Influence of Drying Method on Some Bioactive Compounds and the Composition of Volatile Components in Dried Pink Rock Rose (Cistus creticus L.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Polyphenolic Compounds
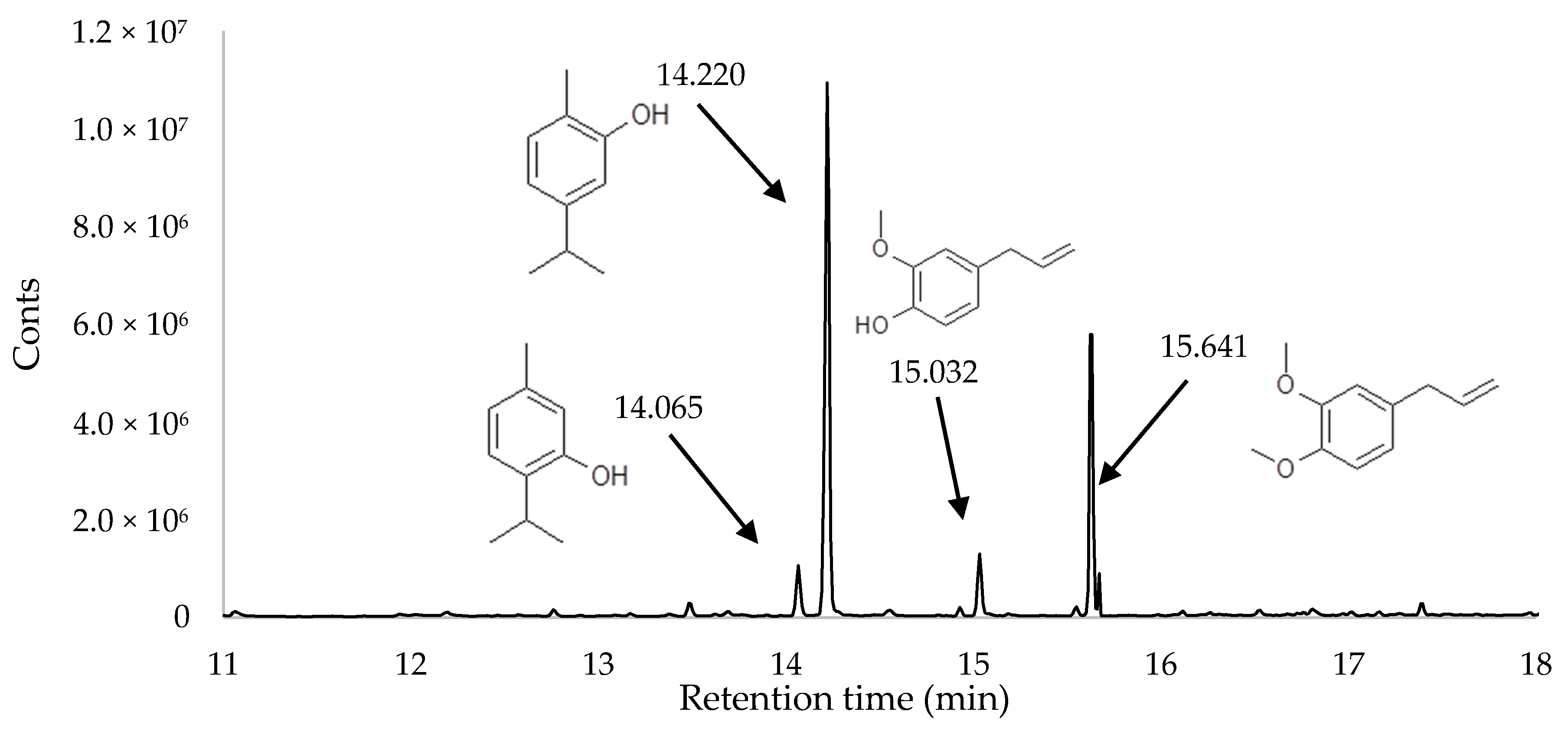
2.2. Chemical Composition of HS-SPME
3. Materials and Methods
3.1. Plant Materials
3.2. Identification and Quantification of Polyphenolic Compounds
3.3. Analysis of Proanthocyanidins by Phloroglucinolysis
3.4. Head Space-Solid Phase Microextraction (HS-SPME)
3.5. Chromatographic Analysis HS-SPME
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Attaguile, G.; Russo, A.; Campisi, A.; Savoca, F.; Acquaviva, R.; Ragusa, N.; Vanella, A. Antioxidant Activity and Protective Effect on DNA Cleavage of Extracts from Cistus incanus L. and Cistus monspeliensis L. Cell Biol. Toxicol. 2000, 16, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Politeo, O.; Maravić, A.; Burčul, F.; Carev, I.; Kamenjarin, J. Phytochemical Composition and Antimicrobial Activity of Essential Oils of Wild Growing Cistus species in Croatia. Nat. Prod. Commun. 2018, 13, 771–774. [Google Scholar] [CrossRef] [Green Version]
- Barrajón-Catalán, E.; Tomás-Menor, L.; Morales-Soto, A.; Bruñá, N.M.; López, D.S.; Segura-Carretero, A.; Micol, V. Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2015; Chapter 74; pp. 649–658. [Google Scholar]
- Vitali, F.; Pennisi, G.; Attaguile, G. Antiproliferative and cytotoxic activity of extracts from Cistus incanus L. and Cistus monspeliensis L. on human prostate cell lines. Nat. Prod. Res. 2011, 25, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic compounds in Cistus incanus herbal infusions–Antioxidant capacity and thermal stability during the brewing process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Kuchta, K.; Tung, N.H.; Ohta, T.; Uto, T.; Raekiansyah, M.; Grötzinger, K.; Rausch, H.; Shoyama, Y.; Rauwald, H.W.; Moritad, K. The old pharmaceutical oleoresin labdanum of Cistus creticus L. exerts pronounced in vitro anti-dengue virus activity. J. Ethnopharmacol. 2019, in press. [Google Scholar] [CrossRef]
