Selection of Optimal Operating Conditions for Extraction of Myrtus Communis L. Essential Oil by the Steam Distillation Method
Abstract
1. Introduction
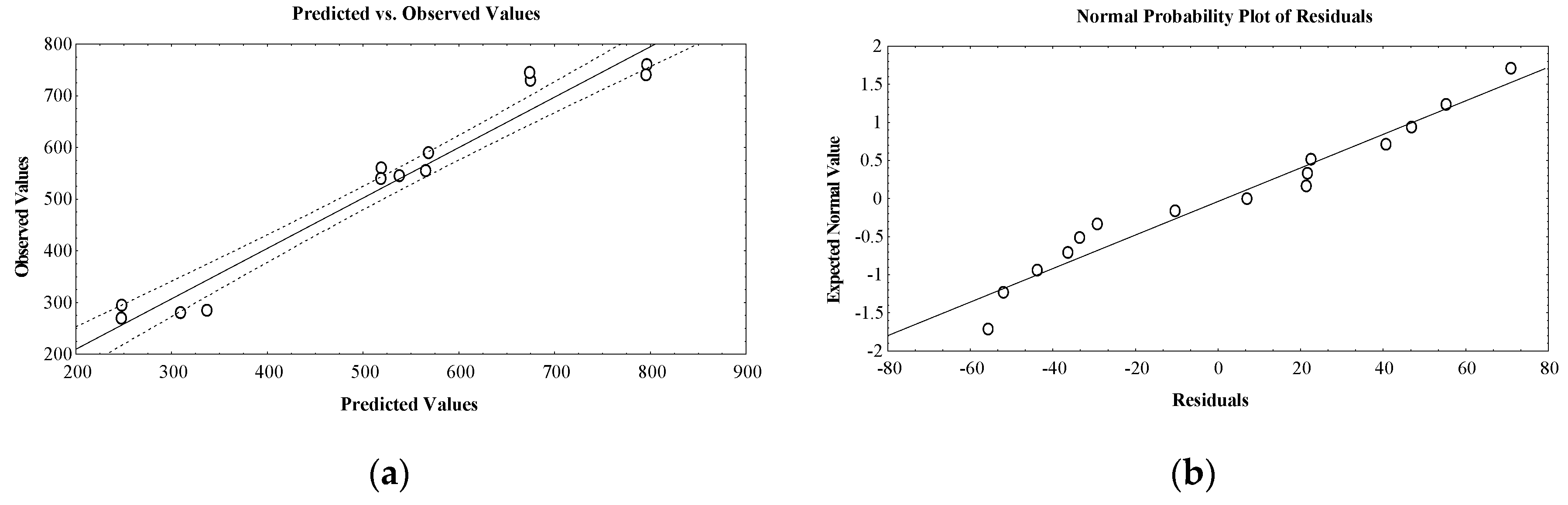
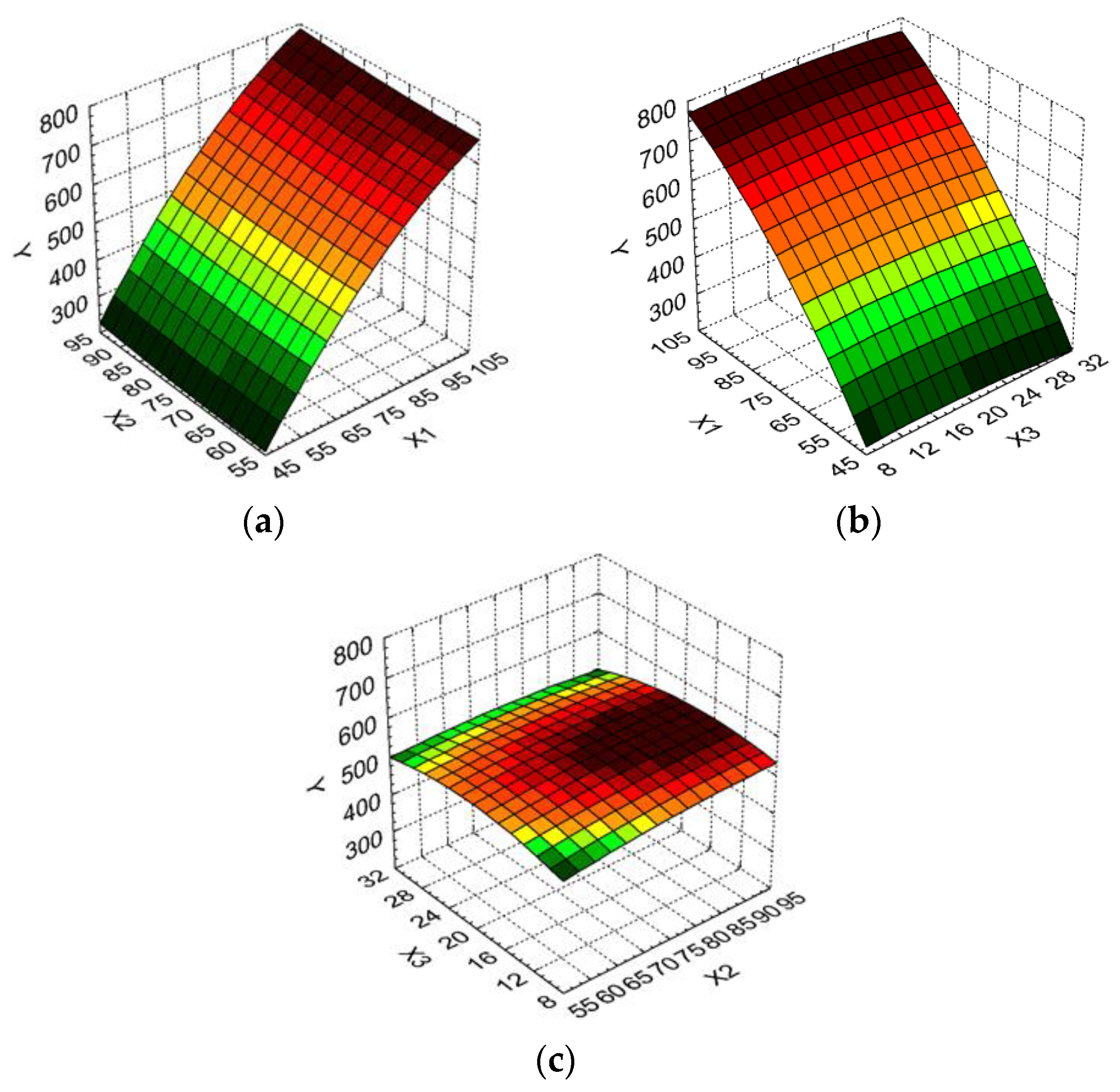
2. Results and Discussion
Design of Experiments and Optimization Technique
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Obtaining Essential Oils
3.2.2. Design of Experiments and Optimization Techniques
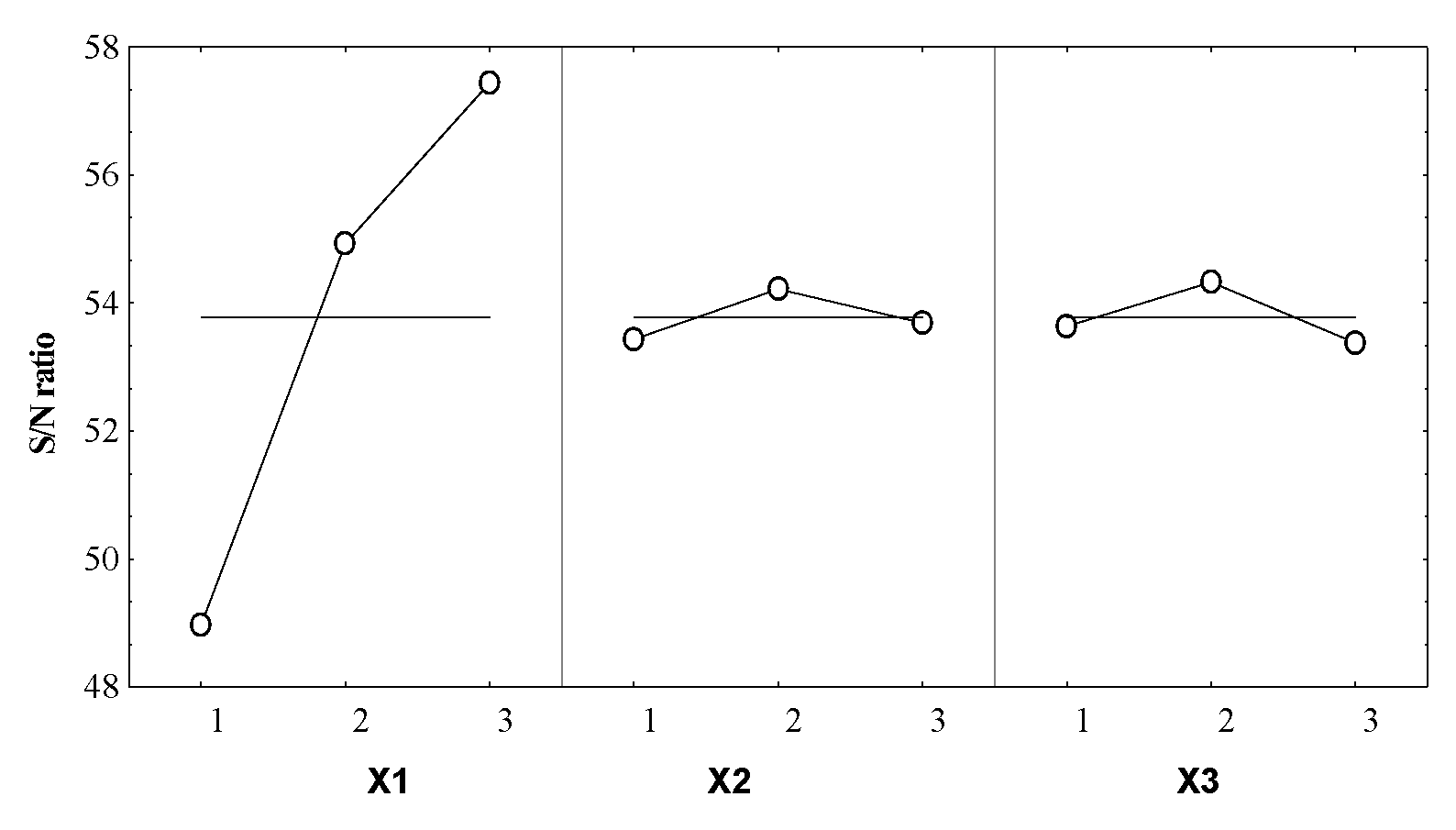
Taguchi Technique
3.2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, E.; Güney, M.; Sadighazadi, S.; Yildirim, H.; Kefayati, S. Volatile Compounds of selected white and black Myrtle (Myrtus Communis L.) types from Mediterranean region of Turkey. J. Med. Plants Res. 2013, 7, 1244–1248. [Google Scholar]
- Wissam, Z.; Ali, A.; Walaa, I. Improvement of polyphenolic content and antioxidant activity of Syrian myrtle berries (Myrtus communis L.) hydro-alcoholic extracts using flavoring additives. Prog. Nutr. 2017, 19, 112–120. [Google Scholar]
- Franco, A.M.; Tocci, N.; Guella, G.; Dell’Agli, M.; Sangiovanni, E.; Perenzoni, D.; Vrhovsek, U.; Mattivi, F. Myrtle Seeds (Myrtus communis L.) as a rich source of the bioactive ellagitannins oenothein B and eugeniflorin D2. ACS Omega 2019, 4, 15966–15974. [Google Scholar] [CrossRef]
- Kanoun, K.; Belyagoubi-Benhammou, N.; Ghembaza, N.; Atik Bekkara, F. Comparative studies on antioxidant activities of extracts from the leaf, stem and berry of Myrtus communis L. Int. Food Res. J. 2014, 21, 1957–1962. [Google Scholar]
- Bouzabata, A.; Cabral, C.; Gonçalves, M.J.; Cruz, M.T.; Bighelli, A.; Cavaleiro, C.; Casanova, J.; Tomi, F.; Salgueiro, L. Myrtus communis L. as source of a bioactive and safe essential oil. Food Chem. Toxicol. 2015, 75, 166–172. [Google Scholar] [CrossRef]
- Bouzabata, A.; Casanova, J.; Bighelli, A.; Cavaleiro, C.; Salgueiro, L.; Tomi, F. The genus Myrtus L. in Algeria: Composition and biological aspects of essential oils from M. communis and M. nivellei: A review. Chem. Biodivers. 2016, 13, 672–680. