Zebrafish-Based Screening Models for the Identification of Anti-Metastatic Drugs
Abstract
:1. Introduction
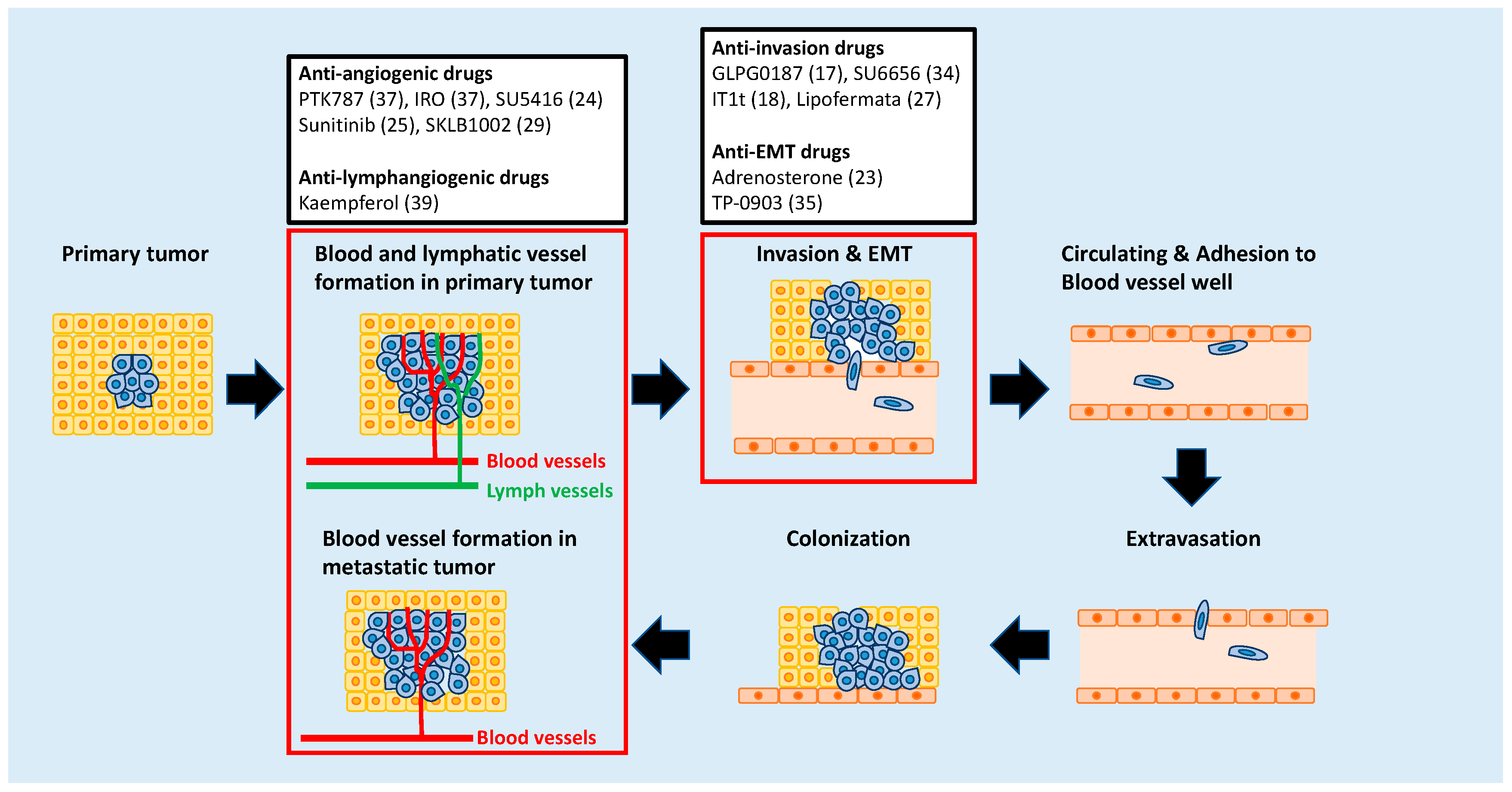
2. Targeting Metastatic Dissemination
3. Targeting the Epithelial-Mesenchymal Transition (EMT) Process
4. Targeting Angiogenesis
5. Targeting Lymphangiogenesis
6. Targeting Tumor Microenvironments
Funding
Acknowledgments
Conflicts of Interest
References
- Fidler, I. The pathogenesis of cancer metastasis: The ’seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Metastasis suppressors alter the signal transduction of cancer cells. Nat. Rev. Cancer 2003, 3, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: The state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Letrado, P.; de Miguel, I.; Lamberto, I.; Díez-Martínez, R.; Oyarzabal, J. Zebrafish: Speeding Up the Cancer Drug Discovery Process. Cancer Res. 2018, 78, 6048–6058. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, L.; Jauhiainen, A.; Kristiansson, E.; Nerman, O.; Larsson, D.G.J. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 2008, 42, 5807–5813. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Sun, S.; Wang, J.; Fei, F.; Dong, Z.; Ke, A.; He, R.; Wang, L.; Zhang, L.; Ji, M.; et al. Canonical Wnt Signaling Remodels Lipid Metabolism in Zebrafish Hepatocytes following Ras Oncogenic Insult. Cancer Res. 2018, 78, 5548–5560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milan, D.J.; Peterson, T.A.; Ruskin, J.N.; Peterson, R.T.; MacRae, C.A. Drugs That Induce Repolarization Abnormalities Cause Bradycardia in Zebrafish. Circulation 2003, 107, 1355–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabezas-Sainz, P.; Guerra-Varela, J.; Carreira, M.; Mariscal, J.; Roel, M.; Rubiolo, J.; Sciara, A.; Botana, L.; López, R.; Sánchez, L. Improving Zebrafish Embryo Xenotransplantation Conditions by Increasing Incubation Temperature and Establishing a Proliferation Index With ZFtool. BMC Cancer 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Schiavone, K.; Tazzyman, S.; Heymann, D.; Chico, T.J. Zebrafish xenograft models of cancer and metastasis for drug discovery. Expert Opin. Drug Discov. 2017, 12, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Trede, N.S. Fish immunology. Curr. Biol. 2009, 19, R678–R682. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Drabsch, Y.; Pujuguet, P.; Ren, J.; van Laar, T.; Zhang, L.; van Dam, H.; Clément-Lacroix, P.; Ten, D.P. Genetic depletion and pharmacological targeting of αv integrin in breast cancer cells impairs metastasis in zebrafish and mouse xenograft models. Breast Cancer Res. 2015, 25, 28. [Google Scholar] [CrossRef]
