Use of Nanomaterial-Based (Micro)Extraction Techniques for the Determination of Cosmetic-Related Compounds
Abstract
1. Introduction
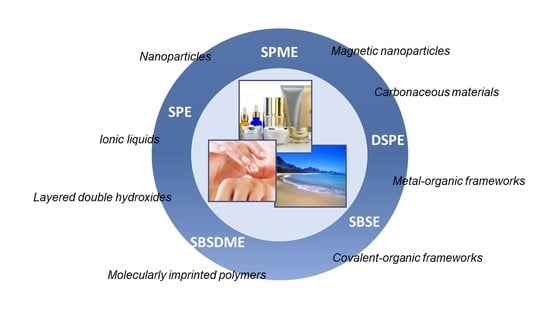
2. (Nano)Materials in Sorbent-Based Microextraction Approaches
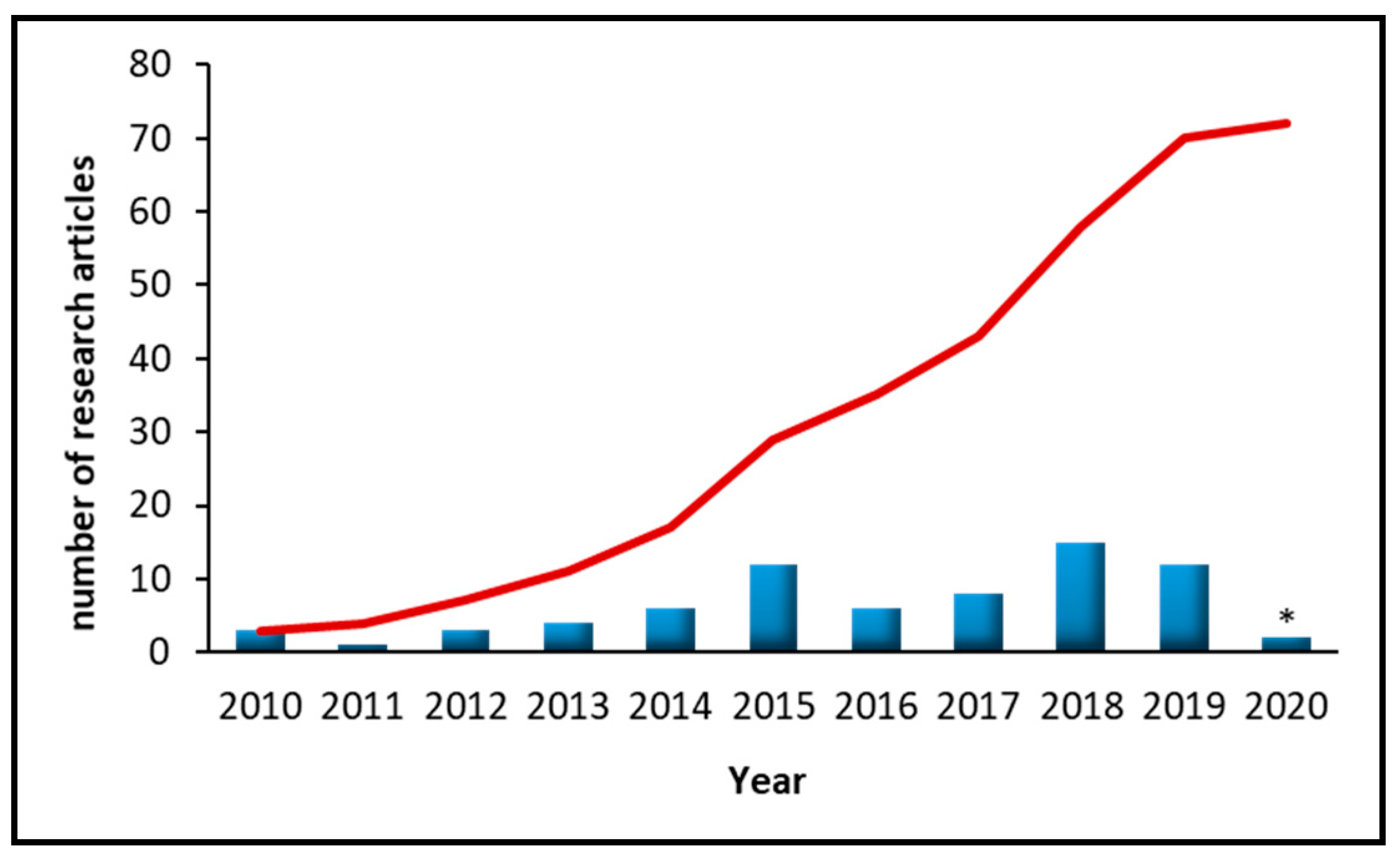
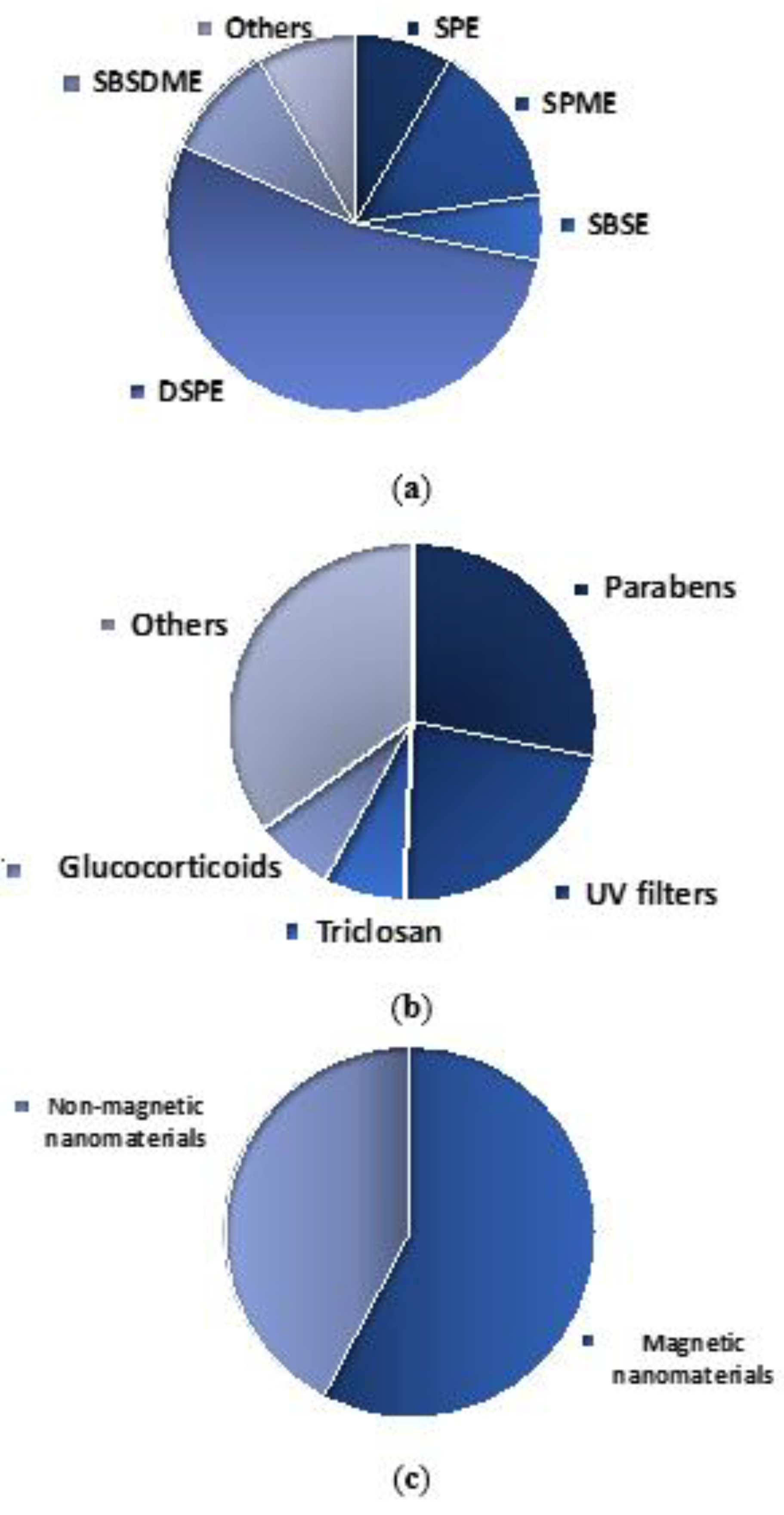
3. Nanomaterials-Based Microextraction Approaches Used for the Determination of Cosmetic-Related Compounds
3.1. Solid Phase Extraction
3.2. Solid Phase Microextraction
3.3. Stir Bar Sorptive Extraction
3.4. Dispersive Solid Phase Extraction
3.5. Stir Bar Sorptive-Dispersive Microextraction
3.6. Other Sorbent-Based Microextraction Approaches
4. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dorato, S. General concepts: Current legislation on cosmetics in various countries. In Analysis of Cosmetics Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–37. ISBN 9780444635082. [Google Scholar]
- Mildau, G. General review of official methods of analysis of cosmetics. In Analysis of Cosmetics Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–83. ISBN 9780444635082. [Google Scholar]
- Bronaugh, R.L.; Maibach, H.I. Percutaneous absorption: Drugs-cosmetics-mechanisms-methodology. In Percutaneous Penetration as It Relates to the Safety Evaluation of Cosmetic Ingredients, 3rd ed.; Yourick, J.J., Bronaugh, R.L., Eds.; Taylor and Francis: Abingdon, UK, 1999; pp. 659–673. ISBN 0-8247-1966-2. [Google Scholar]
- Hewitt, N.J.; Grégoire, S.; Cubberley, R.; Duplan, H.; Eilstein, J.; Ellison, C.; Lester, C.; Fabian, E.; Fernandez, J.; Géniès, C.; et al. Measurement of the penetration of 56 cosmetic relevant chemicals into and though human skin using a standardized protocol. J. Appl. Toxicol. 2019, 40, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharm. 2010, 243, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Fransway, A.F.; Fransway, P.J.; Belsito, D.V.; Yiannias, J.A. Paraben toxicology. Dermatitis 2019, 30, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Hens, L.; Sasco, A.J. Cosmetics as endocrine disruptors: Are they a health risk? Rev. Endocr. Metab. Disord. 2015, 16, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef]
- Haman, C.; Dauchy, X.; Rosin, C.; Munoz, J.F. Occurrence, fate and behaviour of parabens in aquatic environments: A review. Water Res. 2015, 68, 1–11. [Google Scholar] [CrossRef]
- Giokas, D.L.; Chisvert, A.; Salvador, A. Environmental monitoring of cosmetics ingredients. In Analysis of Cosmetics Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 435–547. ISBN 9780444635082. [Google Scholar]
- Murtada, K. Trends in nanomaterial-based solid-phase microextraction with a focus on environmental applications—A review. Trend. Environ. Anal. Chem. 2020, 25, e00077. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Yan, L.; Li, N.; Shi, J.; Jiang, C. Recent developments in detection using noble metal nanoparticles. Crit. Rev. Anal. Chem. 2020, 50, 97–110. [Google Scholar] [CrossRef]
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC Trend. Anal. Chem. 2018, 108, 347–369. [Google Scholar] [CrossRef]
- Reyes-Gallardo, E.M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Magnetic nanoparticles-nylon 6 composite for the dispersive micro solid phase extraction of selected polycyclic aromatic hydrocarbons from water samples. J. Chomatogr. A 2014, 1345, 43–49. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Chisvert, A.; Cárdenas, S.; Lucena, R. Dispersive micro-solid phase extraction. TrAC Trend. Anal. Chem. 2019, 112, 226–233. [Google Scholar] [CrossRef]
- Yang, S.J.; Choi, J.Y.; Chae, H.K.; Cho, J.H.; Nahm, K.S.; Park, C.R. Preparation and enhanced hydro stability and hydrogen storage capacity of CNT@MOF-5 hybrid composite. Chem. Mater. 2009, 21, 1893–1897. [Google Scholar] [CrossRef]
- Reinholds, I.; Jansons, M.; Pugajeva, I.; Bartkevics, V. Recent applications of carbonaceous nanosorbents in solid phase extraction for the determination of pesticides in food sample. Crit. Rev. Anal. Chem. 2019, 49, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Muk, S. Carbon dots optical nanoprobes for biosensors. In Nanobiosensors for Biomolecular Targeting; Gopinath, S.C., Lakshmiriya, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–300. ISBN 9780128139004. [Google Scholar]
- Gutiérrez-Serpa, A.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Metal-organic frameworks as key materials for solid-phase microextraction devices—A review. Separations 2019, 6, 47. [Google Scholar] [CrossRef]
