Ameliorative Effects of Honey, Propolis, Pollen, and Royal Jelly Mixture against Chronic Toxicity of Sumithion Insecticide in White Albino Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Experimental Animals
2.3. Hematological Parameters
2.4. Enzymatic and Biochemical Parameters
2.5. GC-MS Analysis of the HPPJ Mixture
2.6. Statistical Analysis
3. Results
3.1. Mortality and Relative Organ Weight
3.2. Hematological Parameters
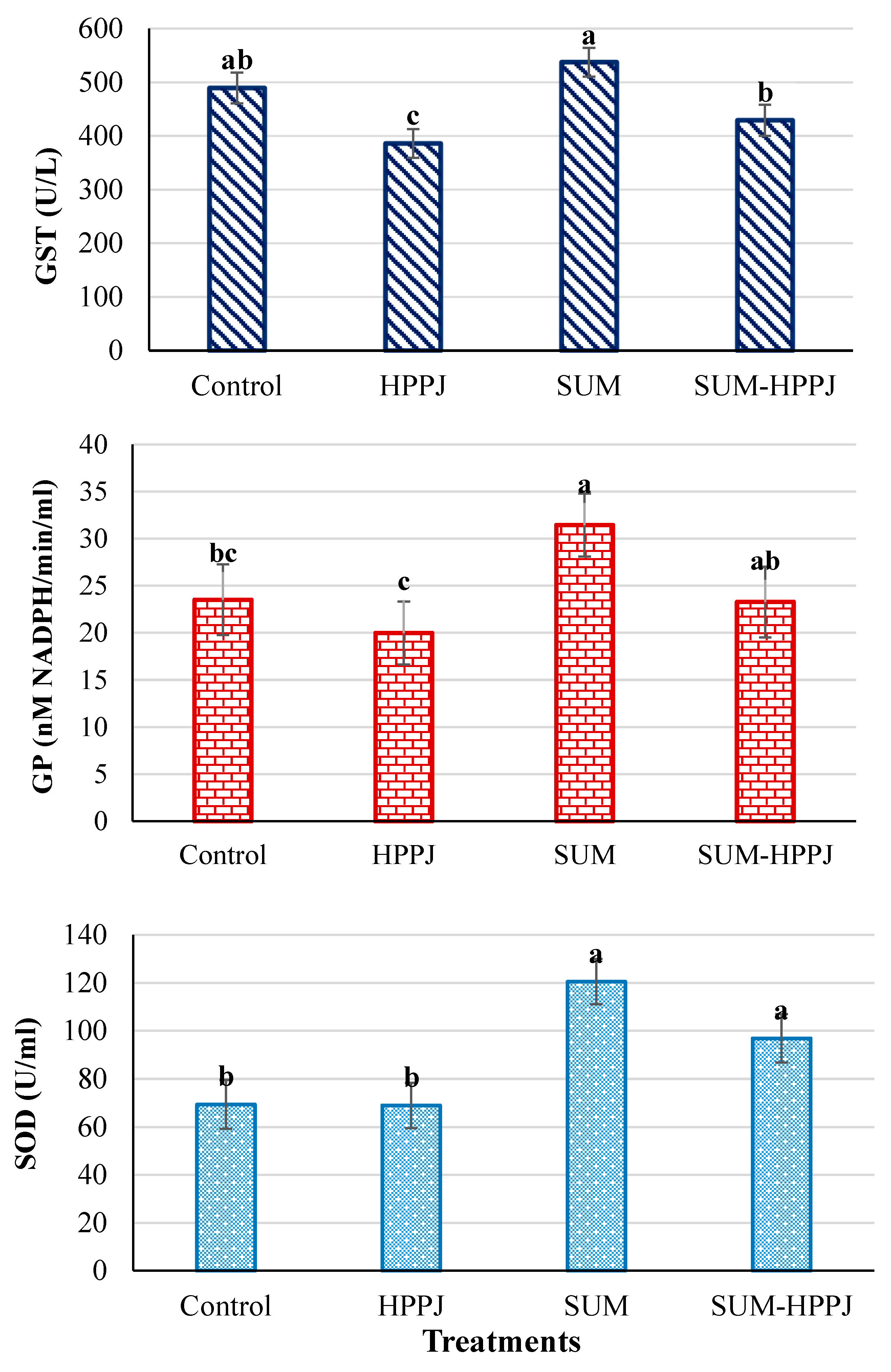
3.3. Serum Antioxidant Enzymes
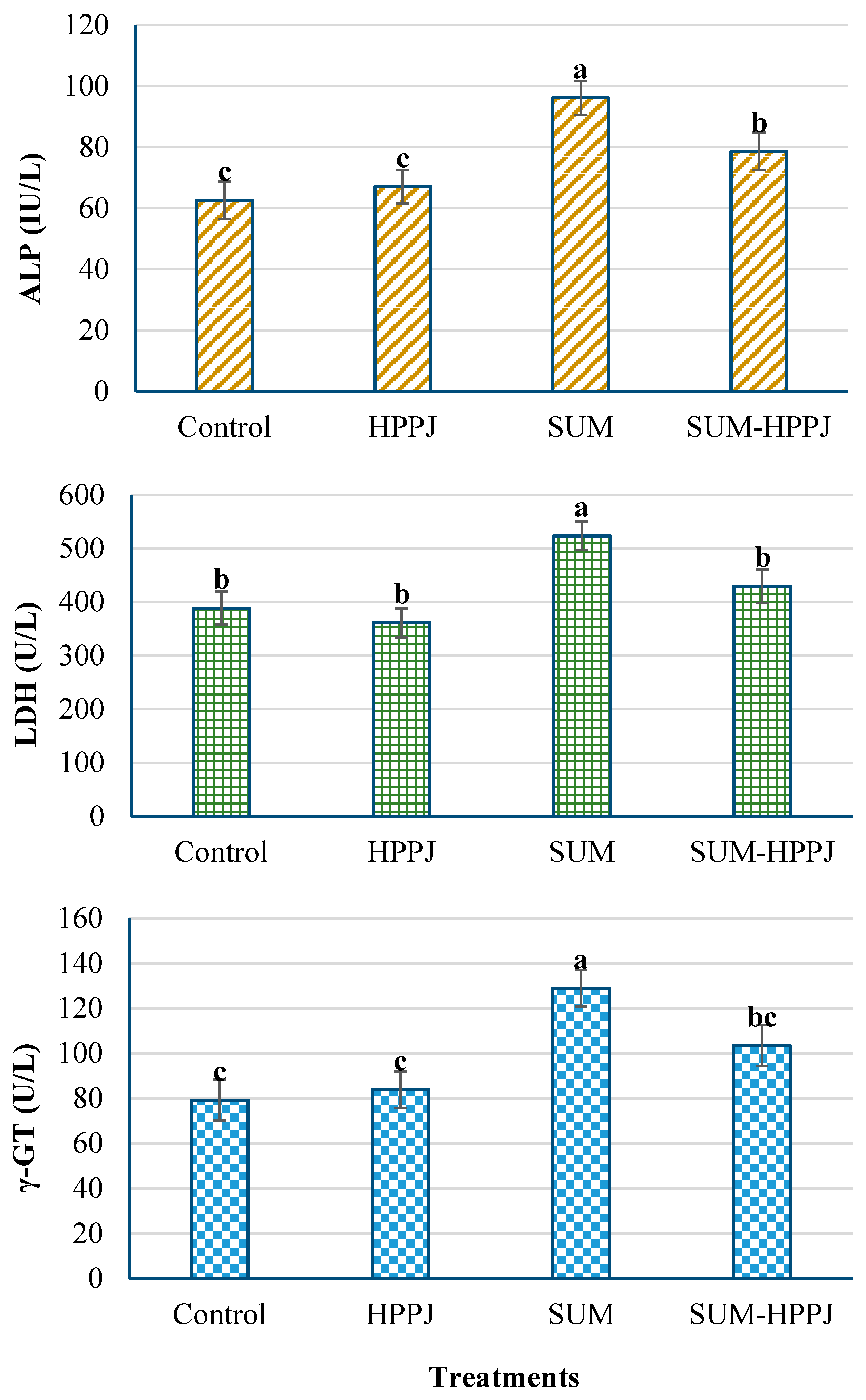
3.4. Serum Hepatic Enzyme Activities
3.5. Serum Renal and Biochemical Parameters
3.6. Chemical Composition of HPPJ Mixture
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bao, L.-J.; Wei, Y.-L.; Yao, Y.; Ruan, Q.-Q.; Zeng, E.Y. Global trends of research on emerging contaminants in the environment and humans: A literature assimilation. Environ. Sci. Pollut. Res. 2015, 22, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rekha, R.S.; Hamid, S. Histopathological effects of pesticide-cholopyrifos on kidney in albino rats. Int. J. Res. Med. Sci. 2013, 1, 465–475. [Google Scholar] [CrossRef]
- Hernández, A.F.; Parrón, T.; Tsatsakis, A.M.; Requena, M.; Alarcón, R.; López-Guarnido, O. Toxic effects of pesticide mixtures at a molecular level: Their relevance to human health. Toxicology 2013, 307, 136–145. [Google Scholar] [CrossRef]
- El-Demerdash, F.M. Lipid peroxidation, oxidative stress and acetylcholinesterase in rat brain exposed to organophosphate and pyrethroid insecticides. Food Chem. Toxicol. 2011, 49, 1346–1352. [Google Scholar] [CrossRef]
- Afshar, S.; Farshid, A.; Heidari, R.; Ilkhanipour, M. Histopathological changes in the liver and kidney tissues of Wistar albino rat exposed to fenitrothion. Toxicol. Ind. Health 2008, 24, 581–586. [Google Scholar] [CrossRef]
- Želježić, D.; Mladinić, M.; Žunec, S.; Vrdoljak, A.L.; Kašuba, V.; Tariba, B.; Živković, T.; Marjanović, A.; Pavičić, I.; Milić, M.; et al. Cytotoxic, genotoxic and biochemical markers of insecticide toxicity evaluated in human peripheral blood lymphocytes and an HepG2 cell line. Food Chem. Toxicol. 2016, 96, 90–106. [Google Scholar] [CrossRef]
