Co-Occurrence of Moniliformin and Regulated Fusarium Toxins in Maize and Wheat Grown in Italy
Abstract
1. Introduction
2. Results and Discussion
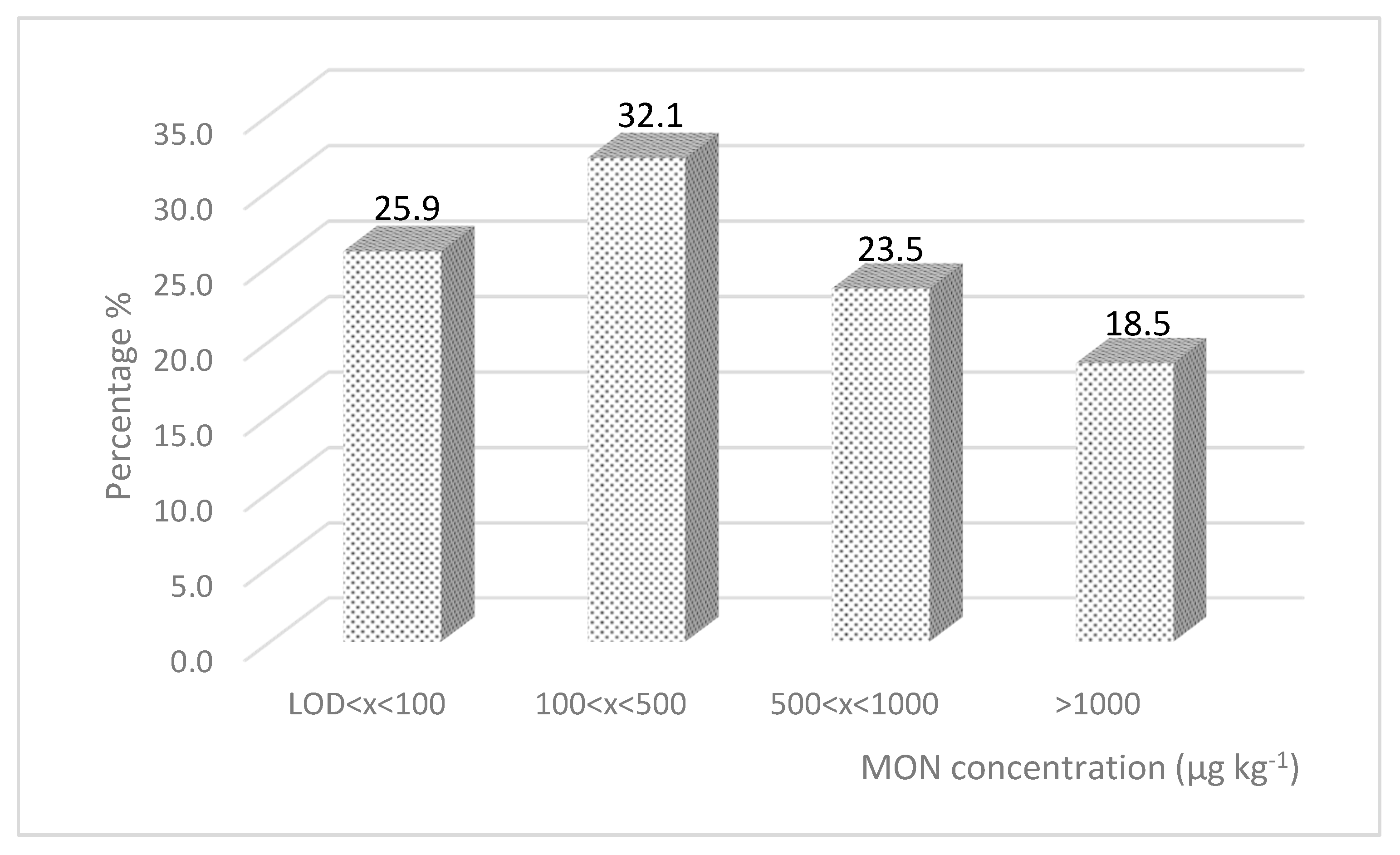
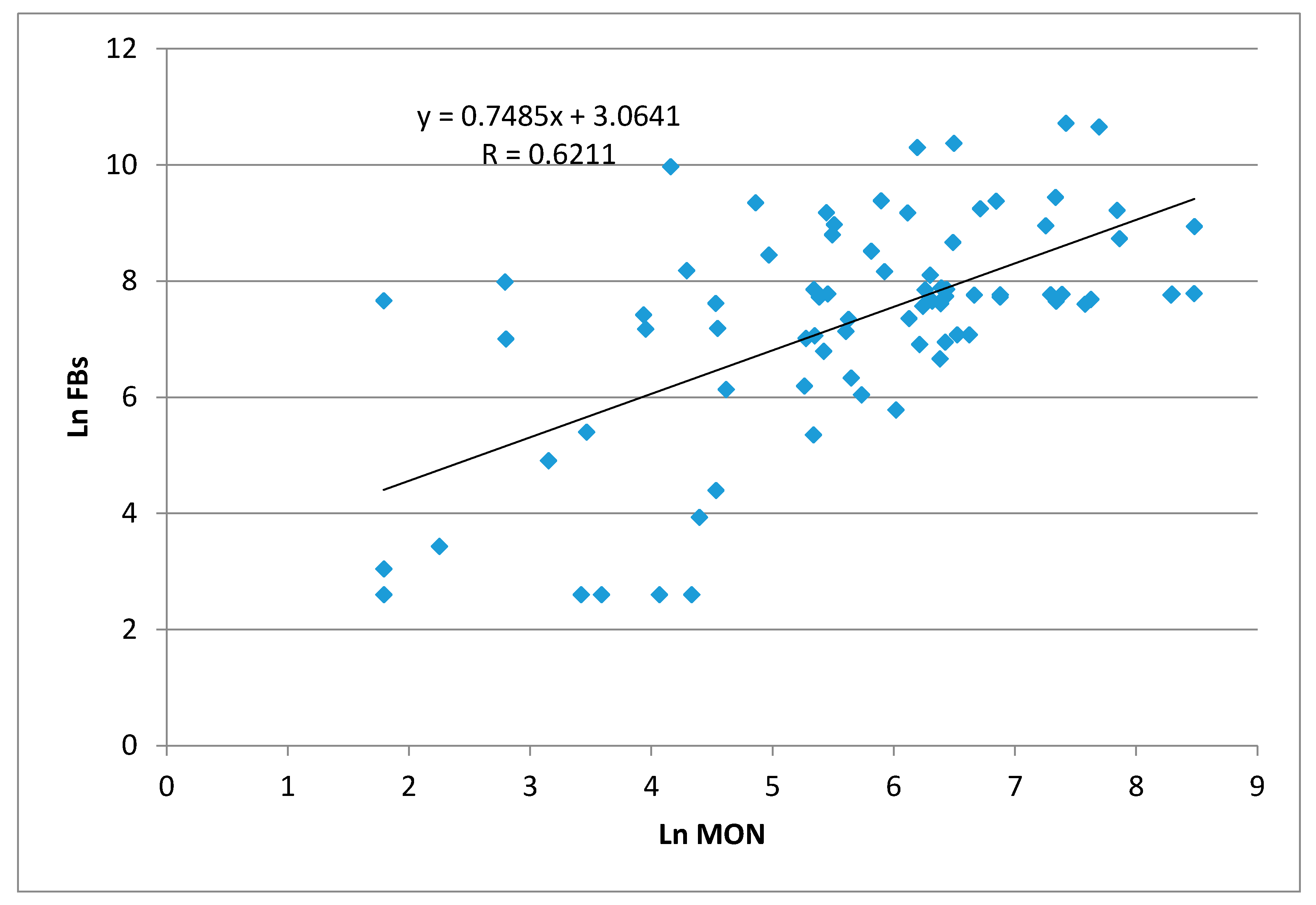
2.1. Maize
2.2. Durum Wheat
2.3. Common Wheat
3. Materials and Methods
3.1. Samples
3.2. Meteorological Data
3.3. Reagents and Standards
3.4. LC-MS/MS Analysis for Moniliformin Determination
3.5. Analysis for Fumonisin and Deoxynivalenol Determination
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Springer, J.P.; Clardy, J.; Cole, R.J.; Kirksey, J.W.; Hill, R.K.; Carlson, R.M.; Isidor, J.L. Structure and synthesis of moniliformin, a novel cyclobutane microbial toxin. J. Am. Chem. Soc. 1974, 96, 2267–2268. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel Contam. Risks to human and animal health related to the presence of moniliformin in food and feed. EFSA J. 2018, 16, 5082. [Google Scholar]
- Rossi, F.; Gallo, A.; Bertuzzi, T. Emerging mycotoxins in the food chain. Mediterr. J. Nutr. Metab. 2020, 13, 7–27. [Google Scholar] [CrossRef]
- Jonsson, M.; Atosuo, J.; Jestoi, M.; Nathanail, A.; Kokkonen, U.; Anttila, M.; Koivisto, P.; Lilius, E.; Peltonen, K. Repeated dose 28-day oral toxicity study of moniliformin in rats. Toxicol. Lett. 2015, 233, 38–44. [Google Scholar] [CrossRef]
- Fremy, J.M.; Alassane-Kpembi, I.; Oswald, I.P.; Cottrill, B.; Van Egmond, H.P. A review on combined effects of moniliformin and co-occurring Fusarium toxins in farm animals. World Mycotoxin J. 2019, 12, 281–291. [Google Scholar] [CrossRef]
- Uhlig, S.; Torp, M.; Jarp, J.; Parich, A.; Gutleb, A.C.; Krska, R. Moniliformin in Norwegian grain. Food Addit. Contam. 2004, 21, 598–606. [Google Scholar] [CrossRef]
- Jestoi, M.; Rokka, M.; Yli-Mattila, T.; Parikka, P.; Rizzo, A.; Peltonen, K. Presence and concentrations of the Fusarium related mycotoxins beauvericin, enniatins, and moniliformin in Finnish grain samples. Food Addit. Contam. 2004, 21, 794–802. [Google Scholar] [CrossRef]
- Van Asselt, E.D.; Azambuja, W.; Moretti, A.; Kastelein, P.; de Rijk, T.C.; Stratakou, I.; van der Fels-Klerx, H.J. A Dutch field survey on fungal infection and mycotoxin concentrations in maize. Food Addit. Contam. 2012, 29, 1556–1565. [Google Scholar] [CrossRef]