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Li, N.; Du, J.; Wu, D.; Liu, J.; Li, N.; Sun, Z.; Li, G.; Wu, Y. Recent advances in facile synthesis and applications of covalent organic framework materials as superior adsorbents in sample pretreatment. TrAC Trend. Anal. Chem. 2018, 108, 154–166. [Google Scholar] [CrossRef]
- Sajid, M.; Basheer, C. Layered double hydroxides: Emerging sorbent materials for analytical extractions. TrAC Trend. Anal. Chem. 2016, 75, 174–182. [Google Scholar] [CrossRef]
- Young, I.R.; Lovell, P.A. Introduction to Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 205–233. ISBN 9781439894156. [Google Scholar]
- Haupt, K.; Linares, A.V.; Bompart, M.; Bui, B.T.S. Molecularly imprinted polymers. In Molecular Imprinting Polymers; Haupt, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–28. ISBN 978-3-642-28420-5. [Google Scholar]
- Ho, T.D.; Canestraro, A.J.; Anderson, J.L. Ionic liquids in solid-phase microextraction: A review. Anal. Chim. Acta 2011, 695, 18–43. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Rocío-Bautista, P.; Pino, V.; Afonso, A.M. Ionic liquids in dispersive liquid-liquid microextraction. TrAC Trend. Anal. Chem. 2013, 51, 87–106. [Google Scholar] [CrossRef]
- Márquez-Sillero, I.; Aguilera-Herrador, E.; Cárdenas, S.; Valcárcel, M. Determination of parabens in cosmetic products using multi-walled carbon nanotubes as solid phase extraction sorbent and corona-charged aerosol detection system. J. Chromatogr. A 2010, 1217, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Du, T.; Kou, H.; Du, X.; Lu, X. Determination of six benzotriazole ultraviolet filters in water and cosmetic samples by graphene sponge-based solid-phase extraction followed by high-performance liquid chromatography. Anal. Bioanal. Chem. 2018, 410, 6955–6962. [Google Scholar] [CrossRef]
- Zhu, R.; Zhao, W.; Zhai, M.; Wei, F.; Cai, Z.; Sheng, N.; Hu, Q. Molecularly imprinted layer-coated silica nanoparticles for selective solid-phase extraction of bisphenol A from chemical cleansing and cosmetics samples. Anal. Chim. Acta 2010, 658, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, X.; Li, J.; Zhu, C.; Liu, M.; Wu, Z.; Liu, L.; Tan, X.; Lei, F. Preparation and application of a molecular capture for safety detection of cosmetics based on surface imprinting and multi-walled carbon nanotube. J. Colloid Interface Sci. 2018, 527, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Li, G.; Luo, Z.; Liu, Z.; Shao, Y.; He, W.; Deng, J.; Luo, X. Carboxylated graphene oxide/polyvinyl chloride as solid-phase extraction sorbent combined with ion chromatography for the determination of sulfonamides in cosmetics. Anal. Chim. Acta 2015, 888, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammad-Zadeh, H.; Falaghi, S.; Rahimpout, E. An innovative nano-sorbent for selective solid-phase extraction and spectrophotometric determination of p-amino benzoic acid in cosmetic products. Int. J. Cosmet. Sci. 2014, 36, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Ara, K.M.; Pandidan, S.; Aliakbari, A.; Raofie, F.; Amini, M.M. Porous-membrane-protected polyaniline-coated SBA-15 nanocomposite micro-solid-phase extraction followed by high-performance liquid chromatography for the determination of parabens in cosmetic products and wastewater. J. Sep. Sci. 2015, 38, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.N.; Yamini, Y.; Asiabi, H. Fabrication of polypyrrole-silver nanocomposite for hollow fiber solid phase microextraction followed by HPLC/UV analysis for determination of parabens in water and beverages samples. J. Food Compost. Anal. 2018, 74, 18–26. [Google Scholar] [CrossRef]
- Ma, M.; Wang, H.; Zhen, Q.; Zhang, M.; Du, X. Development of nitrogen-enriched carbonaceous material coated titania nanotubes array as a fiber coating for solid-phase microextraction of ultraviolet filters in environmental water. Talanta 2017, 167, 118–125. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Liu, H.; Wang, X.; Du, X. Fabrication of a novel Ti–TiO2–ZrO2 fiber for solid phase microextraction followed by high performance liquid chromatography for sensitive determination of UV filters in environmental water samples. Anal. Methods 2014, 6, 8519–8525. [Google Scholar] [CrossRef]