- Rauwald, H.W.; Liebold, T.; Grötzinger, K.; Lehmann, J.; Kuchta, K. Labdanum and Labdanes of Cistus creticus and C. ladanifer: Anti-Borrelia activity and its phytochemical profiling. Phytomedicine 2019, 60, 152977. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Jemia, M.B.; Senatore, F.; Bruno, M.; Menichini, F.; Tundis, R. Chimistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food Chem. Toxicol. 2013, 59, 586–594. [Google Scholar] [CrossRef]
- Mastino, P.A.; Marchetti, M.; Costa, J.; Usaid, M. Interpopulation Variability in the Essential Oil Composition of Cistus creticus subsp. eriocephalus from Sardinia. Chem. Biodivers. 2018, 15, e1800151. [Google Scholar] [CrossRef]
- Demetzos, C.; Anastasaki, T.; Perdetzoglou, D. A Chemometric Interpopulation Study of the Essential Oils of Cistus creticus L. Growing in Crete (Greece). Z. Nat. C 2002, 57, 89–94. [Google Scholar] [CrossRef]
- Stępień, A.E.; Gorzelany, J.; Matłok, N.; Lech, K.; Figiel, A. The effect of drying methods on the energy consumption, bioactive potential and colour of dried leaves of Pink Rock Rose (Cistus creticus). J. Food Sci. Technol. 2019, 56, 2386–2394. [Google Scholar] [CrossRef] [Green Version]
- Calı’n-Sanchez, A.; Figiel, A.; Lech, K.; Szumny, A.; Martı’nez, T.J.; Carbonell-Barrachina, A.A. Drying methods affect the aroma of Origanum majorana L. analyzed by GC-MS and descriptive sensory analysis. Ind. Crops Prod. 2012, 74, 218–227. [Google Scholar]
- Calı’n-Sa´nchez, A.; Figiel, A.; Lech, K.; Szumny, A.; Carbonell-Barrachina, A.A. Effects of drying methods on the composition of thyme (Thymus vulgaris L.) essential oil. Dry Technol. 2013, 31, 224–235. [Google Scholar]
- Kramkowski, R. Ocena jakości suszu z produktów spożywczych [Overall assessment of dried food products]. Masz. Przetwótrwa Płodów Rol. 2001. Pleszew 111–112 [in Polish]. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.ekon-element-000171329895 (accessed on 2 June 2020).