- Tulotta, C.; Stefanescu, C.; Beletkaia, E.; Bussmann, J.; Tarbashevich, K.; Schmidt, T.; Snaar-Jagalska, B. Inhibition of signaling between human CXCR4 and zebrafish ligands by the small molecule IT1t impairs the formation of triple-negative breast cancer early metastases in a zebrafish xenograft model. Dis. Model. Mech. 2016, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Drabsch, Y.; He, S.; Zhang, L.; Snaar-Jagalska, B.; Ten, D.P. Transforming growth factor-β signalling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res. 2013, 15, R106. [Google Scholar] [CrossRef]
- Ye, S.; Liu, Y.; Fuller, A.M.; Katti, R.; Ciotti, G.E.; Chor, S.; Alam, M.Z.; Devalaraja, S.; Lorent, K.; Weber, K.; et al. TGFb and Hippo Pathways Cooperate to Enhance Sarcomagenesis and Metastasis through the Hyaluronan-Mediated Motility Receptor (HMMR). Mol. Cancer Res. 2020, 18, 560–573. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, C.; Calleja, V.; Ferro, R.; Fantin, A.; Riley, A.M.; Potter, B.V.; Brennan, C.H.; Maffucci, T.; Larijani, B.; Falasca, M. A Small Molecule Inhibitor of PDK1/ PLCγ1 Interaction Blocks Breast and Melanoma Cancer Cell Invasion. Sci. Rep. 2016, 20, 26142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, J.; Aryee, D.; Fourtouna, A.; Van der Ent, W.; Kauer, M.; Niedan, S.; Machado, I.; Rodriguez-Galindo, C.; Tirado, O.; Schwentner, R.; et al. Suppression of deacetylase SIRT1 mediates tumor-suppressive NOTCH response and offers a novel treatment option in metastatic Ewing sarcoma. Cancer Res. 2014, 74, 6578–6588. [Google Scholar] [CrossRef] [Green Version]
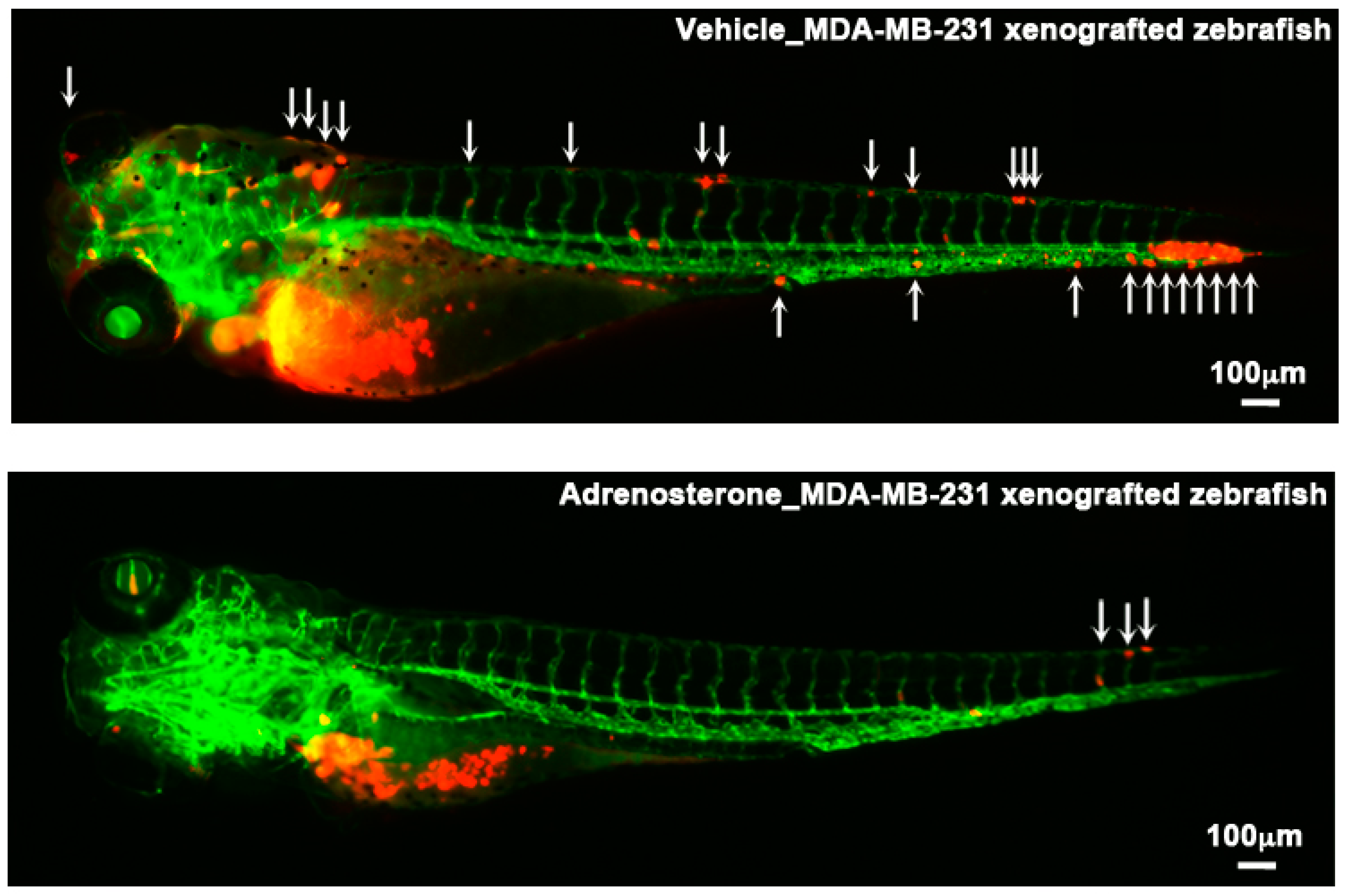
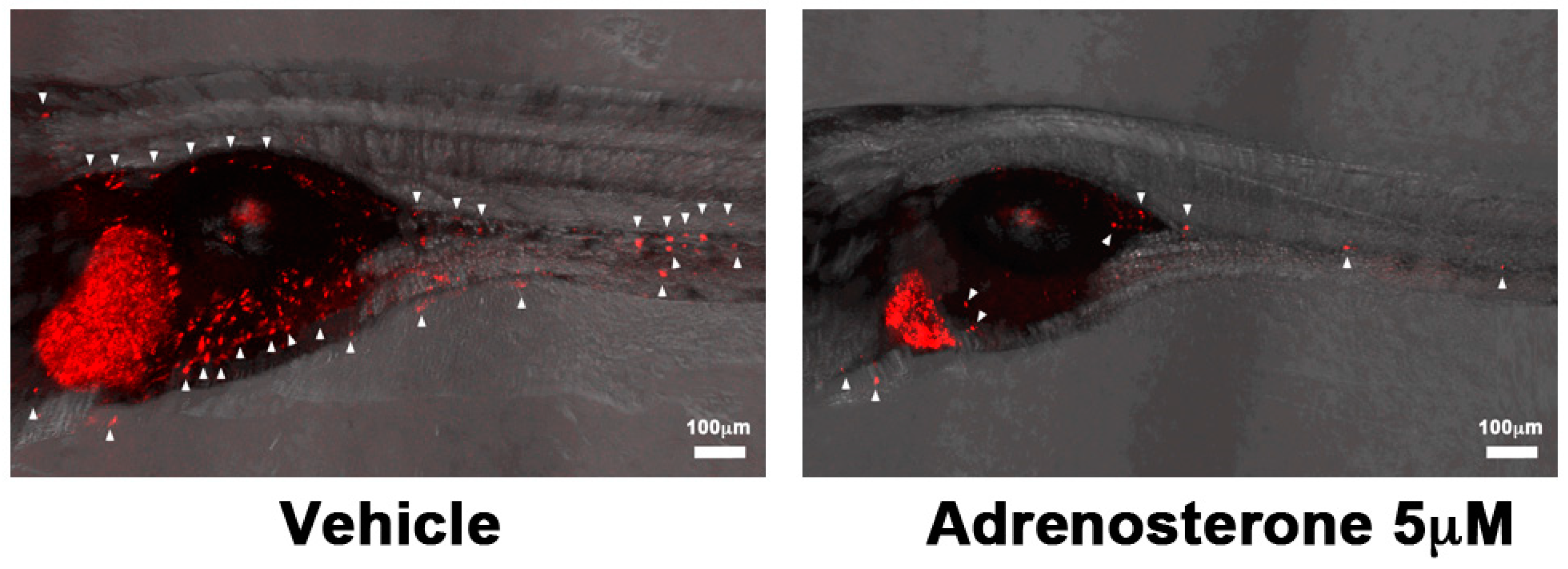
- Nakayama, J.; Lu, J.; Makinoshima, H.; Gong, Z. A Novel Zebrafish Model of Metastasis Identifies the HSD11β1 Inhibitor Adrenosterone as a Suppressor of Epithelial-Mesenchymal Transition and Metastatic Dissemination. Mol. Cancer Res 2020, 18, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Montel, V.; Lester, R.D.; Gonias, S.L.; Klemke, R. High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 17406–17411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Rouh, I.P.; Dahl Jensen, L.; Zhang, D.; Ji, H.; Hauptmann, G.; Ingham, P.; Cao, Y. