Antimicrobials from Venomous Animals: An Overview
Abstract
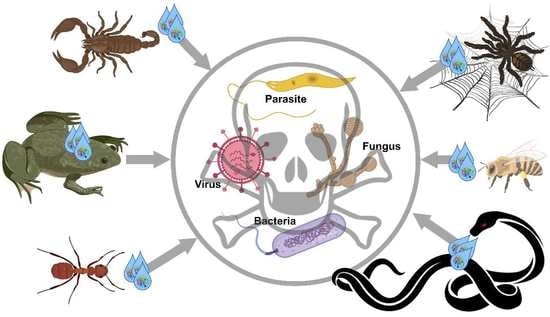
1. Introduction
2. Antimicrobial Activities of Animal Venoms and Secretions
2.1. Antimicrobial Agents from Spiders
2.2. Antimicrobial Agents from Scorpions
2.3. Antimicrobial Agents from Wasps and Bees
2.4. Antimicrobial Agents from Ants
2.5. Antimicrobial Agents from Snakes
2.6. Antimicrobial Agents from Frogs and Toads
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Tackling Antimicrobial Resistance. Eurohealth (Lond); WHO: Geneva, Switzerland, 2020; Volume 26. [Google Scholar]
- Centre for Disease Control. Antibiotic resistance threats in the United States; CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Le, M.N.-T.; Kayama, S.; Yoshikawa, M.; Hara, T.; Kashiyama, S.; Hisatsune, J.; Tsuruda, K.; Onodera, M.; Ohge, H.; Tsuga, K.; et al. Oral colonisation by antimicrobial-resistant Gram-negative bacteria among long-term care facility residents: Prevalence, risk factors, and molecular epidemiology. Antimicrob. Resist. Infect. Control. 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- White, J. Venomous animals: Clinical toxinology. Chall. Life 2010, 100, 233–291. [Google Scholar] [CrossRef]
- Opie, L.H.; Kowolik, H. The discovery of captopril: From large animals to small molecules. Cardiovasc. Res. 1995, 30, 18–25. [Google Scholar] [CrossRef]
- McGivern, J. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial Peptides: Primeval Molecules or Future Drugs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2011, 32, 143–171. [Google Scholar] [CrossRef]
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.; Possani, L.D. Scorpion venom components as potential candidates for drug development. Toxicon 2015, 93, 35–125. [Google Scholar] [CrossRef]
- Hancock, R.E. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156–164. [Google Scholar] [CrossRef]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial Peptides: Versatile Biological Properties. Int. J. Pept. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Boto, A.; De La Lastra, J.P.; González, C.C.; De La Lastra, J.M.P. The Road from Host-Defense Peptides to a New Generation of Antimicrobial Drugs. Molecules 2018, 23, 311. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Platnick, N.I. The World Spider Catalog; American Museum of Natural History: New York, NY, USA, 2011. [Google Scholar]
- Nentwig, W.; Kuhn-Nentwig, L. Spider Venoms Potentially Lethal to Humans. In Spider Ecophysiology; Springer Science and Business Media LLC: Berlin, Germany, 2012; pp. 253–264. [Google Scholar]
- Phartale, N.N.; Kadam, T.A.; Bhosale, H.J.; Karale, M.A.; Gyananath, G. Exploring the antimicrobial potential of Pardosa brevivulva silk. J. Basic Appl. Zool. 2019, 80, 31. [Google Scholar] [CrossRef]
- Al-Kalifawi, E.J.; Kadem, Y.J. The Antimicrobial Activity of Al-Ankabut’s Home (Spider’s Web) Extract. Mesopotamia Environ. J. 2017, Special Issue C, 54–63. [Google Scholar]
- Szymkowiak, P.; Tsiareshyna, M.; Koczura, R. Spider silk of Linothele fallax and Linothele megatheloides (Mygalomorphae, Dipluridae) does not affect the growth of bacteria. Boilogia 2020, 1–5. [Google Scholar] [CrossRef]
- Vassilevski, A.; Kozlov, S.A.; Grishin, E.V. Molecular diversity of spider venom. Biochem. (Moscow) 2009, 74, 1505–1534. [Google Scholar] [CrossRef] [PubMed]
- Benli, M.; Yigit, N. Antibacterial activity of venom from funnel web spider Agelena labyrinthica (Araneae: Agelenidae). J. Venom. Anim. Toxins Incl. Trop. Dis. 2008, 14, 641–650. [Google Scholar] [CrossRef]
- Sutti, R.; Rosa, B.B.; Wunderlich, B.; Júnior, P.I.D.S.; Silva, T.A.A.D.R.E. Antimicrobial activity of the toxin VdTX-I from the spider Vitalius dubius (Araneae, Theraphosidae). Biochem. Biophys. Rep. 2015, 4, 324–328. [Google Scholar] [CrossRef][Green Version]
- Domingos, M.O.; Neves, I.V.; Vigerelli, H.; Pimenta, D.C.; Távora, B.D.C.L.F.; Lemos, T.; Franzolin, M.R.; Marques, V.D.; Barbaro, K.C. The potential of Loxosceles gaucho spider venom to regulate Pseudomonas aeruginosa mechanisms of virulence. Toxicon 2018, 152, 78–83. [Google Scholar] [CrossRef]
- Lazarev, V.N.; Polina, N.F.; Shkarupeta, M.M.; Kostrjukova, E.S.; Vassilevski, A.; Kozlov, S.A.; Grishin, E.V.; Govorun, V.M. Spider Venom Peptides for Gene Therapy of Chlamydia Infection. Antimicrob. Agents Chemother. 2011, 55, 5367–5369. [Google Scholar] [CrossRef]