- Zielinska, M.; Michalska, A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016, 212, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Arabhosseini, A.; Huisman, W.; van Boxtel, A.; Müller, J. Long-term effects of drying conditions on the essential oil and color of tarragon leaves during storage. J. Food Eng. 2007, 79, 561–566. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Pluta, S. The composition of bioactive compounds and antioxidant activity of Saskatoon berry (Amelanchier alnifolia Nutt.) genotypes grown in central Poland. Food Chem. 2017, 235, 234–243. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef]
- Lu, J.; Wei, Y.; Yuan, Q. Preparative separation of punicalagin from pomegranate husk by high-speed countercurrent chromatography. J. Chromatogr. B 2007, 857, 175–179. [Google Scholar] [CrossRef]
- Lachowicz, S.; Michalska, A.; Lech, K.; Majerska, J.; Oszmiański, J.; Figiel, A. Comparison of the effect of four drying methods on polyphenols in saskatoon berry. LWT 2019, 111, 727–736. [Google Scholar] [CrossRef]
- Plaza, M.; Batista, Â.G.; Cazarin, C.B.B.; Sandahl, M.; Turner, C.; Östman, E.; Júnior, M.R.M. Characterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: A pilot clinical study. Food Chem. 2016, 211, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Kolniak-Ostek, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Phytochemical Compounds and Antioxidant Activity in Different Cultivars of Cranberry (Vaccinium Macrocarpon L). J. Food Sci. 2017, 82, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Ahmed, I.; John, M.; Letzel, M.C.; Kuck, D. Stereoselective synthesis of benzylated prodelphinidins and their diastereomers with use of the mitsunobu reaction in the preparation of their gallocatechin precursors. Eur. J. Org. Chem. 2010, 2010, 2544–2554. [Google Scholar] [CrossRef]
- Zhu, M.; Dong, X.; Guo, M. Phenolic profiling of Duchesnea indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules 2015, 20, 22463–22475. [Google Scholar] [CrossRef] [Green Version]
- Matłok, N.; Gorzelany, J.; Stępiń, A.E.; Figiel, A.; Balawejder, M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef] [Green Version]
- Paolini, J.; Falchi, A.; Quilichini, Y.; Desjobert, J.M.; DeCian, M.C.; Varesi, L.; Costa, J. Morphological, chemical and genetic differentiation of two subspecies of Cistus creticus L. (C. creticus subsp. eriocephalus and C. Creticus Subsp. Corsicus). Phytochemistry 2009, 70, 1146–1160. [Google Scholar] [CrossRef]