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc. Natl. Acad. Sci. USA 2009, 106, 19485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, J.; Cao, Z.; Hosaka, K.; Jensen, L.; Yang, H.; Sun, Y.; Zhuang, R.; Liu, Y.; Cao, Y. Invasiveness and metastasis of retinoblastoma in an orthotopic zebrafish tumor model. Sci. Rep. 2015, 14, 10351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Di Martino, J.; Bowman, R.L.; Campbell, N.R.; Baksh, S.C.; Simon-Vermot, T.; Kim, I.S.; Haldeman, P.; Mondal, C.; Yong-Gonzales, V.; et al. Adipocyte-Derived Lipids Mediate Melanoma Progression via FATP Proteins. Cancer Discov. 2018, 8, 1006–1025. [Google Scholar] [CrossRef] [Green Version]
- Savio, M.; Ferraro, D.; Maccario, C.; Vaccarone, R.; Jensen, L.D.; Corana, F.; Mannucci, B.; Bianchi, L.; Cao, Y.; Stivala, L.A. Resveratrol analogue 4,4’-dihydroxy-trans-stilbene potently inhibits cancer invasion and metastasis. Sci. Rep. 2016, 1, 19973. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Cao, Z.; Tian, H.; Shen, G.; Ma, Y.; Xie, H.; Liu, Y.; Zhao, C.; Deng, S.; Yang, Y.; et al. SKLB1002, a novel potent inhibitor of VEGF receptor 2 signaling, inhibits angiogenesis and tumor growth in vivo. Clin. Cancer Res. 2011, 17, 4439–4450. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Huang, L.T.; Wu, J.Q.; He, M.F.; Zhu, S.H.; Zhan, P.; Lv, T.F.; Song, Y. Zebrafish Xenograft Model of Human Lung Cancer for Evaluating Osimertinib Resistance. Biomed. Res. Int. 2019, 27, 3129748. [Google Scholar] [CrossRef]
- Swinney, D.C.; Anthony, J. How were new medicines discovered? Nat. Rev. Drug Discov. 2011, 10, 507–519. [Google Scholar] [CrossRef] [PubMed]
- North, T.E.; Goessling, W.; Walkley, C.R.; Lengerke, C.; Kopani, K.R.; Lord, A.M.; Weber, G.J.; Bowman, T.V.; Jang, I.H.; Grosser, T.; et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 2007, 447, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Cech, J.; Ratanasirintrawoot, S.; Lin, C.Y.; Rahl, P.B.; Burke, C.J.; Langdon, E.; Tomlinson, M.L.; Mosher, J.; Kaufman, C.; et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 2011, 471, 