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Hamdi, N.; Miladi, R.; Abdelkafi, S. Myrtus communis essential oil: Chemical composition and antimicrobial activities against food spoilage pathogens. Chem. Biodivers. 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Mulas, M.; Melis, R.A.M. Essential oil composition of Myrtle (Myrtus communis) leaves. J. Herbs Spices Med. Plants 2011, 17, 21–34. [Google Scholar] [CrossRef]
- Aidi Wannes, W.; Mhamdi, B.; Marzouk, B. Essential oil composition of two Myrtus communis L. varieties grown in North Tunisia. Ital. J. Biochem. 2007, 56, 180–186. [Google Scholar]
- Asif, H.M.; Akram, M.; Uddin, S.; Hasan, Z.; Sami, A.; Iqbal, A.; Tauseef, U.; Bari, A. Myrtus communis L. (Pharmacological activity). J. Med. Plants Res. 2011, 5, 6257–6259. [Google Scholar]
- Nassar, M.I.; Aboutabl, E.S.A.; Ahmed, R.F.; El-Khrisy, E.D.A.; Ibrahim, K.M.; Sleem, A.A. Secondary metabolites and bioactivities of Myrtus communis. Pharmacognosy Res. 2010, 2, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Gençler Özkan, A.M.; Gençler Güray, C.A. Mediterranean: Myrtus communis L. (Myrtle). In Plants and Culture: Seeds of the Cultural Heritage of Europe; Morel, J.-P., Mercuri, A.M., Eds.; Edipuglia: Bari, Italy, 2009; pp. 159–168. ISBN1 8872285747. ISBN2 9788872285749. [Google Scholar]
- Oyedemi, S.O.; Okoh, A.I.; Mabinya, L.V.; Pirochenva, G.; Afolayan, A.J. The proposed mechanism of bactericidal action of eugenol, α-terpineol and g-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr. J. Biotechnol. 2009, 8, 1280–1286. [Google Scholar]
- Aleksic, V.; Mimica-Dukic, N.; Simin, N.; Nedeljkovic, N.S.; Knezevic, P. Synergistic effect of Myrtus communis L. essential oils and conventional antibiotics against multi-drug resistant Acinetobacter baumannii wound isolates. Phytomedicine 2014, 21, 1666–1674. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G. Empirical prediction and validation of antibacterial inhibitory effects of various plant essential oils on common pathogenic bacteria. Int. J. Food Microbiol. 2015, 202, 35–41. [Google Scholar] [CrossRef]
- Curiel, J.A.; Pinto, D.; Marzani, B.; Filannino, P.; Farris, G.A.; Gobbetti, M.; Rizzello, C.G. Lactic acid fermentation as a tool to enhance the antioxidant properties of Myrtus communis berries. Microb. Cell Fact. 2015, 14, 67. [Google Scholar] [CrossRef]