- Li, L.; Guo, R.; Li, Y.; Guo, M.; Wang, X.; Du, X. In situ growth and phenyl functionalization of titania nanoparticles coating for solid-phase microextraction of ultraviolet filters in environmental water samples followed by high performance liquid chromatography–UV detection. Anal. Chim. Acta 2015, 867, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Liu, H.; Wang, X.; Du, X. Electrodeposition of gold nanoparticles onto an etched stainless steel wire followed by a self-assembled monolayer of octanedithiol as a fiber coating for selective solid-phase microextraction. J. Chromatogr. A 2014, 1372, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Huang, X. Online analysis of five organic ultraviolet filters in environmental water samples using magnetism-enhanced monolith-based in-tube solid phase microextraction coupled with high-performance liquid chromatography. J. Chromatogr. A 2017, 1525, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wuang, J.; Liang, W.; Zang, X.; Wang, C.; Wu, Q.; Wang, Z. Single layer graphitic carbon nitride-modified graphene composite as a fiber coating for solid-phase microextraction of polycyclic aromatic hydrocarbons. Microchim. Acta 2017, 184, 2171–2180. [Google Scholar] [CrossRef]
- Tong, S.; Liu, Q.; Li, Y.; Zhou, W.; Jia, Q.; Duan, T. Preparation of porous polymer monolithic column incorporated with graphene nanosheets for solid phase microextraction and enrichment of glucocorticoids. J. Chromatogr. A 2012, 1253, 22–31. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, P.; Zhou, S.; Wang, X.; Du, X. Controlled growth of a porous hydroxyapatite nanoparticle coating on a titanium fiber for rapid and efficient solid-phase microextraction of polar chlorophenols, triclosan and bisphenol A from environmental water. Anal. Methods 2018, 10, 3237–3247. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C.A. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, W.; Liao, X.; Wang, X.; Chen, Z. Covalent immobilization of metal organic frameworks onto chemical resistant poly(ether ketone) jacket for stir bar extraction. Anal. Chim. Acta 2018, 1025, 124–133. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Cárdenas, S. Nanostructured hybrid monolith with integrated stirring for the extraction of UV-filters from water and urine samples. Talanta 2018, 182, 391–395. [Google Scholar] [CrossRef]
- Siritham, C.; Thammakhet-Buranachai, C.; Thavarungkul, P.; Kanatharana, P. A stir foam composed of graphene oxide, poly(ethylene glycol) and natural latex for the extraction of preservatives and antioxidant. Microchim. Acta 2018, 185, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Chang, Q.; Liang, W.; Wu, T.; Wang, C.; Wang, Z. Micro-solid phase extraction of chlorophenols using reduced graphene oxide functionalized with magnetic nanoparticles and graphitic carbon nitride as the adsorbent. Microchim. Acta 2018, 185, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenk, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for determination of pesticide residues in produce. J. Aoac Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Rocío-Bautista, P.; Martínez-Benito, C.; Pino, V.; Pasán, J.; Ayala, J.H.; Ruiz-Pérez, C.; Alfonso, A.M. The metal–organic framework HKUST-1 as efficient sorbent in a vortex-assisted dispersive micro solid-phase extraction of parabens from environmental waters, cosmetic creams, and human urine. Talanta 2015, 139, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rashvand, M.; Vosough, M. Graphene oxide–polyaniline nanocomposite as a potential sorbent for dispersive solid-phase extraction and determination of selected pharmaceutical and personal care products in wastewater samples using HPLC with a diode-array detector. Anal. Methods 2016, 8, 1898–1907. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Q.; Yang, Y.; Huang, J.; Dang, X.; Chen, H. A novel dispersive solid-phase extraction method using metal-organic framework MIL-101 as the adsorbent for the analysis of benzophenones in toner. Talanta 2015, 132, 713–718. [Google Scholar] [CrossRef]
- Gao, R.; Kong, X.; Su, F.; He, X.; Chen, L.; Zhang, Y. Synthesis and evaluation of molecularly imprinted core–shell carbon nanotubes for the determination of triclosan in environmental water samples. J. Chromatogr. A 2010, 1217, 8095–8102. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, N.; Lei, L.; Yang, X.; Zhang, H. Dispersive micro-solid-phase extraction of hormones in liquid cosmetics with metal-organic framework. Anal. Methods 2014, 6, 9435–9445. [Google Scholar] [CrossRef]