518–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo, V.E.; Varshney, G.K.; Lee, M.; Bupp, S.; Xu, L.; Shinn, P.; Crawford, N.P.; Inglese, J.; Burgess, S.M. Phenotype-driven chemical screening in zebrafish for compounds that inhibit collective cell migration identifies multiple pathways potentially involved in metastatic invasion. Dis. Model. Mech. 2015, 8, 565–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, L.; Wang, J.; Morrison, M.A.; Whatcott, C.; Soh, K.K.; Warner, S.; Bearss, D.; Jette, C.A.; Stewart, R.A. Phenotypic chemical screening using a zebrafish neural crest EMT reporter identifies retinoic acid as an inhibitor of epithelial morphogenesis. Dis. Model. Mech. 2016, 9, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.; Bayliss, P.E.; Wood, J.M.; Roberts, T.M. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell 2002, 1, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.C.; Sneed, B.; Haider, J.; Blavo, D.; White, A.; Aiyejorun, T.; Baranowski, T.; Rubinstein, A.L.; Doan, T.N.; Dingledine, R.; et al. Automated, Quantitative Screening Assay for Antiangiogenic Compounds Using Transgenic Zebrafish. Cancer Res. 2007, 67, 11386. [Google Scholar] [CrossRef] [Green Version]
- Camus, S.; Quevedo, C.; Menéndez, S.; Paramonov, I.; Stouten, P.F.; Janssen, R.A.; Rueb, S.; He, S.; Snaar-Jagalska, B.E.; Laricchia-Robbio, L.; et al. Identification of phosphorylase kinase as a novel therapeutic target through high-throughput screening for anti-angiogenesis compounds in zebrafish. Oncogene 2012, 31, 4333–4342. [Google Scholar] [CrossRef] [Green Version]
- Astin, J.W.; Jamieson, S.M.; Eng, T.C.; Flores, M.V.; Misa, J.P.; Chien, A.; Crosier, K.E.; Crosier, P.S. An in vivo antilymphatic screen in zebrafish identifies novel inhibitors of mammalian lymphangiogenesis and lymphatic-mediated metastasis. Mol. Cancer Ther. 2014, 13, 2450–2462. [Google Scholar] [CrossRef] [Green Version]
- Chiang, A.C.; Massagué, J. Molecular Basis of Metastasis. N. Engl. J. Med. 2008, 359, 2814–2823. [Google Scholar] [CrossRef] [Green Version]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domanska, U.M.; Kruizinga, R.C.; Nagengast, W.B.; Timmer-Bosscha, H.; Huls, G.; de Vries, E.G.; Walenkamp, A.M. A review on CXCR4/CXCL12 axis in oncology: No place to hide. Eur. J. Cancer 2013, 49, 219–230. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, Q.; Gerald, W.; Hudis, C.A.; Norton, L.; Smid, M.; Foekens, J.A.; Massagué, J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009, 16, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Boyer, B.; Bourgeois, Y.; Poupon, M.F. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene 2002, 21, 2347–2356. [Google Scholar] [CrossRef] [Green Version]
- Husemann, Y.; Geigl, J.B.; Schubert, F.; Musiani, P.; Meyer, M.; Burghart, E.; Forni, G.; Eils, R.; Fehm, T.; Riethmuller, G.; et al. Systemic spread is an early step in breast cancer. Cancer Cell 2008, 13, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Braun, S.; Vogl, F.D.; Naume, B.; Janni, W.; Osborne, M.P.; Coombes, R.C.; Schlimok, G.; Diel, I.J.; Gerber, B.; Gebauer, G.; et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005, 353, 793–802. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Huang, X.; Zhan, H.; Zeng, Z.; Li, C.; Spitsbergen, J.M.; Meierjohann, S.; Schartl, M.; Gong, Z. Inducible and repressable oncogene-addicted hepatocellular carcinoma in Tet-on xmrk transgenic zebrafish. J. Hepatol. 2012, 56, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T. To differentiate or not—Routes towards metastasis. Nat. Rev. Cancer 2012, 12, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Kröger, C.; Afeyan, A.; Mraz, J.; Eaton, E.N.; Reinhardt, F.; Khodor, Y.L.; Thiru, P.; Bierie, B.; Ye, X.; Burge, C.B.; et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl. Acad. Sci. USA 2019, 116, 7353–7362. [Google Scholar]
- Obradović, M.M.S.; Hamelin, B.; Manevski, N.; Couto, J.P.; Sethi, A.; Coissieux, M.M.; Münst, S.; Okamoto, R.; Kohler, H.; Schmidt, A.; et al. Glucocorticoids promote breast cancer metastasis. Nature 2019, 567, 540–544. [Google Scholar] [CrossRef]
- Soh, K.K.; Kim, W.; Lee, Y.S.; Peterson, P.; Siddiqui-Jain, A.; Warner, S.L.; Bearss, D.J.; Whatcott, C.J. Abstract 235: AXL inhibition leads to a reversal of a mesenchymal phenotype sensitizing cancer cells to targeted agents and immuno-oncology therapies. In Proceedings of the AACR 107th Annual Meeting 2016, New Orleans, LA, USA, 16–20 April 2016. [Google Scholar]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Scott, E.N.; Meinhardt, G.; Jacques, C.; Laurent, D.; Thomas, A.L. Vatalanib: The clinical development of a tyrosine kinase inhibitor of angiogenesis in solid tumours. Expert Opin Investig Drugs 2007, 16, 367–379. [Google Scholar] [CrossRef]