- Kim, K.Y.; Jang, H.H.; Lee, S.-N.; Kim, Y.-S.; An, S. Effects of the myrtle essential oil on the acne skin—clinical trials for Korean women. Biomed. Dermatol. 2018, 2, 28. [Google Scholar] [CrossRef]
- Hennia, A.; Nemmiche, S.; Dandlen, S.; Miguel, M.G. Myrtus communis essential oils: Insecticidal, antioxidant and antimicrobial activities: A review. J. Essent. Oil Res. 2019, 31, 487–545. [Google Scholar] [CrossRef]
- Ansari, K.; Goodarznia, I. Optimization of supercritical carbon dioxide extraction of essential oil from spearmint (Mentha spicata L.) leaves by using Taguchi methodology. J. Supercrit. Fluid 2012, 67, 123–130. [Google Scholar] [CrossRef]
- Tan, Q.L.P.; Kieu, X.N.T.; Kim, N.H.T.; Hong, X.N.T. Application of response surface methodology (RSM) in condition optimization for essential oil production from Citrus latifolia. Emir. J. Food Agric. 2012, 24, 25–30. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wei, S.; Yan, Z. Application of response surface methodology to optimise supercritical carbon dioxide extraction of essential oil from Cyperus rotundus Linn. Food Chem. 2012, 32, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Madhumita, M.; Guha, P.; Nag, A. Extraction of betel leaves (Piper betle L.) essential oil and its bio-active identification: Process optimization, GC-MS analysis and anti-microbial activity. Ind. Crops Prod. 2019, 138, 111578. [Google Scholar] [CrossRef]
- Belhachat, D.; Mekimene, L.; Belhachat, M.; Ferradji, A.; Aid, F. Application of response surface methodology to optimize the extraction of essential oil from ripe berries of Pistacia lentiscus using ultrasonic pretreatment. J. Appl. Res. Med. Aromat. Plants 2018, 9, 132–140. [Google Scholar] [CrossRef]
- Nejad-Sadeghi, M.; Taji, S.; Goodarznia, I. Optimization of supercritical carbon dioxide extraction of essential oil from Dracocephalum kotschyi Boiss: An endangered medicinal plant in Iran. J. Chromatogr. A 2015, 1422, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Thakker, M.R.; Parikh, J.K.; Desai, M.A. Microwave assisted extraction of essential oil from the leaves of Palmarosa: Multi-response optimization and predictive modeling. Ind. Crops Prod. 2016, 86, 311–319. [Google Scholar] [CrossRef]
