Fresh-Cut Bell Peppers in Modified Atmosphere Packaging: Improving Shelf Life to Answer Food Security Concerns
Abstract
:1. Introduction
2. Results
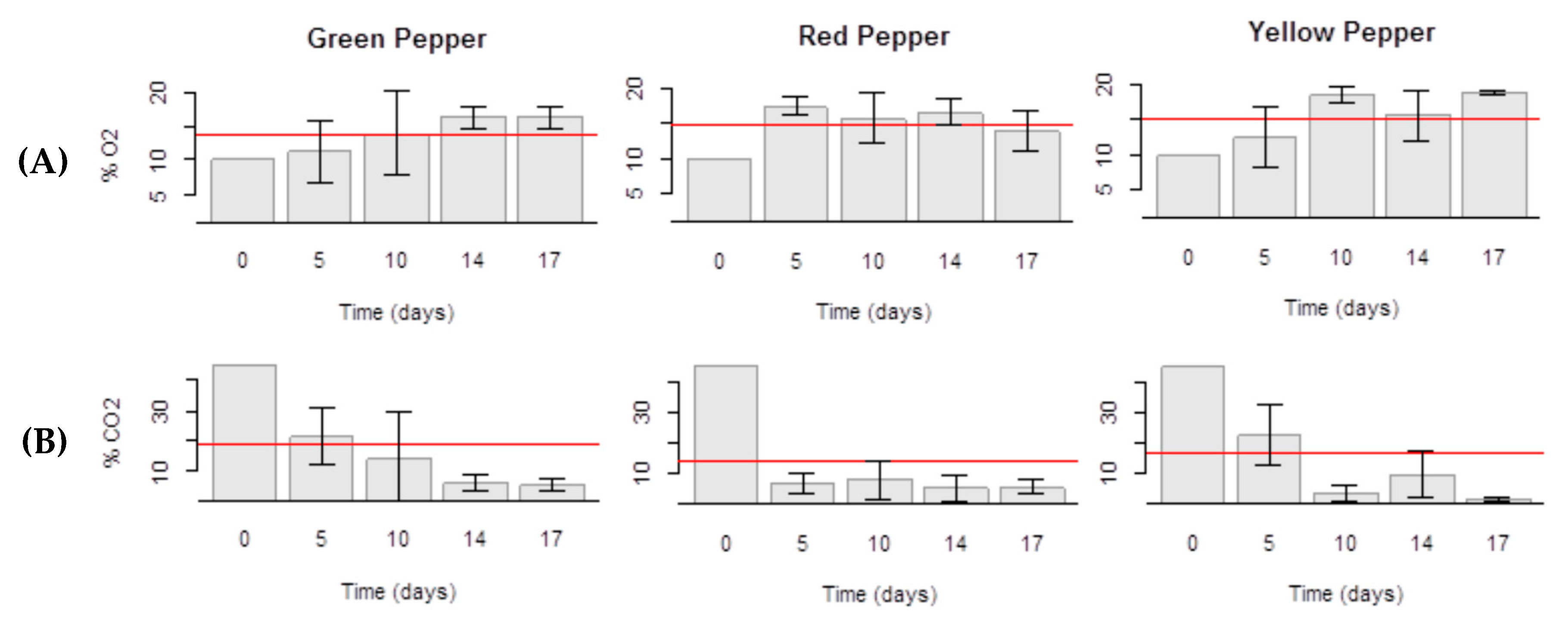
2.1. Packaging and Gas Composition
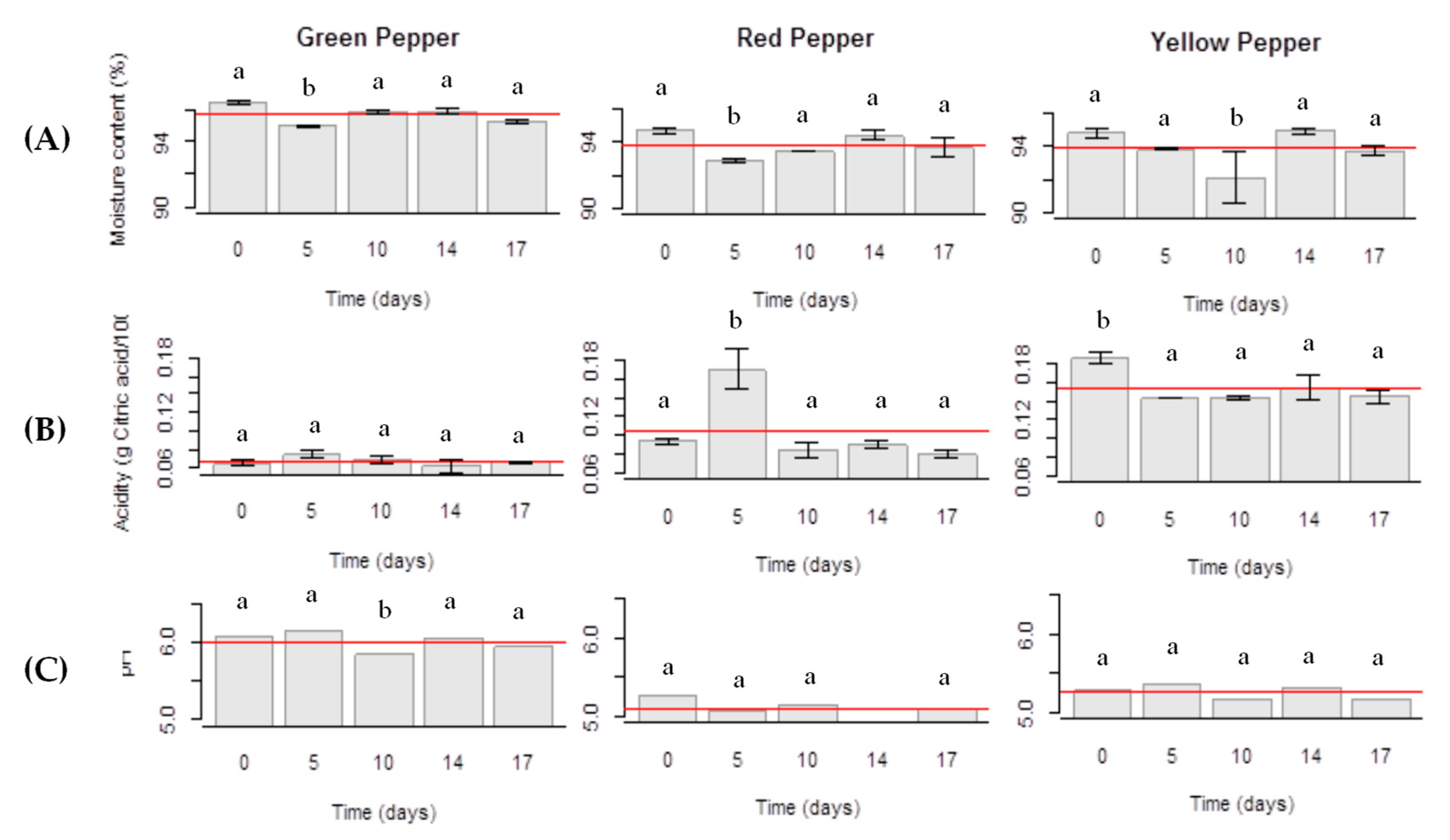
2.2. Moisture and Ash
2.3. Titratable Acidity and pH
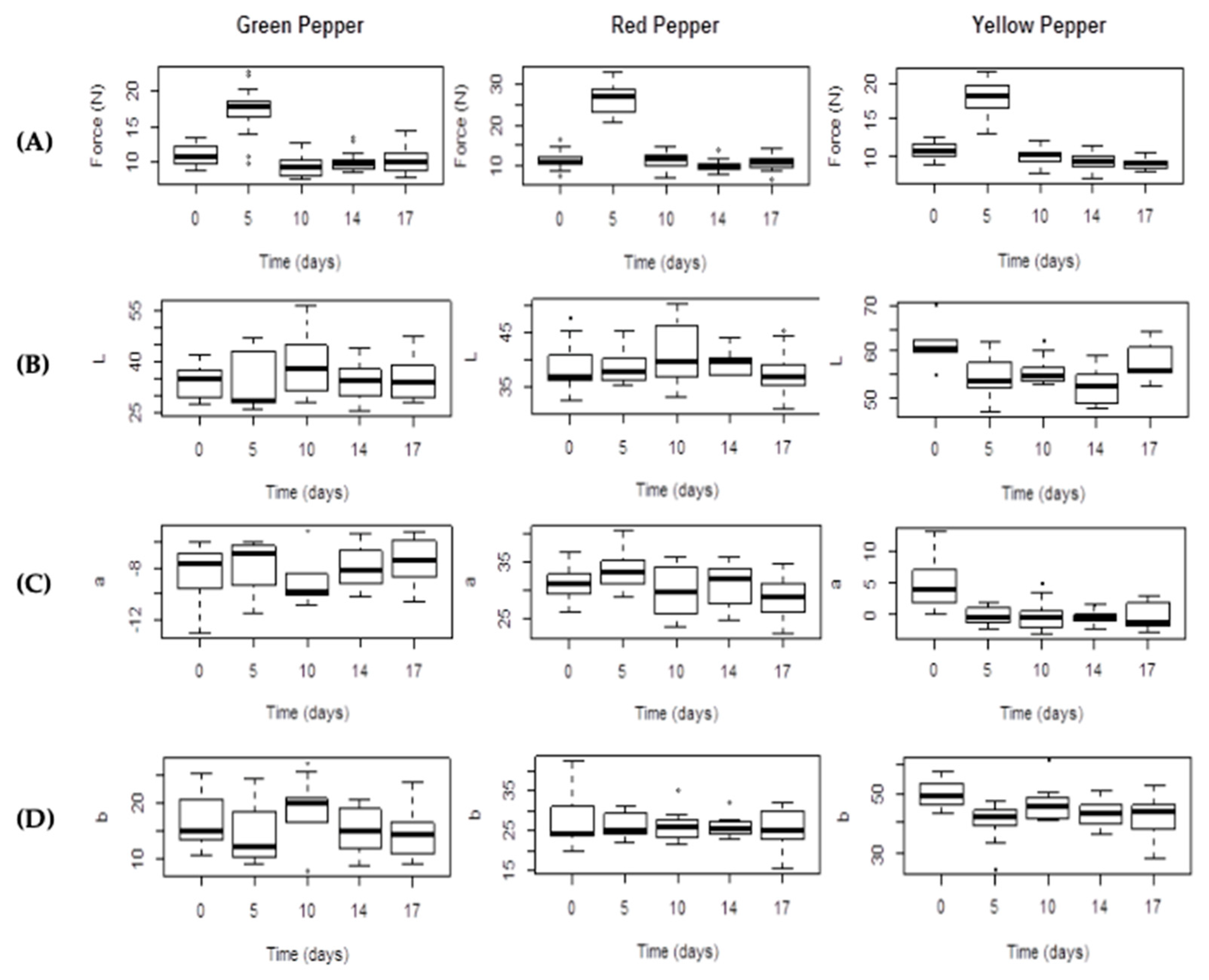
2.4. Texture
2.5. Color Evaluation
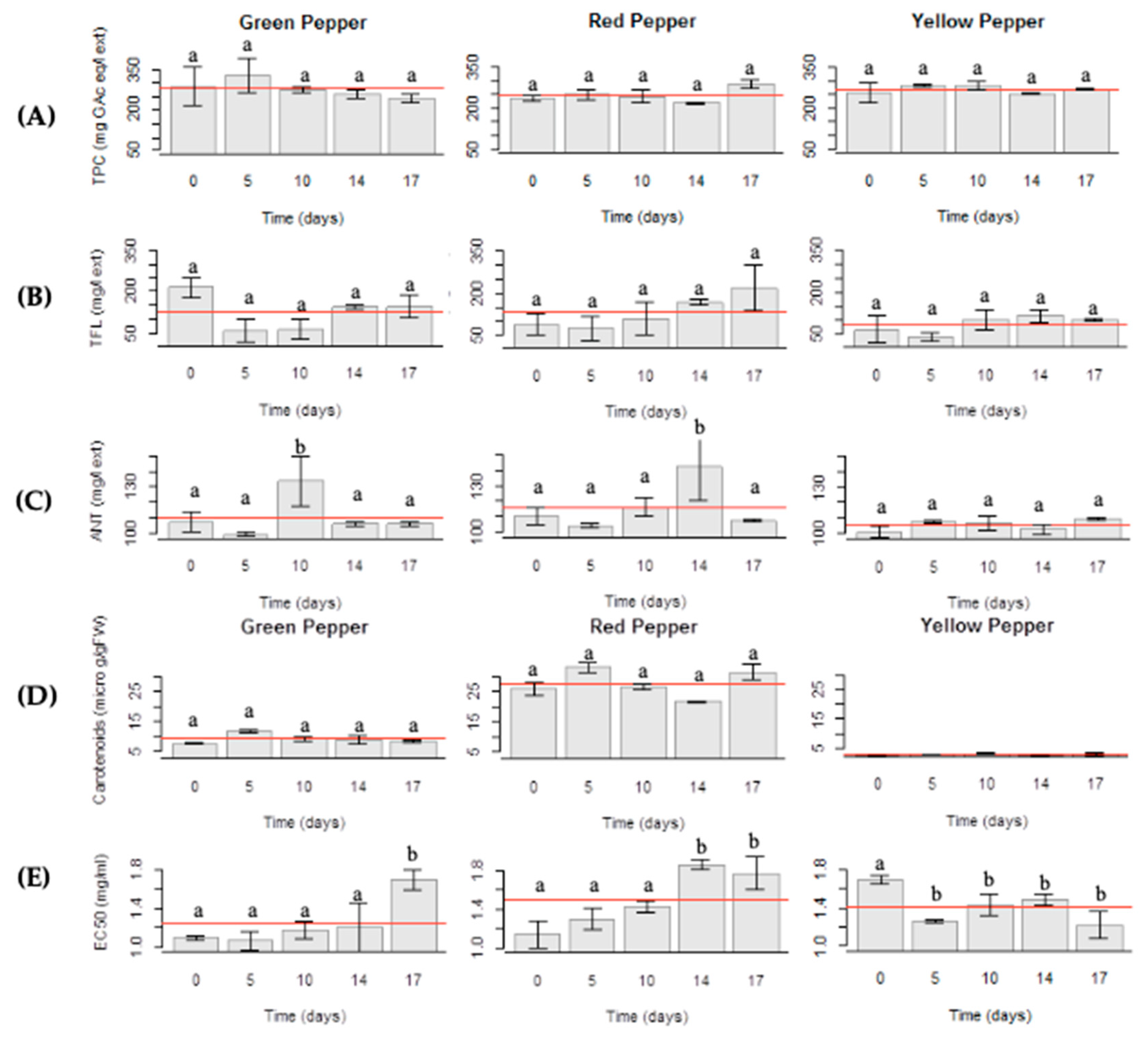
2.6. Total Phenol Content
2.7. Total Flavonoid Content
2.8. Anthocyanin Content
2.9. Carotenoid Content
2.10. Antioxidant Activity and Bioactive Compounds Evaluation
2.11. Microbial Growth Control
2.12. Interplay of Parameters
2.13. Sensory Evaluation
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Minimally Processed Vegetables
4.3. Moisture and Ash Contents
4.4. Titratable Acidity
4.5. pH Analysis
4.6. L*, a*, b*, C* and h* Color
4.7. Texture Analysis
4.8. Extraction of Bioactive Compounds
4.9. Total Phenolic Content (TPC)
4.10. Total Anthocyanin (ANT)
4.11. Total Flavonoid Content (TFL)
4.12. Total Carotenoid (TC)
4.13. Antioxidant Activity-DPPH• Assay
4.14. Total Microorganism Counting
4.15. Sensory Evaluation