- Lee, J.E.; Lim, M.S.; Park, J.H.; Park, C.H.; Koh, H.C. Nuclear NF-κB contributes to chlorpyrifos-induced apoptosis through p53 signaling in human neural precursor cells. Neurotoxicology 2014, 42, 58–70. [Google Scholar] [CrossRef]
- Vanova, N.; Pejchal, J.; Herman, D.; Dlabkova, A.; Jun, D. Oxidative stress in organophosphate poisoning: Role of standard antidotal therapy. J. Appl. Toxicol. 2018, 38, 1058–1070. [Google Scholar] [CrossRef]
- Kumar, S.V.; Fareedullah, M.D.; Sudhakar, Y.; Venkateswarlu, B.; Kumar, E.A. Current review on organophosphorus poisoning. Arch. Appl. Sci. Res. 2010, 2, 199–215. [Google Scholar]
- Orabi, S.H.; Elbialy, B.E.; Shawky, S.M. Ameliorating and hypoglycemic effects of zinc against acute hepatotoxic effect of chlorpyrifos. Sigma 2013, 4, 2. [Google Scholar]
- Durak, D.; Uzun, F.G.; Kalender, S.; Ogutcu, A.; Uzunhisarcikli, M.; Kalender, Y. Malathion-induced oxidative stress in human erythrocytes and the protective effect of vitamins C and E in vitro. Environ. Toxicol. Int. J. 2009, 24, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Othman, N.H. Does Honey Have the Characteristics of Natural Cancer Vaccine? J. Tradit. Complement. Med. 2012, 2, 276–283. [Google Scholar] [CrossRef][Green Version]
- Othman, N.H. Honey and Cancer: Sustainable Inverse Relationship Particularly for Developing Nations—A Review. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Honey as a Potential Natural Anticancer Agent: A Review of Its Mechanisms. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scepankova, H.; Saraiva, J.A.; Estevinho, L.M. Honey Health Benefits and Uses in Medicine. In Bee Products - Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-59689-1. [Google Scholar]
- Baltas, N.; Karaoglu, S.A.; Tarakci, C.; Kolayli, S. Effect of propolis in gastric disorders: Inhibition studies on the growth of Helicobacter pylori and production of its urease. J. Enzyme Inhib. Med. Chem. 2016, 31, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Diab, A.E.A.A.; Abd El-Aziz, E.S.A.; Hendawy, A.A. Immunotoxic effect of (organophosphorous insecticides) (chlorpyrifos, profenofos) and possible ameliorative role of propolis and ginseng. Biosci. Biotechnol. Res. Asia 2013, 10, 645–651. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Effects of Daily Consumption of Honey Solution on Hematological Indices and Blood Levels of Minerals and Enzymes in Normal Individuals. J. Med. Food 2003, 6, 135–140. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from Bee Pollen: Structure, Absorption, Metabolism and Biological Activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Sakai, M.; Inoue, R.; Inoue, H.; Suzuki, N. Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chem. 2001, 75, 237–240. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; Van Den Berg, D.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The Pesticide Manual. A World Compendium, 14th ed.; British Crop Protection Council: Surry, UK, 2006. [Google Scholar]
- NRCNA. Guide for the Care and Use of Laboratory Animals, 8th ed.; All rats were handled in accordance with the standard guide for the care and use of laboratory animals, Ed.; National Research Council of the National Academics. The National Academics Press: Washington, DC, USA, 2011. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef]
- Belfield, A.; Goldberg, D.M. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme 1971, 12, 561–573. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Zöllner, N.; Kirsch, K. Colorimetric method for determination of total lipids. J. Exp. Med. 1962, 135, 545–555. [Google Scholar] [CrossRef]
- Bartels, H.; Böhmer, M.; Heierli, C. Serum kreatininbestimmung ohne enteiweissen. Clin. Chim. Acta 1972, 37, 193–197. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Burtis, C.; Ashwood, E.; Bruns, D.; Saunders, W. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 4th ed.; Saunders: Philadephia, PA, USA, 2005. [Google Scholar]
- Heersink, W.; Hafkenscheid, J.C.M.; Siepel, H.; Van der Enjongekryg, J.; Dijt, C.C.M. Temperature-converting factors for enzymes: Comparison of methods. Enzyme 1980, 25, 333–341. [Google Scholar] [CrossRef]
- SAS Statistical Analysis System. Version 9.3; SAS Institute, Inc.: Cary, NC, USA, 2016.