- Sathish Kumar, R.; Sureshkumar, K.; Velraj, R. Optimization of biodiesel production from Manilkara zapota (L.) seed oil using Taguchi method. Fuel 2015, 140, 90–96. [Google Scholar] [CrossRef]
- Zermane, A.; Larkeche, O.; Meniai, A.-H.; Crampon, C.; Badens, E. Optimization of essential oil supercritical extraction from Algerian Myrtus communis L. leaves using response surface methodology. J. Supercrit. Fluid 2014, 85, 89–94. [Google Scholar] [CrossRef]
- Pereira, P.; Bernardo-Gil, M.G.; João Cebola, M.; Mauricio, E.; Romano, A. Supercritical fluid extracts with antioxidant and antimicrobial activities from myrtle (Myrtus communis L.) leaves. Response surface optimization. J. Supercrit. Fluid. 2013, 83, 57–64. [Google Scholar] [CrossRef]
- Haj Ammar, A.; Zagrouba, F.; Romdhane, M. Optimization of operating conditions of Tunisian myrtle (Myrtus communis L.) essential oil extraction by a hydrodistillation process using a 24 complete factorial design. Flavour Fragr. J. 2010, 25, 503–507. [Google Scholar] [CrossRef]
- Haj Ammar, A.; Zagrouba, F.; Romdhane, M.; Abderrabba, M. Extraction of myrtle (Myrtus communis L.) essential oil from Tunisia by hydrodistillation [Extraction de l’huile essentielle de myrte (Myrtus communis L.) provenant de la Tunisie par hydrodistillation]. Acta Hortic. 2010, 853, 241–250. [Google Scholar] [CrossRef]
- Ghasemi, E.; Raofie, F.; Najafi, N.M. Application of response surface methodology and central composite design for the optimisation of supercritical fluid extraction of essential oils from Myrtus communis L. leaves. Food Chem. 2011, 126, 1449–1453. [Google Scholar] [CrossRef]
- Ghica, M.V.; Albu, M.G.; Leca, M.; Popa, L.; Moisescu, S. Design and optimization of some collagen-minocycline based hydrogels potentially applicable for the treatment of cutaneous wounds infections. Pharmazie 2011, 66, 853–861. [Google Scholar] [PubMed]