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Williams, H.; Wikström, F.; Otterbring, T.; Löfgren, M.; Gustafsson, A. Reasons for household food waste with special attention to packaging. J. Clean Prod. 2012, 24, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Segneanu, A.E.; Grozescu, I.; Cepan, C.; Cziple, F.; Lazar, V.; Velciov, S. Food Security into a Circular Economy. J. Food Sci. Nutr. 2018, 4, 1–3. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Development of a new fresh-like product from ‘Lamuyo’ red bell peppers using hurdle technology. LWT-Food Sci. Technol. 2013, 50, 357–360. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Ayala-Zavala, J.F.; Ruiz-Cruz, S.; Acedo-Félix, E.; Díaz-Cinco, M.E. Effect of temperature and modified atmosphere packaging on overall quality of fresh-cut bell peppers. LTW Food Sci. Technol. 2004, 37, 817–826. [Google Scholar] [CrossRef]
- Jiménez-Monreal, A.M.; García-Diz, L.; Martínez-Tomé, M.; Mariscal, M.; Murcia, M.A. Influence of Cooking Methods on Antioxidant Activity of Vegetables. J. Food Sci. 2009, 74, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulou, H.; Xanthopoulos, G.; Douros, N.; Lambrinos, G. Modified atmosphere packaging storage of green bell peppers: Quality criteria. Biosyst Eng. 2010, 106, 535–543. [Google Scholar] [CrossRef]
- Murcia, M.A.; Jiménez-Monreal, A.M.; García-Diz, L.; Carmona, M.; Maggi, L.; Martínez-Tomé, M. Antioxidant activity of minimally processed (in modified atmospheres), dehydrated and ready-to-eat vegetables. Food Chem. Toxicol. 2009, 47, 2103–2110. [Google Scholar] [CrossRef]