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Juszczak, L.; Florkiewicz, A.; Socha, R.; Gałkowska, D. Effect of honey supplementation with bee products on quality parameters and mineral composition. Emirates J. Food Agric. 2019, 30, 990–997. [Google Scholar]
- Guimarães, N.S.S.; Mello, J.C.; Paiva, J.S.; Bueno, P.C.P.; Berretta, A.A.; Torquato, R.J.; Nantes, I.L.; Rodrigues, T. Baccharis dracunculifolia, the main source of green propolis, exhibits potent antioxidant activity and prevents oxidative mitochondrial damage. Food Chem. Toxicol. 2012, 50, 1091–1097. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Kalender, S.; Uzun, F.G.; Durak, D.; Demir, F.; Kalender, Y. Malathion-induced hepatotoxicity in rats: The effects of vitamins C and E. Food Chem. Toxicol. 2010, 48, 633–638. [Google Scholar] [CrossRef]
- Lasram, M.M.; Lamine, A.J.; Dhouib, I.B.; Bouzid, K.; Annabi, A.; Belhadjhmida, N.; Ben Ahmed, M.; El Fazaa, S.; Abdelmoula, J.; Gharbi, N. Antioxidant and anti-inflammatory effects of N-acetylcysteine against malathion-induced liver damages and immunotoxicity in rats. Life Sci. 2014, 107, 50–58. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mossa, A.-T.H. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Physiol. 2010, 96, 14–23. [Google Scholar] [CrossRef]
- Nagaraju, R.; Joshi, A.K.R.; Rajini, P.S. Organophosphorus insecticide, monocrotophos, possesses the propensity to induce insulin resistance in rats on chronic exposure. J. Diabetes 2015, 7, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Rizzati, V.; Briand, O.; Guillou, H.; Gamet-Payrastre, L. Effects of pesticide mixtures in human and animal models: An update of the recent literature. Chem. Biol. Interact. 2016, 254, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Othman, N.H. Review of the medicinal effects of Tualang honey and a comparison with Manuka honey. Malays. J. Med. Sci. 2013, 20, 6–13. [Google Scholar]
- Sahin, I.; Onbasi, K.; Sahin, H.; Karakaya, C.; Ustun, Y.; Noyan, T. The prevalence of pancreatitis in organophosphate poisonings. Hum. Exp. Toxicol. 2002, 21, 175–177. [Google Scholar] [CrossRef]
- Jo, S.-H.; Son, M.-K.; Koh, H.-J.; Lee, S.-M.; Song, I.-H.; Kim, Y.-O.; Lee, Y.-S.; Jeong, K.-S.; Kim, W.B.; Park, J.-W.; et al. Control of Mitochondrial Redox Balance and Cellular Defense against Oxidative Damage by Mitochondrial NADP + -dependent Isocitrate Dehydrogenase. J. Biol. Chem. 2001, 276, 16168–16176. [Google Scholar] [CrossRef]
- Karami-Mohajeri, S.; Ahmadipour, A.; Rahimi, H.-R.; Abdollahi, M. Adverse effects of organophosphorus pesticides on the liver: A brief summary of four decades of research. Arch. Ind. Hyg. Toxicol. 2017, 68, 261–275. [Google Scholar] [CrossRef]
- Kavitha, B.T.; Shruthi, S.D.; Rai, S.P.; Ramachandra, Y.L. Phytochemical analysis and hepatoprotective properties of Tinospora cordifolia against carbon tetrachloride-induced hepatic damage in rats. J. Basic Clin. Pharm. 2011, 2, 139. [Google Scholar]
- Hoekstra, L.T.; de Graaf, W.; Nibourg, G.A.A.; Heger, M.; Bennink, R.J.; Stieger, B.; van Gulik, T.M. Physiological and Biochemical Basis of Clinical Liver Function Tests. Ann. Surg. 2013, 257, 27–36. [Google Scholar] [CrossRef]