- Yan, L.; E Adams, M. Lycotoxins, Antimicrobial Peptides from Venom of the Wolf Spider Lycosa carolinensis. J. Boil. Chem. 1998, 273, 2059–2066. [Google Scholar] [CrossRef]
- Zhao, H.; Kong, Y.; Wang, H.; Yan, T.; Feng, F.; Bian, J.; Yang, Y.; Yu, H. A defensin-like antimicrobial peptide from the venoms of spider, Ornithoctonus hainana. J. Pept. Sci. 2011, 17, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hou, S.; Li, X.; Wu, M.; Ma, B.; Wang, Z.; Jiang, J.; Deng, M.; Duan, Z.; Tang, X.; et al. Anti-parasitic effect on Toxoplasma gondii induced by a spider peptide lycosin-I. Exp. Parasitol. 2019, 198, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ding, X.; Meng, S.; Liu, C.; Wang, H.; Xia, L.; Liu, Z.; Liang, S. Antimicrobial potential of lycosin-I, a cationic and amphiphilic peptide from the venom of the spider Lycosa singorensis. Curr. Mol. Med. 2013, 13, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Zhu, T.; Xing, M.; Luan, N.; Mwangi, J.; Yan, X.; Mo, G.; Rong, M.-Q.; Li, B.; Lai, R.; et al. An Antiviral Peptide from Alopecosa nagpag Spider Targets NS2B-NS3 Protease of Flaviviruses. Toxins 2019, 11, 584. [Google Scholar] [CrossRef]
- Dos Reis, P.V.M.; Boff, D.; Verly, R.M.; Melo-Braga, M.N.; Cortés, M.E.; Santos, D.M.; Pimenta, A.M.D.C.; Amaral, F.A.; Resende, J.M.; De Lima, M.E. LyeTxI-b, a Synthetic Peptide Derived From Lycosa erythrognatha Spider Venom, Shows Potent Antibiotic Activity in Vitro and in Vivo. Front. Microbiol. 2018, 9, 9. [Google Scholar]
- Shao, J.; Zhang, R.; Ge, X.; Yang, B.; Zhang, J. Analgesic Peptides in Buthus Martensii Karsch: A Traditional Chinese Animal Medicine. Asian J. Tradit. Med. 2007, 2, 45–50. [Google Scholar]
- Ehret-Sabatier, L.; Loew, D.; Goyffon, M.; Fehlbaum, P.; Hoffmann, J.A.; Van Dorsselaer, A.; Bulet, P. Characterization of Novel Cysteine-rich Antimicrobial Peptides from Scorpion Blood. J. Boil. Chem. 1996, 271, 29537–29544. [Google Scholar] [CrossRef]
- Mandard, N.; Sy, D.; Maufrais, C.; Bonmatin, J.-M.; Bulet, P.; Hetru, C.; Vovelle, F. Androctonin, a Novel Antimicrobial Peptide from Scorpion Androctonus Australis: Solution Structure and Molecular Dynamics Simulations in the Presence of a Lipid Monolayer. J. Biomol. Struct. Dyn. 1999, 17, 367–380. [Google Scholar] [CrossRef]
- Díaz-García, A.; Morier-Díaz, L.; Frión-Herrera, Y.; Rodríguez-Sánchez, H.; Caballero-Lorenzo, Y.; Mendoza-Llanes, D.; Riquenes-Garlobo, Y.; A Fraga-Castro, J. In vitro anticancer effect of venom from Cuban scorpion Rhopalurus junceus against a panel of human cancer cell lines. J. Venom Res. 2013, 4, 5–12. [Google Scholar]
- Cociancich, S.; Goyffon, M.; Bontems, F.; Bulet, P.; Bouet, F.; Menez, A.; Hoffmann, J. Purification and Characterization of a Scorpion Defensin, a 4 kDa Antibacterial Peptide Presenting Structural Similarities with Insect Defensins and Scorpion Toxins. Biochem. Biophys. Res. Commun. 1993, 194, 17–22. [Google Scholar] [CrossRef]
- Torres-Larios, A.; Gurrola, G.B.; Zamudio, F.Z.; Possani, L.D. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. JBIC J. Boil. Inorg. Chem. 2000, 267, 5023–5031. [Google Scholar] [CrossRef] [PubMed]
- Uawonggul, N.; Thammasirirak, S.; Chaveerach, A.; Arkaravichien, T.; Bunyatratchata, W.; Ruangjirachuporn, W.; Jearranaiprepame, P.; Nakamura, T.; Matsuda, M.; Kobayashi, M.; et al. Purification and characterization of Heteroscorpine-1 (HS-1) toxin from Heterometrus laoticus scorpion venom. Toxicon 2007, 49, 19–29. [Google Scholar] [CrossRef] [PubMed]
- De Melo, E.T.; Estrela, A.B.; Santos, E.; Machado, P.R.L.; Farias, K.J.S.; Torres, T.M.; Carvalho, E.; Lima, J.P.M.S.; Da Silva-Júnior, A.A.; Barbosa, E.G.; et al. Structural characterization of a novel peptide with antimicrobial activity from the venom gland of the scorpion Tityus stigmurus: Stigmurin. Peptides 2015, 68, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Corzo, G.; Escoubas, P.; Villegas, E.; Barnham, K.J.; He, W.; Norton, R.S.; Nakajima, T. Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator. Biochem. J. 2001, 359, 35. [Google Scholar] [CrossRef]
- Condé, R.; Zamudio, F.Z.; Rodrıéguez, M.H.; Possani, L.D. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000, 471, 165–168. [Google Scholar] [CrossRef]
- El-Bitar, A.M.H.; Sarhan, M.; Abdel-Rahman, M.A.; Quintero-Hernandez, V.; Aoki-Utsubo, C.; Moustafa, M.A.; Possani, L.D.; Hotta, H. Smp76, a Scorpine-Like Peptide Isolated from the Venom of the Scorpion Scorpio Maurus Palmatus, with a Potent Antiviral Activity Against Hepatitis C Virus and Dengue Virus. Int. J. Pept. Res. Ther. 2019, 26, 811–821. [Google Scholar] [CrossRef]