- Liu, G.; Jia, H.; Li, N.; Li, X.; Yu, Z.; Wang, J.; Song, Y. High-fluorescent carbon dots (CDs) originated from China grass carp scales (CGCS) for effective detection of Hg(II) ions. Microchem. J. 2019, 145, 718–728. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Yamini, Y.; Mehdinia, A.; Rouhi, F. Polyaniline-coated Fe3O4 nanoparticles: An anion exchange magnetic sorbent for solid-phase extraction. J. Sep. Sci. 2012, 35, 2256–2265. [Google Scholar] [CrossRef]
- Ghambari, H.; Reyes-Gallardo, E.M.; Lucena, R.; Saraji, M.; Cárdenas, S. Recycling polymer residues to synthesize magnetic nanocomposites for dispersive micro-solid phase extraction. Talanta 2017, 170, 451–456. [Google Scholar] [CrossRef]
- Abbasghorbani, M.; Attaran, A.; Payehghadr, M. Solvent-assisted dispersive micro-SPE by using aminopropyl-functionalized magnetite nanoparticle followed by GC-PID for quantification of parabens in aqueous matrices. J. Sep. Sci. 2013, 36, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, M.M.; Sohaime, N.M.; Yih, B.S.; Saleh, N.M. Magnetite nanoparticles coated with surfactant Sylgard 309 and its application as an adsorbent for paraben extraction from pharmaceutical and water samples. Anal. Methods 2019, 11, 4126–4136. [Google Scholar] [CrossRef]
- Ariffin, M.M.; Azmi, A.H.; Saleh, N.M.; Mohamad, S.; Rozi, S.K. Surfactant functionalization of magnetic nanoparticles: A greener method for parabens determination in water samples by using magnetic solid phase extraction. Microchem. J. 2019, 147, 930–940. [Google Scholar] [CrossRef]
- Casado-Carmona, F.A.; Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Magnetic nanoparticles coated with ionic liquid for the extraction of endocrine disrupting compounds from waters. Microchem. J. 2016, 128, 347–353. [Google Scholar] [CrossRef]
- Mehdinia, A.; Esfandiarnejad, R.; Jabbari, A. Magnetic nanocomposite of self-doped polyaniline–graphene as a novel sorbent for solid-phase extraction. J. Sep. Sci. 2015, 38, 141–147. [Google Scholar] [CrossRef]
- Mehdinia, A.; Bahrami, M.; Mozaffari, S. A comparative study on different functionalized mesoporous silica nanomagnetic sorbents for efficient extraction of parabens. J. Iran Chem. Soc. 2015, 12, 1543–1552. [Google Scholar] [CrossRef]
- Feng, J.; He, X.; Liu, X.; Sun, X.; Li, Y. Preparation of magnetic graphene/mesoporous silica composites with phenyl-functionalized pore-walls as the restricted access matrix solid phase extraction adsorbent for the rapid extraction of parabens from water-based skin toners. J. Chromatogr. A 2016, 1465, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, N.; Ebrahimzadeh, H.; Asgharinezhad, A.A. Preparation of magnetite/multiwalled carbon nanotubes/metal-organic framework composite for dispersive magnetic micro solid phase extraction of parabens and phthalate esters from water samples and various types of cream for their determination with liquid chromatography. J. Chromatogr. A 2019, 1608, 460426. [Google Scholar] [CrossRef]
- Shavar, A.; Soltani, R.; Saraji, M.; Dinari, M.; Alijani, S. Covalent triazine-based framework for micro solid-phase extraction of parabens. J. Chromatogr. A 2018, 1565, 48–56. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Raoov, M.; Yahaya, N.; Salleh, N.M. An ionic liquid loaded magnetically confined polymeric mesoporous adsorbent for extraction of parabens from environmental and cosmetic samples. RSC Adv. 2017, 7, 35832–35844. [Google Scholar] [CrossRef]
- Pastor-Belda, M.; Marín-Soler, L.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Magnetic carbon nanotube composite for the preconcentration of parabens from water and urine samples using dispersive solid phase extraction. J. Chromatogr. A 2018, 1564, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Gashemi, E.; Sillanpää, M. Ultrasound-assisted solid-phase extraction of parabens from environmental and biological samples using magnetic hydroxyapatite nanoparticles as an efficient and regenerable nanosorbent. Microchim. Acta 2019, 186, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, R.; Chen, Z. Metal-organic framework-1210(zirconium/cuprum) modified magnetic nanoparticles for solid phase extraction of benzophenones in soil samples. J. Chromatogr. A 2019, 1607, 460403. [Google Scholar] [CrossRef]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Samperi, R.; Chiozzi, R.Z.; Laganà, A. A new carbon-based magnetic material for the dispersive solid-phase extraction of UV filters from water samples before liquid chromatography–tandem mass spectrometry analysis. Anal. Bioanal. Chem. 2017, 409, 4181–4194. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Kong, X.; Liu, S.; Che, D.; Sun, Z.; Li, G.; Ping, M.; Tang, J.; You, J. Determination of ultraviolet filters in domestic wastewater by LC–MS coupled with polydopamine-based magnetic solid-phase extraction and isotope-coded derivatization. Chromatographia 2018, 81, 1673–1684. [Google Scholar] [CrossRef]