- Avsarogullari, L.; Ikizceli, I.; Sungur, M.; gan Sözüer, E.; Akdur, O.; Yücei, M. Acute Amitraz Poisoning in Adults: Clinical Features, Laboratory Findings, and Management. Clin. Toxicol. 2006, 44, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Karou, S.D.; Tchacondo, T.; Ouattara, L.; Anani, K.; Savadogo, A.; Agbonon, A.; Ben Attaia, M.; de Souza, C.; Sakly, M.; Simpore, J. Antimicrobial, antiplasmodial, haemolytic and antioxidant activities of crude extracts from three selected Togolese medicinal plants. Asian Pac. J. Trop. Med. 2011, 4, 808–813. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Cheng, N.; Gao, H.; Xue, X.; Cao, W.; Sun, L. Antioxidant and hepatoprotective activity of vitex honey against paracetamol induced liver damage in mice. Food Funct. 2015, 6, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Anusha, M.; Venkateswarlu, M.; Prabhakaran, V.; Taj, S.S.; Kumari, B.P.; Ranganayakulu, D. Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian J. Pharmacol. 2011, 43, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.-T.H.; Refaie, A.A.; Ramadan, A. Effect of Exposure to Mixture of Four Organophosphate Insecticides. Res. J. Environ. Toxicol. 2011, 5, 323–335. [Google Scholar] [CrossRef]
- Carter, C.J. Glutamine, glutamine synthetase and Huntington’s disease. Lancet 1981, 317, 1427–1428. [Google Scholar] [CrossRef]
- Tuchscherer, M.; Otten, W.; Kanitz, E.; Gräbner, M.; Tuchscherer, A.; Bellmann, O.; Rehfeldt, C.; Metges, C.C. Effects of inadequate maternal dietary protein: Carbohydrate ratios during pregnancy on offspring immunity in pigs. BMC Vet. Res. 2012, 8, 232. [Google Scholar] [CrossRef]
- Attia, A.M.; Nasr, H.M. Dimethoate-induced changes in biochemical parameters of experimental rat serum and its neutralization by black seed (Nigella sativa L.) oil. Slovak J. Anim. Sci. 2009, 42, 87–94. [Google Scholar]
- Kamath, V.; Rajini, P.S. Altered glucose homeostasis and oxidative impairment in pancreas of rats subjected to dimethoate intoxication. Toxicology 2007, 231, 137–146. [Google Scholar] [CrossRef]
- Abdel-Ghany, R.; Mohammed, E.; Anis, S.; Barakat, W. Impact of Exposure to Fenitrothion on Vital Organs in Rats. J. Toxicol. 2016, 2016, 1–18. [Google Scholar] [CrossRef]
- Nowroozi, M.R.; Ayati, M.; Amini, E.; Radkhah, K.; Jamshidian, H.; Delpazir, A.; Ghasemi, F.; Kanafi, A.R. Assessment of testicular perfusion prior to sperm extraction predicts success rate and decreases the number of required biopsies in patients with non-obstructive azoospermia. Int. Urol. Nephrol. 2015, 47, 53–58. [Google Scholar] [CrossRef]
- Pohanka, M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef]
- Nassar, A.M.K. Acetylcholinesterase: A Universal Toxicity Biomarker. J. Agric. Environ. Sci. 2016, 15, 28. [Google Scholar]
- Elhalwagy, M.E.; Darwish, N.S.; Shokry, D.A.; El-Aal, A.G.A.; Abd-Alrahman, S.H.; Nahas, A.-A.; Ziada, R.M. Garlic and alpha lipoic supplementation enhance the immune system of albino rats and alleviate implications of pesticides mixtures. Int. J. Clin. Exp. Med. 2015, 8, 7689–7700. [Google Scholar]