- Ghica, M.V.; Popa, L.; Saramet, G.; Leca, M.; Lupuliasa, D.; Moisescu, S. Optimization of the pharmaceutical products and process design applying Taguchi quality engineering principles. Farmacia 2011, 59, 321–328. [Google Scholar]
- Ghica, M.V.; Albu, M.G.; Popa, L.; Moisescu, S. Response surface methodology and Taguchi approach to assess the combined effect of formulation factors on minocycline delivery from collagen sponges. Pharmazie 2013, 68, 340–348. [Google Scholar] [PubMed]
- Ghica, M.V.; Albu Kaya, M.G.; Dinu-Pîrvu, C.-E.; Lupuleasa, D.; Udeanu, D.I. Development, optimization and in vitro/in vivo characterization of collagen-dextran spongious wound dressings loaded with flufenamic acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef]
- Ghannadi, A.; Dezfuly, N. Essential oil analysis of the leaves of Persian true myrtle. Int. J. Med. Arom. Plants 2011, 1, 48–50. [Google Scholar]
- Walle, M.; Walle, B.; Zerihun, L.; Makonnen, E. Sedative-hypnotic like effect of the essential oil from the leaves of Myrtus communis on mice. Am. J. Biomed. Life Sci. 2014, 2, 70–77. [Google Scholar] [CrossRef][Green Version]
- Senatore, F.; Formisano, C.; Napolitano, F.; Rigano, D.; Özcan, M. Chemical composition and antibacterial activity of essential oil of Myrtus communis L. growing wild in Italy and Turkey. J. Essent. Oil Bear. Plants 2013, 9, 162–169. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Barra, A.; Angioni, A.; Sarritzu, E.; Pirisi, F.M. Chemical composition of volatiles in Sardinian myrtle (Myrtus communis L.) alcoholic extracts and essential oils. J. Agric. Food. Chem. 2006, 54, 1420–1426. [Google Scholar] [CrossRef]
- Boelens, M.H.; Jimènez, R. The chemical composition of Spanish myrtle oils. Part II. J. Essent. Oil Res. 1992, 4, 349–353. [Google Scholar] [CrossRef]
- Koukos, P.K.; Papadopoulou, K.I.; Papagiannopoulos, A.D.; Patiaka, D.T. Chemicals from Greek forestry biomass: Constituents of the leaf oil of Myrtus communis grown in Greece. J. Essent. Oil Res. 2001, 13, 245–246. [Google Scholar] [CrossRef]
Sample Availability: No samples of the compounds are available from the authors. |
| Trials No. | Input Variables Coded Level (Physical Level) | Response | |||
|---|---|---|---|---|---|
| X1 (%) | X2 (min) | X3 (mm) | Y (mL) | ||