- Jost, L.M.; Gschwind, H.P.; Jalava, T.; Wang, Y.; Guenther, C.; Souppart, C.; Rottmann, A.; Denner, K.; Waldmeier, F.; Gross, G.; et al. Metabolism and disposition of vatalanib (PTK787/ZK-222584) in cancer patients. Drug Metab. Dispos. 2006, 34, 1817–1828. [Google Scholar] [CrossRef] [Green Version]
- Los, M.; Roodhart, J.M.; Voest, E.E. Target practice: Lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist 2007, 12, 443–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, A.; Padhani, A.; Hayes, C.; Kakkar, A.J.; Leach, M.; Trigo, J.M.; Scurr, M.; Raynaud, F.; Phillips, S.; Aherne, W.; et al. A Phase I Study of the Angiogenesis Inhibitor SU5416 (Semaxanib) in Solid Tumours, Incorporating Dynamic Contrast MR Pharmacodynamic End Points. Br. J. Cancer 2005, 93, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Heymach, J.V.; Desai, J.; Manola, J.; Davis, D.W.; McConkey, D.J.; Harmon, D.; Ryan, D.P.; Goss, G.; Quigley, T.; Van den Abbeele, A.D.; et al. Phase II study of the antiangiogenic agent SU5416 in patients with advanced soft tissue sarcomas. Clin. Cancer Res. 2004, 10, 5732–5740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockhart, A.C.; Cropp, G.F.; Berlin, J.D.; Donnelly, E.; Schumaker, R.D.; Schaaf, L.J.; Hande, K.R.; Fleischer, A.C.; Hannah, A.L.; Rothenberg, M.L. Phase I/pilot study of SU5416 (semaxinib) in combination with irinotecan/bolus 5-FU/LV (IFL) in patients with metastatic colorectal cancer. Am. J. Clin. Oncol. 2006, 29, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, E.C. Cutting off cancer’s supply lines. Nature 2009, 458, 686–687. [Google Scholar] [CrossRef]
- Viviane, M.; Michael, D. Lymphangiogenesis and cancer metastasis. J. Cell. Mol. Med. 2009, 13, 1405–1416. [Google Scholar]
- Okuda, K.S.; Astin, J.W.; Misa, J.P.; Flores, M.V.; Crosier, K.E.; Crosier, P.S. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 2012, 139, 2381–2391. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Muhammad, I.; Bahare, S.; Javad, S.-R.; Tanweer, A.G.; Farhan, S.; Ali, I.; Muhammad, S.; Fokou, P.V.T.; Arshad, M.U.; Haroon, K.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar]
