RETRACTED: Nanoformulated Ajwa (Phoenix Dactylifera) Bioactive Compounds Improve the Safety of Doxorubicin without Compromising Its Anticancer Efficacy in Breast Cancer
Abstract
1. Introduction
2. Results
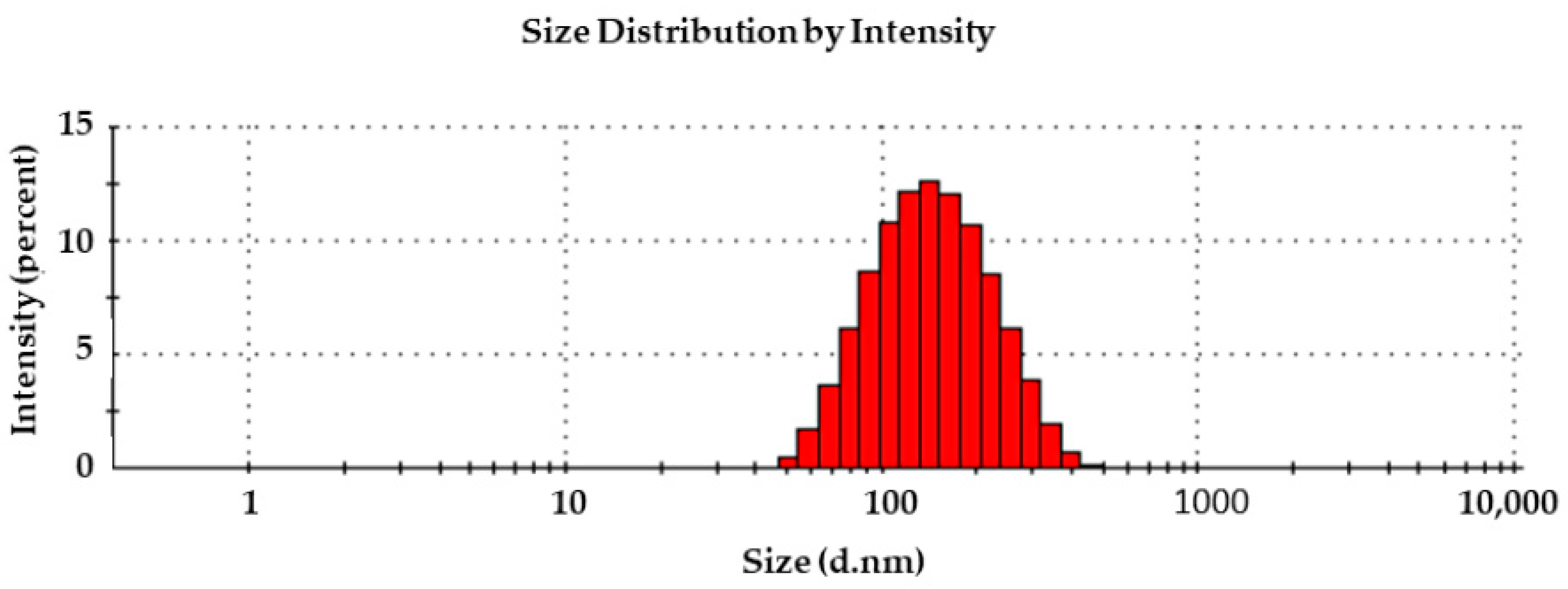
2.1. RQ-NPs Size and Entrapping Efficiency
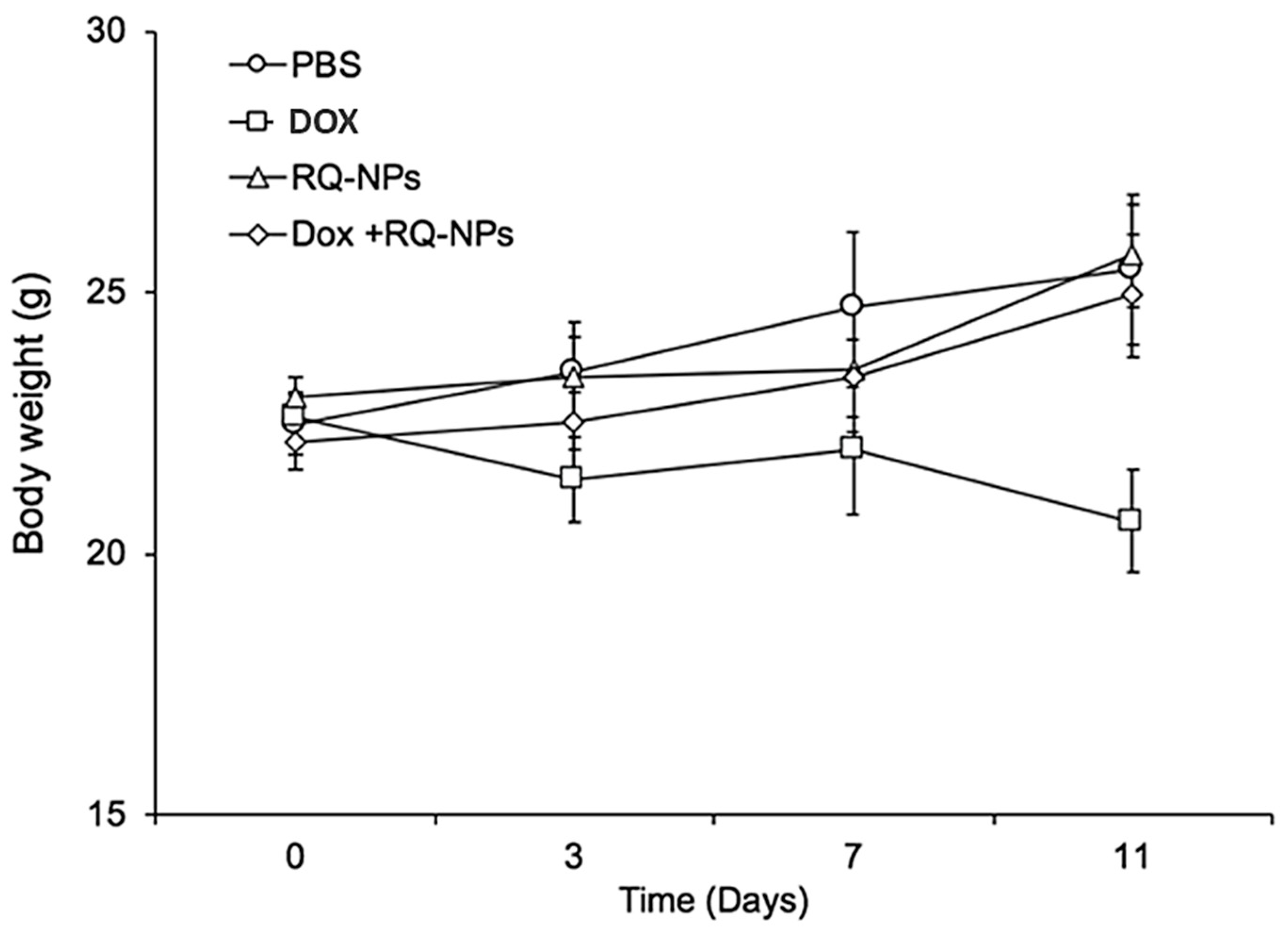
2.2. Effect of DOX and RQ-NPs on Body Weight
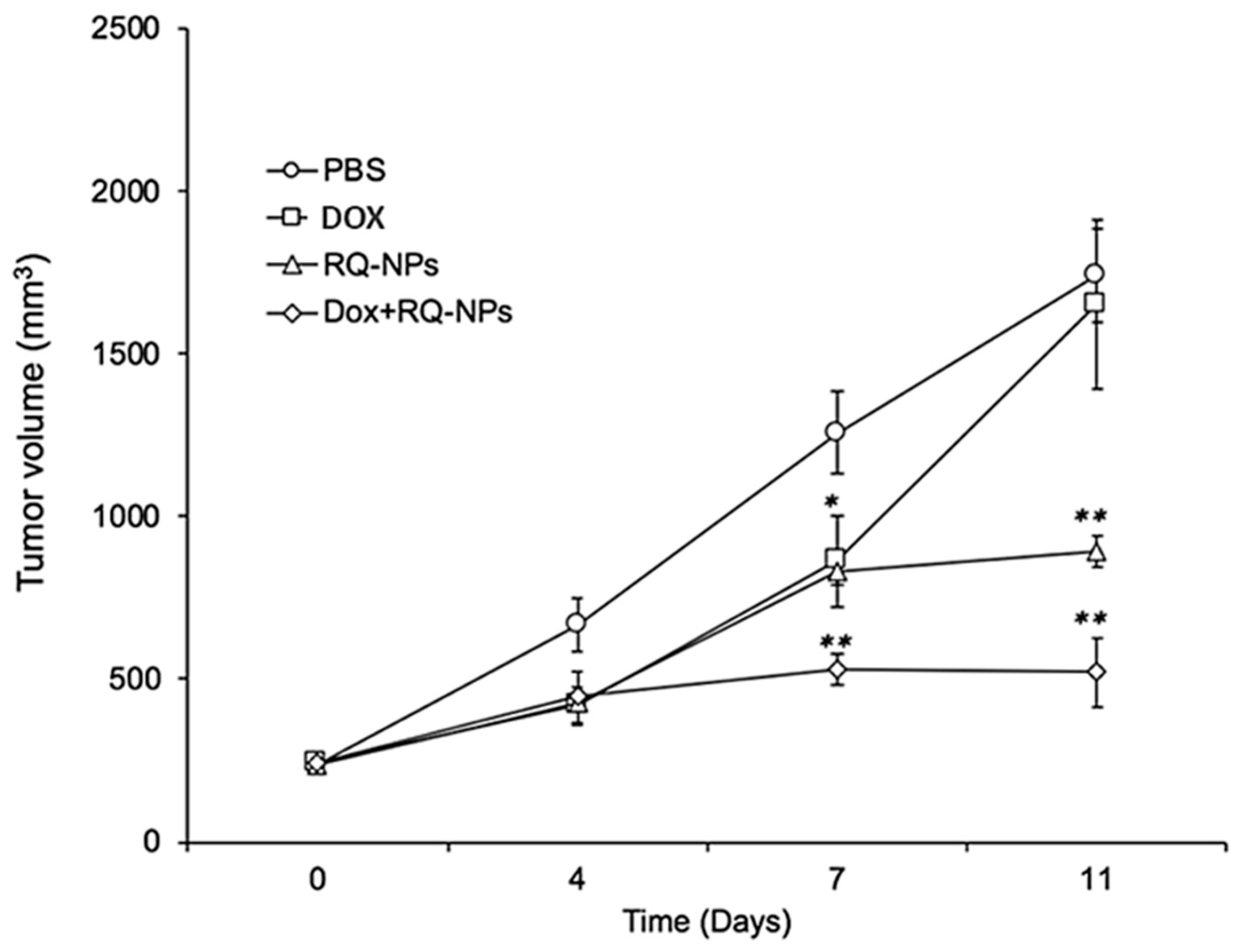
2.3. Effect of DOX and RQ-NPs on Tumor Volume
2.4. Effect of DOX and RQ-NPs on Tumor Weight
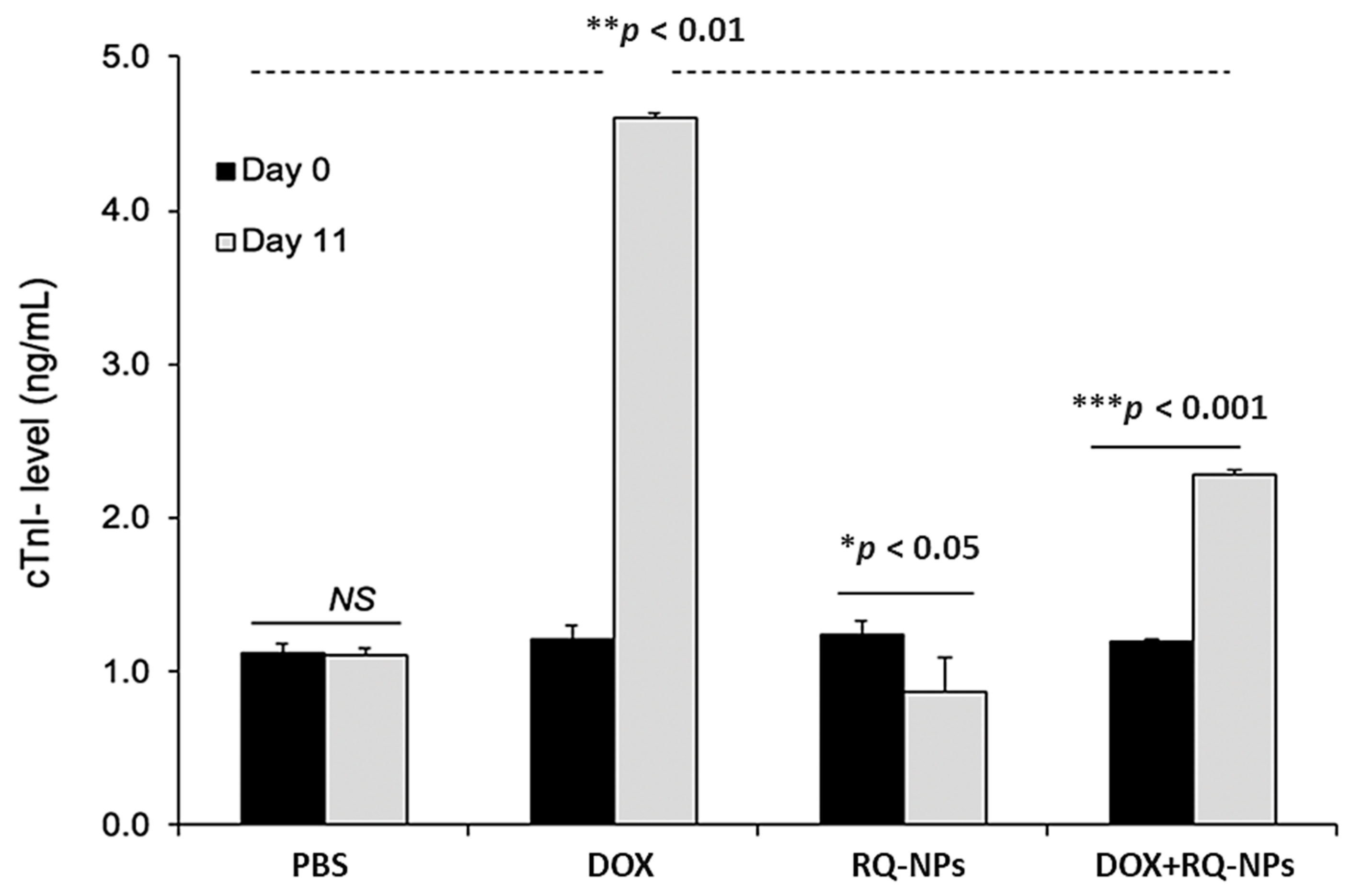
2.5. Effect of DOX and RQ-NPs on Plasma cTn-I Levels
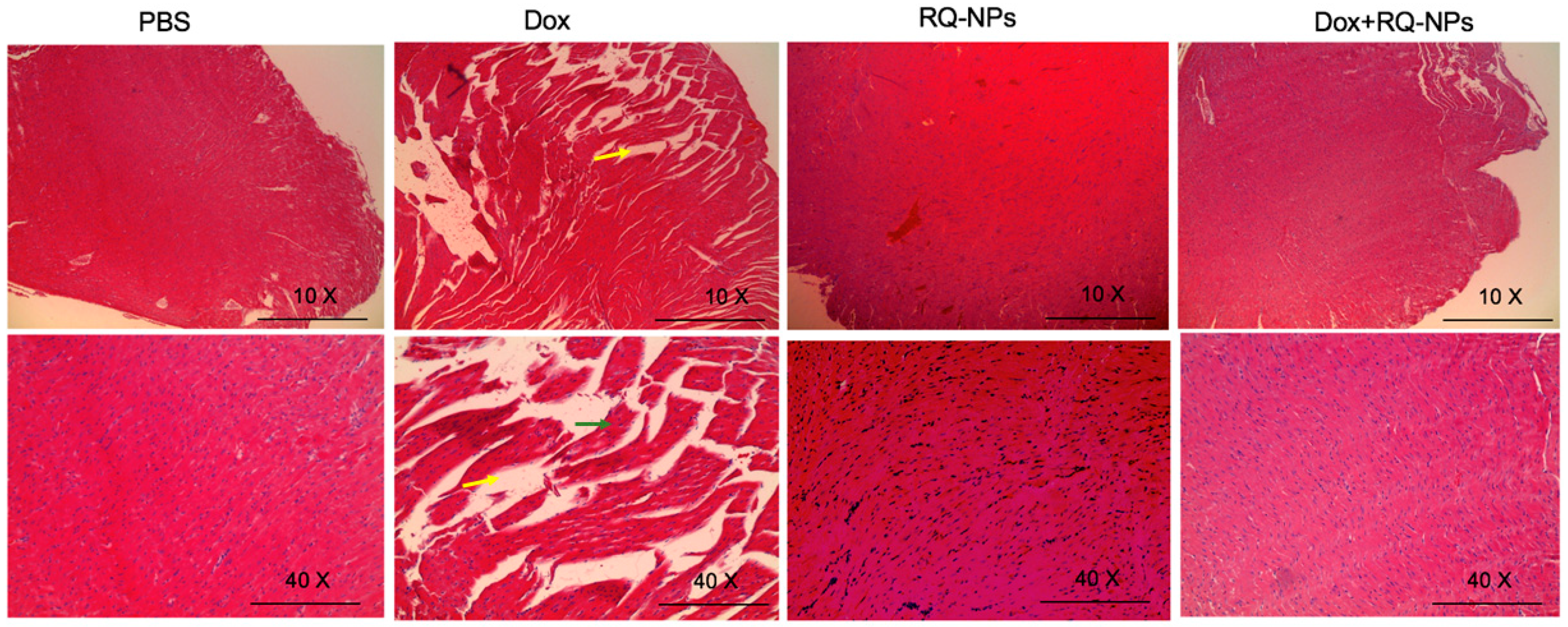
2.6. Effect of DOX and RQ-NPs on Heart Histopathology
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Nanoformulation, Characterization, and Entrapment Efficiency
4.4. Animals
4.5. Tumor Implantation and Treatment
4.6. Determination of cTn-I Levels
4.7. Histopathological Examination
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The story of mcf-7 breast cancer cell line: 40 years of experience in research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Soule, H.D.; Vazquez, J.; Long, A.; Albert, S.; Brennan, M. A Human Cell Line From a Pleural Effusion Derived From a Breast Carcinoma 2. Jnci J. Natl. Cancer Inst. 1973, 51, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, E.E.; McDaniel, R.E.; Maximov, P.Y.; Fan, P.; Jordan, V.C. Models and mechanisms of acquired antihormone resistance in breast cancer: Significant clinical progress despite limitations. Horm. Mol. Biol. Clin. Investig. 