- Herrera, M.; van Dam, R.; Spanjer, M.; de Stoppelaar, J.; Mol, H.; de Nijs, M.; López, P. Survey of moniliformin in wheat- and corn-based products using a straightforward analytical method. Mycotoxin Res. 2017, 33, 333–341. [Google Scholar] [CrossRef]
- Jajic, I.; Dudaš, T.; Krstovic, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savic, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin in Serbian Maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Effect of fungicide application to control Fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J. 2015, 8, 499–510. [Google Scholar] [CrossRef]
- Scarpino, V.; Blandino, M.; Negre, M.; Rayneri, A.; Vanara, F. Moniliformin analysis in maize samples from North-West Italy using multifunctional clean-up columns and the LC-MS/MS detection method. Food Addit. Contam. 2013, 30, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Beccari, G.; Prodi, A.; Senatore, M.T.; Balmas, V.; Tini, F.; Onofri, A.; Pedini, L.; Sulyok, M.; Brocca, L.; Covarelli, L. Cultivation Area Aspects the Presence of Fungal Communities and Secondary Metabolites in Italian Durum Wheat Grains. Toxins 2020, 12, 97. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Rastelli, S.; Mulazzi, A.; Pietri, A. LC-MS/MS and LC-UV Determination of Moniliformin by Adding Lanthanide Ions to the Mobile Phase. Toxins 2019, 11, 570. [Google Scholar] [CrossRef]
- Scarpino, V.; Vanara, F.; Rayneri, A.; Blandino, M. Fate of moniliformin during different large-scale maize dry-milling processes. LWT Food Sci. Technol. 2020, 123, 109098. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Kosa, J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Sulyok, M. Mycotoxins in maize harvested in Serbia in the period 2012–2015. Part 2: Non-regulated mycotoxins and other fungal metabolites. Food Chem. 2020, 317, 126409. [Google Scholar] [CrossRef]
- Shala-Mayrhofer, V.; Varga, E.; Marjakaj, R.; Berthiller, F.; Musolli, A.; Berisha, D.; Kelmendi, B.; Lemmens, M. Investigations on Fusarium spp. and their mycotoxins causing Fusarium ear rot of maize in Kosovo. Food Addit. Contam. Part B 2013, 6, 237–243. [Google Scholar] [CrossRef]