| Obs. | Pre. | ||||
| 1 | 1 (50) | 1 (60) | 1 (10) | 280 | 309 |
| 2 | 3 (100) | 1 (60) | 1 (10) | 730 | 675 |
| 3 | 1 (50) | 3 (90) | 1 (10) | 295 | 248 |
| 4 | 3 (100) | 3 (90) | 1 (10) | 760 | 796 |
| 5 | 1 (50) | 1 (60) | 3 (30) | 275 | 308 |
| 6 | 3 (100) | 1 (60) | 3 (30) | 745 | 674 |
| 7 | 1 (50) | 3 (90) | 3 (30) | 270 | 247 |
| 8 | 3 (100) | 3 (90) | 3 (30) | 740 | 795 |
| 9 | 1 (50) | 2 (75) | 2 (20) | 285 | 336 |
| 10 | 3 (100) | 2 (75) | 2 (20) | 750 | 793 |
| 11 | 2 (75) | 1 (60) | 2 (20) | 545 | 538 |
| 12 | 2 (75) | 3 (90) | 2 (20) | 590 | 568 |
| 13 | 2 (75) | 2 (75) | 1 (10) | 560 | 519 |
| 14 | 2 (75) | 2 (75) | 3 (30) | 540 | 518 |
| 15 | 2 (75) | 2 (75) | 2 (20) | 555 | 565 |
| Responses | Sources of Variation | Sum of Squares | df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Y | Regression | 4704598 | 4 | 1176150 2232 | 526.94 | <0.0001 |
| Residual | 24552 | 11 | ||||
| Total | 4729150 | 15 |
| Run Order | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S/N (dB) | 48.94 | 57.26 | 49.36 | 57.61 | 48.78 | 57.44 | 48.62 | 57.38 | 49.0 | 57.50 | 54.72 | 55.41 | 54.96 | 54.64 | 54.88 |
| Control Factors (Independent Variables) | Y | |
|---|---|---|
| “Larger—the—Better” | Effect Size | |
| X1 | 3 | 3.662 |
| X2 | 2 | 0.438 |
| X3 | 2 | 0.545 |
| S/N expected (dB) | 58.42 | |
| S/N observed (dB) | 57.50 | |
| RT | Compound Name | SI | RSI | CAS Number | % |
|---|---|---|---|---|---|
| 12.99 | α-Phellandrene | 931 | 970 | 1529-99-3 | 0.24 |
| 13.38 | α-Pinene | 985 | 987 | 80-56-8 | 33.14 |
| 14.19 | cis-Ocimene | 681 | 786 | 6874-10-8 | 0.06 |
| 15.49 | Δ-3-Carene | 976 | 976 | 13466-78-9 | 0.43 |
| 15.96 | β-Pinene | 674 | 810 | 127-91-3 | 0.09 |
| 16.82 | γ-Terpinene | 958 | 978 | 99-85-4 | 0.29 |
| 18.08 | Eucalyptol | 984 | 985 | 470-82-6 | 55.09 |
| 20.73 | α-Terpineolene | 875 | 923 | 586-62-9 | 0.17 |
| 21.50 | Linalool | 978 | 980 | 78-70-6 | 1.79 |
| 24.44 | trans-Pinocarveol | 897 | 956 | 547-61-5 | 0.13 |
| 26.43 | α-Terpineol | 782 | 936 | 10482-56-1 | 0.06 |
| 27.00 | Terpinen-4-ol | 907 | 966 | 562-74-3 | 0.15 |
| 28.00 | β-Fenchyl alcohol | 938 | 955 | 470-08-6 | 3.20 |