- Nöthlings, U.; Murphy, S.P.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Flavonols and pancreatic cancer risk: The multiethnic cohort study. Am. J. Epidemiol. 2007, 166, 924–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, J.A. Therapeutic targeting of the tumor microenvironment. Cancer Cell 2005, 7, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patton, E.E.; Widlund, H.R.; Kutok, J.L.; Kopani, K.R.; Amatruda, J.F.; Murphey, R.D.; Berghmans, S.; Mayhall, E.A.; Traver, D.; Fletcher, C.D.; et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 2005, 15, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilmann, S.; Ratnakumar, K.; Langdon, E.; Kansler, E.; Kim, I.; Campbell, N.R.; Perry, E.; McMahon, A.; Kaufman, C.; van Rooijen, E.; et al. A Quantitative System for Studying Metastasis Using Transparent Zebrafish. Cancer Res. 2015, 75, 4272–4282. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.S.; Heilmann, S.; Kansler, E.; Zhang, Y.; Zimmer, M.; Ratnakumar, K.; Bowman, R.L.; Simon-Vermot, T.; Fennell, M.; Garippa, R.; et al. Microenvironment-derived factors driving metastatic plasticity in melanoma. Nat. Commun. 2017, 9, 14343. [Google Scholar] [CrossRef] [Green Version]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef]
| Drug | Targeting Molecule | Targeting Molecular Event | Cancer Cell | Recipient Zebrafish | Inoculation Site | Reference |
|---|---|---|---|---|---|---|
| GLPG0187 | αv integrin | Invasion | MDA-MB-231 (Breast) | Tg(Fli1: EGFP) | Duct of Cuvier | [17] |
| IT1t | CXCR4 | Invasion | MDA-MB-231 (Breast) | Tg(Fli1: EGFP) | Duct of Cuvier | [18] |
| SB431542 | TGFβR1 | Invasion | MDA-MB-231 (Breast) | Tg(Fli1: EGFP) | Duct of Cuvier | [19] |
| SB525334 | TGFβR1 | Invasion | KIA (UPS) | Tg(Fli1: EGFP) | Yolk sac | [20] |
| 2-O-Bn-InsP5 | PDK1 | Invasion | MDA-MB-231 (Breast) | Tg(kdrl: HsHRAS-mCherry)s896 | Duct of Cuvier | [21] |
| Tenovin-6 | SIRT1/2 | Migration | TC252 (Ewing sarcoma) and A673 (Ewing sarcoma) | Tg(Fli1: EGFP) | Duct of Cuvier | [22] |
| Adrenosterone | HSD11β1 | EMT | HCCLM3 (Liver) and MDA-MB-231 (Breast) | Tg(kdrl: EGFP) | Duct of Cuvier | [23] |
| SU5416 | VEGFR | Angiogenesis | MDA-MB-435 (Skin) | Tg(Fli1: EGFP) | Peritoneal cavity | [24] |
| Sunitinib | VEGF | Angiogenesis | T241 (Thyroid) | Tg(Fli1: EGFP) | Perivitelline space | [25] |
| Sunitinib | VEGF | Invasion | SJmRBL-8 (Retinoblastoma) | Tg(Fli1: EGFP) | Eye | [26] |
