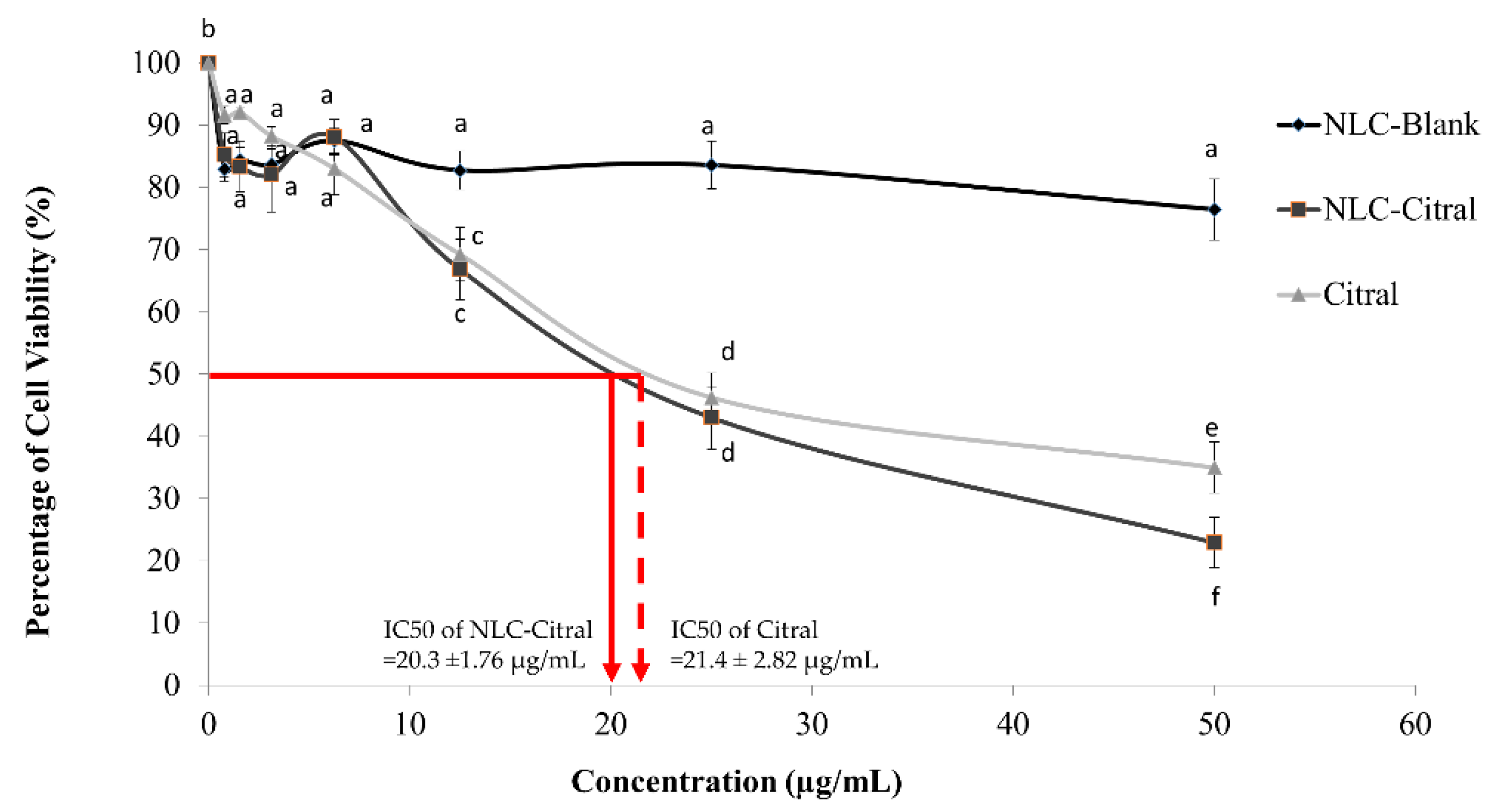
2.1. NLC-Citral Inhibited the Proliferation of 4T1 Cells In Vitro
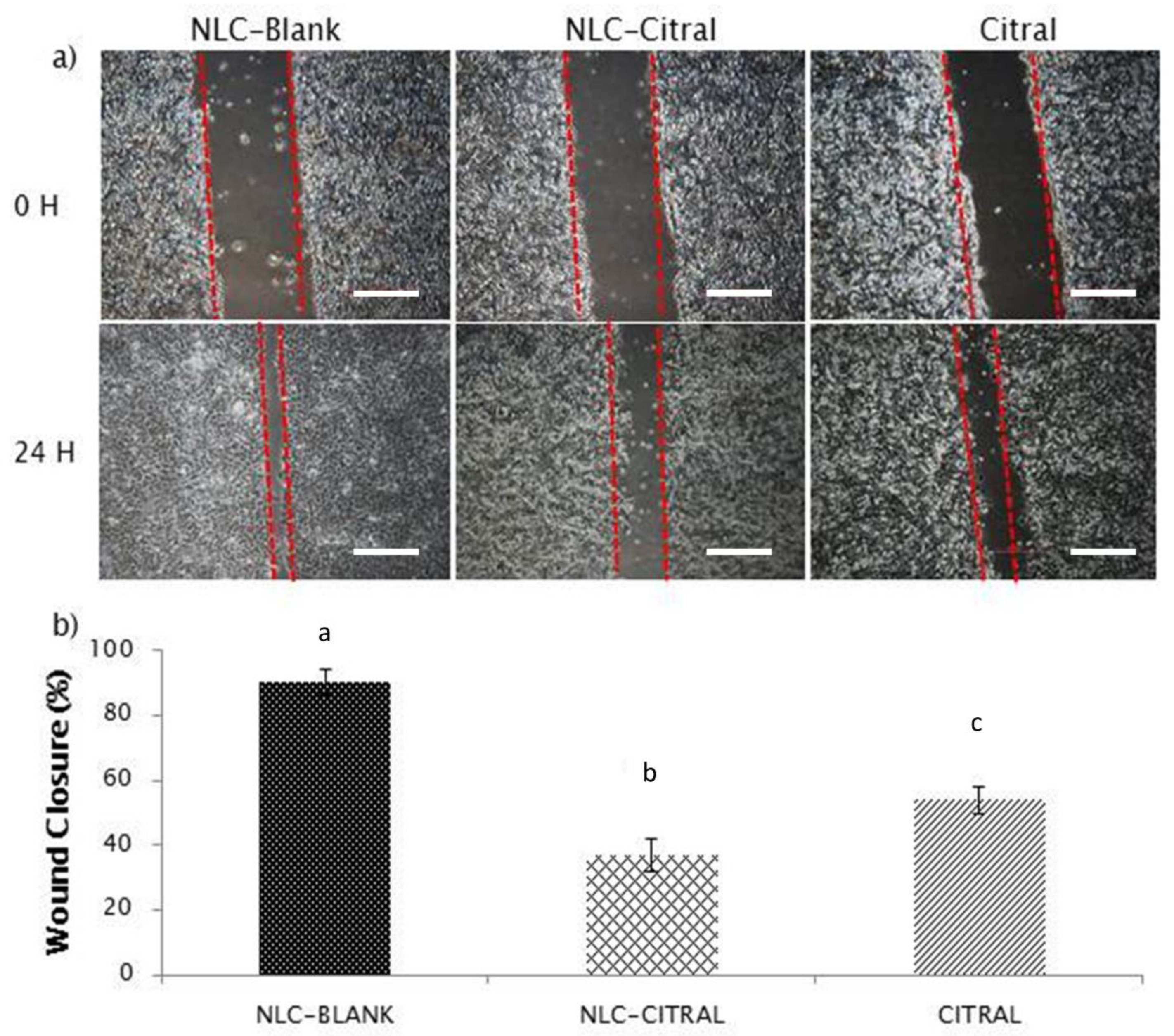
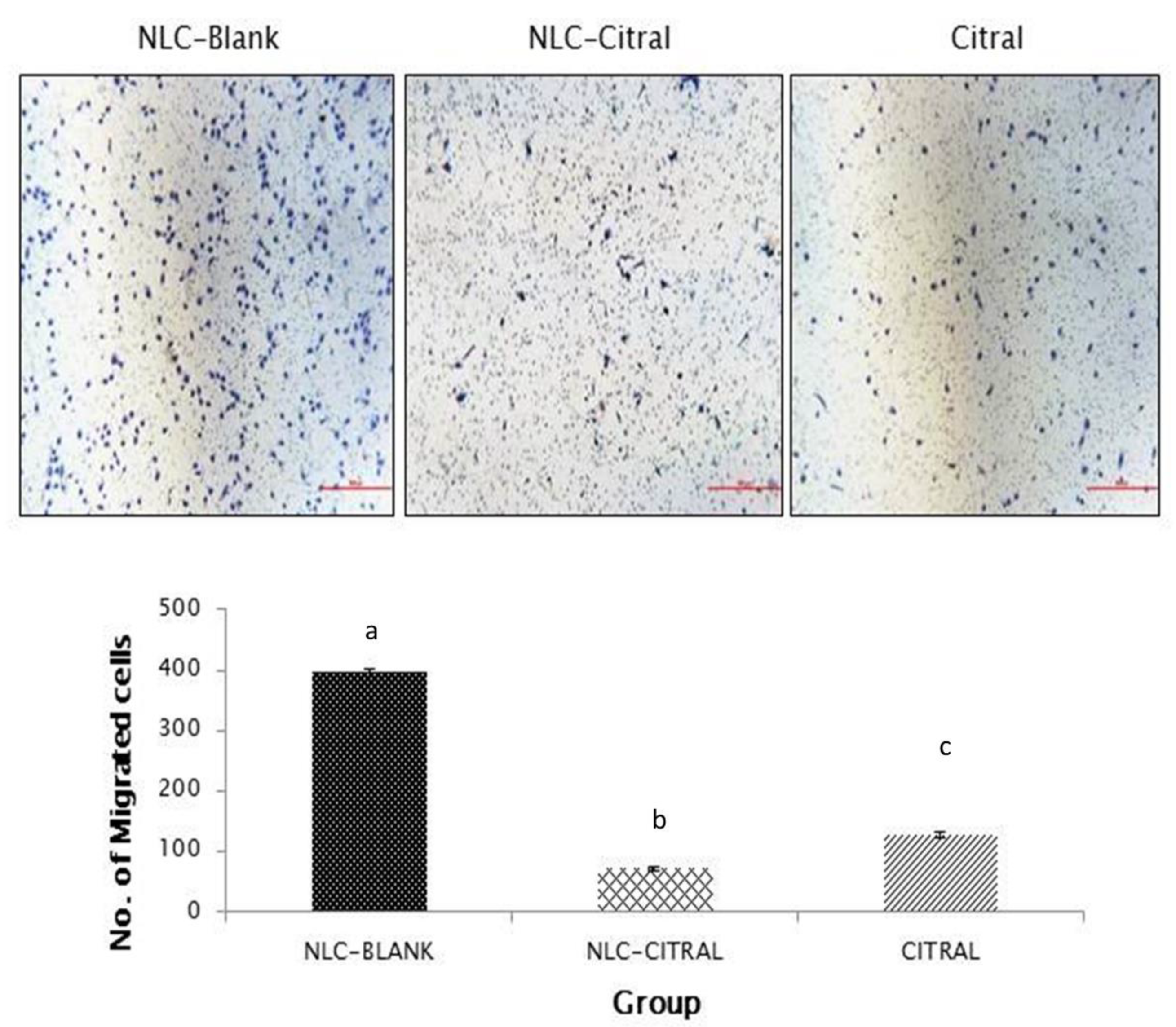
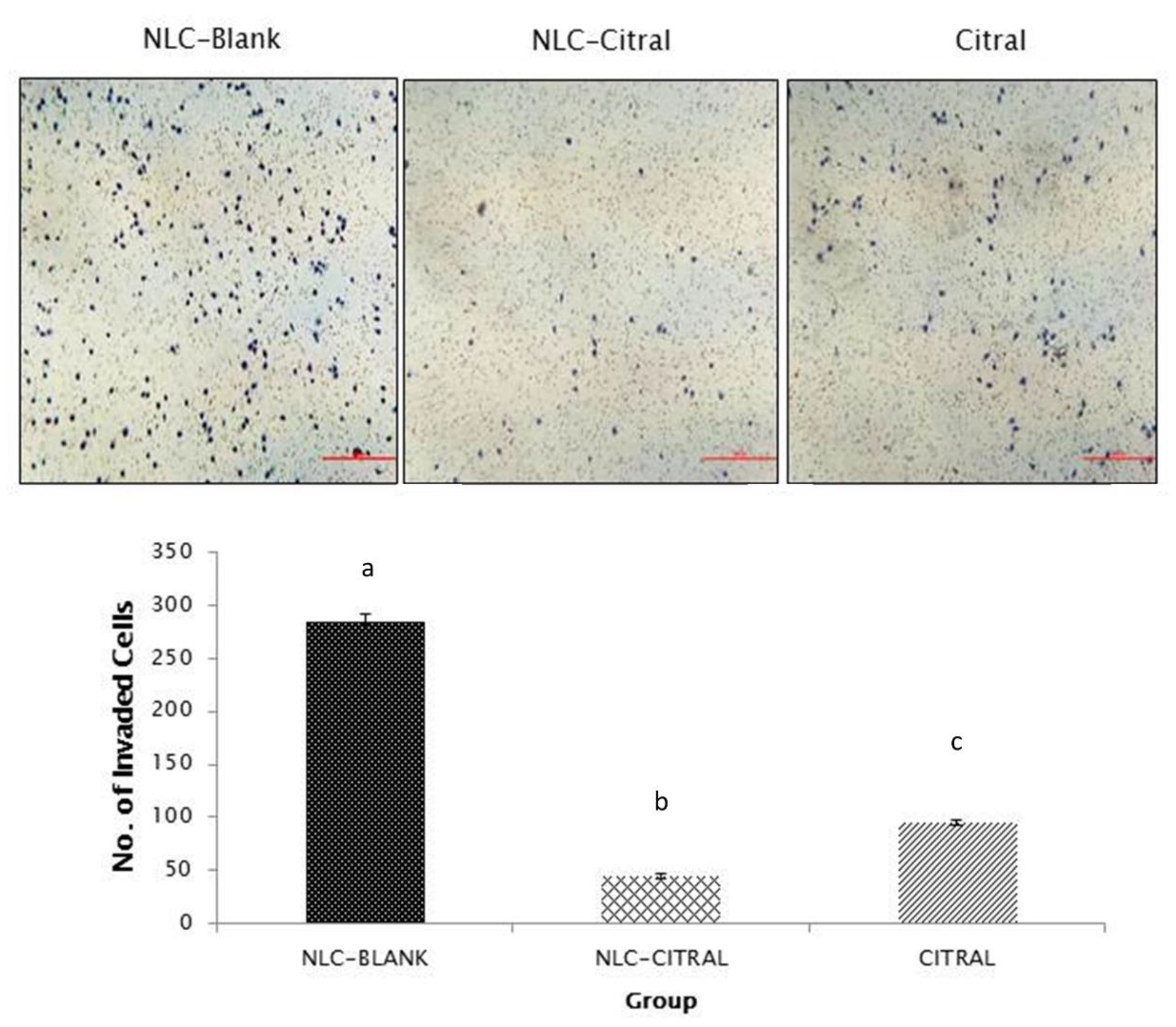
2.2. NLC-Citral Reduced the Migration and Invasion of 4T1 Cells In Vitro
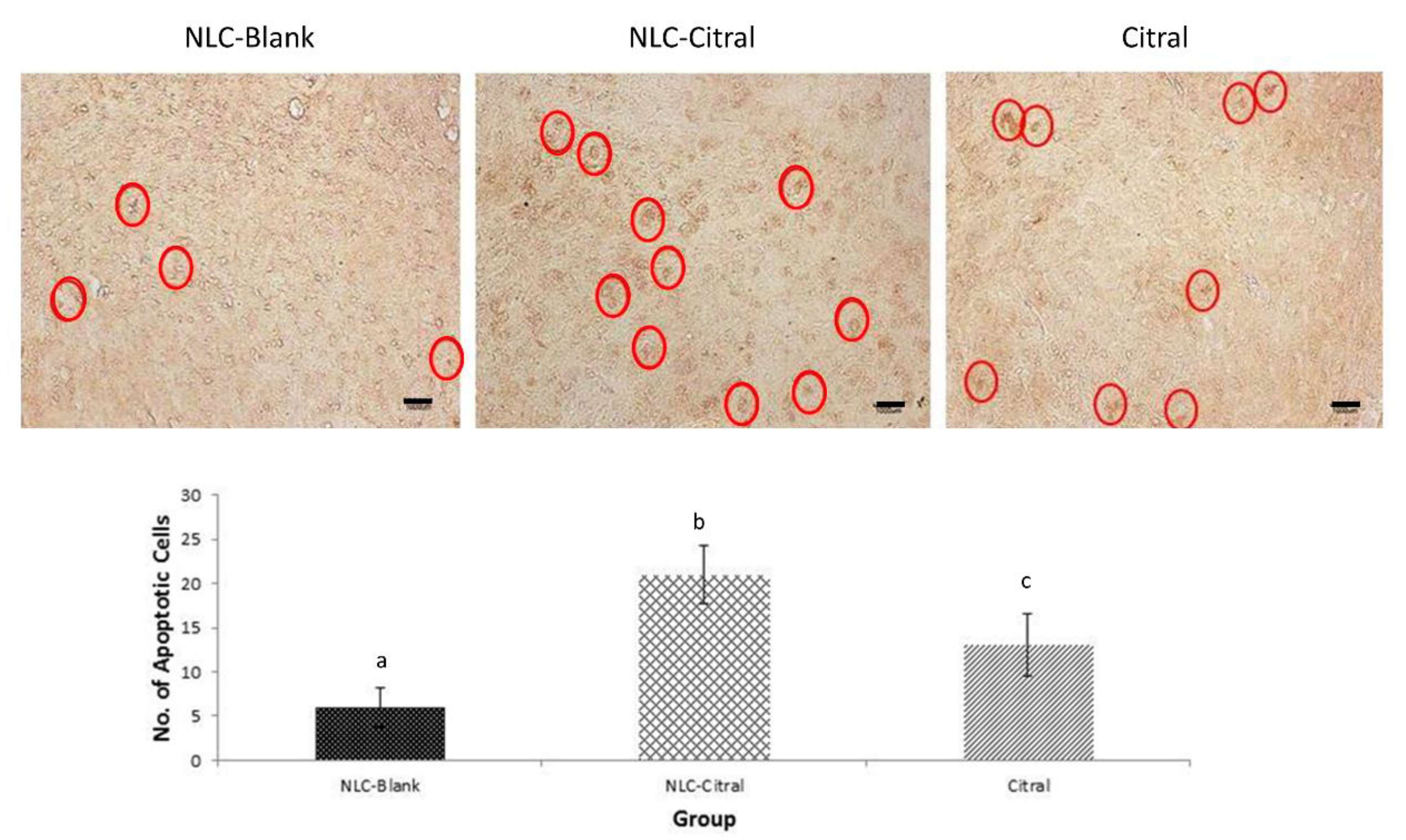
2.3. NLC-Citral Induced Apoptosis in the 4T1 Tumor
2.4. NLC-Citral Regulated Serum Cytokines Expressions (IL-10 and IL-1β)
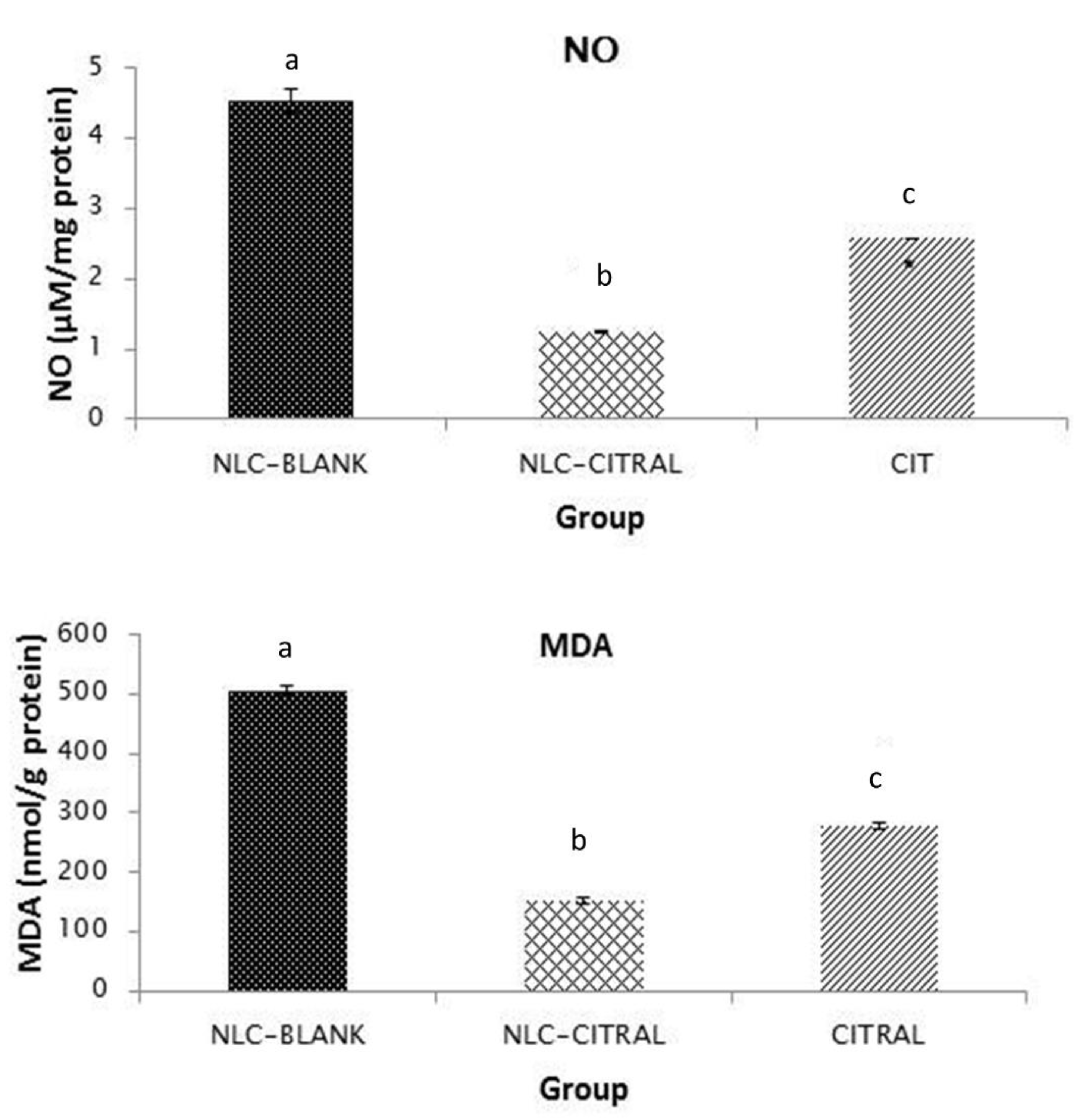
2.5. NLC-Citral Decreased Nitric Oxide and Lipid Peroxidation Levels in 4T1 Tumor
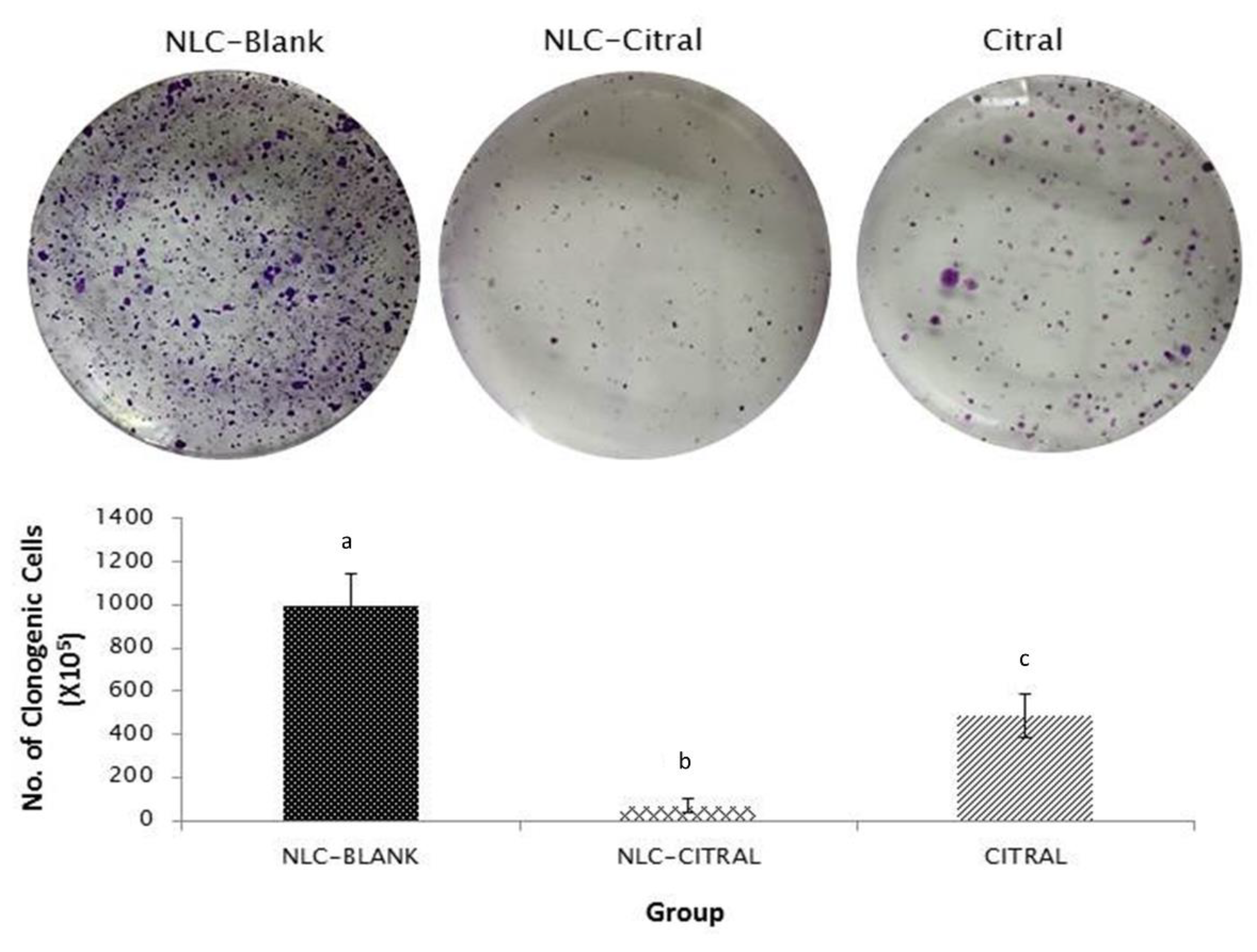
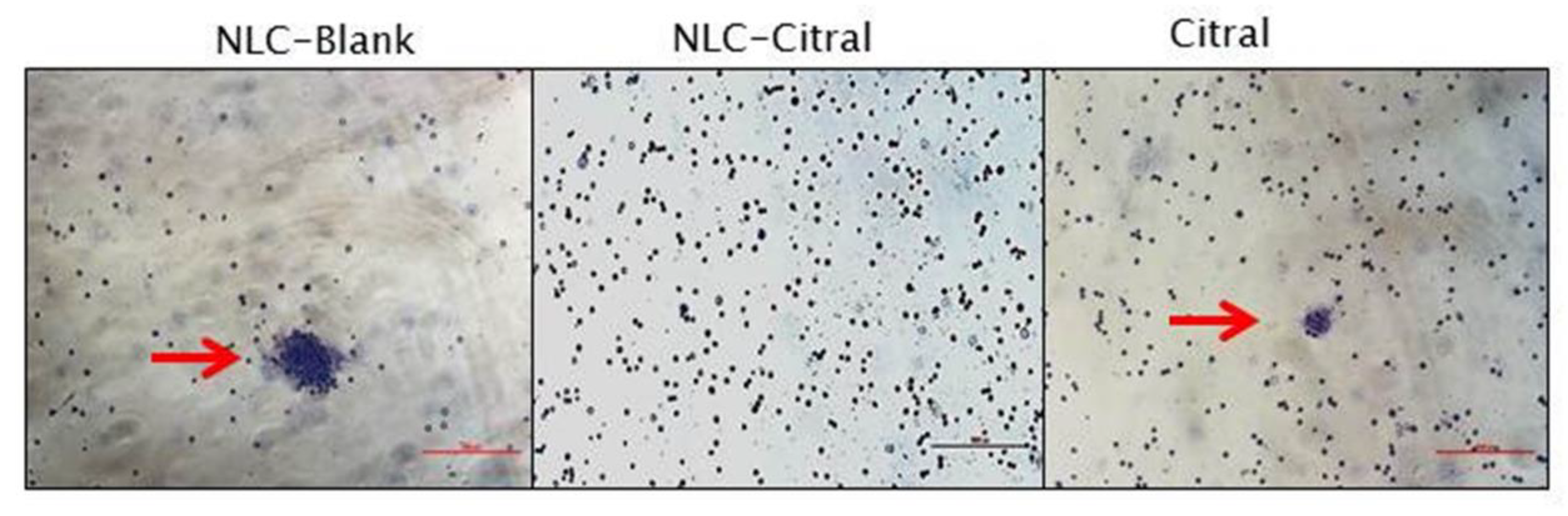
2.6. NLC-Citral Possessed Anti-Metastatic Effects on 4T1 Cells
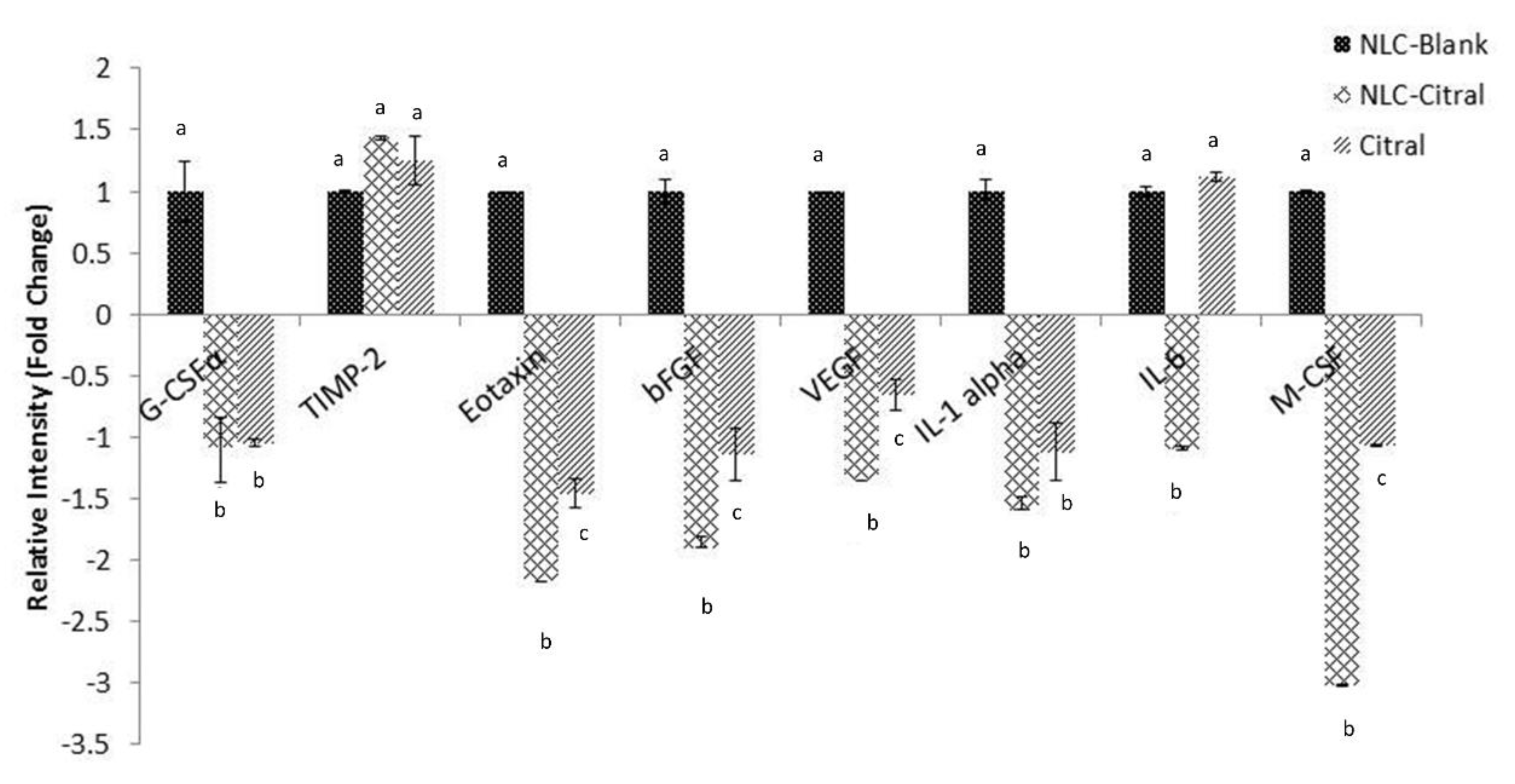
2.7. Regulation of the Metastasis-Related Genes and Proteins Expression
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of NLC-Citral
4.2. Cell Culture Condition
4.3. MTT Assay
4.4. In Vitro Scratch Assay
4.5. In Vitro Transwell Migration and Invasion Assay
4.6. Animals
4.7. Animals Grouping and Treatment
4.8. TUNEL Assay
4.9. Nitric Oxide Detection Assay
4.10. Malondialdehyde Detection
4.11. Serum Cytokine Detection
4.12. Clonogenic Assay
4.13. Bone Marrow Smear
4.14. Quantitative Real-Time PCR Assay of Metastatic Related Genes for In Vivo Tumor
4.15. Proteome Profiler Mouse Angiogenesis and Metastasis Protein for In Vivo Tumor