- Román, I.P.; Chisvert, A.; Canals, A. Dispersive solid-phase extraction based on oleic acid-coated magnetic nanoparticles followed by gas chromatography-mass spectrometry for UV-filter determination in water samples. J. Chromatogr. A 2011, 1218, 2467–2475. [Google Scholar] [CrossRef]
- Giokas, D.L.; Zhu, Q.; Pan, Q.; Chisvert, A. Cloud point–dispersive μ-solid phase extraction of hydrophobic organic compounds onto highly hydrophobic core–shell Fe2O3@C magnetic nanoparticles. J. Chromatogr. A 2012, 1251, 33–39. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, X.; Feng, F.; Dang, X.; Huang, J.; Chen, H. Magnetic solid-phase extraction of triclosan using core-shell Fe3O4@MIL-100 magnetic nanoparticles, and its determination by HPLC with UV detection. Microchim. Acta 2016, 183, 2467–2472. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.; Huang, L.; Lin, Z.; Cai, Z. Core-shell structured magnetic covalent organic framework nanocomposites for triclosan and triclocarban adsorption. ACS Appl. Mater. Interfaces 2019, 11, 22492–22500. [Google Scholar] [CrossRef]
- Li, F.; Cai, C.; Cheng, J.; Zhou, H.; Ding, K.; Zhang, L. Extraction of endocrine disrupting phenols with iron-ferric oxide core-shell nanowires on graphene oxide nanosheets, followed by their determination by HPLC. Microchim. Acta 2015, 182, 2503–2511. [Google Scholar] [CrossRef]
- Jiang, X.; Cheng, J.; Zhou, H.; Li, F.; Wung, W.; Ding, K. Polyaniline-coated chitosan-functionalized magnetic nanoparticles: Preparation for the extraction and analysis of endocrine-disrupting phenols in environmental water and juice samples. Talanta 2015, 141, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhang, B.; Guo, P.; Chen, G.; Chang, C.; Fu, Q. Facile preparation of magnetic molecularly imprinted polymers for the selective extraction and determination of dexamethasone in skincare cosmetics using HPLC. J. Sep. Sci. 2018, 41, 2441–2452. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Li, J.; Wu, Z.; Wang, F.; Liu, L.; Tan, X.; Lei, F. Selective separation and determination of glucocorticoids in cosmetics using dual-template magnetic molecularly imprinted polymers and HPLC. J. Colloid Interf. Sci. 2017, 504, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Xia, L.; Xiao, X.; Li, G. Magnetic metal-organic frameworks-101 functionalized with graphite-like carbon nitride for the efficient enrichment of glucocorticoids in cosmetics. J. Chromatogr. A 2019, 1606, 460382. [Google Scholar] [CrossRef]
- Khani, R.; Sobhani, S.; Yari, T. Magnetic dispersive micro solid-phase extraction of trace Rhodamine B using imino-pyridine immobilized on iron oxide as nanosorbent and optimization by Box–Behnken design. Microchem. J. 2019, 146, 471–478. [Google Scholar] [CrossRef]
- Bagheri, H.; Daliri, R.; Roostaie, A. A novel magnetic poly(aniline-naphthylamine)-based nanocomposite for micro solid phase extraction of rhodamine B. Anal Chim. Acta 2013, 794, 38–46. [Google Scholar] [CrossRef]
- Tarigh, G.D.; Shemirani, F. Magnetic multi-wall carbon nanotube nanocomposite as an adsorbent for preconcentration and determination of lead (II) and manganese (II) in various matrices. Talanta 2013, 115, 744–750. [Google Scholar] [CrossRef]
- Xia, L.; Chen, X.; Xiao, X.; Li, G. Magnetic-covalent organic polymer solid-phase extraction coupled with high-performance liquid chromatography for the sensitive determination of fluorescent whitening agents in cosmetics. J. Sep. Sci. 2018, 41, 3733–3741. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Y.; Li, J.J.; Su, X.M.; Wu, Z.Y.; Li, P.F.; Lei, F.H.; Tan, X.C.; Shi, Z.W. Synthesis of magnetic molecularly imprinted polymers for the selective separation and determination of metronidazole in cosmetic samples. Anal. Bioanal. Chem. 2015, 407, 3875–3880. [Google Scholar] [CrossRef]