2012, 9, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Farshad, H.; Zarghi, A.; Kobarfard, F.; Zendehdel, R.; Nakhjavani, M.; Arfaiee, S.; Zebardast, T.; Mohebi, S.; Anjidani, N.; Ashtarinezhad, A.; et al. Remarks in Successful Cellular Investigations for Fighting Breast Cancer Using Novel Synthetic Compounds. In Breast Cancer—Focusing Tumor Microenvironment, Stem Cells and Metastasis; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Gest, C.; Joimel, U.; Huang, L.; Pritchard, L.-L.; Petit, A.; Dulong, C.; Buquet, C.; Hu, C.-Q.; Mirshahi, P.; Laurent, M.; et al. Rac3 induces a molecular pathway triggering breast cancer cell aggressiveness: Differences in MDA-MB-231 and MCF-7 breast cancer cell lines. BMC Cancer 2013, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharm. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Lu, T.; Finkel, T. Free radicals and senescence. Exp. Cell Res. 2008, 314, 1918–1922. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Santos, R.; Cardoso, S.; Correia, S.; Oliveira, P.; Santos, M.; Moreira, P. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Goehe, R.W.; Lotrionte, M.; Mezzaroma, E.; Sumner, E.T.; Biondi-Zoccai, G.G.L.; Seropian, I.M.; Van Tassell, B.W.; Loperfido, F.; Palazzoni, G.; et al. Comparative Cardiac Toxicity of Anthracyclines In Vitro and In Vivo in the Mouse. PLoS ONE 2013, 8, e58421. [Google Scholar] [CrossRef]
