Effect of the Addition of Amine in Organophosphorus Compounds on Molecular Structuration of Ionic Liquids–Application to Solvent Extraction
Abstract
1. Introduction
2. Material and Methods
3. Results and Discussion
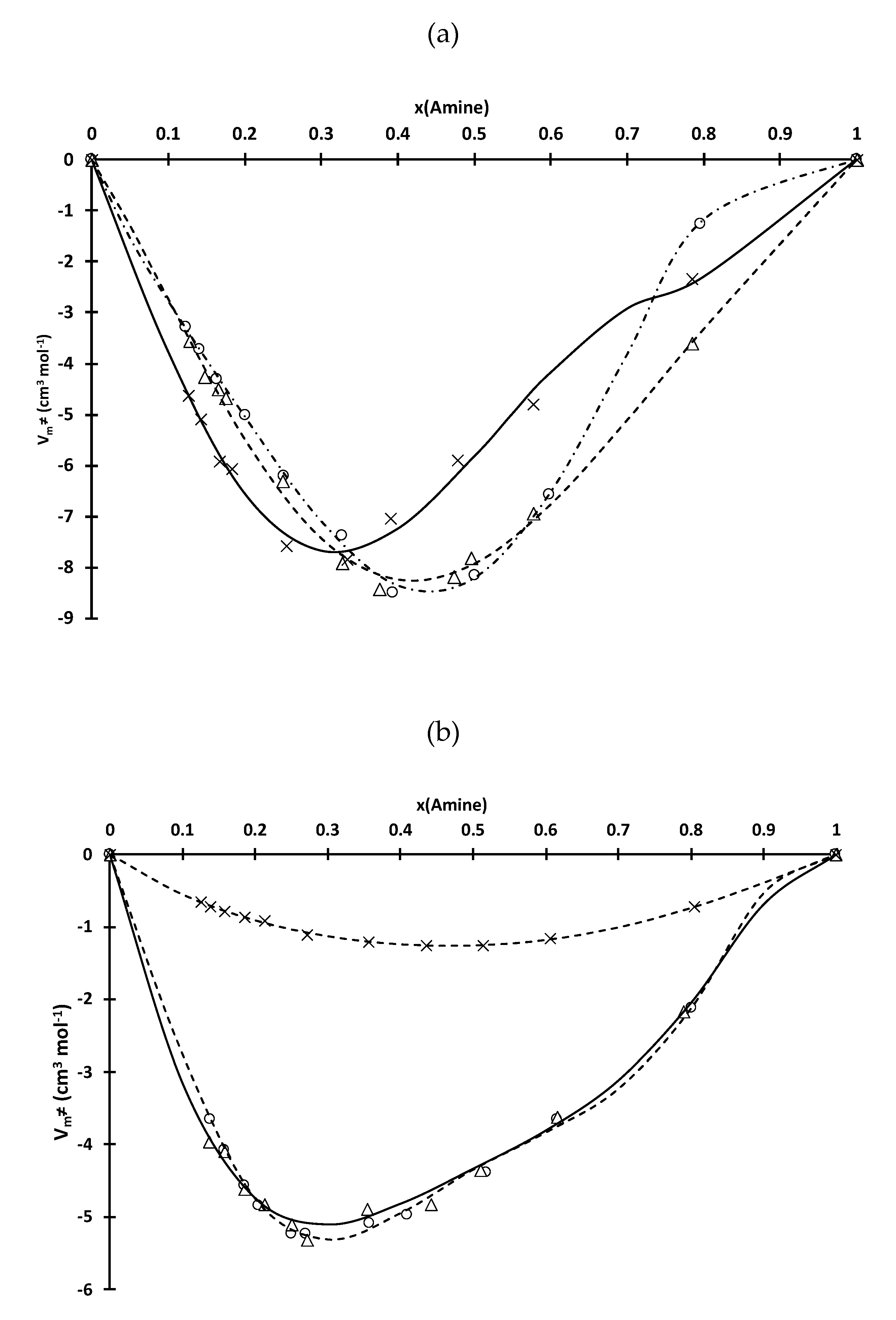
3.1. Physicochemical Properties
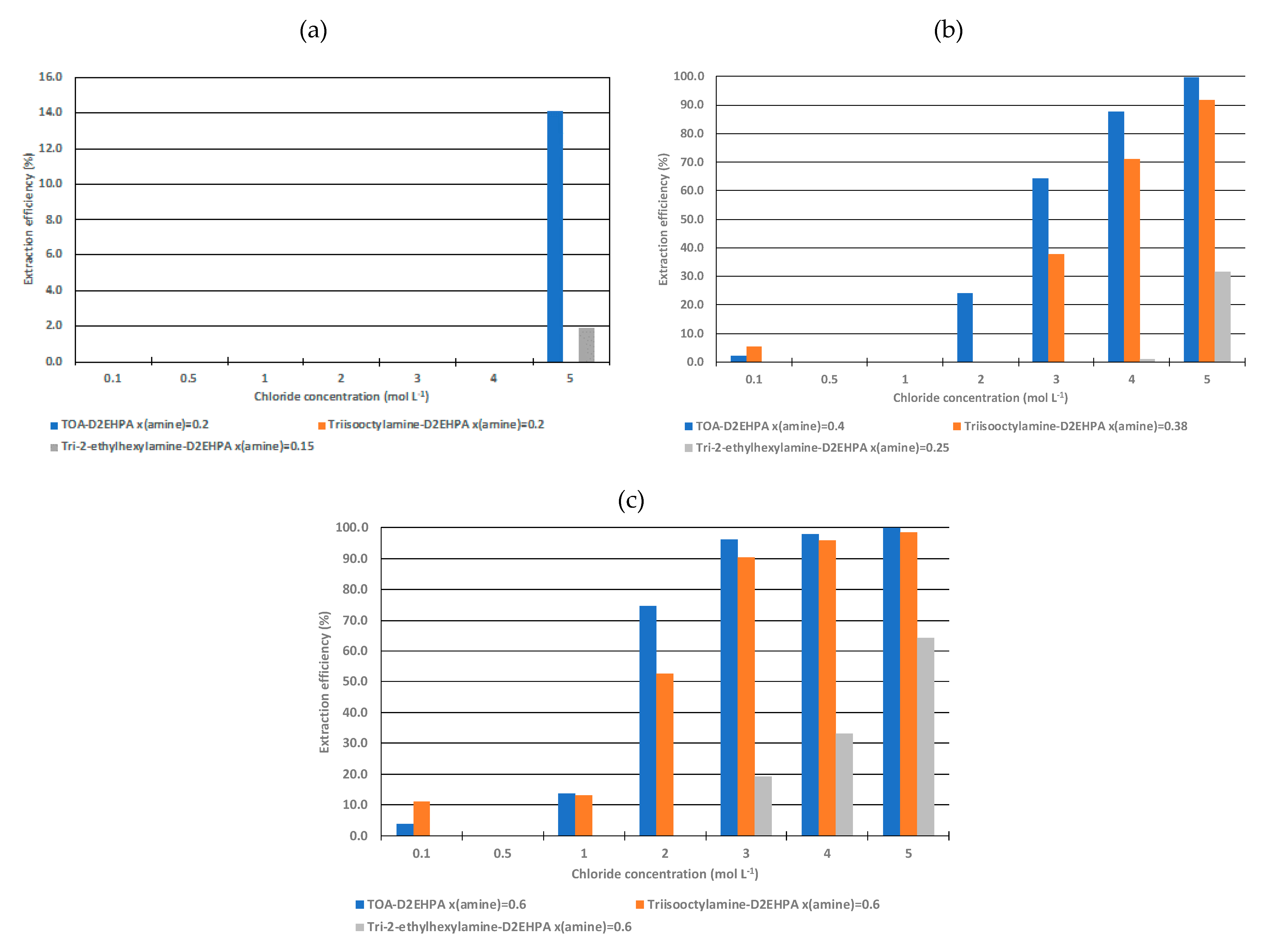
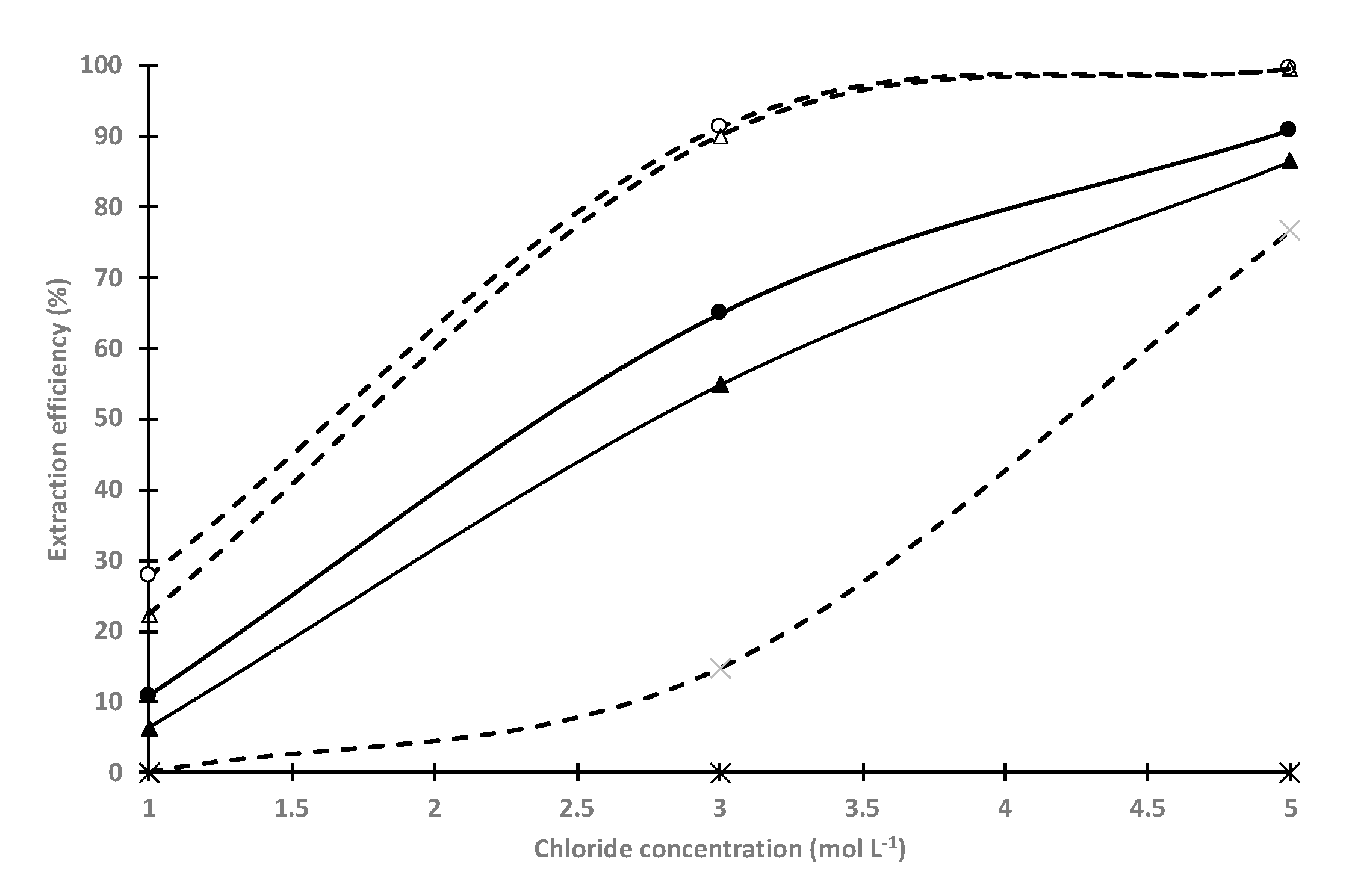
3.2. Effect of Amine Molar Fraction on the Liquid-Liquid Extraction Properties of the Ionic Liquids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chagnes, A. Fundamentals in Electrochemistry and Hydrometallurgy. In Lithium Process Chemistry: Resources, Extractions, Batteries and Recycling, 1st ed.; Chagnes, A., Swiatowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 41–80. [Google Scholar]
- Prestianni, A.; Joubert, L.; Chagnes, A.; Cote, G.; Adamo, C. A Density Functional Theory Study of Uranium (VI) Nitrate Monoamides Complexes. Phys. Chem. Chem. Phys. 2011, 13, 19371–19377. [Google Scholar] [CrossRef]
- Love, J.; Miguirditchian, M.; Chagnes, A. New Insights into the Recovery of Strategic and Critical Metals by Solvent Extraction. In Ion Exchange and Solvent Extraction, 1st ed.; Moyer, B.A., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–44. [Google Scholar]
