Antitumor Activity of New Olivacine Derivatives
Abstract
1. Introduction
2. Results and Discussion
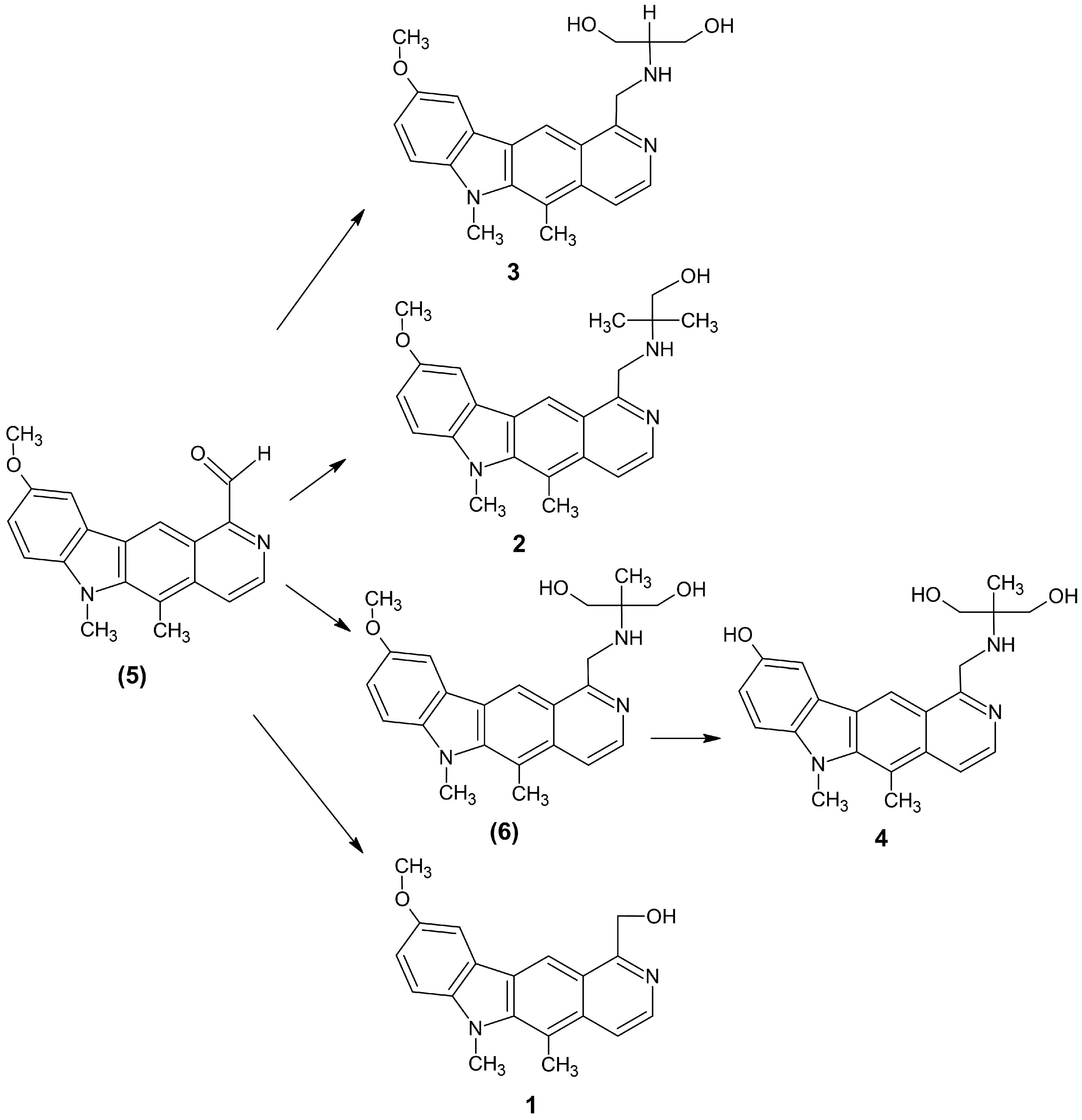
2.1. Chemistry
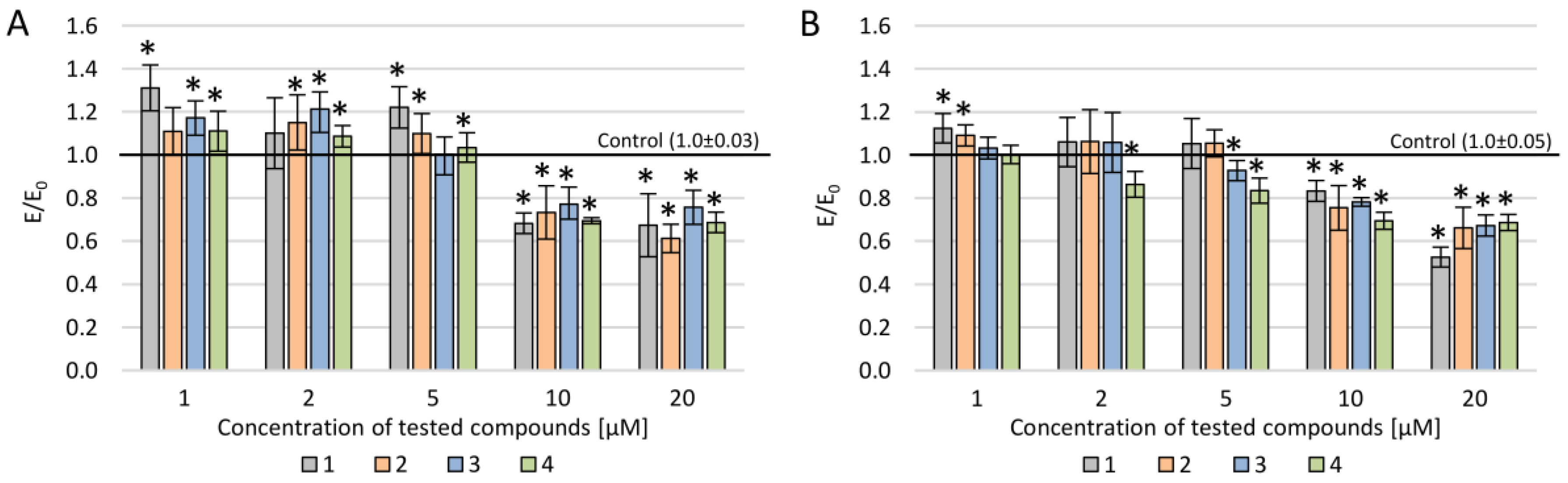
2.2. Biological Evaluation
3. Materials and Methods
3.1. Tested Compounds
3.2. Cell Lines and Conditions
3.3. DCF-DA Assay
3.4. MTT Assay
3.5. Accumulation of Rhodamine 123
3.6. Detection of Apoptosis
3.7. Proliferation Inhibition—Mitotic Index
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gębarowska, E.; Zabel, M.; Majewski, A.; Kołodziej, J. Evaluation of individual sensitivity to cytostatic drugs of in vitro cultured lung tumor cells. Folia Histochem. Cytobiol. 1999, 33, 135–136. [Google Scholar]
- Shoemaker, R.H. The NCI60 human tumor cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, N.; Nagarajan, R. Total synthesis of ellipticine quinones, olivacine, and calothrixin b. J. Org. Chem. 2014, 79, 736–741. [Google Scholar] [CrossRef]
- Monnot, M.; Mauffret, O.; Simon, V.; Lescot, E.; Psaume, B.; Saucier, J.M.; Charra, M.; Belehradek, J.; Fermandjian, S. DNA-drug recognition, and effects on topoisomerase II-mediated cytotoxicity. A three-mode binding model for ellipticine derivatives. J. Biol. Chem. 1991, 66, 1820–1829. [Google Scholar]
- Fosse, P.; Rene, B.; Charra, M.; Paoletti, C.; Saucier, J.M. Stimulation of topoisomerase II-mediated DNA cleavage by ellipticine derivatives: Structure-activity relationship. Mol. Pharmacol. 1992, 42, 590–595. [Google Scholar] [PubMed]
- Froelich-Ammon, S.J.; Patchan, M.W.; Osheroff, N.; Thompson, R.B. Topoisomerase II binds to ellipticine in the absence or presence of DNA: Chaeacterization of enzyme drug interactions by fluorescence spectroscopy. J. Biol. Chem. 1995, 270, 14998–15004. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, C.; Chen, L.; Sebti, S.; Chen, J. Rescue of mutant p53 transcription function by ellipticine. Oncogene 2003, 22, 4478–4487. [Google Scholar] [CrossRef]
- Sugikawa, E.; Hosoi, T.; Yazaki, N.; Gamanuma, M.; Nakanishi, N.; Ohashi, M. Mutant p53 mediated induction of cell cycle arrest and apoptosis at G1 phase by 9-hydroxyellipticine. Anticancer Res. 1999, 19, 3099–3108. [Google Scholar]