- Maidatsi, K.V.; Chatzimitakos, T.G.; Sakkas, V.A.; Stalikas, C.D. Octyl-modified magnetic graphene as a sorbent for the extraction and simultaneous determination of fragrance allergens, musks and phthalates in aqueous samples by gas chromatography with mass spectrometry. J. Sep. Sci. 2015, 38, 3758–3765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lai, H.; Li, G.; Hu, Y. CoFe2O4@HNT/AuNPs for rapid magnetic solid-phase extraction and efficient SERS detection of complex samples all-in-one. Anal. Chem. 2020, 92, 4607–4613. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Chisvert, A.; Giokas, D.L.; Salvador, A. Development of stir bar sorptive-dispersive microextraction mediated by magnetic nanoparticles and its analytical application to the determination of hydrophobic organic compounds in aqueous media. J. Chromatogr. A 2014, 1362, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Chisvert, A.; Giokas, D.L.; Salvador, A. Determination of ultraviolet filters in bathing waters by stir bar sorptive–dispersive microextraction coupled to thermal desorption–gas chromatography–mass spectrometry. Talanta 2016, 147, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Chisvert, A.; Moyano, C.; Giokas, D.L.; Salvador, A. Expanding the application of stir bar sorptive-dispersive microextraction approach to solid matrices: Determination of ultraviolet filters in coastal sand samples. J. Chromatogr. A 2019, 1564, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Chisvert, A.; Giokas, D.L.; Salvador, A. Stir bar sorptive-dispersive microextraction mediated by magnetic nanoparticles–nylon 6 composite for the extraction of hydrophilic organic compounds in aqueous media. Anal. Chim. Acta 2016, 926, 63–71. [Google Scholar] [CrossRef]
- Grau, J.; Benedé, J.L.; Serrano, J.; Segura, A.; Chisvert, A. Stir bar sorptive-dispersive microextraction for trace determination of triphenyl and diphenyl phosphate in urine of nail polish users. J. Chromatogr. A 2019, 1593, 9–16. [Google Scholar] [CrossRef]
- Miralles, P.; van Gemert, I.; Chisvert, A.; Salvador, A. Stir bar sorptive-dispersive microextraction mediated by magnetic nanoparticles-metal organic framework composite: Determination of N-nitrosamines in cosmetic products. J. Chromatogr. A 2019, 1604, 460465. [Google Scholar] [CrossRef]
- Vállez-Gomis, V.; Grau, J.; Benedé, J.L.; Chisvert, A.; Salvador, A. Reduced graphene oxide-based magnetic composite for trace determination of polycyclic aromatic hydrocarbons in cosmetics by stir bar sorptive dispersive microextraction. J. Chromatogr. A 2020, 1624, 461229. [Google Scholar] [CrossRef]
- Makkliang, F.; Kanatharana, P.; Thavarungkul, P.; Thammakhet-Buranachai, C. A miniaturized monolith-MWCNTs-COOH multi-stir-rod micro extractor device for trace parabens determination in cosmetic and personal care products. Talanta 2018, 184, 429–436. [Google Scholar] [CrossRef]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Determination of parabens in waters by magnetically confined hydrophobic nanoparticle microextraction coupled to gas chromatography/mass spectrometry. Microchem. J. 2013, 110, 643–648. [Google Scholar] [CrossRef]
- Wang, H.; Cocovi-Solberg, D.J.; Hu, B.; Miró, M. 3D-Printed microflow injection analysis platform for online magnetic nanoparticle sorptive extraction of antimicrobials in biological specimens as a front end to liquid chromatographic assays. Anal. Chem. 2017, 89, 12541–12549. [Google Scholar] [CrossRef] [PubMed]
- Fresco-Cala, B.; Cárdenas, S. Preparation of macroscopic carbon nanohorn-based monoliths in polypropylene tips by medium internal phase emulsion for the determination of parabens in urine samples. Talanta 2019, 198, 295–301. [Google Scholar] [CrossRef]
- Wang, L.; Zang, X.; Wang, C.; Wang, Z. Graphene oxide as a micro-solid-phase extraction sorbent for the enrichment of parabens from water and vinegar samples. J. Sep. Sci. 2014, 37, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, S.; Furton, K.G.; Santana-Rodríguez, J.J. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples. Anal. Bioanal. Chem. 2015, 407, 8137–8150. [Google Scholar] [CrossRef] [PubMed]
| Analyte(s) a | Matrix | Extraction Technique b | Material/Composite c | Instrumental Technique d | LOD (ng L−1) | RSD (%) | RR (%) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Parabens | Cosmetic | SPE | MWCNT | C-CAD | 500–2100 | <7.6 | 96–104 | 2010 | [28] |
| GCCs | Cosmetic | SPE | MWCNT-MIP | LC-UV | 5000 | <2.1 | 83–106 | 2010 | [30] |
| p-aminobenzoic acid | Cosmetic | SPE | NI-Zn-LDH | UV | 3780 | 1.2 | 96–101 | 2014 | [33] |
| Sulphonamides | Cosmetic | SPE | GO-PVC | LC-UV | 3400–7100 | <7.6 | 88–102 | 2015 | [32] |