- Artese, A.; Dourdain, S.; Arrachart, G.; Pellet-Rostaing, S.; Boubals, N. Use of Bifunctional Compounds N, P for Extracting Uranium from Aqueous Solutions of Nitric Acid. In Proceedings of the JNRC-RANC, Budapest, Hungary, 5–10 May 2019. [Google Scholar]
- Nash, K.L.; Choppin, G.R. Separations Chemistry for Actinide Elements: Recent Developments and Historical Perspective. Sep. Sci. Technol. 1997, 32, 255–274. [Google Scholar] [CrossRef]
- Wilson, A.; Bailey, P.; Tasker, P.; Turkington, J.; Grant, R.; Love, J. Solvent Extraction: The Coordination Chemistry Behind Extractive Metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.N.; Reddy, B.R.; Kantam, M.L.; Kumar, J.R.; Lee, J.Y. Synergistic Solvent Extraction of Neodymium(III) from Chloride Solutions Using a Mixture of Triisooctylamine and Bis(2,4,4-Trimethylpentyl) Monothiophosphinic Acid. Sep. Sci. Technol. 2014, 49, 130–136. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, H.S.; Lee, M.S. Solvent Extraction of Pr and Nd from Chloride Solution by The Mixtures of Cyanex 272 and Amine Extractants. Hydrometallurgy 2014, 150, 61–67. [Google Scholar] [CrossRef]
- Banda, R.; Jeon, H.S.; Lee, M.S. Separation of Ce and La from Synthetic Chloride Leach Solution of Monazite Sand by Precipitation and Solvent Extraction. Metall. Mater. Trans. B 2014, 45, 2009–2017. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, H.S.; Lee, M.S. Extraction of Hydrochloric Acid with Binary Mixtures of Tertiary Amine and Organophosphorus Acid and Analysis of the Interaction between the Constituents of these Mixtures. Hydrometallurgy 2015, 155, 44–50. [Google Scholar] [CrossRef]
- Jiang, D.; Song, N.; Liao, S.; Lian, Y.; Ma, J.; Jia, Q. Study on the Synergistic Extraction of Vanadium by Mixtures of Acidic Organophosphorus Extractants and Primary Amine N1923. Sep. Purif. Technol. 2015, 156, 835–840. [Google Scholar] [CrossRef]
- Jia, Q.; Zhan, C.; Li, D.; Niu, C. Extraction of Zinc(II) and Cadmium(II) by Using Mixtures of Primary Amine N1923 and Organophosphorus Acids. Sep. Sci. Technol. 2005, 39, 1111–1123. [Google Scholar] [CrossRef]
- Kim, C.J.; Rajesh Kumar, J.; Kim, J.-S.; Lee, J.Y.; Yoon, H.-S. Solvent Extraction Studies on Uranium using Amine Based Extractants and Recovery from Low Grade Ore Leach Liquors. J. Braz. Chem. Soc. 2012, 23, 1254–1264. [Google Scholar] [CrossRef]
- Grigorieva, N.A.; Pavlenko, N.I.; Pashkov, G.L.; Fleitlikh, I.Yu.; Nikiforova, L.K. Investigation of the State of Bis(2,4,4-Trimethylpentyl)Dithiophosphinic Acid in Nonane in the Presence of Electron Donor Additives. Solvent Extr. Ion Exch. 2010, 28, 510–525. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, M.S. Effect of the Diluents on the Interaction between Components in the Binary Mixtures of Organophosphorus Acid and Tertiary Amine. J. Mol. Liq. 2016, 220, 41–48. [Google Scholar] [CrossRef]
- Abumandour, E.-S.; Mutelet, F.; Alonso, S. Performance of an Absorption Heat Transformer Using New Working Binary Systems Composed of ionic liquid and water. Appl. Therm. Eng. 2016, 94, 579–589. [Google Scholar] [CrossRef]
- Syzova, N.; Eyal, A.M.; Vitner, A.; Hazan, B. Interactions between the Components of ABC Extractants and Extraction of Monocarboxylic Acids by these Extractants. Solvent Extr. Ion Exch. 2004, 22, 31–49. [Google Scholar] [CrossRef]
- Cholico Gonzalez, D.; Avila-Rodriguez, M.; Antonio Reyes-Aguilerad, J.; Cote, G.; Chagnes, A. Rheological behaviour of Cyphos IL101-Cyanex 272 Binary Mixtures between 288.15K and 343.15K. J. Mol. Liq. 2012, 169, 27–32. [Google Scholar]
- Liu, Y.; Lee, M.S. Determination of Viscosity and Dielectric Constant for Studying the Interactions in Binary Mixtures of Organophosphorus Acid and Tertiary Amine. J. Mol. Liq. 2016, 222, 233–238. [Google Scholar] [CrossRef]
- Omelchuk, K.; Szczepański, P.; Shrotre, A.; Haddad, M.; Chagnes, A. Effects of Structural Changes of New Organophosphorus Cationic Exchangers on Solvent Extraction of Cobalt, Nickel and Manganese from Acidic Chloride Media. RSC Adv. 2017, 7, 5660–5668. [Google Scholar] [CrossRef]