- Rout, P.K.; Rao, Y.R.; Sree, A.; Naik, S.N. Composition of essential oil, concrete, absolute, wax and headspace volatiles of Murrarya paniculata (Linn.) Jack flowers. Flavour Fragr. J. 2007, 22, 352–357. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Peak No | Tentative Compounds | [M − H]− (m/z)/[M − H]− MS/MS (m/z) | Δmax [nm] | Rt [min] | VMD | CPD/VMFD | CD 40 °C | CD 50 °C | CD 60 °C | FD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flavonols | 17 | Myricetin-hexoside-di-rhamnoside | 771/609/317 | 355 | 6.03 | 4.5 ± 0.1 b | 5.8 ± 0.1 c | 6.0 ± 0.1 d | 5.5 ± 0.1 c | 5.6 ± 0.1 c | 3.2 ± 0.1 a |
| 18 | Quercetin-di-hexoside | 625/463/301 | 356 | 6.59 | 19.0 ± 0.4 b | 19.7 ± 0.4 b | 19.1 ± 0.4 b | 19.7 ± 0.4 b | 19.4 ± 0.4 b | 17.3 ± 0.3 a | |
| 19 | Myricetin-3-O-galactoside | 479/317 | 358 | 6.69 | 9.1 ± 0.2 a | 10.5 ± 0.2 b | 12.5 ± 0.2 c | 12.2 ± 0.2 c | 12.6 ± 0.3 c | 10.1 ± 0.2 b | |
| 20 | Myricetin-3-O-glucoside | 479/317 | 354 | 6.78 | 12.8 ± 0.3 b | 12.9 ± 0.3 b | 12.3 ± 0.2 b | 12.1 ± 0.2 b | 15.1 ± 0.3 | 8.8 ± 0.2 a | |
| 21 | Luteolin-hexoside-di rhamnoside | 739/577/285 | 350 | 7.06 | 1.1 ± 0.01 a | 1.1 ± 0.01 a | 1.1 ± 0.01 a | 1.0 ± 0.01 a | 1.1 ± 0.01 a | 1.2 ± 0.01 a | |
| 22 | Luteolin-dihexoside-dirhamnoside | 901/739/285 | 350 | 7.30 | 3.5 ± 0.1 a | 3.6 ± 0.1 a | 3.6 ± 0.1 a | 3.5 ± 0.1 a | 4.0 ± 0.1 b | 3.3 ± 0.1 a | |
| 23 | Quercetin-3-O-rutinoside | 609/301 | 353 | 7.37 | 55.9 ± 1.1 b | 58.4 ± 1.2 b | 55.6 ± 1.1 b | 58.4 ± 1.2 b | 57.2 ± 1.1 b | 44.6 ± 0.9 a | |
| 24 | Myricetin-3-O-rhamnoside | 463/317 | 350 | 7.44 | 969.6 ± 19.4 b | 1032.2 ± 20.6 c | 964.4 ± 19.3 b | 968.8 ± 19.4 b | 1046 ± 20.9 c | 631.5 ± 12.6 a | |
| 25 | Quercetin-3-O-galactoside | 463 | 350 | 7.55 | 2.1 ± 0.01 a | 2.9 ± 0.1 b | 3.0 ± 0.1 b | 3.4 ± 0.1 c | 2.9 ± 0.1 b | 3.8 ± 0.1 c | |
| 26 | Quercetin-3-O-glucoside | 463 | 350 | 7.70 | 1.2 ± 0.01 a | 1.7 ± 0.01 b | 1.2 ± 0.01 a | 1.7 ± 0.01 b | 1.8 ± 0.01 b | 1.4 ± 0.01 a | |
| 27 | Luteolin-3-O-rutinoside | 593/475/285 | 349 | 8.09 | 1.3 ± 0.01 a | 1.3 ± 0.01 a | 1.4 ± 0.01 a | 1.3 ± 0.01 a | 1.3 ± 0.01 a | 1.4 ± 0.01 a | |
| 28 | Quercetin-3-O-(600-acetyl)arabinopyranoside | 475/301 | 351 | 8.19 | 2.3 ± 0.01 a | 2.5 ± 0.01 a | 3.2 ± 0.1 b | 2.6 ± 0.1 a | 2.7 ± 0.1 a | 3.1 ± 0.1 b | |
| 29 | Quercetin-rhamnoside-dihexoside | 771/625/301 | 351 | 8.33 | 2.3 ± 0.01 a | 2.7 ± 0.1 b | 2.7 ± 0.1 b | 3.1 ± 0.1 c | 3.1 ± 0.1 c | 2.4 ± 0.01 a | |