| Benzotriazole UV stabilizers | Cosmetic and environmental | SPE | GO | LC-UV | 20–80 | <8.1 | 89–105 | 2018 | [29] |
| BPA | Cosmetic | SPE | SiO2@MIP | LC-FLD | 229 | <9 | 87–97 | 2018 | [31] |
| Analyte(s) a | Matrix | Extraction Technique b | Material/Composite c | Instrumental Technique d | LOD (ng L−1) | RSD (%) | RR (%) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| GCCs | Cosmetic | SPME | BMA-EDMA-rGO | LC-MS | 130–1930 | <14 | 84–104 | 2012 | [43] |
| UV filters | Environmental | SPME | Ti-TiO2/ZrO2 | LC-UV | 32–82 | <11 | 77–114 | 2014 | [38] |
| UV filters | Environmental | SPME | Co-S-AuNPs | LC-UV | 25–56 | <9.4 | 92–106 | 2014 | [40] |
| Parabens | Cosmetic and environmental | SPME | SBA-15/PANI-p-TSA | GC-FID | 80–400 | <7 | 82–108 | 2015 | [35] |
| UV filters | Environmental | SPME | Ph-TiO2-Ti | LC-UV | 0.1–50 | <9.1 | 86–106 | 2015 | [39] |
| UV filters | Environmental | SPME | PANI/TiO2NTs/Ti | LC-UV | 30–50 | <7.7 | 86–113 | 2017 | [37] |
| UV filters | Environmental | SPME | PIL-MCC/MNPs | LC-UV | 40–260 | <10 | 71–119 | 2017 | [41] |
| PAHs | Cosmetic | SPME | g-C3N4@rGO | GC-MS | 1.0–2.0 | <12 | 70–118 | 2017 | [42] |
| Parabens | Environmental | SPME | PPY-AgNPs | LC-UV | 10 | <4.5 | 94–104 | 2018 | [36] |
| TCS, BPA and CPs | Environmental | SPME | HAP@SiO2 | LC-UV | 12–14 | <8.2 | 90–110 | 2018 | [44] |
| Analyte(s) a | Matrix | Extraction Technique b | Material/Composite c | Instrumental Technique d | LOD (ng L−1) | RSD (%) | RR (%) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Parabens | Cosmetic and biological | SBSE | MIL-68 | LC-MS/MS | 1–2 | <9.7 | 73–104 | 2018 | [46] |
| UV filters | Environmental | SBSE | CNH/MA | LC-UV | 100–1000 | <7.9 | 71–124 | 2018 | [47] |
| MI, BHT, BHA | Cosmetic | SBSE | GO-PEG-PANNL | GC-MS | 500–5000 | <3 | 84–107 | 2018 | [48] |
| CPs | Cosmetic | SBSE | Fe3O4-rGO/g-C3N4 | LC-UV | 200–300 ng kg−1 | <12 | 85–104 | 2018 | [49] |
| Analyte(s) a | Matrix | Extraction Technique b | Material/Composite c | Instrumental Technique d | LOD (ng L−1) e | RSD (%) | RR (%) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| TCS | Environmental | DSPE | MWCNT@MIP | LC-UV | n.r. | <12 | 91–95 | 2010 | [54] |
| UV filters | Environmental | (M) DSPE | CoFe2O4@oleic acid | GC-MS | 0.2–6 | <16 | 74–119 | 2011 | [74] |
| Parabens | Environmental | (M) DSPE | Fe3O4@PANI | LC-UV | 300–400 | <2.4 | 86–109 | 2012 | [57] |
| UV filters | Environmental | CP (M) DSPE | Fe2O3@C-PSx | LC-UV | 1430–7500 | <14.9 | 89–97 | 2012 | [75] |
| Parabens | Environmental | (M) DSPE | Fe3O4-AP | GC-PID | 50–300 | <8 | 87–103 | 2013 | [59] |
| Rhodamine B | Cosmetic and environmental | (M) DSPE | Fe3O4@PAN | Fl | 100 | <8.2 | 94–99 | 2013 | [84] |
| Pb (II) Mn (II) | Cosmetic and biological | (M) DSPE | Fe3O4-MWCNTs | AA | 600–1000 | <4.3 | n.r. | 2013 | [85] |
| Hormones | Cosmetic | DSPE | MIL-101(Cr) | LC-UV | 360–910 | <6.1 | 93–102 | 2014 | [55] |
| Parabens | Cosmetic, biological and environmental | DSPE | HKUST-1 | LC-UV | 1500–2600 | <15 | 57–101 | 2015 | [51] |
| UV filters | Cosmetics | DSPE | MIL-101 | LC-UV | 900–1200 | <10 | 94–105 | 2015 | [53] |
| Parabens | Cosmetic | (M) DSPE | Fe3O4@PANI-rGO | GC-FID | 1200–2800 | <7.9 | 89–101 | 2015 | [63] |
| Parabens | Cosmetic | (M) DSPE | Fe3O4-G-mSiO2-Ph | LC-UV | 10,000–25,000 | <5.61 | 79–106 | 2015 | [64] |
| TCS and BPA | Environmental | (M) DSPE | Fe-Fe2O3/GO | LC-UV | 80–100 | <7.5 | 85–93 | 2015 | [78] |
| TCS, BPA and CPs | Environmental | (M) DSPE | Fe3O4@PANI | LC-UV | 100–130 | <6.6 | 85–107 | 2015 | [79] |
| Metronidazole | Cosmetic | (M) DSPE | Fe3O4@MIP | LC-UV | 3000 | <5.20 | 91–104 | 2015 | [87] |
| Musks, phthalates and allergens | Environmental | (M) DSPE | Fe3O4-rGO-OCT | GC-MS | 0.29–3.2 | <9.4 | 83–105 | 2015 | [88] |
| Parabens | Environmental | DSPE | GO-PANI | LC-UV | 50–1800 | <11.5 | 74–120 | 2016 | [52] |
| Parabens and UV filters | Environmental | (M) DSPE | Fe3O4@MIM-PF6 | LC-MS/MS | 260–1350 | <8.3 | 87–99 | 2016 | [62] |
| Parabens | Cosmetic | (M) DSPE | Fe3O4@SiO2 | GC-FID | 200–900 | <5.6 | 85–107 | 2016 | [65] |
| TCS | Cosmetic | (M) DSPE | Fe3O4-MIL-100 | LC-UV | 30,000 ng Kg−1 | <5.5 | 91–101 | 2016 | [76] |
| Parabens | Environmental | (M) DSPE | CoFe2O4-PS | LC-MS | 50–150 | <8.5 | 81–105 | 2017 | [58] |
| Parabens | Cosmetic and environmental | (M) DSPE | Fe3O4@βCD-BMIM-Cl | LC-UV | 20–90 | <14.9 | 80–117 | 2017 | [68] |