- Elliott, P. Pathogenesis of Cardiotoxicity Induced by Anthracyclines. Semin. Oncol. 2006, 33, 2–7. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Oyinloye, B.E.; Sepehrimanesh, M.; Allah, M.A.G.; Abu-Reidah, I.; Shaheen, H.M.; Razeghian-Jahromi, I.; Alsenosy, A.E.-W.A.; Noreldin, A.E.; Al Jaouni, S.K.; et al. Date palm (phoenix dactylifera): Novel findings and future directions for food and drug discovery. Curr. Drug Discov. Technol. 2019, 16, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Rajab, A.R.; Elkablawy, M.A.; Sheik, B.Y. Antioxidant and Tissue-Protective Studies on Ajwa Extract: Dates from Al Madinah Almonwarah, Saudia Arabia. Egypt. J. Forensic Sci. Appl. Toxicol. 2012, 12, 201–223. [Google Scholar] [CrossRef]
- Khan, F.; Ahmed, F.; Pushparaj, P.N.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; AlQahtani, M.; Gauthaman, K. Ajwa Date (Phoenix dactylifera L.) Extract Inhibits Human Breast Adenocarcinoma (MCF7) Cells In Vitro by Inducing Apoptosis and Cell Cycle Arrest. PLoS ONE 2016, 11, e0158963. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Ahmad, R.; Khan, M.A.; Upadhyay, S.; Husain, I.; Srivastava, A.N. Cytostatic and Anti-tumor Potential of Ajwa Date Pulp against Human Hepatocellular Carcinoma HepG2 Cells. Sci. Rep. 2019, 9, 245. [Google Scholar] [CrossRef]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef]
- Mousa, D.S.; El-Far, A.H.; Saddiq, A.A.; Sudha, T.; Mousa, S.A. Nanoformulated Bioactive Compounds Derived from Different Natural Products Combat Pancreatic Cancer Cell Proliferation. Int. J. Nanomed. 2020, 15, 2259–2268. [Google Scholar] [CrossRef]