- Mialkowski, C.; Chagnes, A.; Carré, B.; Willmann, P.; Lemordant, D. Thermodynamic Properties of Binary Liquid Mixtures Containing Dimethyl Carbonate and γ-butyrolactone. J. Chem. Thermodyn. 2002, 34, 1845–1854. [Google Scholar] [CrossRef]
- Liu, Y.; Seung Lee, M. Separation of Cobalt and Nickel from Chloride Leach Solution of Nickel Laterite Ore by Solvent Extraction. Geosystem Eng. 2016, 19, 214–221. [Google Scholar] [CrossRef]
- Marcus, Y.Z.; Sengupta, A.K. Ion Exchange and Solvent Extraction; Marcel Dekker Inc.: New York, NY, USA, 2020. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
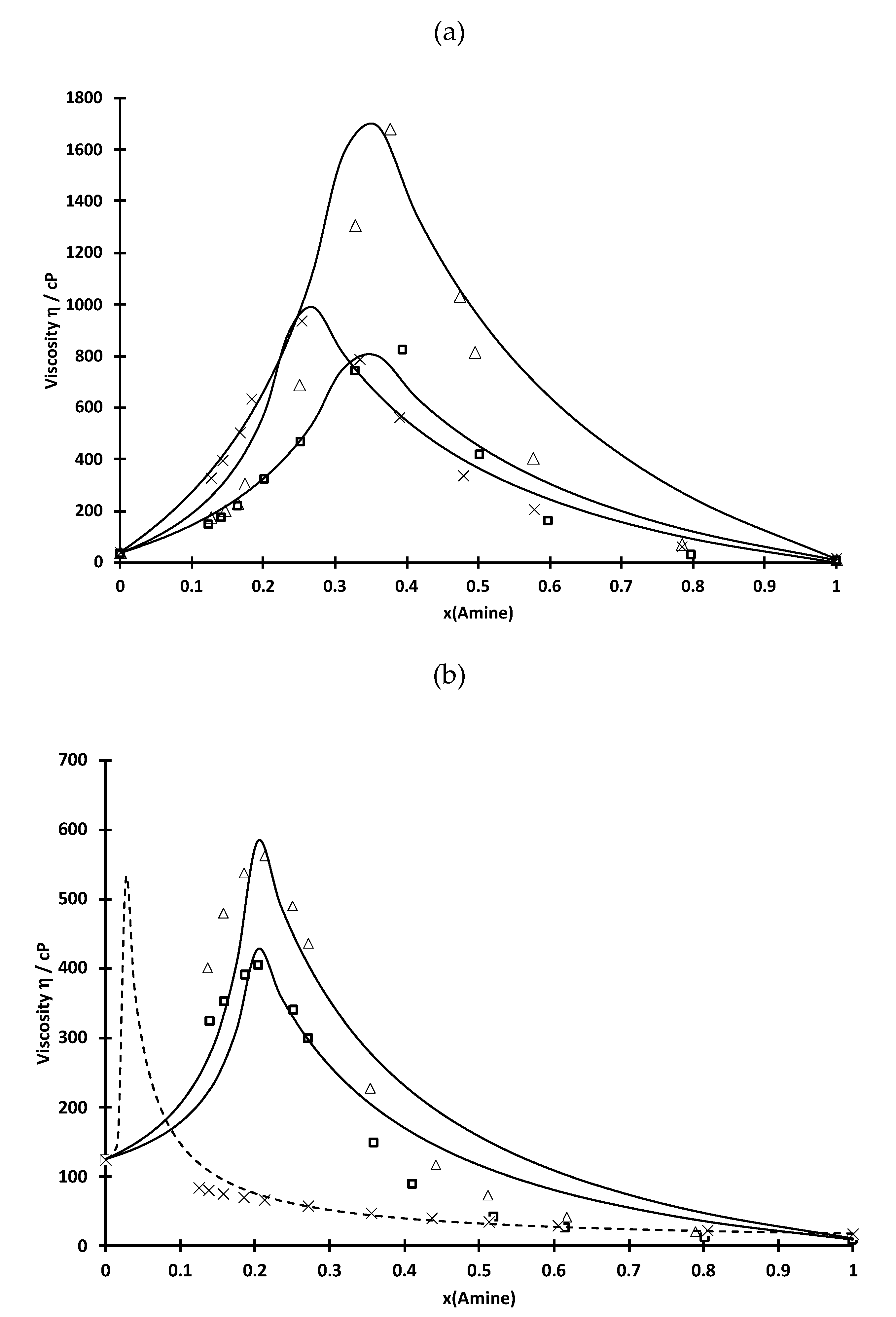
| D2EHPA-TEHA | D2EHPA-TIOA | D2EHPA-TOA | ||||
|---|---|---|---|---|---|---|
| x (amine) | η (cP) | ρ (g·L−1) | η (cP) | ρ (g·L−1) | η (cP) | ρ (g·L−1) |
| 0.00 | 36.3 | 0.972 | 36.3 | 0.972 | 36.1 | 0.972 |
| 0.13 | 324.7 | 0.960 | 172.9 | 0.956 | 147.3 | 0.955 |
| 0.14 | 395.1 | 0.958 | 202.2 | 0.955 | 176.9 | 0.953 |
| 0.17 | 502.9 | 0.956 | 226.7 | 0.954 | 220.7 | 0.950 |
| 0.19 | 633.9 | 0.953 | 304.8 | 0.950 | 325.2 | 0.945 |
| 0.25 | 934.0 | 0.944 | 686.7 | 0.940 | 470.4 | 0.938 |
| 0.33 | 788.4 | 0.930 | 1305.1 | 0.930 | 744.5 | 0.927 |