| LY294002 | PI3-kinase | Invasion | MDA-MB-231 (Breast) | Tg(Fli1: EGFP) | Duct of Cuvier | [19] |
| GM6001 | MMPs | Invasion | MDA-MB-231 (Breast) | Tg(Fli1: EGFP) | Duct of Cuvier | [19] |
| Lipofermata | FATP | Invasion | ZMEL1 (Skin) | Casper | Subcutaneous tissue | [27] |
| DHS | Unknown | Invasion | LLC (Lung) | Tg(Fli1: EGFP) | Perivitelline cavity | [28] |
| SKLB1002 | VEGFR2 | Angiogenesis | B16-F10 (Skin) | Tg(Fli1: EGFP) | Perivitelline space | [29] |
| Osimertinib | EGFR | Angiogenesis | H1975 (Lung) | Tg(Fli1: EGFP) | Duct of Cuvier | [30] |
| Drug | Targeting Molecule | Targeting Molecular Event | Screening Platform | # of Drugs Subjected to the Screen | Reference |
|---|---|---|---|---|---|
| SU6656 | Src | Migration | Tg(cldnb: EGFP) | 2960 compounds from the LOPAC1280 library, the NatProd library, and the GSK Published Kinase Inhibitor Set (PKIS) | [34] |
| Adrenosterone | HSD11β1 | EMT | Tg(Fabp10A: mCherry-T2A-Twist1-ERT2) × Tg(fabp10a: TA; TRE:xmrk; krt4: GFP) | 68 chemicals from the Prestwick Chemical Library | [23] |
| TP-0903 | AXL receptor | EMT | Tg(snai1b: GFP) | Not applicable | [35] |
| PTK787 | VEGFR | Angiogenesis | WT | Not applicable | [36] |
| IRO | Unknown | Angiogenesis | Tg(VEGFR2: GRCFP) | 1280 chemicals from the LOPAC1280 library | [37] |
| F11 | PhKG1 | Angiogenesis | Tg (fli1: EGFP) | 288 chemicals | [38] |
| Kaempferol | VEGFR2/3 | Lymphangiogenesis | Tg(lyve1: EGFP) | 1120 chemicals from the Prestwick Chemical Library | [39] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, J.; Makinoshima, H. Zebrafish-Based Screening Models for the Identification of Anti-Metastatic Drugs. Molecules 2020, 25, 2407. https://doi.org/10.3390/molecules25102407
Nakayama J, Makinoshima H. Zebrafish-Based Screening Models for the Identification of Anti-Metastatic Drugs. Molecules. 2020; 25(10):2407. https://doi.org/10.3390/molecules25102407
Chicago/Turabian StyleNakayama, Joji, and Hideki Makinoshima. 2020. "Zebrafish-Based Screening Models for the Identification of Anti-Metastatic Drugs" Molecules 25, no. 10: 2407. https://doi.org/10.3390/molecules25102407
APA StyleNakayama, J., & Makinoshima, H. (2020). Zebrafish-Based Screening Models for the Identification of Anti-Metastatic Drugs. Molecules, 25(10), 2407. https://doi.org/10.3390/molecules25102407