- Dai, C.; Ma, Y.; Zhao, Z.; Zhao, R.; Wang, Q.; Wu, Y.; Cao, Z.; Li, W. Mucroporin, the First Cationic Host Defense Peptide from the Venom of Lychas mucronatus. Antimicrob. Agents Chemother. 2008, 52, 3967–3972. [Google Scholar] [CrossRef]
- Du, Q.; Hou, X.; Wang, L.; Zhang, Y.; Xi, X.; Wang, H.; Zhou, M.; Duan, J.; Wei, M.; Chen, T.; et al. AaeAP1 and AaeAP2: Novel Antimicrobial Peptides from the Venom of the Scorpion, Androctonus aeneas: Structural Characterisation, Molecular Cloning of Biosynthetic Precursor-Encoding cDNAs and Engineering of Analogues with Enhanced Antimicrobial and Anticancer Activities. Toxins 2015, 7, 219–237. [Google Scholar] [CrossRef]
- Parente, A.; Daniele-Silva, A.; Furtado, A.A.; Melo, M.A.; Lacerda, A.F.; Queiroz, M.; Moreno, C.J.G.; Santos, E.; Rocha, H.A.O.; Barbosa, E.G.; et al. Analogs of the Scorpion Venom Peptide Stigmurin: Structural Assessment, Toxicity, and Increased Antimicrobial Activity. Toxins 2018, 10, 161. [Google Scholar] [CrossRef]
- Moreno, M.; Giralt, E. Three Valuable Peptides from Bee and Wasp Venoms for Therapeutic and Biotechnological Use: Melittin, Apamin and Mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Shim, S.-R.; Rhee, H.Y.; Park, H.-J.; Jung, W.-S.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; Cho, K.-H.; Park, S.-U. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Park. Relat. Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.M.; Heneine, L.G.D.; Pesquero, J.L.; Albuquerque, M.L.D. Pharmaceutical Composition Containin an Apitoxin Fraction and Use Thereof. WO2011041865A1, 2011. [Google Scholar]
- Jalaei, J.; Fazeli, M.; Rajaian, H.; Shekarforoush, S.S. In vitro antibacterial effect of wasp (Vespa orientalis) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.; Swaby, S. Antimicrobial Effects of Wasp (Vespa Orientalis) Venom. Egypt. Pharm. J. 2018, 17, 218. [Google Scholar]
- Han, S.; Lee, K.; Yeo, J.; Baek, H.; Park, K. Antibacterial and Anti-Inflammatory Effects of Honeybee (Apis Mellifera) Venom against Acne-Inducing Bacteria. J. Med. Plants Res. 2010, 4, 459–464. [Google Scholar]
- Ghabanchi, J.; Bazargani, A.; Afkar, M.D.; Foroushan, S.B.; Aein, S.D. In Vitro Assessment of Anti-Streptococcus Mutans Potential of Honey; Short Communication. Iran. Red. Crescent Med. J. 2010, 12, 61–64. [Google Scholar]
- Critchfield, J.W.; Butera, S.T.; Folks, T.M. Inhibition of HIV Activation in Latently Infected Cells by Flavonoid Compounds. AIDS Res. Hum. Retroviruses 1996, 12, 39–46. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Lee, W.-H.; Zhang, Y. Antimicrobial peptides from the venom gland of the social wasp Vespa tropica. Toxicon 2013, 74, 151–157. [Google Scholar] [CrossRef]
- Turillazzi, S.; Mastrobuoni, G.; Dani, F.R.; Moneti, G.; Pieraccini, G.; La Marca, G.; Bartolucci, G.; Perito, B.; Lambardi, D.; Cavallini, V.; et al. Dominulin A and B: Two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-ion trap. J. Am. Soc. Mass Spectrom. 2006, 17, 376–383. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuca, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Leandro, L.F.; Mendes, C.A.; Casemiro, L.A.; Vinhólis, A.H.; Cunha, W.; De Almeida, R.; Martins, C.H.G. Antimicrobial activity of apitoxin, melittin and phospholipase A2 of honey bee (Apis mellifera) venom against oral pathogens. Anais da Academia Brasileira de Ciências 2015, 87, 147–155. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, N.; Park, K.; Han, S.-M.; Yeo, J.-H.; Seo, H.S. Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Socarras, K.; Theophilus, P.A.S.; Torres, J.P.; Gupta, K.; Sapi, E. Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiotics 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Vinhote, J.F.C.; Lima, D.B.; Menezes, R.; Mello, C.P.; De Souza, B.M.; Havt, A.; Palma, M.S.; Dos Santos, R.P.; De Albuquerque, E.L.; Freire, V.N.; et al. Trypanocidal activity of mastoparan from Polybia paulista wasp venom by interaction with TcGAPDH. Toxicon 2017, 137, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Neto, L.M.; Neves, R.C.; Gonçalves, J.C.; Trentini, M.M.; Mucury-Filho, R.; Smidt, K.S.; Fensterseifer, I.C.; Silva, O.N.; Lima, L.D.; et al. Evaluation of the antimicrobial activity of the mastoparan Polybia-MPII isolated from venom of the social wasp Pseudopolybia vespiceps testacea (Vespidae, Hymenoptera). Int. J. Antimicrob. Agents 2017, 49, 167–175. [Google Scholar] [CrossRef]