- Miller, C.M.; O’Sullivan, E.C.; McCarthy, F.O. Novel 11-substituted ellipticines as potent anticancer agents with divergent activity against cancer cells. Pharmaceuticals 2019, 12, 90. [Google Scholar] [CrossRef]
- Malonne, H.; Atassi, G. DNA topoisomerase targeting drugs: Mechanisms of action and perspectives. Anticancer. Drugs 1997, 8, 811–822. [Google Scholar] [CrossRef]
- Le Mée, S.; Pierré, A.; Markovits, J.; Atassi, G.; Jacquemin-Sablon, A.; Saucier, J.M. S16020-2, a new highly cytotoxic antitumor olivacine derivative: DNA interaction and DNA topoisomerase II inhibition. Mol. Pharmacol. 1998, 53, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Sotelo, E. 1,5-Dimethyl-6H-pyridazino[4–5-b]carbazole, a 3-aza bioisoster of the antitumor alkaloid olivacine. Chem. Pharm. Bull. 2002, 50, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Pichard-Garcia, L.; Weaver, R.J.; Eckett, N.; Scarfe, G.; Fabre, J.M.; Lucas, C.; Maurel, P. The olivacine derivative S 16020 (9-hydroxy-5,6-dimethyl-N-[2-(dimethylamino)ethyl)-6H-pyrido(4,3-b) -carbazole-1-carboxamide) induces CYP1A and its own metabolism in human hepatocytes in primary culture. Drug Metab. Dispos. 2004, 32, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Jasztold-Howorko, R.; Tylińska, B.; Bbiaduń, O.; Barowski, T.G.; Gasiorowski, K. New pyridocarbazole derivatives. Synthesis and their in vitro anticancer activity. Acta Pol. Pharm.—Drug Res. 2013, 70, 823–832. [Google Scholar]
- Moreira, H.; Szyjka, A.; Gasiorowski, K. Chemopreventive activity of celastrol in drug-resistant human colon carcinoma cell cultures. Oncotarget 2018, 9, 21211–21233. [Google Scholar] [CrossRef]
- Pierré, A.; Léonce, S.; Pérez, V.; Atassi, G. Circumvention of P-glycoprotein-mediated multidrug resistance by S16020–2: Kinetics of uptake and efflux in sensitive and resistant cell lines. Cancer Chemother. Pharmacol. 1998, 42, 454–460. [Google Scholar] [CrossRef]
- Juan, E.; Le Vée, M.; Mayati, A.; Denizot, C.; Parmentier, Y.; Fardel, O. Evaluation of P-glycoprotein inhibitory potential using a rhodamine 123 accumulation assay. Pharmaceutics 2016, 8, 12. [Google Scholar] [CrossRef]
- Palko-Labuz, A.; Sroda-Pomianek, K.; Uryga, A.; Kostrzewa-Suslow, E.; Michalak, K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and drug-resistant LoVo/Dx cells. Biomed. Pharmacother. 2017, 88, 232–241. [Google Scholar] [CrossRef]