| 30 | Myricetin deohyhexoside-hexoside | 771/625/317 | 351 | 8.43 | 18.2 ± 0.4 a | 20.8 ± 0.4 b | 20.1 ± 0.4 b | 21.2 ± 0.4 b | 24.2 ± 0.5 c | 18.8 ± 0.4 a | |
| 31 | Quercetin-3-O-rhamnoside | 447/301 | 350 | 8.51 | 23.9 ± 0.5 b | 26.0 ± 0.5 c | 24.9 ± 0.5 b | 25.8 ± 0.5 c | 28.4 ± 0.6 d | 20.5 ± 0.4 a | |
| 32 | Isorhamnetin-3-O-glucoside | 477/315 | 359 | 8.62 | 2.3 ± 0.01 a | 2.3 ± 0.01 a | 2.0 ± 0.01 a | 3.2 ± 0.1 c | 3.6 ± 0.1 c | 2.5 ± 0.1 b | |
| 33 | Kaempferl-3-O-diglucoside | 609/285 | 370 | 9.04 | 2.0 ± 0.01 b | 2.2 ± 0.01 b | 1.8 ± 0.01 b | 2.0 ± 0.01 b | 2.9 ± 0.1 c | 1.3 ± 0.01 a | |
| 34 | Quercetin-deoxyhexoside-hexoside | 755/609/301 | 369 | 9.10 | 1.1 ± 0.01 a | 1.2 ± 0.01 a | 1.1 ± 0.01 a | 1.1 ± 0.01 a | 1.2 ± 0.01 a | 1.0 ± 0.01 a | |
| 35 | Myricetin-3-O-rutinoside | 625/479/317 | 363 | 9.51 | 2.3 ± 0.01 a | 2.4 ± 0.01 a | 2.5 ± 0.1 a | 2.6 ± 0.1 b | 2.9 ± 0.1 c | 2.3 ± 0.01 a | |
| 36 | Kaempferl-3-O-rutinoside | 593/285 | 354 | 10.95 | 12.0 ± 0.2 a | 14.1 ± 0.3 c | 13.4 ± 0.3 b | 14.3 ± 0.3 c | 14.1 ± 0.3 c | 12.8 ± 0.3 a | |
| 37 | Kaempferl-3-O-rutinoside | 593/285 | 354 | 11.20 | 2.7 ± 0.1 c | 2.4 ± 0.01 b | 2.5 ± 0.1 b | 2.5 ± 0.01 b | 2.8 ± 0.1 c | 2.2 ± 0.01 a | |
| Sum of flavonols (mg 100 g−1) | 1149.3 b | 1226.7 b | 1154.6 b | 1165.8 b | 1252.9 b | 793.6 a | |||||
| Sum of flavonols (g 100 g−1) | 1.2 b | 1.2 b | 1.2 b | 1.2 b | 1.3 b | 0.8 a | |||||
| Flavan-3-ols | 1 | Gaolloyl glucose | 331 | 266 | 2.08 | 156.98 ± 3.14 b | 110.85 ± 2.22 a | 95.83 ± 1.92 a | 113.93 ± 2.28 a | 108.82 ± 2.18 a | 100.7 ± 2.01 a |
| 2 | Gallocatechintrimer | 913/609/423/305 | 280 | 2.74 | 22.35 ± 0.45 c | 23.39 ± 0.47 c | 24.1 ± 0.48 c | 20.98 ± 0.42 b | 20.24 ± 0.4 b | 10.51 ± 0.21 a | |
| 3 | Gallocatechin dimer | 609/423/305 | 270 | 2.83 | 18.42 ± 0.37 b | 20.17 ± 0.4 c | 20.73 ± 0.41 c | 17.42 ± 0.35 b | 17.97 ± 0.36 b | 6.78 ± 0.14 a | |
| 4 | (+)-catechin | 289 | 270 | 3.14 | 31.68 ± 0.63 b | 36.95 ± 0.74 c | 36.52 ± 0.73 c | 31.57 ± 0.63 b | 32.92 ± 0.66 b | 26.02 ± 0.52 a | |
| 6 | Gallocatechin | 305 | 270 | 3.31 | 172.89 ± 3.46 c | 188.3 ± 3.77 c | 166.84 ± 3.34 b | 169.61 ± 3.39 b | 156.73 ± 3.13 b | 72.16 ± 1.44 a | |
| 7 | Prodelphinidin dimer | 593/305 | 286 | 3.57 | 44.79 ± 0.9 a | 48.48 ± 0.97 b | 49.31 ± 0.99 b | 48.18 ± 0.96 b | 49.37 ± 0.99 b | 76.48 ± 1.53 c | |
| 13 | (−)-epicatechin | 289 | 277 | 4.78 | 92.29 ± 1.85 b | 118.81 ± 2.38 c | 115.73 ± 2.31 c | 111.45 ± 2.23 c | 102.35 ± 2.05 b | 73.88 ± 1.48 a | |