- Roy, M.K.; Takenaka, M.; Isobe, S.; Tsushida, T. Antioxidant potential, anti-proliferative activities, and phenolic content in water-soluble fractions of some commonly consumed vegetables: Effects of thermal treatment. Food Chem. 2007, 103, 106–114. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolic compounds, ascorbic acid, carotenoids and antioxidant properties of green, red and yellow bell peppers. J. Food Agric. Environ. 2003, 1, 22–27. [Google Scholar]
- Singh, R.; Giri, S.K.; Kotwaliwale, N. Shelf-life enhancement of green bell pepper (Capsicum annuum L.) under active modified atmosphere storage. Food Packag Shelf Life. 2014, 1, 101–112. [Google Scholar] [CrossRef]
- Fernández-León, M.F.; Fernández-León, A.M.; Lozano, M.; Ayuso, M.C.; Amodio, M.L.; Colelli, G.; González-Gómez, D. Retention of quality and functional values of broccoli ‘Parthenon’ stored in modified atmosphere packaging. Food Control. 2013, 31, 302–313. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Chisari, M.; Patané, C. Polyphenol oxidase, total phenolics and ascorbic acid changes during storage of minimally processed ‘California Wonder’ and ‘Quadrato d’Asti’ sweet peppers. LWT Food Sci Technol. 2012, 49, 192–196. [Google Scholar] [CrossRef]