| 0.39 | 561.5 | 0.918 | 1679.8 | 0.923 | 824.1 | 0.917 |
| 0.48 | 335.7 | 0.900 | 1027.9 | 0.901 | 420.3 | 0.898 |
| 0.58 | 204.2 | 0.882 | 402.9 | 0.886 | 163.5 | 0.878 |
| 0.79 | 61.7 | 0.847 | 71.2 | 0.848 | 31.4 | 0.837 |
| 1.00 | 17.5 | 0.815 | 11.0 | 0.813 | 8.3 | 0.808 |
| Cyanex-TEHA | Cyanex-TIOA | Cyanex-TOA | ||||
|---|---|---|---|---|---|---|
| x (amine) | η (cP) | ρ (g·L−1) | η (cP) | ρ (g·L−1) | η (cP) | ρ (g·L−1) |
| 0.00 | 124.2 | 0.815 | 124.9 | 0.813 | 124.6 | 0.808 |
| 0.13 | 83.8 | 0.832 | 400.0 | 0.834 | 325.0 | 0.829 |
| 0.15 | 80.3 | 0.850 | 480.0 | 0.853 | 353.2 | 0.850 |
| 0.18 | 75.6 | 0.859 | 537.0 | 0.866 | 390.4 | 0.862 |
| 0.20 | 70.3 | 0.867 | 562.5 | 0.874 | 404.7 | 0.875 |
| 0.24 | 65.8 | 0.875 | 489.9 | 0.883 | 340.1 | 0.881 |
| 0.27 | 56.5 | 0.885 | 435.8 | 0.895 | 299.7 | 0.893 |
| 0.36 | 46.6 | 0.891 | 227.4 | 0.897 | 149.9 | 0.896 |
| 0.43 | 39.7 | 0.894 | 116.6 | 0.901 | 89.9 | 0.901 |
| 0.51 | 34.4 | 0.897 | 73.2 | 0.904 | 43.2 | 0.902 |
| 0.61 | 29.4 | 0.900 | 41.4 | 0.906 | 26.4 | 0.905 |
| 0.80 | 22.1 | 0.901 | 20.4 | 0.909 | 13.5 | 0.906 |
| 1.00 | 17.7 | 0.916 | 10.9 | 0.916 | 8.3 | 0.916 |
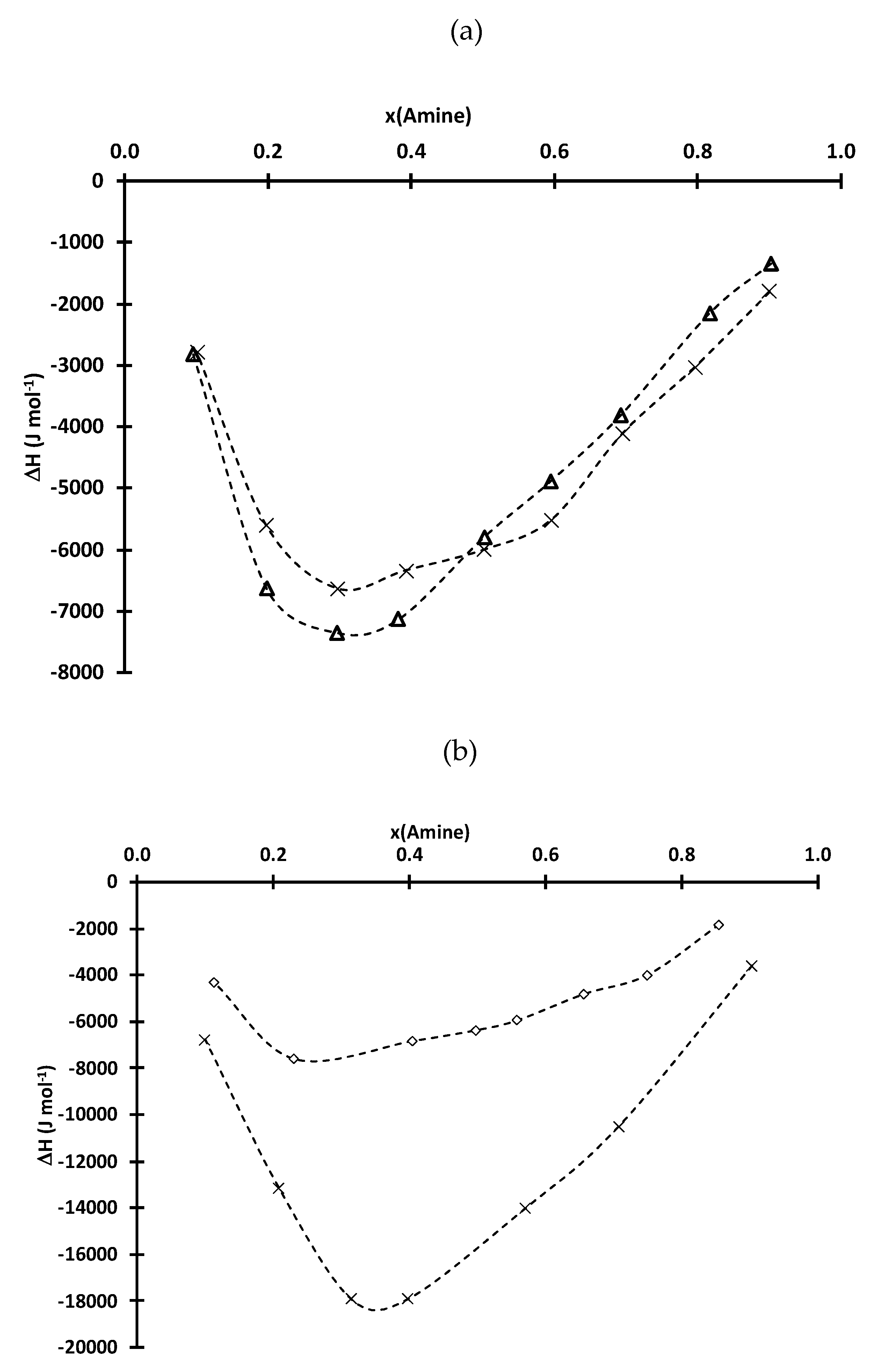
| D2EHPA | Cyanex 272 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TEHA | TIOA | TOA | TIOA | TOA | |||||