- Pereira, A.V.; De Barros, G.; Pinto, E.G.; Tempone, A.G.; Orsi, R.D.O.; Santos, L.D.; Calvi, S.; Ferreira, R.; Pimenta, D.C.; Barraviera, B. Melittin induces in vitro death of Leishmania (Leishmania) infantum by triggering the cellular innate immune response. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 1. [Google Scholar] [CrossRef]
- Yu, A.-R.; Kim, J.-J.; Park, G.-S.; Oh, S.-M.; Han, C.-S.; Lee, M.Y. The Antifungal Activity of Bee Venom against Dermatophytes. J. Appl. Boil. Chem. 2012, 55, 7–11. [Google Scholar] [CrossRef]
- Park, J.; Kwon, O.; An, H.-J.; Park, K.K. Antifungal Effects of Bee Venom Components on Trichophyton rubrum: A Novel Approach of Bee Venom Study for Possible Emerging Antifungal Agent. Ann. Dermatol. 2018, 30, 202–210. [Google Scholar] [CrossRef]
- Sample, C.J.; Hudak, K.E.; Barefoot, B.E.; Koci, M.D.; Wanyonyi, M.S.; Abraham, S.; Staats, H.F.; Ramsburg, E. A mastoparan-derived peptide has broad-spectrum antiviral activity against enveloped viruses. Peptides 2013, 48, 96–105. [Google Scholar] [CrossRef]
- Uddin, B.; Lee, B.-H.; Nikapitiya, C.; Kim, J.-H.; Kim, T.-H.; Lee, H.-C.; Kim, C.G.; Lee, J.-S.; Kim, C.-J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef]
- Latreille, P.A. Genera Crustaceorum et Insectorum Secundum Ordinem in Familias Disposita, iconibus exemplisque plurimis explicata; A. Koenig: Paris, France, 2013. [Google Scholar] [CrossRef]
- Schmidt, J.O. Biochemistry of Insect Venoms. Annu. Rev. Èntomol. 1982, 27, 339–368. [Google Scholar] [CrossRef]
- Torres, A.; Quinet, Y.; Havt, A.; Rdis-Baptista, G.; Martins, A.M.C. Molecular Pharmacology and Toxinology of Venom from Ants. In An Integrated View of the Molecular Recognition and Toxinology - From Analytical Procedures to Biomedical Applications; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Kou, J.; Ni, Y.; Li, N.; Wang, J.; Liu, L.; Jiang, Z.-H. Analgesic and anti-inflammatory activities of total extract and individual fractions of Chinese medicinal ants Polyrhachis lamellidens. Boil. Pharm. Bull. 2005, 28, 176–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Badr, G.; Garraud, O.; Daghestani, M.; Al-Khalifa, M.; Richard, Y. Human breast carcinoma cells are induced to apoptosis by samsum ant venom through an IGF-1-dependant pathway, PI3K/AKT and ERK signaling. Cell. Immunol. 2012, 273, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Orivel, J.; Redeker, V.; Le Caer, J.-P.; Krier, F.; Revol-Junelles, A.-M.; Longeon, A.; Chaffotte, A.; Dejean, A.; Rossier, J. Ponericins, New Antibacterial and Insecticidal Peptides from the Venom of the Ant Pachycondyla goeldii. J. Boil. Chem. 2001, 276, 17823–17829. [Google Scholar] [CrossRef] [PubMed]
- Pluzhnikov, K.A.; Kozlov, S.A.; Vassilevski, A.; Vorontsova, O.V.; Feofanov, A.V.; Grishin, E.V. Linear antimicrobial peptides from Ectatomma quadridens ant venom. Biochimie 2014, 107, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Zelezetsky, I.; Pag, U.; Antcheva, N.; Sahl, H.-G.; Tossi, A. Identification and optimization of an antimicrobial peptide from the ant venom toxin pilosulin. Arch. Biochem. Biophys. 2005, 434, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Zelezetsky, I.; Pacor, S.; Pag, U.; Papo, N.; Shai, Y.; Sahl, H.-G.; Tossi, A. Controlled alteration of the shape and conformational stability of α-helical cell-lytic peptides: Effect on mode of action and cell specificity. Biochem. J. 2005, 390, 177–188. [Google Scholar] [CrossRef]
- Perito, B.; Cremonini, M.; Montecchi, T.; Turillazzi, S. A Preliminary Study on the Antimicrobial Activity of Sting Secretion and Gastral Glands of the Acrobat Ant Crematogaster Scutellaris. Bull. Insectol. 2018, 71, 97–101. [Google Scholar]
- Benmoussa, K.; Authier, H.; Prat, M.; Alaeddine, M.; Lefèvre, L.; Rahabi, M.C.; Bernad, J.; Aubouy, A.; Bonnafé, E.; Leprince, J.; et al. P17, an Original Host Defense Peptide from Ant Venom, Promotes Antifungal Activities of Macrophages through the Induction of C-Type Lectin Receptors Dependent on LTB4-Mediated PPARγ Activation. Front. Immunol. 2017, 8, 8. [Google Scholar] [CrossRef]
- Téné, N.; Bonnafé, E.; Berger, F.; Rifflet, A.; Guilhaudis, L.; Ségalas-Milazzo, I.; Pipy, B.; Coste, A.; Leprince, J.; Treilhou, M. Biochemical and biophysical combined study of bicarinalin, an ant venom antimicrobial peptide. Peptides 2016, 79, 103–113. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 2 May 2020).