- Pinho, A.I.; Wallau, G.L.; Nunes, M.E.M.; Leite, N.F.; Tintino, S.R.; da Cruz, L.C.; da Cunha, F.A.B.; da Costa, J.G.M.; Douglas Melo Coutinho, H.; Posser, T.; et al. Fumigant Activity of the Psidium guajava Var. Pomifera (Myrtaceae) Essential Oil in Drosophila melanogaster by Means of Oxidative Stress. Oxid. Med. Cell. Longev. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Silveyra, M.X.; Candela, A.; Compañ, A.; Clària, J.; Jover, R.; Pérez-Mateo, M.; Felipo, V.; Martínez, S.; Galcerán, J. Changes in liver and plasma acetylcholinesterase in rats with cirrhosis induced by bile duct ligation. Hepatology 2006, 43, 444–453. [Google Scholar] [CrossRef]
- Vaidya, V.K. Horizontal transfer of antimicrobial resistance by extended-spectrum β lactamase-producing enterobacteriaceae. J. Lab. Physicians 2011, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Burnett, W. An assessment of the value of serum cholinesterase as a liver function test and in the diagnosis of jaundice. Gut 1960, 1, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Soltaninejad, K.; Abdollahi, M. Current opinion on the science of organophosphate pesticides and toxic stress: A systematic review. Med. Sci. Monit. 2009, 15, RA75–RA90. [Google Scholar] [PubMed]
- Fortunato, J.J.; Agostinho, F.R.; RÉus, G.Z.; Petronilho, F.C.; Dal-Pizzol, F.; Quevedo, J. Lipid peroxidative damage on malathion exposure in rats. Neurotox. Res. 2006, 9, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Mongi, S.; Mahfoud, M.; Amel, B.; Kamel, J.; Abdelfattah, E.F. Protective effects of vitamin C against haematological and biochemical toxicity induced by deltamethrin in male Wistar rats. Ecotoxicol. Environ. Saf. 2011, 74, 1765–1769. [Google Scholar] [CrossRef]
- Hundekari, I.; Suryakar, A.; Rathi, D. Acute organo-phosphorus pesticide poisoning in North Karnataka, India: Oxidative damage, haemoglobin level and total leukocyte. Afr. Health Sci. 2013, 13, 129–136. [Google Scholar] [CrossRef]
- Al-Qayim, A.J.M.; Ghali, L.; Al-Azwai, T. Comparative effects of propolis and malic acid on hematological parameters of aluminum exposed male rats. Glob. J. Biosci. Biotechnol. 2014, 3, 6–11. [Google Scholar]
- Hassan, H.A.; Yousef, M.I. Ameliorating effect of chicory (Cichorium intybus L.)-supplemented diet against nitrosamine precursors-induced liver injury and oxidative stress in male rats. Food Chem. Toxicol. 2010, 48, 2163–2169. [Google Scholar] [CrossRef]
- Southam, A.D.; Lange, A.; Hines, A.; Hill, E.M.; Katsu, Y.; Iguchi, T.; Tyler, C.R.; Viant, M.R. Metabolomics Reveals Target and Off-Target Toxicities of a Model Organophosphate Pesticide to Roach (Rutilus rutilus): Implications for Biomonitoring. Environ. Sci. Technol. 2011, 45, 3759–3767. [Google Scholar] [CrossRef]
- Pakzad, M.; Fouladdel, S.; Nili-Ahmadabadi, A.; Pourkhalili, N.; Baeeri, M.; Azizi, E.; Sabzevari, O.; Ostad, S.N.; Abdollahi, M. Sublethal exposures of diazinon alters glucose homostasis in Wistar rats: Biochemical and molecular evidences of oxidative stress in adipose tissues. Pestic. Biochem. Physiol. 2013, 105, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Malekirad, A.A.; Faghih, M.; Mirabdollahi, M.; Kiani, M.; Fathi, A.; Abdollahi, M. Neurocognitive, Mental Health, and Glucose Disorders in Farmers Exposed to Organophosphorus Pesticides. Arch. Ind. Hyg. Toxicol. 2013, 64, 1–8. [Google Scholar] [CrossRef]