| x(amine) | H (J·mol−1) | x(amine) | H (J·mol−1) | x(amine) | H (J·mol−1) | x(amine) | H (J·mol−1) | x(amine) | H (J·mol−1) |
| 0.11 | −4331.0 | 0.20 | −10,498.8 | 0.10 | −6780.1 | 0.10 | −2794.0 | 0.10 | −2824.5 |
| 0.23 | −7605.3 | 0.30 | −12,514.6 | 0.21 | −13,137.3 | 0.20 | −5611.7 | 0.20 | −6640.6 |
| 0.41 | −6831.8 | 0.38 | −14,455.3 | 0.31 | −17,921.9 | 0.30 | −6640.2 | 0.30 | −7346.5 |
| 0.50 | −6375.6 | 0.49 | −14,446.2 | 0.40 | −17,900.4 | 0.39 | −6342.3 | 0.38 | −7132.9 |
| 0.56 | −5950.2 | 0.59 | −13,809.4 | 0.57 | −14,032.3 | 0.50 | −6006.0 | 0.50 | −5794.3 |
| 0.66 | −4811.6 | 0.67 | −12,617.9 | 0.71 | −10,513.4 | 0.60 | −5513.4 | 0.59 | −4888.8 |
| 0.75 | −3993.5 | 0.78 | −8095.2 | 0.90 | −3609.2 | 0.70 | −4110.0 | 0.69 | −3814.1 |
| 0.86 | −1828.6 | 0.89 | −4299.0 | 0.80 | −3027.1 | 0.82 | −2147.2 | ||
| 0.90 | −1802.3 | 0.90 | −1349.5 | ||||||
| D2EHPA | Cyanex 272 | |||||
|---|---|---|---|---|---|---|
| TOA | TIOA | TEHA | TOA | TIOA | TEHA | |
| A | −28.164 | 0 | 0 | 31.791 | 7.294 | 0 |
| B | 3.242 | −12.909 | −30.578 | 3.910 | 4.519 | 0.832 |
| C | 47.528 | 11.669 | −12.034 | −22.358 | −12.723 | −0.362 |
| D | 18.944 | 15.831 | 33.124 | 12.240 | 12.272 | 9.594 |
| E | −32.904 | −31.707 | −23.300 | −17.542 | −17.657 | −5.050 |
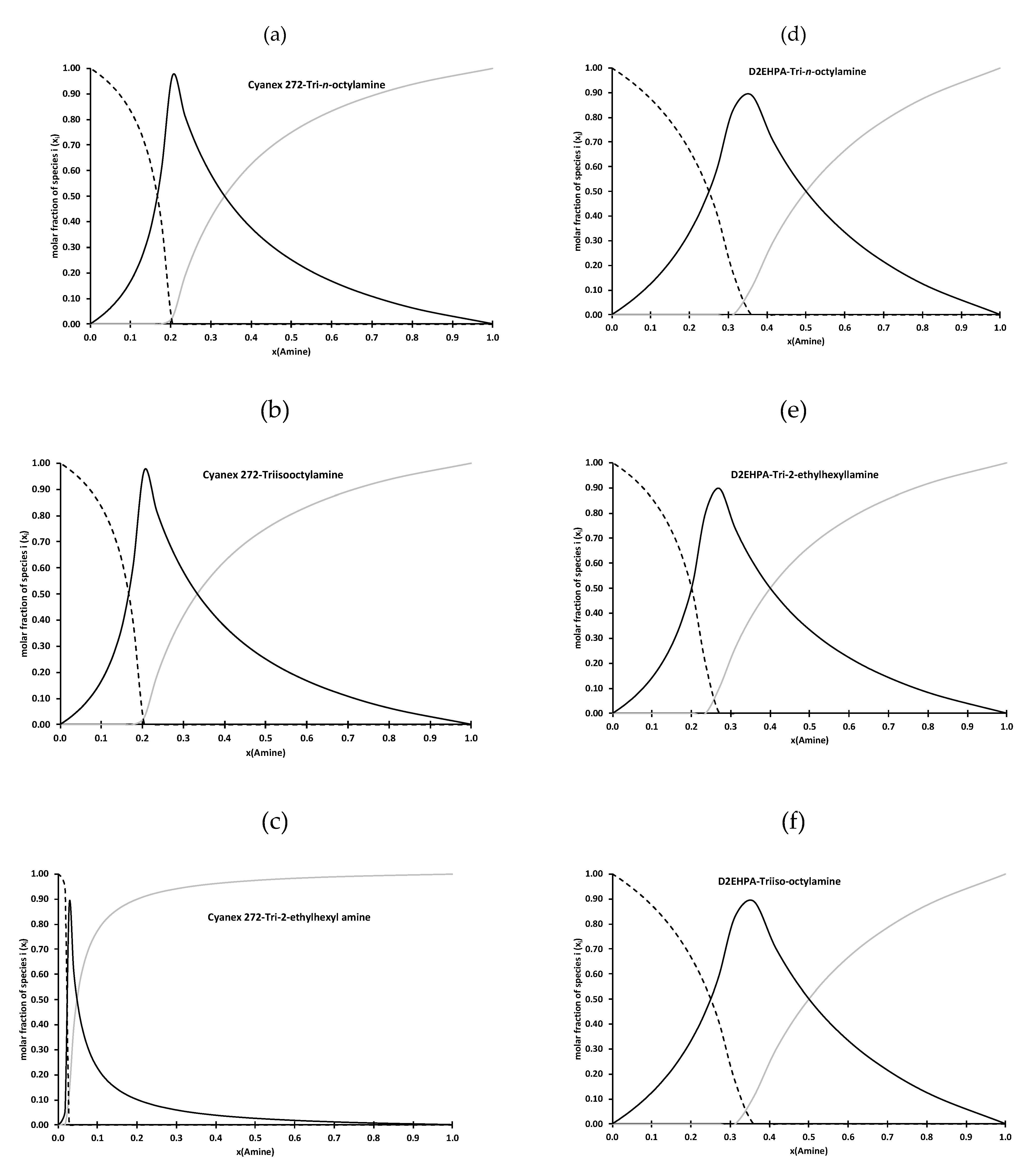
| D2EHPA | Cyanex 272 | |