- Scarpino, V.; Rayneri, A.; Vanara, F.; Scopel, C.; Causin, R.; Blandino, M. Relationship between European Corn Borer injury, Fusarium proliferatum and F. subglutinans infection and moniliformin contamination in maize. Field Crops Res. 2015, 183, 69–78. [Google Scholar] [CrossRef]
- Janse van Rensburg, B.; Mc Laren, N.W.; Flett, B.C. Grain colonization by fumonisin-producing Fusarium spp. and fumonisin synthesis in South African commercial maize in relation to prevailing weather conditions. Crop Protect. 2017, 102, 129–136. [Google Scholar] [CrossRef]
- De la Campa, R.; Hooker, D.C.; Miller, J.D.; Schaafsma, A.W.; Hammond, B.G. Modeling effects of environment, insect damage, and Bt genotypes on fumonisin accumulation in maize in Argentina and the Philippines. Mycopathologia 2005, 159, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Srobárová, A.; Moretti, A.; Ferracane, R.; Ritieni, A.; Logrieco, A. Toxigenic Fusarium species of Liseola section in pre-harvest maize ear rot, and associated mycotoxins in Slovakia. Eur. J. Plant Pathol. 2002, 108, 299–306. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Camardo Leggieri, M.; Battilani, P.; Pietri, A. Co-occurrence of type A and B trichothecenes and zearalenone in wheat grown in northern Italy over the years 2009–2011. Food Addit. Contam. Part B 2014, 7, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Cendoya, E.; Nichea, M.J.; Monge, M.P.; Sulyok, M.; Chiacchiera, S.M.; Ramirez, M.L. Fumonisin occurrence in wheat-based products from Argentina. Food Addit. Contam. Part B 2019, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, T.; Ritieni, A.; Galvano, F.; Amodio Cocchieri, R. Natural co-occurrence of deoxynivalenol and fumonisins B1 and B2 in Italian marketed foodstuffs. Food Addit. Contam. 2003, 20, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Pascale, M.; Bottalico, A.; Pancaldi, D.; Perrone, G.; Visconti, A. Occurrence of deoxynivalenol in cereals from experimental fields in various Italian regions. Petria 2002, 12, 123–129. [Google Scholar]