| UV filters | Environmental | (M) DSPE | Fe3O4-GCB | LC-MS/MS | 1–4 | <15 | 81–115 | 2017 | [72] |
| GCCs | Cosmetic | (M) DSPE | Fe3O4@dtMIP | LC-UV | 15,000 | <2.6 | 87–102 | 2017 | [81] |
| Parabens | Cosmetic, biological and environmental | (M) DSPE | Fe3O4@COF | LC-UV | 20 | <4.9 | 86–102 | 2018 | [67] |
| Parabens | Biological and environmental | (M) DSPE | Fe3O4-MWCNTs | GC-MS | 30–2000 | <9.2 | 81–119 | 2018 | [69] |
| UV filters | Environmental | (M) DSPE | Fe3O4@PDA | LC-MS | 60–130 | <3 | 95–104 | 2018 | [73] |
| GCCs | Cosmetic | (M) DSPE | Fe3O4@MIP | LC-UV | 50,000 | <2.7 | 94–98 | 2018 | [80] |
| Whitening agents | Cosmetic | (M) DSPE | Fe3O4@COF | LC-FLD | 0.1 | <5.5 | 78–105 | 2018 | [86] |
| Hg(II) | Cosmetic and environmental | DSPE | CDs | Fl | 2800 | <3.4 | 91–117 | 2019 | [56] |
| Parabens | Environmental | (M) DSPE | Fe3O4@sylgard 309 | LC-UV | 20,000–30,000 | <11.4 | 60–120 | 2019 | [60] |
| Parabens | Environmental | (M) DSPE | Fe3O4@DC193C | LC-UV | 2300–6300 | <10.2 | 86–118 | 2019 | [61] |
| Parabens and phthalates | Environmental | (M) DSPE | Fe3O4-MWCNTs-MIL-101 | LC-UV | 30–150 | <7.5 | 38–71 | 2019 | [66] |
| Parabens | Biological and environmental | (M) DSPE | γ-Fe2O3@HAP | GC-MS | 5000–10,000 | <4.2 | 95–106 | 2019 | [70] |
| UV filters | Environmental | (M) DSPE | Fe3O4-1210 (Zr/Cu) | LC-UV | 10–20 | <3.6 | 88–114 | 2019 | [71] |
| TCS and TCC | Biological | (M) DSPE | Fe3O4@COF | UPLC-MS/MS | 5–20 | n.r. | 93–109 | 2019 | [77] |
| GCCs | Cosmetic | (M) DSPE | Fe3O4-MIL-101/g-C3N4 | UPLC-MS/MS | 2 | <5.5 | 77–113 | 2019 | [82] |
| Rhodamine B | Cosmetic | (M) DSPE | γ-Fe2O3@imino-pyridine | Fl | 1600 | <2.7 | 91–97 | 2019 | [83] |
| 4,4′-thioaniline | Cosmetic | (M) DSPE | CoFe2O4@HNTs-Au-NPs | SERS | 26,000 | <10 | 72–104 | 2020 | [89] |
| Analyte(s) a | Matrix | Extraction Technique b | Material/Composite c | Instrumental Technique d | LOD (ng L−1) | RSD (%) | RR (%) e | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| UV filters | Environmental | SBSDME | CoFe2O4@oleic acid | LC-UV | 2400–30,600 | <11 | 79–120 | 2014 | [90] |
| UV filters | Environmental | SBSDME | CoFe2O4@oleic acid | LC-UV | 1600–2900 | <12 | 90–115 | 2016 | [91] |
| UV filters | Environmental | SBSDME | CoFe2O4-nylon 6 | TD-GC-MS | 13–148 | <11 | 0–116 | 2016 | [93] |
| UV filters | Environmental | SBSDME | CoFe2O4@oleic acid | GC-MS | 10–550 ng kg−1 | <14 | 91–110 | 2019 | [92] |
| TPP and DPP | Biological | SBSDME | CoFe2O4-Strata X-AW | LC-MS/MS | 1.9–6.3 | <8 | 81–111 | 2019 | [94] |
| N-Nitrosamines | Cosmetic | SBSDME | CoFe2O4-MIL-101 | LC-MS/MS | 60–300 | <13.9 | 96–109 | 2019 | [95] |
| PAHs | Cosmetic | SBSDME | CoFe2O4-rGO | GC-MS | 20–2500 | <10 | n.r. | 2020 | [96] |
| Analyte(s) a | Matrix | Extraction Technique b | Material/Composite c | Instrumental Technique d | LOD (ng L−1) | RSD (%) | RR (%) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Parabens | Environmental | MCE | Fe3O4-C18 | GC-MS | 23.2–86.1 | <7.1 | 96–106 | 2013 | [98] |
| Parabens | Environmental | μSPE | GO | GC-MS | 5–10 | <9.5 | 85–106 | 2014 | [101] |
| Benzotriazole UV stabilizers | Environmental | FPSE | PDMS | UPLC-MS/MS | 6.01–60.7 | <29.2 | 35–99 | 2015 | [102] |
| Parabens + TCS | Biological | Microflow injection | magnetic SPE PANI chip | LC-UV | 1100–4500 | <11 | 84–117 | 2017 | [99] |
| Parabens | Cosmetic | Rotative SPME | MWCNTs-COOH | LC-UV | 630–800 | <5.8 | 83–103 | 2018 | [97] |
| Parabens | Biological | DPX | CNH monolith | LC-UV | 1000–7000 | <16 | 80–116 | 2019 | [100] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grau, J.; Benedé, J.L.; Chisvert, A. Use of Nanomaterial-Based (Micro)Extraction Techniques for the Determination of Cosmetic-Related Compounds. Molecules 2020, 25, 2586. https://doi.org/10.3390/molecules25112586
Grau J, Benedé JL, Chisvert A. Use of Nanomaterial-Based (Micro)Extraction Techniques for the Determination of Cosmetic-Related Compounds. Molecules. 2020; 25(11):2586. https://doi.org/10.3390/molecules25112586
Chicago/Turabian StyleGrau, José, Juan L. Benedé, and Alberto Chisvert. 2020. "Use of Nanomaterial-Based (Micro)Extraction Techniques for the Determination of Cosmetic-Related Compounds" Molecules 25, no. 11: 2586. https://doi.org/10.3390/molecules25112586
APA StyleGrau, J., Benedé, J. L., & Chisvert, A. (2020). Use of Nanomaterial-Based (Micro)Extraction Techniques for the Determination of Cosmetic-Related Compounds. Molecules, 25(11), 2586. https://doi.org/10.3390/molecules25112586