- Sudha, T.; El-Far, A.H.; Mousa, D.S.; Mousa, S.A. Resveratrol and Its Nanoformulation Attenuate Growth and the Angiogenesis of Xenograft and Orthotopic Colon Cancer Models. Molecules 2020, 25, 1412. [Google Scholar] [CrossRef]
- El-Far, A.; Al Jaouni, S.; Li, W.; Mousa, S. Protective Roles of Thymoquinone Nanoformulations: Potential Nanonutraceuticals in Human Diseases. Nutrients 2018, 10, 1369. [Google Scholar] [CrossRef]
- Bussing, A.; Bischof, M.; Hatzmann, W.; Bartsch, F.; Soto-Vera, D.; Fronk, E.-M.; Gmeindl, M. Prevention of surgery-induced suppression of granulocyte function by intravenous application of a fermented extract from Viscum album L. in breast cancer patients. Anticancer Res. 2005, 25, 4753–4757. [Google Scholar]
- Shu, Y.; Xie, B.; Liang, Z.; Chen, J. Quercetin reverses the doxorubicin resistance of prostate cancer cells by downregulating the expression of c-met. Oncol. Lett. 2017, 15, 2252–2258. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, S.; Xue, X.; Zhu, X.; Song, S.; Wang, B.; Jiang, L.; Qin, M.; Liang, H.; Gao, L. Quercetin and doxorubicin co-delivery using mesoporous silica nanoparticles enhance the efficacy of gastric carcinoma chemotherapy. Int. J. Nanomed. 2018, 13, 5113–5126. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Wu, G.; Su, C.; Dong, Y.; Zhang, K.; Li, J.; Sun, X.; Li, Y.; Chen, X.; Feng, C. pH-sensitive amphiphilic chitosan-quercetin conjugate for intracellular delivery of doxorubicin enhancement. Carbohydr. Polym. 2019, 223, 115072. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 2016, 7, 32184–32199. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, S.; Zhao, Q.; Wang, B.; Wang, X.; Li, K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed. Pharm. 2018, 100, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Kardami, E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B. Doxorubicin-Induced Cardiac Mitochondrionopathy. Pharm. Toxicol. 2003, 93, 105–115. [Google Scholar] [CrossRef]
- Kavazis, A.N.; Morton, A.B.; Hall, S.E.; Smuder, A.J. Effects of doxorubicin on cardiac muscle subsarcolemmal and intermyofibrillar mitochondria. Mitochondrion 2017, 34, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Oxidized phospholipids in Doxorubicin-induced cardiotoxicity. Chem. Biol. Interact. 2019, 303, 35–39. [Google Scholar] [CrossRef]
- Morris, P.G.; Chen, C.; Steingart, R.; Fleisher, M.; Lin, N.; Moy, B.; Come, S.; Sugarman, S.; Abbruzzi, A.; Lehman, R.; et al. Troponin I and C-Reactive Protein Are Commonly Detected in Patients with Breast Cancer Treated with Dose-Dense Chemotherapy Incorporating Trastuzumab and Lapatinib. Clin. Cancer Res. 2011, 17, 3490–3499. [Google Scholar] [CrossRef]
- Shi, Y.; Su, X.; Cui, H.; Yu, L.; Du, H.; Han, Y. Combination of quercetin and Adriamycin effectively suppresses the growth of refractory acute leukemia. Oncol. Lett. 2019. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Luo, Y.; You, J.; Yin, D.; Xu, Q.; He, H.; He, M. Quercetin protects cardiomyocytes against doxorubicin-induced toxicity by suppressing oxidative stress and improving mitochondrial function via 14-3-3γ. Toxicol. Mech. Methods 2019, 29, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Parikh, M.; Shah, H.; Gandhi, T. Modulation of Nrf2 by quercetin in doxorubicin-treated rats. Heliyon 2020, 6, e03803. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Chen, L.; Lu, Q.; Sharma, S.; Li, L.; Morimoto, S.; Wang, G. Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br. J. Pharm. 2014, 171, 4440–4454. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Hu, R.-Y.; Chou, H.-C. Quercetin-induced cardioprotection against doxorubicin cytotoxicity. J. Biomed. Sci. 2013, 20, 95. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, L.; Ma, J.; Lu, L.; Wang, X.; Ren, J.; Yang, J. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1904–1911. [Google Scholar] [CrossRef]
- Mandziuk, S.; Baj, T.; Sieniawska, E.; Dudka, J.; Gieroba, R.; Iwan, M.; Glowniak, K. Protective effect of Mutellina purpurea polyphenolic compounds in doxorubicin-induced toxicity in H9c2 cardiomyocytes. Drug Chem. Toxicol. 2015, 38, 1–8. [Google Scholar] [CrossRef]
- Hariri, W.; Sudha, T.; Bharali, D.J.; Cui, H.; Mousa, S.A. Nano-Targeted Delivery of Toremifene, an Estrogen Receptor-α Blocker in Prostate Cancer. Pharm. Res. 2015, 32, 2764–2774. [Google Scholar] [CrossRef]