- Conesa, A.; Artés-Hernández, F.; Geysen, S.; Nicolaï, B.; Artés, F. High oxygen combined with high carbon dioxide improvesmicrobial and sensory quality of fresh-cut peppers. Postharvest Biol. Technol. 2007, 43, 230–237. [Google Scholar] [CrossRef]
- Manolopoulou, H.; Lambrinos, G.; Xanthopoulos, G. Active modified atmosphere packaging of fresh-cut bell peppers: Effect on quality indices. J. Food Res. 2012, 1, 148–158. [Google Scholar] [CrossRef]
- Gavara, R.; Catalá, R.; Hernández-Muñoz, P. Extending the shelf-life of fresh-cut produce through active packaging. Stewart Postharvest Rev. 2009, 5, 1–6. [Google Scholar]
- Saltveit, M.E. A Summary of ca requirements and recommendations for vegetables. Acta Hortic. 2001, 600, 723–727. [Google Scholar] [CrossRef]
- Saltveit, M.E. Is it possible to find an optimal controlled atmosphere? Postharvest Biol. Technol. 2003, 27, 3–13. [Google Scholar] [CrossRef]
- Manolopoulou, E.; Varzakas, T. Effect of Storage Conditions on the Sensory Quality, Colour and Texture of Fresh-Cut Minimally Processed Cabbage with the Addition of Ascorbic Acid, Citric Acid and Calcium Chloride. Food Nutr Sci. 2011, 2, 956–963. [Google Scholar] [CrossRef] [Green Version]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- HPA. Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods Placed on the Market; Health Protection Agency: London, UK, 2009; Volume 7, pp. 1–33.