- Beccari, G.; Colasante, V.; Tini, F.; Senatore, M.T.; Prodi, A.; Sulyok, M.; Covarelli, L. Causal agents of Fusarium head blight of durum wheat (Triticum durum Desf.) in Central Italy and their in vitro biosynthesis of secondary metabolites. Food Microbiol. 2018, 70, 17–27. [Google Scholar] [CrossRef]
- Leggieri, M.C.; Van Der Fels-Klerx, H.J.; Battilani, P. Cross-validation of predictive models for deoxynivalenol in wheat at harvest. World Mycotoxin J. 2013, 6, 389–397. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. Forest Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Pietri, A.; Bertuzzi, T. Simple Phosphate Buffer Extraction for the Determination of Fumonisins in Masa, Maize, and Derived Products. Food Anal. Methods 2012, 5, 1088–1096. [Google Scholar] [CrossRef]
- Clewer, A.G.; Scarisbrick, D.H. Practical Statistics and Experimental Design for Plant and Crop Science; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
Sample Availability: Samples of maize, durum and common wheat are available from the authors. |


| MON | FBs | DON | |
|---|---|---|---|
| Field 1 (Castagnole, TO, Piedmont) | |||
| Positives (%) | 100 | 100 | 100 |
| Mean ± std. dev. | 408 ± 234 A,B | 1491 ± 815 A | 149 ± 180 a |
| Median | 372 | 1180 | 103 |
| Range | 93–751 | 780–3510 | 44–614 |
| Field 2 (Chivasso, TO, Piedmont) | |||
| Positives (%) | 78 | 33 | 44 |
| Mean ± std. dev. | 71 ± 94 C | 121 ± 185 B | <LOD c |
| Median | 35 | <LOD | <LOD |
| Range | <LOD-307 | <LOD-420 | <LOD |
| Field 3 (Bergamo, BG, Lombardy) | |||
| Positives (%) | 100 | 100 | 78 |
| Mean ± std. dev. | 2255 ± 1610 A | 2227 ± 157 A | 28.6 ± 70.3 b,c |
| Median | 1953 | 2290 | <LOD |
| Range | 592–4800 | 2000–2400 | <LOD-216 |
| Field 4 (Fontanella, BG, Lombardy) | |||
| Positives (%) | 89 | 100 | 78 |
| Mean ± std. dev. | 592 ± 494 A,B | 3296 ± 3542 A | 42 ± 87 a,b,c |
| Median | 543 | 2260 | <LOD |
| Range | <LOD-1529 | 1000–12,600 | <LOD-273 |
| Field 5 (S. Angelo Lod., LO, Lombardy) | |||
| Positives (%) | 100 | 100 | 89 |
| Mean ± std. dev. | 1231 ± 1550 A,B | 6803 ± 3327 A | 397 ± 780 a,b |
| Median | 622 | 6610 | <LOD |