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 202. Ca Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Naidu, M.U.R.; Ramana, G.V.; Rani, P.U.; Mohan, I.K.; Suman, A.; Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis-complicating the treatment of cancer. Neoplasia 2004, 6, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A.; Uzzo, R.; Amato, R.J.; Ellis, G.K.; Hakimian, B.; Roodman, G.D.; Smith, M.R. The science and practice of bone health in oncology: Managing bone loss and metastasis in patients with solid tumors. J. Natl. Compr. Canc. Netw. 2009. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Bilancio, A.; Migliaccio, A. The androgen receptor in breast cancer. Front Endocrinol. 2018, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Pulaski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol 2001, 39, 20.2.1–20.2.16. [Google Scholar] [CrossRef]
- Nigjeh, S.E.; Yeap, S.K.; Nordin, N.; Rahman, H.; Rosli, R. In vivo anti-tumor effects of citral on 4T1 breast cancer cells via induction of apoptosis and downregulation of aldehyde dehydrogenase activity. Molecules 2019, 24, 3241. [Google Scholar] [CrossRef] [PubMed]
- Nigjeh, S.E.; Yeap, S.K.; Nordin, N.; Kamalideghan, B.; Ky, H.; Rosli, R. Citral induced apoptosis in MDA-MB-231 spheroid cells. BMC Complement Altern. Med. 2018, 18, 56. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, L.L.; Du, Y.Z.; You, J.; Hu, F.Q.; Zeng, S. Preparation and characteristics of nanostructured lipid carriers for control-releasing progesterone by melt-emulsification. Colloids Surf. B Biointerfaces 2007, 60, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Yeap, S.K.