- Rima, M.; Naini, S.A.; Karam, M.; Sadek, R.; Sabatier, J.-M.; Fajloun, Z. Vipers of the Middle East: A Rich Source of Bioactive Molecules. Molecules 2018, 23, 2721. [Google Scholar] [CrossRef]
- Munawar, A.; Ali, S.A.; Akrem, A.; Betzel, C. Snake Venom Peptides: Tools of Biodiscovery. Toxins 2018, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-Y.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B. Snake venom toxins: Toxicity and medicinal applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181. [Google Scholar] [CrossRef] [PubMed]
- Oguiura, N.; Boni-Mitake, M.; Affonso, R.; Zhang, G. In vitro antibacterial and hemolytic activities of crotamine, a small basic myotoxin from rattlesnake Crotalus durissus. J. Antibiot. 2011, 64, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.F.C.; Dantas, R.T.; Toyama, M.H.; Filho, E.D.; Zara, F.J.; Queiroz, M.G.; Nogueira, N.A.P.; Oliveira, M.; Toyama, D.D.O.; Monteiro, H.S.A.; et al. Antibacterial and antiparasitic effects of Bothrops marajoensis venom and its fractions: Phospholipase A2 and l-amino acid oxidase. Toxicon 2010, 55, 795–804. [Google Scholar] [CrossRef]
- Accary, C.; Hraoui-Bloquet, S.; Hamze, M.; Mallem, Y.; Omar, F.; Sabatier, J.-M.; Desfontis, J.-C.; Fajloun, Z. Protein Content Analysis and Antimicrobial Activity of the Crude Venom of Montivipera bornmuelleri; a Viper from Lebanon. Infect. Disord. Drug Targets 2014, 14, 49–55. [Google Scholar] [CrossRef]
- Rima, M.; Accary, C.; Haddad, K.; Sadek, R.; Hraoui-Bloquet, S.; Desfontis, J.C.; Fajloun, Z. Identification of L-amino acid oxidase (Mb-LAAO) with antibacterial activity in the venom of Montivipera bornmuelleri, a viper from Lebanon. Infect. Disord. Drug Targets 2013, 13, 337–343. [Google Scholar] [CrossRef]
- Accary, C.; Mantash, A.; Mallem, Y.; Fajloun, Z.; Assem, E. Separation and Biological Activities of Phospholipase A2 (Mb-PLA2) from the Venom of Montivipera bornmuelleri, a Lebanese Viper. J. Liq. Chromatogr. Relat. Technol. 2014, 38, 833–839. [Google Scholar] [CrossRef]
- Lee, M.L.; Tan, N.H.; Fung, S.-Y.; Sekaran, S.D. Antibacterial action of a heat-stable form of l-amino acid oxidase isolated from king cobra (Ophiophagus hannah) venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 237–242. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Ho, B.; Chow, V.T. Purification, characterization and bactericidal activities of basic phospholipase A2 from the venom of Agkistrodon halys (Chinese pallas). Biochimie 2008, 90, 1372–1388. [Google Scholar] [CrossRef]
- Zhao, F.; Lan, X.-Q.; Du, Y.; Chen, P.-Y.; Zhao, J.; Zhao, F.; Lee, W.-H.; Zhang, Y. King Cobra Peptide OH-CATH30 as a Potential Candidate Drug through Clinic Drug-Resistant Isolates. Zool. Res. 2018, 39, 87. [Google Scholar]
- Wang, Y.; Zhang, Z.; Chen, L.; Guang, H.; Li, Z.; Yang, H.; Li, J.; You, D.; Yu, H.; Lai, R. Cathelicidin-BF, a Snake Cathelicidin-Derived Antimicrobial Peptide, Could Be an Excellent Therapeutic Agent for Acne Vulgaris. PLoS ONE 2011, 6, e22120. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yue, J.; Xiong, Y.; Wang, W.; Yu, S.; Wang, H. In vitro activities of small peptides from snake venom against clinical isolates of drug-resistant Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 2003, 22, 172–174. [Google Scholar] [CrossRef]
- Nair, D.G.; Fry, B.G.; Alewood, P.; Kumar, P.P.; Kini, R.M. Antimicrobial activity of omwaprin, a new member of the waprin family of snake venom proteins. Biochem. J. 2007, 402, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Yamane, E.S.; Bizerra, F.C.; Oliveira, E.B.; Moreira, J.T.; Rajabi, M.; Nunes, G.L.; De Souza, A.O.; Da Silva, I.D.; Yamane, T.; Karpel, R.L.; et al. Unraveling the antifungal activity of a South American rattlesnake toxin crotamine. Biochimie 2013, 95, 231–240. [Google Scholar] [CrossRef]
- Costa, B.A.; Sanches, L.; Gomide, A.B.; Bizerra, F.; Mas, C.D.; Oliveira, E.B.; Perez, K.R.; Itri, R.; Oguiura, N.; Hayashi, M.A.F. Interaction of the Rattlesnake Toxin Crotamine with Model Membranes. J. Phys. Chem. B 2014, 118, 5471–5479. [Google Scholar] [CrossRef]