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Castro, S.M.; Saraiva, J.A.; Lopes-da-Silva, J.A.; Delgadillo, I.; Loey, A.V.; Smout, C.; Hendrickx, M. Effect of thermal blanching and of high-pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chem. 2008, 107, 1436–1449. [Google Scholar] [CrossRef]
- Rao, T.V.R.; Gol, N.B.; Shah, K.K. Effect of postharvest treatments and storage temperatures on the quality and shelf life of sweet pepper (Capsicum annum L.). Sci Hortic. 2011, 132, 18–26. [Google Scholar] [CrossRef]
- Renu, R.; Chidanand, D.V. Effect of modified atmosphere storage conditions on biochemical parameters of bell peppers. Int J. Agric. Food Sci Technol. 2013, 4, 915–922. [Google Scholar]
- Sahoo, N.R.; Bal, L.M.; Pal, U.S.; Sahoo, D. A comparative study on the effect of packaging material and storage environment on shelf life of fresh bell-pepper. J. Food Meas Charact. 2014, 8, 164–170. [Google Scholar] [CrossRef]
- Sharma, K.D.; Cardona, J.A.; Sibomana, M.S.; Herrera, N.G.S.; Nampeera, E.; Fallik, E. Quality attributes of modified atmosphere apckages bell pepper (Capsicum annuum L.) during storage. J. Nutr Food Res. Technol. 2018, 1, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Bayogan, E.R.; Salvilla, R.; Majomot, A.M.C.; Acosta, J. Shelf life of two sweet pepper (Capsicum annuum) cultivars stored at ambient and evaporative cooling conditions. South. Western J. Hortic Biol Environ. 2017, 8, 1–15. [Google Scholar]
- Meng, X.; Zhang, M.; Adhikari, B. Extending shef-life of fresh-cut green peppers using pressurized argon treatment. Postharvest Biol. Technol. 2012, 71, 13–20. [Google Scholar] [CrossRef]
- Tano, K.; Nevry, R.K.; Koussémon, M.; Oulé, M.K. The effects of different storage temperatures on the quality of fresh bell peppers (Capsicum annuum L.). Agric. J. 2008, 3, 157–162. [Google Scholar]
- Pilon, L.; Oetterer, M.; Gallo, C.R.; Spoto, M.H.F. Shelf life of minimally processed carrot and green pepper. Food Sci Technol. 2006, 26, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Castro, S.M.; Van Loey, A.; Saraiva, J.A.; Smout, C.; Hendrickx, M. Process stability of Capsicum annuum pectin methylesterase in model systems, pepper puree and intact pepper tissue. Eur Food Res. Technol. 2005, 221, 452–458. [Google Scholar] [CrossRef]
- Estrada, B.; Bernal, M.A.; Diaz, J.; Pomar, F.; Merino, F. Fruit Development in Capsicum annuum: Changes in Capsaicin, Lignin, Free Phenolics, and Peroxidase Patterns. J. Agric. Food Chem. 2000, 48, 6234–6239. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, P.M.A.; Stan, S. The effect of washing on physicochemical changes in packaged, sliced green peppers. Int. J. Food Sci. Technol. 2004, 39, 43–51. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, M.; Adhikari, B. Prediction of storage quality of fresh-cut green peppers using artificial neural network. Int. J. Food Sci. Tecnol. 2012, 47, 1586–1592. [Google Scholar] [CrossRef]
- Yamane, H.; Konno, K.; Sabelis, M.; Takabayashi, J.; Sassa, T.; Oikawa, H. Chemical Defense and Toxins of Plants. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2010; pp. 339–385. [Google Scholar]
- Camelo, A.F.L.; Gómez, P.A. Comparison of color indexes for tomato ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Piga, A.; Del Caro, A.; Pinna, I.; Agabbio, M. Changes in ascorbic acid, polyphenol content and antioxidant activity in minimally processed cactus pear fruits. LWT Food Sci. Technol. 2003, 36, 257–262. [Google Scholar] [CrossRef]
- Barbosa, C.D.A.C.B. Legumes minimamente processados e pré-cozidos mantidos em atmosfera modificada. Avaliação Sensorial, Físico-Química/Compostos Bioativos e Microbiológica. Evolução da Qualidade ao Longo do Armazenamento com Apoio da Quimiometria. Ph.D. Thesis, University of Porto, Porto, Portugal, October 2014. [Google Scholar]
- AOAC–Official Method of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersbur, ML, USA, 2012.