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; Rahman, H.S.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Characterization and toxicity of citral incorporated with the nanostructured lipid carrier. PeerJ 2018, 6, e3916. [Google Scholar] [CrossRef]
- Su, J.; Zhang, N.; Ho, P.C. Evaluation of the pharmacokinetics of all-trans-retinoic acid (ATRA) in Wistar rats after intravenous administration of ATRA loaded into tributyrin submicron emulsion and its cellular activity on caco-2 and HepG2 cell lines. J. Pharm. Sci. 2008, 97, 2844–2853. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci. Rep. 2019, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Andrais, B.; Kumar, P.; Murray, D. The growing complexity of cancer cell response to DNA-damaging agents: Caspase 3 mediates cell death or survival? Int. J. Mol. Sci. 2016, 17, 708. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Zheng, F.; Yang, X.; Yu, A.; Zhai, G. Nanostructured lipid carriers for oral delivery of baicalin: In vitro and in vivo evaluation. Colloid Surf. A Phys. Eng. Asp. 2015, 466, 154–159. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Olopade, O.I. MYC and breast cancer. Genes Cancer 2010, 1, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef]
- Hickok, J.R.; Thomas, D.D. Nitric oxide and cancer therapy: The emperor has NO clothes. Curr. Pharm. Des. 2010, 16, 381–391. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef]