| Range | 143–4811 | 2360–11,890 | <LOD-2080 |
| Field 6 (Arquà Polesine, RO, Veneto) | |||
| Positives (%) | 100 | 100 | 89 |
| Mean ± std. dev. | 487 ± 545 B,C | 5492 ± 4850 A | 18 ± 26 b,c |
| Median | 245 | 2930 | <LOD |
| Range | 8–1613 | 30–11,800 | <LOD-83 |
| Field 7 (S. Bellino, RO, Veneto) | |||
| Positives (%) | 100 | 100 | 100 |
| Mean ± std. dev. | 135 ± 98 B,C | 3383 ± 6797 A | 35 ± 89 b,c |
| Median | 92 | 1100 | <LOD |
| Range | 15–283 | 50–21,320 | <LOD-271 |
| Field 8 (Pozzuolo, UD, Friuli VG) | |||
| Positives (%) | 89 | 100 | 78 |
| Mean ± std. dev. | 633 ± 853 B,C | 3547 ± 3474 A | <LOD b,c |
| Median | 276 | 1540 | <LOD |
| Range | <LOD-2593 | 25–9650 | <LOD-29 |
| Field 9 (Mirandola, MO, Emilia-Romagna) | |||
| Positives | 100 | 100 | 67 |
| Mean ± std. dev. | 777 ± 680 A,B | 17,458 ± 18,880 A | <LOD b,c |
| Median | 488 | 4981 | <LOD |
| Range | 192–2190 | 323–43,297 | <LOD-38 |
| MON | DON | |
|---|---|---|
| Field 1 (Rieti, RI, Latium) | ||
| Positives (%) | 20 | 100 |
| Mean ± std. dev. | 6.7 ± 4.6 B,C | 305 ± 155 A |
| Median | <LOD | 297 |
| Range | <LOD-18 | 123–498 |
| Field 2 (Barbaruta, GR, Tuscany) | ||
| Positives (%) | 80 | 100 |
| Mean ± std. dev. | 519 ± 438 A | 222 ± 156 A,B |
| Median | 423 | 224 |
| Range | <LOD-1500 | 18–476 |
| Field 3 (Montelibretti, ROME, Latium) | ||
| Positives (%) | 50 | 100 |
| Mean ± std. dev. | 83 ± 104 A,B,C | 83 ± 88 B,C,D |
| Median | 44 | 49 |
| Range | <LOD-291 | <LOD-291 |
| Field 4 (Marciano, AR, Tuscany) | ||
| Positives (%) | 40 | 90 |
| Mean ± std. dev. | 14 ± 23 B,C | 160 ± 129 A,B,C |
| Median | <LOD | 136 |
| Range | <LOD-78 | <LOD-408 |
| Field 5 (Viterbo, VT, Latium) | ||
| Positives (%) | 70 | 90 |
| Mean ± std. dev. | 23 ± 26 B,C | <LOD E |
| Median | 8 | <LOD |
| Range | <LOD-132 | <LOD-27 |
| Field 6 (Tarquinia, VT, Latium) | ||
| Positives (%) | 40 | 100 |
| Mean ± std. dev. | 27 ± 41 B,C | 32 ± 33 C,D,E |
| Median | <LOD | 22 |
| Range | <LOD-132 | <LOD-101 |
| Field 7 (Alberese, GR, Tuscany) | ||
| Positives (%) | 60 | 90 |
| Mean ± std. dev. | 196 ± 320 A,B | 38 ± 71 D,E |
| Median | 12 | <LOD |
| Range | <LOD-951 | <LOD-236 |