- Saxena, A.; Bawa, A.S.; Raju, P.S. Use of modified atmosphere packaging to extent shef-life of minimally processed jackfruit (Artocarpus heterophyllus L.) bulbs. J. Food. Eng. 2008, 87, 455–466. [Google Scholar] [CrossRef]
- Porter, Y. Antioxidant properties of green broccoli and purple-sprouting broccoli under different cooking conditions. Biosci. Horiz. 2012, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Hamauzu, Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. Available online: https://www.ajevonline.org/content/16/3/144.article-info (accessed on 18 November 2019).
- Li, H.; Deng, Z.; Zhu, H.; Hu, C.; Liu, R.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops. Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Yuan, G.F.; Sun, B.; Yuan, J.; Wang, Q.M. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B. 2009, 10, 580–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- ISO 6887-1:1999 Microbiology of Food and Animal Feeding Stuffs—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination, Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions; International Standardization Organization ed.: Geneve, Switzerland, 1999; pp. 1–5.
- ISO 4833:2003 Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Microorganisms—Colony-Count Technique at 30 Degrees C; International Standardization Organization ed.: Geneve, Switzerland, 2003; pp. 1–9. [Google Scholar]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices, 3rd ed.; Elsevier Academic Publisher: San Diego, CA, USA, 2004; p. 408. [Google Scholar]
- Alves, M.R.; Oliveira, M.B. Monitorization of consumer and naïf panels in the sensory evaluation of two types of potato chips by predictive biplots applied to generalized procrustes and three-way Tucker-1 analysis. J. Chemom. 2005, 19, 564–574. [Google Scholar] [CrossRef]
- Alves, M.R. Evaluation of the predictive power of biplot axes to automate the construction and layout of biplots based on the accuracy of direct readings from common outputs of multivariate analyses: 1. application to principal component analysis. J. Chemom. 2012, 26, 180–190. [Google Scholar] [CrossRef]
- Barbosa, C.; Oliveira, M.B.P.P.; Alves, M.R. Chemometrics in Food Authentication; Oliveira, M.B.P.P., Mafra, I., Amaral, J., Eds.; Current Topics in Food Authentication Transworld Research Network, Publisher: Kerala, India, 2011; pp. 237–268. [Google Scholar]
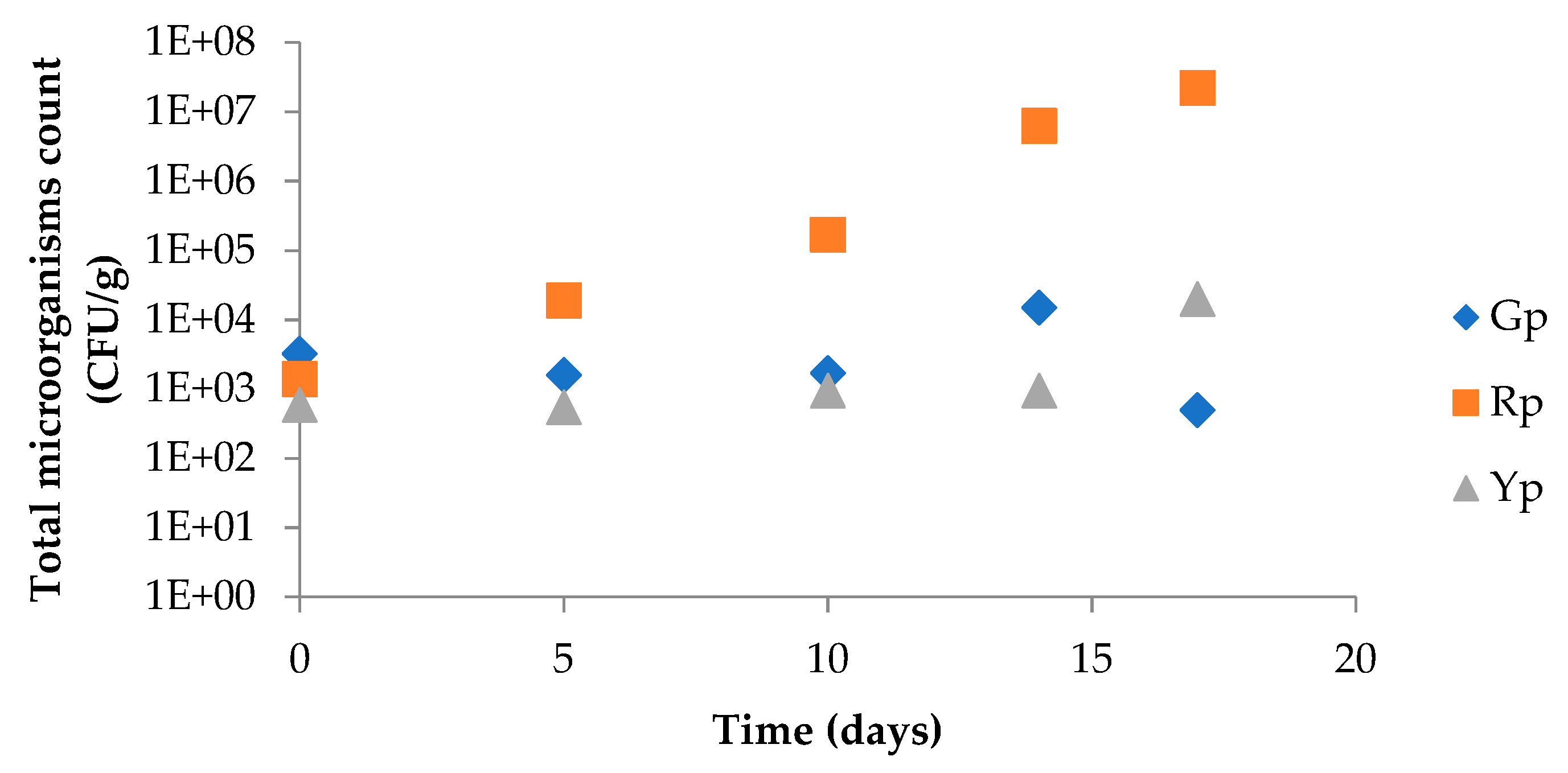

| Bell Pepper | Days | L* | a* | b* | Cb | hc |
|---|---|---|---|---|---|---|
| Gp | 0 | 33.70 ± 0.03 | −8.58 ± 0.12 | 16.9 3± 0.02 | 18.98 ± 0.12 | 116.87 ± 0.10 |
| 5 | 33.60 ± 0.02 | −7.78 ± 0.08 | 14.40 ± 0.01 | 16.37 ± 0.18 | 118.38 ± 0.07 | |
| 10 | 39.38 ± 0.03 | −8.94 ± 0.04 | 18.65 ± 0.04 | 20.68 ± 0.03 | 115.59 ± 0.06 | |