- Lerebours, F.; Vacher, S.; Andrieu, C.; Espie, M.; Marty, M.; Lidereau, R.; Bieche, I. NF-kappa B genes have a major role in inflammatory breast cancer. Bmc Cancer 2008, 8, 41. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, X.; Liu, Q.; Zhang, X.; Bartels, C.E.; Geller, D.A. Nitric oxide production upregulates Wnt/b-catenin signaling by inhibiting dickkopf-1. Cancer Res. 2013, 73, 1–12. [Google Scholar] [CrossRef]
- Vahora, H.; Khan, M.A.; Alalami, U.; Hussain, A. The potential role of nitric oxide in halting cancer progression through chemoprevention. J. Cancer Prev. 2016, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.E.; Abu, N.; Rahman, H.S.; Ky, H.; Ho, W.Y.; Lim, K.L.; How, C.W.; Rasedee, A.; Alitheen, N.B.; Yeap, S.K. Nanostructured lipid carrier improved in vivo anti-tumor and immunomodulatory effect of Zerumbone in 4T1 challenged mice. Rsc Adv. 2015, 5, 22066–22074. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef] [PubMed]
- Samant, R.S.; Shevde, L.A. Recent advances in anti-angiogenic therapy of cancer. Oncotarget 2011, 2, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Dluzniewski, P.J.; Wang, M.H.; Zheng, S.L.; De Marzo, A.M.; Drake, C.G.; Fedor, H.L.; Partin, A.W.; Han, M.; Fallin, M.D.; Xu, J.; et al. Variation in IL10 and other genes involved in the immune response and in oxidation and prostate cancer recurrence. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1774–1782. [Google Scholar] [CrossRef]