| Field 8 (Libertinia, CT, Sicily) | ||
| Positives (%) | 0 | 100 |
| Mean ± std. dev. | <LOD C | 111 ± 42 A,B |
| Median | <LOD | 112 |
| Range | <LOD | 43–181 |
| MON | DON | |
|---|---|---|
| Field 1 (Cigliano, VC, Piedmont) | ||
| Positives (%) | 31.2 | 93.7 |
| Mean ± std. dev. | 15 ± 17 A,B | 1740 ± 1398 A |
| Median | <LOD | 1714 |
| Range | <LOD-51 | <LOD-4200 |
| Field 2 (S. Angelo Lod., LO, Lombardy) | ||
| Positives (%) | 25 | 100 |
| Mean ± std. dev. | 17 ± 28 A,B | 1445 ± 787 A |
| Median | <LOD | 1333 |
| Range | <LOD-90 | 342–2600 |
| Field 3 (Conselice, RA, Emilia-Romagna) | ||
| Positives (%) | 0 | 43.7 |
| Mean ± std. dev. | <LOD B | 90 ± 140 B |
| Median | <LOD | <LOD |
| Range | <LOD | <LOD-452 |
| Field 4 (Barbaruta, GR, Tuscany) | ||
| Positives (%) | 37.5 | 50 |
| Mean ± std. dev. | 41 ± 87 A | 96 ± 125 B |
| Median | <LOD | 43 |
| Range | <LOD-349 | <LOD-412 |
| Field 5 (Foggia, FG, Apulia) | ||
| Positives (%) | 0 | 0 |
| Mean ± std. dev. | <LOD B | <LOD C |
| Median | <LOD | <LOD |
| Range | <LOD | <LOD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuzzi, T.; Giorni, P.; Rastelli, S.; Vaccino, P.; Lanzanova, C.; Locatelli, S. Co-Occurrence of Moniliformin and Regulated Fusarium Toxins in Maize and Wheat Grown in Italy. Molecules 2020, 25, 2440. https://doi.org/10.3390/molecules25102440
Bertuzzi T, Giorni P, Rastelli S, Vaccino P, Lanzanova C, Locatelli S. Co-Occurrence of Moniliformin and Regulated Fusarium Toxins in Maize and Wheat Grown in Italy. Molecules. 2020; 25(10):2440. https://doi.org/10.3390/molecules25102440
Chicago/Turabian StyleBertuzzi, Terenzio, Paola Giorni, Silvia Rastelli, Patrizia Vaccino, Chiara Lanzanova, and Sabrina Locatelli. 2020. "Co-Occurrence of Moniliformin and Regulated Fusarium Toxins in Maize and Wheat Grown in Italy" Molecules 25, no. 10: 2440. https://doi.org/10.3390/molecules25102440
APA StyleBertuzzi, T., Giorni, P., Rastelli, S., Vaccino, P., Lanzanova, C., & Locatelli, S. (2020). Co-Occurrence of Moniliformin and Regulated Fusarium Toxins in Maize and Wheat Grown in Italy. Molecules, 25(10), 2440. https://doi.org/10.3390/molecules25102440