- Purohit, V.; Simeone, D.M.; Lyssiotis, C.A. Metabolic regulation of redox balance in cancer. Cancers (Basel) 2019, 11, 955. [Google Scholar] [CrossRef]
- Babu, K.R.; Tay, Y. The Yin-Yang regulation of reactive oxygen species and microRNAs in cancer. Int. J. Mol. Sci. 2019, 20, 5335. [Google Scholar] [CrossRef]
- Moreira, H.; Szyjka, A.; Paliszkiewicz, K.; Barg, E. Prooxidative Activity of Celastrol Induces Apoptosis, DNA Damage, and Cell Cycle Arrest in Drug-Resistant Human Colon Cancer Cells. Oxid. Med. Cell. Longev. 2019, 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.K. Gene network analysis of oxidative stress-mediated drug sensitivity in resistant ovarian carcinoma cells. Pharmacogenom. J. 2010, 10, 94–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szuławska, A.; Czyz, M. Molecular mechanisms of anthracyclines action. Postępy Higieny Medycyny Doświadczalnej (Online) 2006, 60, 78–100. [Google Scholar]
- Wartenberg, M.; Ling, F.C.; Müschen, M.; Klein, F.; Acker, H.; Gassmann, M.; Petrat, K.; Pütz, V.; Hescheler, J.; Sauer, H. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 2003, 17, 503–505. [Google Scholar] [CrossRef]
- Tylinska, B.; Jasztold-Howorko, R.; Kowalczewska, K.; Szczaurska-Nowak, K.; Gbarowski, T.; Wietrzyk, J. Design, synthesis and analysis of anticancer activity of new SAR-based S16020 derivatives. Acta Pol. Pharm.—Drug Res. 2018, 75, 1313–1320. [Google Scholar] [CrossRef]
- Singh, M.P.; Hill, G.C.; Péoc’h, D.; Rayner, B.; Imbach, J.L.; Lown, J.W. High-field NMR and restrained molecular modeling studies on a DNA heteroduplex containing a modified apurinic abasic site in the form of covalently linked 9-aminoellipticine. Biochemistry 1994, 33, 10271–10285. [Google Scholar] [CrossRef]
- Chu, Y.; Hsu, M.T. Ellipticine increase the superhelical density of intracellular SV40 DNA by intercalation. Nucleic Acids Res. 1992, 20, 4033–4038. [Google Scholar] [CrossRef]
- El-Deiry, W.S. The role of p53 in chemosensitivity and radiosensitivity. Oncogene 2003, 22, 7486–7495. [Google Scholar] [CrossRef]
- Wiman, K.G. Strategies for therapeutic targeting of the p53 pathway in cancer. Cell Death Differ. 2006, 13, 921–926. [Google Scholar] [CrossRef]