- Baumgarten, S.C.; Frasor, J. Minireview: Inflammation: An instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol. Endocrinol. 2012, 26, 360–3671. [Google Scholar] [CrossRef]
- Helbig, G.; Christopherson, K.W.; Bhat-Nakshatri, P.; Kumar, S.; Kishimoto, H.; Miller, K.D.; Broxmeyer, H.E.; Nakshatri, H. NF-kB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J. Biol. Chem. 2003, 278, 21631–21638. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Rashad, Y.A.; Elkhodary, T.R.; El-Gayar, A.M.; Eissa, L.A. Evaluation of serum levels of HER2, MMP-9, nitric oxide, and total antioxidant capacity in Egyptian breast cancer patients: Correlation with clinico-pathological parameters. Sci. Pharm. 2013, 82, 129–145. [Google Scholar] [CrossRef]
- Carrillo-de Santa Pau, E.; Carrillo Arias, F.; Caso Pelaez, E.; Muguruza Trueba, I.; Sanchez Hernandez, I.; Munoz Molina, G.M.; Moreno Balsalobre, R.; Sacristan Lopez, S.; Gomez-Pinillos, A.; Toledo Lobo Mdel, V. Vascular endothelial growth factor (VEGF) serum levels are associated with survival in early stages of lung cancer patients. Cancer Investig. 2010, 28, 393–398. [Google Scholar] [CrossRef]
- Sosnoski, D.M.; Krishnan, V.; Kraemer, W.J.; Dunn-Lewis, C.; Mastro, A.M. Changes in cytokines of the bone microenvironment during breast cancer metastasis. Int. J. Breast Cancer 2012, 2012, 160265. [Google Scholar] [CrossRef] [PubMed]