| 14 | 33.83 ± 0.05 | −7.81 ± 0.12 | 15.26 ± 0.08 | 17.15 ± 0.05 | 117.09 ± 0.02 | |
| 17 | 34.58 ± 0.04 | −7.49 ± 0.05 | 14.95 ± 0.11 | 16.72 ± 0.02 | 116.63 ± 0.08 | |
| Rp | 0 | 38.79 ± 0.02 | 31.37 ± 0.03 | 27.81 ± 0.03 | 41.92 ± 0.01 | 41.56 ± 0.01 |
| 5 | 38.99 ± 0.08 | 33.73 ± 0.08 | 26.35 ± 0.06 | 42.80 ± 0.04 | 37.99 ± 0.02 | |
| 10 | 41.32 ± 0.07 | 30.02 ± 0.16 | 26.40 ± 0.04 | 39.98 ± 0.06 | 41.32 ± 0.06 | |
| 14 | 39.70 ± 0.08 | 30.89 ± 0.09 | 26.15 ± 0.13 | 40.48 ± 0.12 | 40.25 ± 0.04 | |
| 17 | 37.61 ± 0.04 | 28.79 ± 0.08 | 25.36 ± 0.11 | 38.37 ± 0.07 | 41.37 ± 0.03 | |
| Yp | 0 | 61.39 ± 0.08 | 4.98 ± 0.09 | 49.98 ± 0.06 | 50.22 ± 0.15 | 84.31 ± 0.02 |
| 5 | 54.51 ± 0.06 | −0.09 ± 0.17 | 40.64 ± 0.07 | 40.64 ± 0.08 | 90.12 ± 0.06 | |
| 10 | 55.79 ± 0.03 | −0.09 ± 0.12 | 46.47 ± 0.08 | 46.47 ± 0.04 | 90.10 ± 0.01 | |
| 14 | 52.47 ± 0.05 | −0.21 ± 0.09 | 43.46 ± 0.04 | 43.46 ± 0.03 | 90.27 ± 0.04 | |
| 17 | 57.24 ± 0.07 | −0.46 ± 0.04 | 41.82 ± 0.02 | 41.82 ± 0.02 | 90.62 ± 0.07 |
| Capsicum annuum L. | Experiment Details: | Shelf-Life a | Ref |
|---|---|---|---|
| Fresh-cut green bell peppers cv Twingo F1; Southern Greece | MAP; 5 and 10 °C, 90% RH Initial conditions-P: 22% O2; 2–5% CO2 Packaging: LDPE-60, MDPE-30 and PVC | 14 days at 5 °C LDPE-60 film | [6] |
| Fresh-cut green bell peppers cv Swarma harvested from the Central Institute of Agricultural Engineering, Bhopal, India | Active Packages: P1 (4.5% O2; 7.8% CO2); P2 (4.7% O2; 7.5% CO2) 8 ± 1 °C, 95 ± 3% RH Packaging system designed by the authors using permeable polymeric films (38 μm thick polypropylene (PP) with ten perforations of 0.3 mm made using a needle). | 49 days at 8 °C, 95% RH P1 and P2 | [10] |
| Fresh-cut green bell peppers cv Twingo F1; Southern Greece | MAP; 0 and 5 °C, 90% RH P1: 5% O2; 10% CO2; P2: 5% O2; 15% CO2 Packaging: 20 μm thick HDPE (0.96g cm−3) packages | 10 days 0 °C P1 and P2 | [14] |
| Mature green bell peppers, obtained at R. Ranga Swamy farm (green house), Thanjavur, India | Initial conditions: 20% O2; 0.1% CO2 33 °C, 65% RH; 20 °C, 75% RH; 8°C, 95% RH Packaging and storage conditions: Corrugated fiber board boxes; Diffusion channel system with different lengths (10, 1.5 and 25 cm) | 10 days (33 °C/65% RH) 28 days (8 °C/95% RH) 25cm diffusion channel length | [24] |
| Mature green bell peppers, Orissa University of Agriculture and Technology, Bhubaneswar, Odisha, India | P: 9.0–9.8% O2; 3.3–4.1% CO2 23–35 °C, 35–75% RH and 4–6 °C, 45% RH Packaging: MAP with LDPE (Low-density Polyethylene), MAP with PP, MAP in perforated LDPE films, MAP in perforated PP films-five holes of 0.3 mm diameter in each side of the film, shrink packaging with Biaxially Oriented PP (BOPP) film and vacuum packaging with PP film. | 20 days 4–6 °C, 45% RH MAP with PP film and vacuum packaging with PP film | [25] |