| 16 | Gallocatechin-(4α-8)-catechin | 593/289 | 291 | 5.78 | 12.61 ± 0.25 a | 13.71 ± 0.27 b | 22.34 ± 0.45 c | 13.01 ± 0.26 b | 10.48 ± 0.21 a | 16.39 ± 0.33 b | |
| Polymers proanthocyanidins | 497.56 ± 9.95 a | 511.31 ± 10.23 a | 563.95 ± 11.28 b | 553.74 ± 11.07 b | 508.4 ± 10.17 a | 570.98 ± 11.42 b | |||||
| Sum of flavan-3-ols (mg 100 g−1) | 1049.8 b | 1072.0 b | 1095.4 b | 1080.0 b | 1007.3 a | 953.9 a | |||||
| Sum of flavan-3-ols (g 100 g−1) | 1.1 b | 1.1 b | 1.1 b | 1.1 b | 1.0 a | 1.0 | |||||
| Hydrolyzed tannins | 5 | bis-HHDP-glucose | 783/481/301 | 218 | 3.25 | 237.0 ± 4.7 b | 263.7 ± 5.3 c | 256 ± 5.1 c | 231.6 ± 4.6 b | 221.9 ± 4.4 b | 89.4 ± 1.8 a |
| 8 | Ellagic acid rhamnoside | 633/593/301 | 208 | 3.58 | 20.1 ± 0.4 b | 20.7 ± 0.4 b | 24.4 ± 0.5 c | 19.4 ± 0.4 a | 19.1 ± 0.4 a | 18.8 ± 0.4 a | |
| 9 | Ellagitannin | 933/783/609/423/305/301 | 208 | 3.79 | 15.8 ± 0.3 b | 16.7 ± 0.3 b | 16.2 ± 0.3 b | 14.3 ± 0.3 b | 16.6 ± 0.3 b | 5.7 ± 0.1 a | |
| 10 | bis-HHDP-glucose | 783/481/301 | 226 | 4.15 | 26.9 ± 0.5 b | 33.7 ± 0.7 c | 28.2 ± 0.6 b | 29.7 ± 0.6 c | 24.3 ± 0.5 b | 16.4 ± 0.3 a | |
| 11 | Punicalagin isomer | 1083/781/601/301 | 250 | 4.63 | 10.0 ± 0.2 a | 19.6 ± 0.4 b | 20.4 ± 0.4 b | 17.6 ± 0.4 b | 12.3 ± 0.2 a | 9.3 ± 0.2 a | |
| 12 | Cornusiin B | 1085/783/451/301 | 240 | 4.75 | 24.3 ± 0.5 b | 27.2 ± 0.5 b | 27.6 ± 0.6 b | 26.5 ± 0.5 b | 27.5 ± 0.5 b | 17.7 ± 0.4 a | |
| 14 | Cornusiin B | 1085/783/451/301 | 240 | 4.87 | 42.8 ± 0.9 b | 67.8 ± 1.4 c | 67.4 ± 1.3 c | 68.5 ± 1.4 c | 46.5 ± 0.9 b | 30.5 ± 0.6 a | |
| 15 | Ellagic acid rutinoside | 609/463/301 | 241 | 5.49 | 18.9 ± 0.4 b | 23.6 ± 0.5 c | 25.3 ± 0.5 c | 21.4 ± 0.4 b | 20.6 ± 0.4 b | 3.7 ± 0.1 a | |
| Sum of hydrolyzed tannins (mg 100 g−1) | 395.8 b | 473.1 c | 465.5 c | 429.0 b | 388.8 b | 191.5 a | |||||
| Sum of hydrolyzed tannins (g 100 g−1) | 0.4 b | 0.5 c | 0.5 c | 0.4 b | 0.4 b | 0.2 a | |||||
| Sum of phenolic compounds (mg 100 g−1) | 2594.7 b | 2771.7 b | 2715.4 b | 2674.8 b | 2649.0 b | 1938.0 a | |||||
| Sum of phenolic compounds (g 100 g−1) | 2.6 b | 2.8 b | 2.7 b | 2.7 b | 2.7 b | 1.4 a | |||||
| No. | Ordinary Substance Name | Systematic Substance Name | RT [min] | Peak Share in the Chromatogram [%] | No CAS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| VMD | CPD/VMFD | CD 40 °C | CD 50 °C | CD 60 °C | FD | |||||
| 1 | linalool | (±)-3,7-Dimethyl-1,6-octadien-3-ol, (±)-3,7-Dimethyl-3-hydroxy-1,6-octadiene | 10.99 | trace | trace | trace | trace | trace | trace | 78-70-6 |