- Eubank, T.D.; Roberts, R.D.; Khan, M.; Curry, J.M.; Nuovo, G.J.; Kuppusamy, P.; Marsh, C.B. Granulocyte-macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res. 2009, 69, 2133–2140. [Google Scholar] [CrossRef]
- Agarwal, S.; Lakoma, A.; Chen, Z.; Hicks, J.; Metelitsa, L.S.; Kim, E.S.; Shohet, J.M. G-CSF promotes neuroblastoma tumorigenicity and metastasis via STAT3-dependent cancer stem cell activation. Cancer Res. 2015, 75, 2566–2579. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, Z.; Li, Y.; Zhu, C.; Sun, C.; Wang, A.; Zheng, H.; Sun, R. Expression of survivin, PTEN and BFGF in lung cancer progression tissue microarray. Chin. J. Cancer Res. 2004, 16, 297–301. [Google Scholar] [CrossRef]
- Nikzad, S.; Hashemi, B.; Hassan, Z.M.; Mozdarani, H. The cell survival of F10B16 melanoma and 4T1 breast adenocarcinoma irradiated to gamma radiation using the MTT assay based on two different calculation methods. J. Biomed. Phys. Eng. 2013, 3, 29–36. [Google Scholar]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Zulfadli, A.J.; Omar, A.R.; Sulaiman, M.R.; Abdullah, M.P.; Alitheen, N.B. Flavokawain A induces apoptosis in MCF-7 and MDA-MB231 and inhibits the metastatic process in vitro. PLoS ONE 2014, 9, e105244. [Google Scholar] [CrossRef]
- Schaeffer, D.; Somarelli, J.A.; Hanna, G.; Palmer, G.M.; Garcia-Blanco, M.A. Cellular migration and invasion uncoupled: Increased migration is not an inexorable consequence of epithelial-to-mesenchymal transition. Mol. Cell Biol. 2014, 34, 