|---|---|---|
| TOA | a = 2 R3NHL(HL) ηIP = 900 cP | a = 4 R3NHL(HL)3 ηIP = 440 cP |
| TIOA | a = 2 R3NHL(HL) ηIP=1900 cP | a = 4 R3NHL(HL)3 ηIP = 600 cP |
| TEHA | a = 3 R3NHL(HL)2 ηIP = 1100 cP | _ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gmar, S.; Mutelet, F.; Chagnes, A. Effect of the Addition of Amine in Organophosphorus Compounds on Molecular Structuration of Ionic Liquids–Application to Solvent Extraction. Molecules 2020, 25, 2584. https://doi.org/10.3390/molecules25112584
Gmar S, Mutelet F, Chagnes A. Effect of the Addition of Amine in Organophosphorus Compounds on Molecular Structuration of Ionic Liquids–Application to Solvent Extraction. Molecules. 2020; 25(11):2584. https://doi.org/10.3390/molecules25112584
Chicago/Turabian StyleGmar, Soumaya, Fabrice Mutelet, and Alexandre Chagnes. 2020. "Effect of the Addition of Amine in Organophosphorus Compounds on Molecular Structuration of Ionic Liquids–Application to Solvent Extraction" Molecules 25, no. 11: 2584. https://doi.org/10.3390/molecules25112584
APA StyleGmar, S., Mutelet, F., & Chagnes, A. (2020). Effect of the Addition of Amine in Organophosphorus Compounds on Molecular Structuration of Ionic Liquids–Application to Solvent Extraction. Molecules, 25(11), 2584. https://doi.org/10.3390/molecules25112584