- Gomes, V.M.; Carvalho, A.; Da Cunha, M.; Keller, M.; Bloch, C.; Deolindo, P.; Alves, E. Purification and characterization of a novel peptide with antifungal activity from Bothrops jararaca venom. Toxicon 2005, 45, 817–827. [Google Scholar] [CrossRef]
- Bandeira, I.C.J.; Bandeira-Lima, D.; Mello, C.P.; Pereira, T.P.; Menezes, R.; Sampaio, T.L.; Falcao, C.B.; Radis-Baptista, G.; Martins, A.M.C. Antichagasic effect of crotalicidin, a cathelicidin-like vipericidin, found in Crotalus durissus terrificus rattlesnake’s venom gland. Parasitology 2017, 145, 1059–1064. [Google Scholar] [CrossRef]
- Maluf, S.E.C.; Mas, C.D.; Oliveira, E.; Melo, P.; Carmona, A.; Gazarini, M.; Hayashi, M. Inhibition of malaria parasite Plasmodium falciparum development by crotamine, a cell penetrating peptide from the snake venom. Peptides 2016, 78, 11–16. [Google Scholar] [CrossRef]
- Da Mata, É.C.G.; Mourao, C.; Rangel, M.; Schwartz, E. Antiviral activity of animal venom peptides and related compounds. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 3. [Google Scholar] [CrossRef]
- Cecilio, A.B.; Caldas, S.; De Oliveira, R.A.; Santos, A.S.B.; Richardson, M.; Naumann, G.B.; Schneider, F.S.; Alvarenga, V.G.; Estevao-Costa, M.; Fuly, A.L.; et al. Molecular Characterization of Lys49 and Asp49 Phospholipases A2 from Snake Venom and Their Antiviral Activities against Dengue virus. Toxins 2013, 5, 1780–1798. [Google Scholar] [CrossRef]
- Thankappan, B.; Angayarkanni, J. Biological characterization of omw1 and omw2: Antimicrobial peptides derived from omwaprin. 3 Biotech 2019, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Tan, C.K.; Hashimi, S.M.; Zulfiker, A.H.M.; Good, D.; Wei, M.Q. Toad Glandular Secretions and Skin Extractions as Anti-Inflammatory and Anticancer Agents. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, X.; Li, Z.; Zhou, A.; Tiffany-Castiglioni, E.; Xie, L.; Qian, Y. Identification of anti-tumor components from toad venom. Oncol. Lett. 2017, 14, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; He, X.; Hu, J.; Kamau, P.; Lai, R.; Rao, D.; Luo, L. Bv8-Like Toxin from the Frog Venom of Amolops jingdongensis Promotes Wound Healing via the Interleukin-1 Signaling Pathway. Toxins 2019, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Filho, G.A.C.; Schwartz, C.A.; Resck, I.S.; Murta, M.M.; Lemos, S.S.; Castro, M.S.; Kyaw, C.; Pires, O.R.; Leite, J.R.S.A.; Bloch, C.; et al. Antimicrobial activity of the bufadienolides marinobufagin and telocinobufagin isolated as major components from skin secretion of the toad Bufo rubescens. Toxicon 2005, 45, 777–782. [Google Scholar] [CrossRef]
- Tempone, A.G.; Pimenta, D.C.; Lebrun, I.; Sartorelli, P.; Taniwaki, N.N.; De Andrade, H.F.; Antoniazzi, M.M.; Jared, C. Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the toad Rhinella jimi parotoid macrogland secretion. Toxicon 2008, 52, 13–21. [Google Scholar] [CrossRef]
- Conlon, J.M.; Sonnevend, A.; Davidson, C.; Smith, D.D.; Nielsen, P.F. The ascaphins: A family of antimicrobial peptides from the skin secretions of the most primitive extant frog, Ascaphus truei. Biochem. Biophys. Res. Commun. 2004, 320, 170–175. [Google Scholar] [CrossRef]
- Dourado, F.S.; Leite, J.R.S.A.; Silva, L.P.; Melo, J.A.; Bloch, C.; Schwartz, E. Antimicrobial peptide from the skin secretion of the frog Leptodactylus syphax. Toxicon 2007, 50, 572–580. [Google Scholar] [CrossRef]
- Li, B.; Lyu, P.; Xie, S.; Qin, H.; Pu, W.; Xu, H.; Chen, T.; Shaw, C.; Ge, L.; Kwok, H.F. LFB: A Novel Antimicrobial Brevinin-Like Peptide from the Skin Secretion of the Fujian Large Headed Frog, Limnonectes fujianensi. Biomolecules 2019, 9, 242. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Shen, J.-H.; Jin, Y.; Lee, W.-H.; Zhang, Y. Maximins S, a novel group of antimicrobial peptides from toad Bombina maxima. Biochem. Biophys. Res. Commun. 2005, 327, 945–951. [Google Scholar] [CrossRef]
- Yang, H.-L.; Shen, Z.-Q.; Liu, X.; Kong, Y. Two novel antimicrobial peptides from skin venoms of spadefoot toad Megophrys minor. Chin. J. Nat. Med. 2016, 14, 294–298. [Google Scholar] [CrossRef]