| 2 | decanal | capraldehyde | 12.79 | trace | trace | trace | trace | trace | trace | 112-31-2 |
| 3 | thymoquinone | 2-isopropyl-5-methyl-1,4-benzoquinone | 13.48 | 1.65 ± 0.3 a | 1.94 ± 0.4 c | 1.75 ± 0.2 b | 1.80 ± 0.3 b | 1.49 ± 0.1 a | 1.64 ± 0.2 a | 490-91-5 |
| 4 | thymol | 2-isopropyl-5-methylphenol | 14.06 | 5.37 ± 0.6 a | 5.04 ± 0.5 a | 5.50 ± 0.6 a | 6.05 ± 0.3 b | 5.51 ± 0.7 a | 5.51 ± 0.2 a | 89-83-8 |
| 5 | carvacrol | 5-isopropyl-2-methylphenol | 14.22 | 58.8 ± 1.1 a | 61.9 ± 1.3 c | 58.47 ± 1.7 a | 62.66 ± 1.6 c | 60.26 ± 1.7 b | 59.48 ± 1.4 b | 499-75-2 |
| 6 | alpha-terpinyl acetate | 2-(4-methyl-3-cyclohexen-1-yl)-2-propanyl acetate | 14.92 | trace | trace | 1.31 ± 0.1 a | trace | trace | trace | 80-26-2 |
| 7 | eugenol | 4-allyl-1-hydroxy-2-methoxybenzene | 15.03 | 6.72 ± 0.5 b | 6.91 ± 0.3 b | 6.92 ± 0.1 b | 4.551 ± 0.3 a | 6.55 ± 0.7 b | 6.32 ± 0.4 b | 97-53-0 |
| 8 | methyl eugenol | 4-allyl veratrole | 15.64 | 24.63 ± 0.8 a | 24.14 ± 0.9 a | 24.60 ± 1.3 a | 24.89 ± 0.7 a | 24.55 ± 0.6 a | 24.31 ± 0.3 a | 93-15-2 |
| 9 | dihydroactinidiolide | (S)-dihydroactinidiolide | 17.39 | trace | trace | trace | trace | trace | trace | 17092-92-1 |
| TOTAL | 97.21 a | 99.99 b | 98.55 a | 99.96 b | 98.36 a | 97.27 a | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matłok, N.; Lachowicz, S.; Gorzelany, J.; Balawejder, M. Influence of Drying Method on Some Bioactive Compounds and the Composition of Volatile Components in Dried Pink Rock Rose (Cistus creticus L.). Molecules 2020, 25, 2596. https://doi.org/10.3390/molecules25112596
Matłok N, Lachowicz S, Gorzelany J, Balawejder M. Influence of Drying Method on Some Bioactive Compounds and the Composition of Volatile Components in Dried Pink Rock Rose (Cistus creticus L.). Molecules. 2020; 25(11):2596. https://doi.org/10.3390/molecules25112596
Chicago/Turabian StyleMatłok, Natalia, Sabina Lachowicz, Józef Gorzelany, and Maciej Balawejder. 2020. "Influence of Drying Method on Some Bioactive Compounds and the Composition of Volatile Components in Dried Pink Rock Rose (Cistus creticus L.)" Molecules 25, no. 11: 2596. https://doi.org/10.3390/molecules25112596
APA StyleMatłok, N., Lachowicz, S., Gorzelany, J., & Balawejder, M. (2020). Influence of Drying Method on Some Bioactive Compounds and the Composition of Volatile Components in Dried Pink Rock Rose (Cistus creticus L.). Molecules, 25(11), 2596. https://doi.org/10.3390/molecules25112596