| Fresh-cut red bell peppers var Kannon harvested from the Arava Desert of Israel | Initial conditions—P: 21% O2; 0.3% CO2 1.5 °C; 7 °C and 17 °C, 94% RH Packaging: Xtend® (XF) and Polyethylene (PE) films | 14 days at 7 °C, 94% RH XF packaging | [26] |
| Mature green bell peppers; Cultivars–Smooth Cayenne1; Sultan2 Bangkerohan, Davao City, Philippines | Ambient Conditions: (28.74 ± 0.94 °C, 65.68 ± 7.43% RH; 13.62 KPa VPD) *; Evaporative cooling (EC): (23.91 ± 3.85 °C, 93.84 ± 9.33% RH; 1.79 KPa VPD) ** The EC was covered with two layers of jute sack (burlap) as walls of the EC cabinet. The sack wall was constantly bathed with water from a container placed on top of the EC cabinet to keep it moistened. | 1* 9 days 1** 18 days 2* 7 days 2** 15 days | [27] |
| Fresh-cut green bell peppers; Jiangsu, China | Pressurized argon treatments (2–6 MPa/1 h) P: 5% O2; 8% CO2; 4 °C, 90% RH Packaging: Polystyrene packages; Pressurized Argon Treatments (PAT) | 12 days (PAT of 4 MPa/1h); 4 °C | [28] |
| Red bell peppers in ripening stage, from green to red color, obtained at local distributor of Abidjan, Côte d’Ivoire | 6, 16, 21 and 30 ± 2 °C; 20 days Packaging: Polyethylene perforated sealed bags (30 μm thick with 20 perforations of 5 mm in diameter. | 12 days at 6 °C | [29] |
| Fresh-cut green bell peppers obtained at local distributor of São Paulo, Brazil (22°42′ S; 47°38′ W; 546 m) | P: 2% O2; 10% CO2; 88% N2; 1 ± 1 °C; 21 days Packaging: BOPP/LDPE plastic bags. | 14 days at 1 ± 1 °C | [30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, C.; Machado, T.B.; Alves, M.R.; Oliveira, M.B.P.P. Fresh-Cut Bell Peppers in Modified Atmosphere Packaging: Improving Shelf Life to Answer Food Security Concerns. Molecules 2020, 25, 2323. https://doi.org/10.3390/molecules25102323
Barbosa C, Machado TB, Alves MR, Oliveira MBPP. Fresh-Cut Bell Peppers in Modified Atmosphere Packaging: Improving Shelf Life to Answer Food Security Concerns. Molecules. 2020; 25(10):2323. https://doi.org/10.3390/molecules25102323
Chicago/Turabian StyleBarbosa, Carla, Thelma B. Machado, Manuel Rui Alves, and Maria Beatriz P. P. Oliveira. 2020. "Fresh-Cut Bell Peppers in Modified Atmosphere Packaging: Improving Shelf Life to Answer Food Security Concerns" Molecules 25, no. 10: 2323. https://doi.org/10.3390/molecules25102323
APA StyleBarbosa, C., Machado, T. B., Alves, M. R., & Oliveira, M. B. P. P. (2020). Fresh-Cut Bell Peppers in Modified Atmosphere Packaging: Improving Shelf Life to Answer Food Security Concerns. Molecules, 25(10), 2323. https://doi.org/10.3390/molecules25102323