- Michael Conlon, J.; Galadari, S.; Raza, H.; Condamine, E. Design of Potent, Non-toxic Antimicrobial Agents Based upon the Naturally Occurring Frog Skin Peptides, Ascaphin-8 and Peptide XT-7. Chem. Biol. Drug Des. 2008, 72, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A. Handbook of Biologically Active Peptides, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar] [CrossRef]
- Malgieri, G.; Avitabile, C.; Palmieri, M.; D’Andrea, L.D.; Isernia, C.; Romanelli, A.; Fattorusso, R. Structural Basis of a Temporin 1b Analogue Antimicrobial Activity against Gram Negative Bacteria Determined by CD and NMR Techniques in Cellular Environment. ACS Chem. Boil. 2015, 10, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Reiling, S.; Zarena, D.; Wang, G. Host defense antimicrobial peptides as antibiotics: Design and application strategies. Curr. Opin. Chem. Boil. 2017, 38, 87–96. [Google Scholar] [CrossRef]
| Animal | Peptide | Primary Sequence | Activity | Accession Number/Reference |
|---|---|---|---|---|
| Spiders | ||||
| Lycosa carolinensis | Lycotoxins-I | NIWLTALKFLGKHAAHLAKQQLSKLC | Antibacterial Antifungal | P61507.1 |
| Lycotoxins-II | NKIKWFKTMKSIAKFIAKEQMKKHLGGEC | P61508.1 | ||
| Ornithoctonus hainana | Oh-defensin | NMLCKLSMFGAVLGVPACAIDCLPMGKTGGSCEGGVCGCRKLTFKILWDKKFGC | [26] | |
| Alopecosa nagpag | Av-LCTX-An1a | NMETAHVFLLSFLLLCVFAVDLIEAGFGCPLDQMQCHNHCQSVRYRGGYCTNFLKMTCKCYGC | Antiviral | QGD15041 |
| Lycosa erythrognatha | LyeTxI | NXIWLTALKFLGKNLGKLAKQQLAKLXC | Antibacterial | 6CL3_A |
| Scorpions | ||||
| Androctonus australis | Androctonin | NRSVCRQIKICRRRGGCYYKCTNRPYC | Antibacterial Antifungal | P56684.1 |
| Leiurus quinquestriatus | Defensin | NGFGCPLNQGACHRHCRSIRRRGGYCAGFFKQTCTCYRNC | Antibacterial | P41965.1 |
| Hadrurus aztecus | Hadrurin | NGILDTIKSIASKVWNSKTVQDLKRKGINWVANKLGVSPQAAC | P82656.1 | |
| Pandinus imperator | Pandinin 1 | NGKVWDWIKSAAKKIWSSEPVSQLKGQVLNAAKNYVAEKIGATPTC | P83239.1 | |
| Pandinin 2 | NFWGALAKGALKLIPSLFSSFSKKDC | Antibacterial Antifungal | P83240.1 | |
| Scorpine | NMNSKLTALIFLGLIAIAYCGWINEEKIQKKIDERMGNTVLGGMAKAIVHKMAKNEFQCMANMDMLGNCEKHCQTSGEKGYCHGTKCKCGTPLSYC | Antimalarial Antibacterial | P56972.1 | |
| Scorpio maurus palmatus | Smp76 | NGWINEKKMQQKIDEKIGKNIIGGMAKAVIHKMAKNEFQCVANVDTLGNCKKHCAKTTGEKGYCHGTKCKCGIELSYC | Antiviral | [41] |
| Lychas mucronatus | Mucroporin | NMKVKFLLAVFLIVLVVTDHCHALFGLIPSLIGGLVSAFKGRRKRQMEARFEPQNRNYRKRELDLEKLFANMPDYC | Antibacterial | ACF93401.1 |
| Wasps and Bees | ||||
| Polistes dominulus | Dominulin-A | NINWKKIAEVGGKILSSLC | Antibacterial | P0C1M6.1 |
| Dominulin-B | NINWKKIAEIGKQVLSALC | P0C1M7.1 | ||
| Apis mellifera | Melittin | NMKFLVNVALVFMVVYISYIYAAPEPEPAPEPEAEADAEADPEAGIGAVLKVLTTGLPALISWIKRKRQQGC | Antibacterial Antiparasitic Antiviral | AFI40556.1 |
| Polybia paulista | Mastoparan | NIDWKKLLDAAKQILC | Antibacterial Antiparasitic | P0C1Q4.1 |
| Synthetic peptide | Mastoporan-derived peptide (MP7-NH2) | NINLKALAALAKALL-NH2C | Antiviral | [64] |
| Ants | ||||
| Pachycondyla goeldii | Ponericin-L1 | NLLKELWTKMKGAGKAVLGKIKGLLC | Antibacterial | P82421.1 |
| Ponericin-L2 | NLLKELWTKIKGAGKAVLGKIKGLLC | P82422.1 | ||
| Ponericin-G1 | NGWKDWAKKAGGWLKKKGPGMAKAALKAAMQC | Antibacterial Antifungal | P82414.1 | |
| Ponericin-G2 | NGWKDWLKKGKEWLKAKGPGIVKAALQAATQC | P82415.1 | ||
| Ponericin-G3 | NGWKDWLNKGKEWLKKKGPGIMKAALKAATQC | P82416.1 | ||
| Ponericin-G4 | NDFKDWMKTAGEWLKKKGPGILKAAMAAATC | P82417.1 | ||
| Ponericin-G5 | NGLKDWVKIAGGWLKKKGPGILKAAMAAATQC | P82418.1 | ||
| Ponericin-G6 | NGLVDVLGKVGGLIKKLLPC | P82419.1 | ||
| Ponericin-G7 | NGLVDVLGKVGGLIKKLLPGC | P82420.1 | ||
| Ponericin-W1 | NWLGSALKIGAKLLPSVVGLFKKKKQC | P82423.1 | ||
| Ponericin-W2 | NWLGSALKIGAKLLPSVVGLFQKKKKC | P82424.1 | ||
| Ponericin-W3 | NGIWGTLAKIGIKAVPRVISMLKKKKQC | P82425.1 | ||
| Ponericin-W4 | NGIWGTALKWGVKLLPKLVGMAQTKKQC | P82426.1 | ||
| Ponericin-W5 | NFWGALIKGAAKLIPSVVGLFKKKQC | P82427.1 | ||
| Ponericin-W6 | NFIGTALGIASAIPAIVKLFKC | Antibacterial | P82428.1 | |
| Myrmecia pilosula | Pilosulin-1 | NMKLSCLLLTLTIIFVLTIVHAPNVEAKDLADPESEAVGFADAFGEADAVGEADPNAGLGSVFGRLARILGRVIPKVAKKLGPKVAKVLPKVMKEAIPMAVEMAKSQEEQQPQC | Antibacterial Antifungal | Q07932.1 |