- Lasram, M.M.; El-Golli, N.; Lamine, A.J.; Douib, I.B.; Bouzid, K.; Annabi, A.; El Fazaa, S.; Abdelmoula, J.; Gharbi, N. Changes in glucose metabolism and reversion of genes expression in the liver of insulin-resistant rats exposed to malathion. The protective effects of N-acetylcysteine. Gen. Comp. Endocrinol. 2015, 215, 88–97. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Mishra, J. Effects of fenthion on the blood and tissue chemistry of a teleost fish (Heteropneustes fossilis). J. Comp. Pathol. 1983, 93, 27–31. [Google Scholar] [CrossRef]
- Omotayo, E.O.; Gurtu, S.; Sulaiman, S.A.; Wahab, M.S.A.; Sirajudeen, K.N.S.; Salleh, M.S.M. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2010, 80, 74. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, M.S.M.D.; Gurtu, S. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. In Proceedings of the Annales d’endocrinologie; Elsevier: New York, NY, YSA, 2010; Volume 71, pp. 291–296. [Google Scholar]
- Batumalaie, K.; Zaman Safi, S.; Mohd Yusof, K.; Shah Ismail, I.; Devi Sekaran, S.; Qvist, R. Effect of Gelam Honey on the Oxidative Stress-Induced Signaling Pathways in Pancreatic Hamster Cells. Int. J. Endocrinol. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Han, X.Y.; Hu, J.N.; Wang, Z.; Wei, S.N.; Zheng, S.W.; Wang, Y.P.; Li, W. 5-HMF attenuates liver fibrosis in CCL4-plus-alcohol-induced mice by suppression of oxidative stress. J. Nutr. Sci. Vitaminol. (Tokyo) 2017, 63, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qu, X.N.; Han, Y.; Zheng, S.W.; Wang, J.; Wang, Y.P. Ameliorative effects of 5-hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on alcoholic liver oxidative injury in mice. Int. J. Mol. Sci. 2015, 16, 2446–2457. [Google Scholar] [CrossRef] [PubMed]
- Russ, M.; Jauk, S.; Wintersteiger, R.; Andrä, M.; Brcic, I.; Ortner, A. Investigation of antioxidative effects of a cardioprotective solution in heart tissue. Mol. Cell. Biochem. 2019, 461, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.; Lee, J.; Moraes-Vieira, P.M.; Donaldson, C.J.; Sontheimer, A.; Aryal, P.; Wellenstein, K.; Kolar, M.J.; Nelson, A.T.; Siegel, D.; et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab. 2018, 27, 419–427. [Google Scholar] [CrossRef]
| Group | Liver ± SE | Kidney ± SE | Heart ± SE | Brain ± SE | Spleen ± SE | Lung ± SE | Testes ± SE |
|---|---|---|---|---|---|---|---|
| Control | 3.11c ± 0.105 | 0.73bc ± 0.042 | 0.38a ± 0.026 | 0.713b ± 0.036 | 0.27a ± 0.059 | 0.73a ± 0.045 | 1.13a ± 0.062 |
| HPPJ | 3.36bc ± 0.105 | 0.60c ± 0.042 | 0.34a ± 0.026 | 0.704b ± 0.032 | 0.33a ± 0.046 | 0.64a ± 0.045 | 1.17a ± 0.062 |
| SUM | 3.64ab ± 0.105 | 0.82ab ± 0.042 | 0.40a ± 0.023 | 0.928a ± 0.032 | 0.26a ± 0.046 | 0.76a ± 0.040 | 1.06a ± 0.062 |
| SUM-HPPJ | 3.99a ± 0.122 | 0.94a ± 0.042 | 0.44a ± 0.026 | 0.932a ± 0.036 | 0.24a ± 0.051 | 0.79a ± 0.045 | 0.76b ± 0.071 |
| Group | WBC ± SE | RBC ± SE | HGB ± SE | HCT ± SE | MCV ± SE | MCH ± SE | MCHC ± SE | PLT ± SE |
|---|---|---|---|---|---|---|---|---|
| Control | 11.20a ± 0.285 | 5.54ab ± 0.188 | 10.78ab ± 0.198 | 34.28a ± 0.980 | 59.47b ± 2.530 | 18.68b ± 0.716 | 31.43b ± 0.428 | 566.8a ± 38.81 |
| HPPJ | 10.33ab ± 0.247 | 6.05a ± 0.146 | 11.08a ± 0.177 | 34.24a ± 0.877 | 56.58b ± 2.263 | 18.32b ± 0.640 | 32.37ab ± 0.383 | 579.2a ± 34.71 |