3486–3499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, K.E.; Wilgus, T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound Care (New Rochelle) 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Garcia, Y.J.; Rodríguez-Malaver, A.J.; Peñaloza, N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J. Neurosci. Methods 2005, 144, 127–135. [Google Scholar] [CrossRef]
- Chang, H.; Wei, J.W.; Tao, Y.L.; Ding, P.R.; Xia, Y.F.; Gao, Y.H.; Xiao, W.W. CCR6 is a predicting biomarker of radiosensitivity and potential target of radiosensitization in rectal cancer. Cancer Res. Treat. 2018, 50, 1203–1213. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Yeap, S.K.; Abdul Samad, N.; Andas, R.J.; Muhammad Nadzri, N.; Anasamy, T.; et al. Acute toxicity study of zerumbone-loaded nanostructured lipid carrier on BALB/c mice model. Biomed. Res. Int. 2014, 2014, 563930. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Zeenathul, N.A.; Chartrand, M.S.; Yeap, S.K.; Abdul, A.B.; Tan, S.W.; Othman, H.H.; Ajdari, Z.; et al. Antileukemic effect of zerumbone-loaded nanostructured lipid carrier in WEHI-3B cell-induced murine leukemia model. Int. J. Nanomed. 2015, 10, 1649–1666. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the NLC-citral is available from the authors. |

| Gene Name | Accession Number | Sequence of Primers |
|---|---|---|
| Mmp-9 | NM_013599.3 | F: 5′-GCCGACTTTTGTGGTCTTCC-3′ R: 5′-GGTACAAGTATGCCTCTGCCA-3′ |
| iNOS | NM_010927.3 | F: 5′-GCACCGAGATTGGAGTTC-3′ R: 5′-GAGCACAGCCACATTGAT-3′ |