- Bykov, V.J.N.; Issaeva, N.; Shilov, A.; Hultcrantz, M.; Pugacheva, E.; Chumakov, P.; Bergman, J.; Wiman, K.G.; Selivanova, G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat. Med. 2002, 8, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Tong, J.H.M.; Chan, A.W.H.; Yu, J.; Kang, W.; To, K.F. Targeting the oncogenic p53 mutants in colorectal cancer and other solid tumors. Int. J. Mol. Sci. 2019, 20, 5999. [Google Scholar] [CrossRef] [PubMed]
- Gębarowski, T.; Wiatrak, B.; Gębczak, K.; Tylińska, B.; Gąsiorowski, K. Effect of new olivacine derivatives on p53 protein level. Pharmacol. Rep. 2020, 72, 214–224. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 2, 3, and 4 are available from the authors. |


| Cell Line | IC50 [µM] | |||
|---|---|---|---|---|
| Compound | LoVo | LoVo/DX | NHDF | |
| 1 | 4.84 ± 1.03 | 16.42 ± 0.49 | 74.03 ± 5.12 | |
| 2 | 13.50 ± 0.80 | 20.03 ± 3.86 | 58.78 ± 2.14 | |
| 3 | 15.43 ± 0.26 | 22.21 ± 0.79 | 41.08 ± 4.77 | |
| 4 | 9.37 ± 0.13 | 19.36 ± 2.43 | 52.30 ± 2.43 | |
| Ellipticine | 4.28 ± 0.53 | 18.16 ± 0.34 | 22.45 ± 3.14 | |
| Cell Line | Cell Line | |||
|---|---|---|---|---|
| Compound | LoVo | LoVo/DX | NHDF | |
| 1 | 2.3 ± 0.9 | 4.2 ± 0.9 | 20.3 ± 7.8 | |
| 2 | 5.8 ± 2.9 | 6.5 ± 1.9 | 23.5 ± 12.6 | |
| 3 | 7.4 ± 2.9 | 12.5 ± 2.2 | 21.4 ± 1.6 | |
| 4 | 3.2 ± 0.7 | 4.7 ± 1.0 | 18.9 ± 2.1 | |
| Ellipticine | 3.4 ± 1.0 | 7.0 ± 3.7 | 21.2 ± 7.8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piasny, J.; Wiatrak, B.; Dobosz, A.; Tylińska, B.; Gębarowski, T. Antitumor Activity of New Olivacine Derivatives. Molecules 2020, 25, 2512. https://doi.org/10.3390/molecules25112512
Piasny J, Wiatrak B, Dobosz A, Tylińska B, Gębarowski T. Antitumor Activity of New Olivacine Derivatives. Molecules. 2020; 25(11):2512. https://doi.org/10.3390/molecules25112512
Chicago/Turabian StylePiasny, Janusz, Benita Wiatrak, Agnieszka Dobosz, Beata Tylińska, and Tomasz Gębarowski. 2020. "Antitumor Activity of New Olivacine Derivatives" Molecules 25, no. 11: 2512. https://doi.org/10.3390/molecules25112512
APA StylePiasny, J., Wiatrak, B., Dobosz, A., Tylińska, B., & Gębarowski, T. (2020). Antitumor Activity of New Olivacine Derivatives. Molecules, 25(11), 2512. https://doi.org/10.3390/molecules25112512