| SUM | 7.73c ± 0.247 | 3.96c ± 0.146 | 9.98b ± 0.198 | 31.94a ± 0.877 | 81.10a ± 2.263 | 25.79a ± 0.640 | 31.85b ± 0.383 | 253.8b ± 34.71 |
| SUM-HPPJ | 9.87b ± 0.285 | 5.28b ± 0.163 | 10.50ab ± 0.198 | 31.25a ± 0.980 | 59.50b ± 2.530 | 20.00b ± 0.716 | 33.63a ± 0.428 | 522.5a ± 38.81 |
| Group | Glucose ± SE | Total Protein ± SE | Total Lipids ± SE | Uric Acid ± SE | Creatinine ± SE | AChE ± SE |
|---|---|---|---|---|---|---|
| Control | 60.80b ± 5.462 | 7.54a ± 0.477 | 229.75a ± 13.795 | 2.93b ± 0.255 | 0.667c ± 0.094 | 20.94a ± 0.662 |
| HPPJ | 62.39b ± 4.885 | 6.60b ± 0.427 | 211.90a ± 12.338 | 3.16b ± 0.228 | 0.859c ± 0.084 | 20.23a ± 0.592 |
| SUM | 77.31a ± 4.885 | 5.54c ± 0.427 | 97.47c ± 12.338 | 4.61a ± 0.228 | 2.000a ± 0.084 | 13.15c ± 0.592 |
| SUM-HPPJ | 67.50b ± 5.462 | 6.53b ± 0.477 | 166.71b ± 13.795 | 3.91ab ± 0.255 | 1.078b ± 0.094 | 18.13b ± 0.662 |
| Chemical Component | % in the Extract of Total |
|---|---|
| HMF | 24.47 |
| Ketone, methyl-2-methyl-1,3-oxothiolan-2-yl | 12.84 |
| Linoleic acid (9,12-Octadecadienoic acid) | 10.19 |
| 4H-Pyran-4-one | 8.57 |
| 4-Hydroxy-3-methyl-2-butanone1-Propanol-1-D1 | 5.59 |
| Triulose | 4.32 |
| α-d-Mannofuranoside | 2.81 |
| N1, N1-Dimethyl-N2-n-butylformamidine | 2.58 |
| D-Mannitol | 2.28 |
| 2-Hydroxy-2-cyclopenten-1-one | 1.95 |
| Glyceraldehyde | 1.73 |
| Propane, 1-isothiocyanato-4H-pyran-4-one | 1.21 |
| Lanosta-7,9(11)-diene-3, beta-18,20-triol-3,18-diacetate | 1.16 |
| Flavone, 5-hydroxy-7-methoxy | 1.07 |
| Distannoxane, hexabutyl | 0.86 |
| Palmitic acid | 0.79 |
| D-Glucitol | 0.46 |
| α-D-Galactopyranose | 0.44 |
| d-Glycero-d-heptose | 0.29 |
| 3-Deoxy-d-mannoic lactone | 0.28 |
| 2,5-Dihydroxy-3,6-dimethylhydroxy-1,4-dioxane | 0.18 |
| Heptadecanoic acid | 0.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, A.M.K.; Salim, Y.M.M.; Eid, K.S.A.; Shaheen, H.M.; Saati, A.A.; Hetta, H.F.; Elmistekawy, A.; Batiha, G.E.-S. Ameliorative Effects of Honey, Propolis, Pollen, and Royal Jelly Mixture against Chronic Toxicity of Sumithion Insecticide in White Albino Rats. Molecules 2020, 25, 2633. https://doi.org/10.3390/molecules25112633
Nassar AMK, Salim YMM, Eid KSA, Shaheen HM, Saati AA, Hetta HF, Elmistekawy A, Batiha GE-S. Ameliorative Effects of Honey, Propolis, Pollen, and Royal Jelly Mixture against Chronic Toxicity of Sumithion Insecticide in White Albino Rats. Molecules. 2020; 25(11):2633. https://doi.org/10.3390/molecules25112633
Chicago/Turabian StyleNassar, Atef M.K., Yehia M.M. Salim, Khalid S.A. Eid, Hazem M. Shaheen, Abdullah A. Saati, Helal F. Hetta, Amr Elmistekawy, and Gaber El-Saber Batiha. 2020. "Ameliorative Effects of Honey, Propolis, Pollen, and Royal Jelly Mixture against Chronic Toxicity of Sumithion Insecticide in White Albino Rats" Molecules 25, no. 11: 2633. https://doi.org/10.3390/molecules25112633
APA StyleNassar, A. M. K., Salim, Y. M. M., Eid, K. S. A., Shaheen, H. M., Saati, A. A., Hetta, H. F., Elmistekawy, A., & Batiha, G. E.-S. (2020). Ameliorative Effects of Honey, Propolis, Pollen, and Royal Jelly Mixture against Chronic Toxicity of Sumithion Insecticide in White Albino Rats. Molecules, 25(11), 2633. https://doi.org/10.3390/molecules25112633