| Tetramorium bicarinatum | P17 | NMKLSFLSLALATIFVMAIIYAPQMEARASSDADADAAASADADADALAEASALFKEILEKIKAKLGKKC | Antifungal | AIO11144.1 |
| Bicarinalin | NMKLSFLSLVLAIILVMALMYTPHAEAKAWADADADATAAADADADAVADALADAVAKIKIPWGKVKDFLVGGMKAVGKKC | Antibacterial Antifungal Antiparasitic | W8GNV3.1 | |
| Snakes | ||||
| Crotalus durissus | Crotamine | NMKILYLLFAFLFLAFLSEPGNAYKQCHKKGGHCFPKEKICLPPSSDFGKMDCRWRWKCCKKGSGKC | Antibacterial | AAF34911.1 |
| Bothrops marajoensis | L-AAO | NAHDGNPLEECFREDDEEFFLEIAKNGLTATSNPKRVVIVC | Antibacterial Antifungal Antiparasitic | P0CJ40.1 |
| Bungarus fasciatus | Cathelicidin | NMEGFFWKTLLVVGALAIAGTSSLPHKPLIYEEAVDLAVSIYNSKSGEDSLYRLLEAVSPPKWDPLSESNQELNFTMKETVCLVAEERSLEECDFQEDGVVMGCTGYYFFGESPPVVVLTCKPVGEEGEQKQEEGNEEEKEVEEEEQEEDEKDQPRRVKRFKKFFRKLKKSVKKRAKEFFKKPRVIGVSIPFC | Antibacterial | ACI22652.1 |
| Oxyuranus microlepidotus | Omwaprin | NKDRPKKPGLCPPRPQKPCVKECKNDDSCPGQQKCCNYGCKDECRDPIFVGC | P83952.1 | |
| Crotalus durissus terrificus | Crotamine | NMKILYLLFAFLFLAFLSEPGNAYKQCHKKGGHCFPKEKICLPPSSDFGKMDCRWRWKCCKKGSGKC | Antifungal Antiparasitic | AAF34911.1 |
| Crotalicidin | NMQGFFWKTWLVLAVCGTPASLAHRPLSYGEALELAVSVYNGKAGEASLYRLLEAVPQPEWDPSSEGSQQLNFTLKETACQVEEERSLEECGFQEDGVVLECTGYYFFGETPPVVVLSCVPVGGVEEEEEEEEEEQKAEAENDEEVEKEKGDEEKDQPKRVKRFKKFFKKVKKSVKKRLKKIFKKPMVIGVTIPFC | Antiparasitic | U5KJM4.1 | |
| Frogs and Toads | ||||
| Ascaphus truei | Ascaphin-1 | NGFRDVLKGAAKAFVKTVAGHIANC | Antibacterial | P0CJ25.1 |
| Ascaphin-2 | NGFRDVLKGAAKQFVKTVAGHIANIC | P0CJ26.1 | ||
| Ascaphin-3 | NGFRDVLKGAAKAFVKTVAGHIANIC | P0CJ27.1 | ||
| Ascaphin-4 | NGFKDWIKGAAKKLIKTVAANIANQC | P0CJ28.1 | ||
| Ascaphin-5 | NGIKDWIKGAAKKLIKTVASHIANQC | P0CJ29.1 | ||
| Ascaphin-6 | NGFKDWIKGAAKKLIKTVASSIANEC | P0CJ30.1 | ||
| Ascaphin-7 | NGFKDWIKGAAKKLIKTVASAIANQC | P0CJ31.1 | ||
| Ascaphin-8 | NGFKDLLKGAAKALVKTVLFC | P0CJ32.1 | ||
| Leptodactylus syphax | Syphaxin | NGVLDILKGAAKDLAGHVATKVINKIC | P85279.1 | |
| Limnonectes fujianensis | LFB | NGLFSVVKGVLKGVGKNVSGSLLDQLKCKISGGCC | Antibacterial Antifungal | [108] |
| Bombina maxima1 | Maximin-1 | NMNFKYIVAVSFLLASAYARSEENDEQSLSQRDVLEEESLREIRGIGTKILGGVKTALKGALKELASTYANGKRTAEEHEVMKRLEAVMRDLDSLDYPEEAAERETRSFNQEEIANLFTKKEKRILGPVISTIGGVLGGLLKNLGC | P83080.1 | |
| Maximin-2 | NMNFKYIVAVSFLIASAYARSEENDEQSLSQRDVLEEESLREIRGIGTKILGGVKTALKGALKELASTYVNGKRTAEDHEVMKRLEAVMRDLDSLDYPEEAAERETRGFNQEEIANLFTKKEKRILGPVISTIGGVLGGLLKNLGC | P83081.1 | ||
| Maximin-3 | NMNFKYIVAVSFLIASAYARSVQNDEQSLSQRDVLEEESLREIRGIGGKILSGLKTALKGAAKELASTYLHRRRTAEEHEVMKRLEAVMRDLDSLDYPEEASERETRGFNQDEIANLFTKKEKRILGPVLSMVGSALGGLIKKIGC | P83082.1 | ||
| Maximin-4 | NMNFKYIIAVSFFIASAYARSEEKDVQSLSQRDVLEEESLREIRGIGGVLLSAGKAALKGLAKVLAEKYANGKRTAEDHEVMKRLEAVMRDLDSLDHPEEASERETRGFNQEEIANLFTKKEKRILGPVLGLVGNALGGLIKKIGC | P83083.1 | ||
| Maximin-5 | NMNFKYIVAVSFLIASAYARSVQNDEQSLSQRDVLEEESLREIRSIGAKILGGVKTFFKGALKELASTYLQRKRTAEEQHEVMKRLEAVMRDLDSLDHPEEASEREIRGFNQEEIANLFTKKEKRILGPVISKIGGVLGGLLKNLGC | P83084.1 | ||
| Megophrys minor | Megin 1 | NFLKGCWTKWYSLKPKCPF-NH2C | [110] | |
| Megin 2 | NFFVLKFLLKWAGKVGLEHLACKFKNWCC | [110] | ||
| Rana temporaria | Temporin-A | NFLPLIGRVLSGILC | Antibacterial | P56917.2 |
| Temporin-B | NLLPIVGNLLKSLLC | 6GIL_A | ||
| Temporin-C | NLLPILGNLLNGLLC | P56918.2 | ||
| Temporin-E | NVLPIIGNLLNSLLC | P56920.2 | ||
| Temporin-F | NFLPLIGKVLSGILC | P56921.2 | ||
| Temporin-K | NLLPNLLKSLLC | P56923.2 | ||
| Temporin-L | NFVQWFSKFLGRILC | P57104.1 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. https://doi.org/10.3390/molecules25102402
Yacoub T, Rima M, Karam M, Sabatier J-M, Fajloun Z. Antimicrobials from Venomous Animals: An Overview. Molecules. 2020; 25(10):2402. https://doi.org/10.3390/molecules25102402
Chicago/Turabian StyleYacoub, Tania, Mohamad Rima, Marc Karam, Jean-Marc Sabatier, and Ziad Fajloun. 2020. "Antimicrobials from Venomous Animals: An Overview" Molecules 25, no. 10: 2402. https://doi.org/10.3390/molecules25102402
APA StyleYacoub, T., Rima, M., Karam, M., Sabatier, J.-M., & Fajloun, Z. (2020). Antimicrobials from Venomous Animals: An Overview. Molecules, 25(10), 2402. https://doi.org/10.3390/molecules25102402