| 33.73 | trans-Pinocarvyl acetate | 781 | 931 | 1686-15-3 | 0.12 |
| 35.27 | 3(10)-Caren-4-ol, acetoacetic acid ester | 862 | 862 | NA | 1.08 |
| 36.50 | α-Terpinenyl acetate | 958 | 988 | 80-26-2 | 1.93 |
| 38.47 | β-Elemene | 759 | 891 | 515-13-9 | 0.04 |
| 39.15 | Linalyl acetate | 747 | 876 | 115-95-7 | 0.14 |
| 40.14 | trans-Caryophyllene | 955 | 970 | 87-44-5 | 0.19 |
| 41.96 | α-Humulene | 872 | 940 | 6753-98-6 | 0.08 |
| 48.07 | Caryophyllene oxide | 738 | 893 | 1139-30-6 | 0.06 |
| Input Variables | Coded Symbol | Coded and Uncoded Variation Levels | ||
| Low (1) | Middle (2) | High (3) | ||
| Boiler occupancy rate, (%) | X1 | 50 | 75 | 100 |
| Duration of distillation, (min) | X2 | 60 | 75 | 90 |
| Particle size, (mm) | X3 | 10 | 20 | 30 |
| Response | Coded Symbol | Constraint | ||
| Essential oil volume (mL) | Y | Maximize | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, D.A.; Ghica, M.V.; Dănilă, E.; Öztürk, Ş.; Türkmen, M.; Albu Kaya, M.G.; Dinu-Pîrvu, C.-E. Selection of Optimal Operating Conditions for Extraction of Myrtus Communis L. Essential Oil by the Steam Distillation Method. Molecules 2020, 25, 2399. https://doi.org/10.3390/molecules25102399
Kaya DA, Ghica MV, Dănilă E, Öztürk Ş, Türkmen M, Albu Kaya MG, Dinu-Pîrvu C-E. Selection of Optimal Operating Conditions for Extraction of Myrtus Communis L. Essential Oil by the Steam Distillation Method. Molecules. 2020; 25(10):2399. https://doi.org/10.3390/molecules25102399
Chicago/Turabian StyleKaya, Durmuş Alpaslan, Mihaela Violeta Ghica, Elena Dănilă, Şevket Öztürk, Musa Türkmen, Mădălina Georgiana Albu Kaya, and Cristina-Elena Dinu-Pîrvu. 2020. "Selection of Optimal Operating Conditions for Extraction of Myrtus Communis L. Essential Oil by the Steam Distillation Method" Molecules 25, no. 10: 2399. https://doi.org/10.3390/molecules25102399
APA StyleKaya, D. A., Ghica, M. V., Dănilă, E., Öztürk, Ş., Türkmen, M., Albu Kaya, M. G., & Dinu-Pîrvu, C.-E. (2020). Selection of Optimal Operating Conditions for Extraction of Myrtus Communis L. Essential Oil by the Steam Distillation Method. Molecules, 25(10), 2399. https://doi.org/10.3390/molecules25102399