| Nf-κb | NM_0086892 | F: 5′- CCTGCTTCTGGAGGGTGATG -3′ R: 5′- GCCGCTATATGCAGAGGTGT -3′ |
| C-myc | NM_010849.4 | F: 5′-TGATGTGGTGTCTTGGAGAA-3′ R: 5′-CGTAGTTGTGCTGGTGAGTG-3′ |
| Icam1 | NM_010493.2 | F: 5′- TGCTCAGGTATCCATCCATCC-3′ R: 5′- ACGGTGCCACAGTTCTCAA-3′ |
| GAPDH | NM_008084.3 | F: 5′-GAAGGTGGTGAAGCAGGCATC-3′ R: 5′-GAAGGTGGAAGAGTGGGAGTT-3′ |
| ACTB | NM_007393.3 | F: 5′-TTCCAGCCTTCCTTCTTG-3′ R: 5′- GGAGCCAGAGCAGTAATC-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Mohamad, N.E.; Abu, N.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model. Molecules 2020, 25, 2670. https://doi.org/10.3390/molecules25112670
Nordin N, Yeap SK, Rahman HS, Zamberi NR, Mohamad NE, Abu N, Masarudin MJ, Abdullah R, Alitheen NB. Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model. Molecules. 2020; 25(11):2670. https://doi.org/10.3390/molecules25112670
Chicago/Turabian StyleNordin, Noraini, Swee Keong Yeap, Heshu Sulaiman Rahman, Nur Rizi Zamberi, Nurul Elyani Mohamad, Nadiah Abu, Mas Jaffri Masarudin, Rasedee Abdullah, and Noorjahan Banu Alitheen. 2020. "Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model" Molecules 25, no. 11: 2670. https://doi.org/10.3390/molecules25112670
APA StyleNordin, N., Yeap, S. K., Rahman, H. S., Zamberi, N. R., Mohamad, N. E., Abu, N., Masarudin, M. J., Abdullah, R., & Alitheen, N. B. (2020). Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model. Molecules, 25(11), 2670. https://doi.org/10.3390/molecules25112670