- Bharali, D.J.; Sudha, T.; Cui, H.; Mian, B.M.; Mousa, S.A. Anti-CD24 nano-targeted delivery of docetaxel for the treatment of prostate cancer. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 263–273. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent anti-proliferative and pro-apoptotic effects of (−)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1619–1626. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (RQ-NPs) are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godugu, K.; El-Far, A.H.; Al Jaouni, S.; Mousa, S.A. RETRACTED: Nanoformulated Ajwa (Phoenix Dactylifera) Bioactive Compounds Improve the Safety of Doxorubicin without Compromising Its Anticancer Efficacy in Breast Cancer. Molecules 2020, 25, 2597. https://doi.org/10.3390/molecules25112597
Godugu K, El-Far AH, Al Jaouni S, Mousa SA. RETRACTED: Nanoformulated Ajwa (Phoenix Dactylifera) Bioactive Compounds Improve the Safety of Doxorubicin without Compromising Its Anticancer Efficacy in Breast Cancer. Molecules. 2020; 25(11):2597. https://doi.org/10.3390/molecules25112597
Chicago/Turabian StyleGodugu, Kavitha, Ali H. El-Far, Soad Al Jaouni, and Shaker A. Mousa. 2020. "RETRACTED: Nanoformulated Ajwa (Phoenix Dactylifera) Bioactive Compounds Improve the Safety of Doxorubicin without Compromising Its Anticancer Efficacy in Breast Cancer" Molecules 25, no. 11: 2597. https://doi.org/10.3390/molecules25112597
APA StyleGodugu, K., El-Far, A. H., Al Jaouni, S., & Mousa, S. A. (2020). RETRACTED: Nanoformulated Ajwa (Phoenix Dactylifera) Bioactive Compounds Improve the Safety of Doxorubicin without Compromising Its Anticancer Efficacy in Breast Cancer. Molecules, 25(11), 2597. https://doi.org/10.3390/molecules25112597







