Cinnamic Acid Conjugates in the Rescuing and Repurposing of Classical Antimalarial Drugs
Abstract
1. Introduction
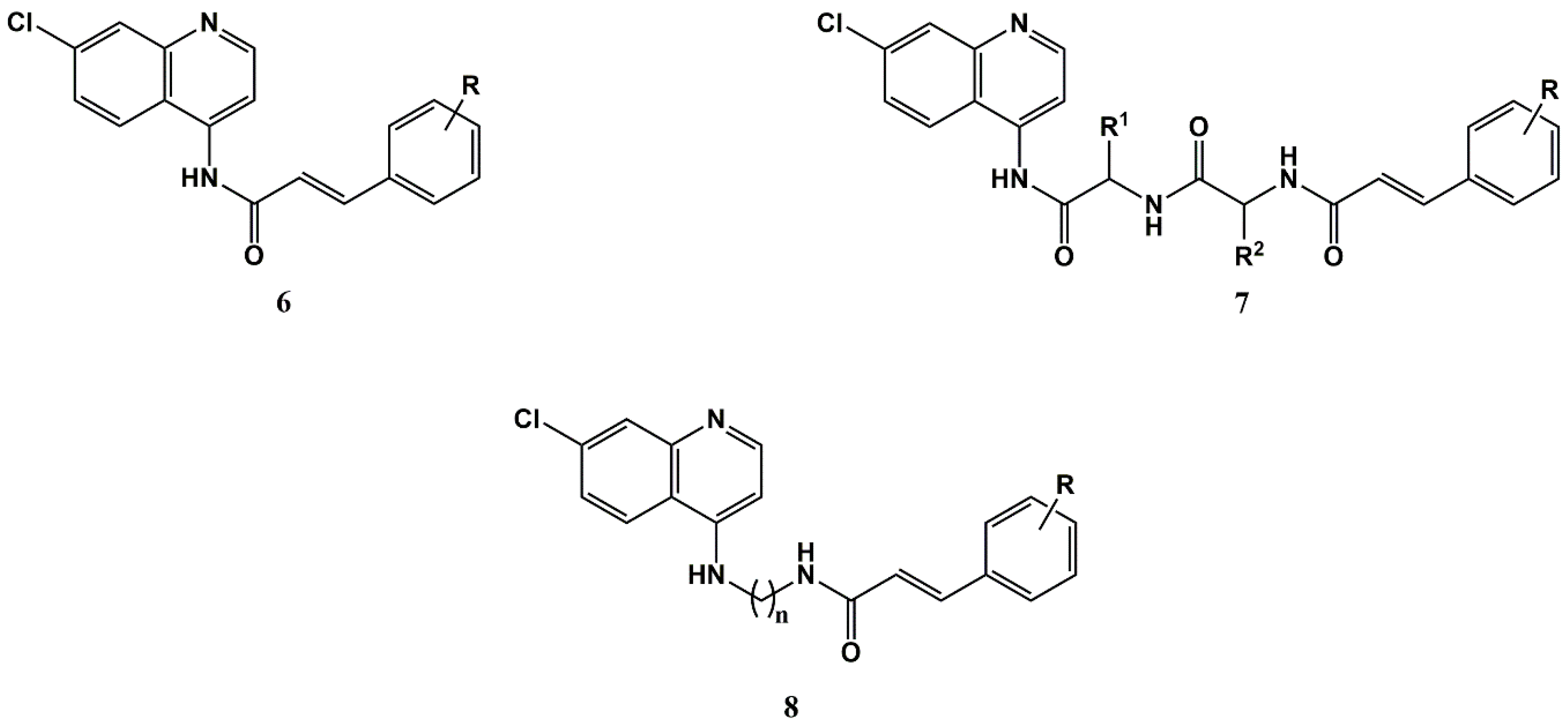
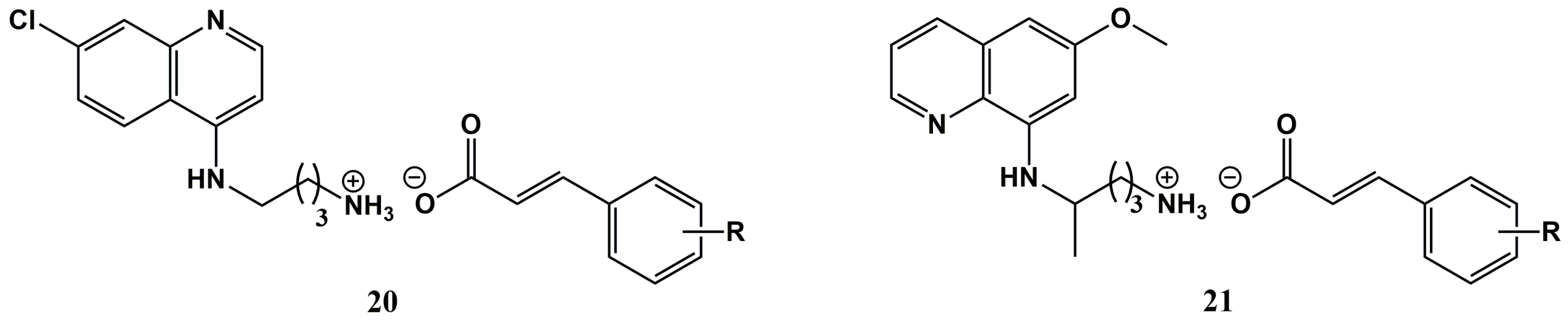
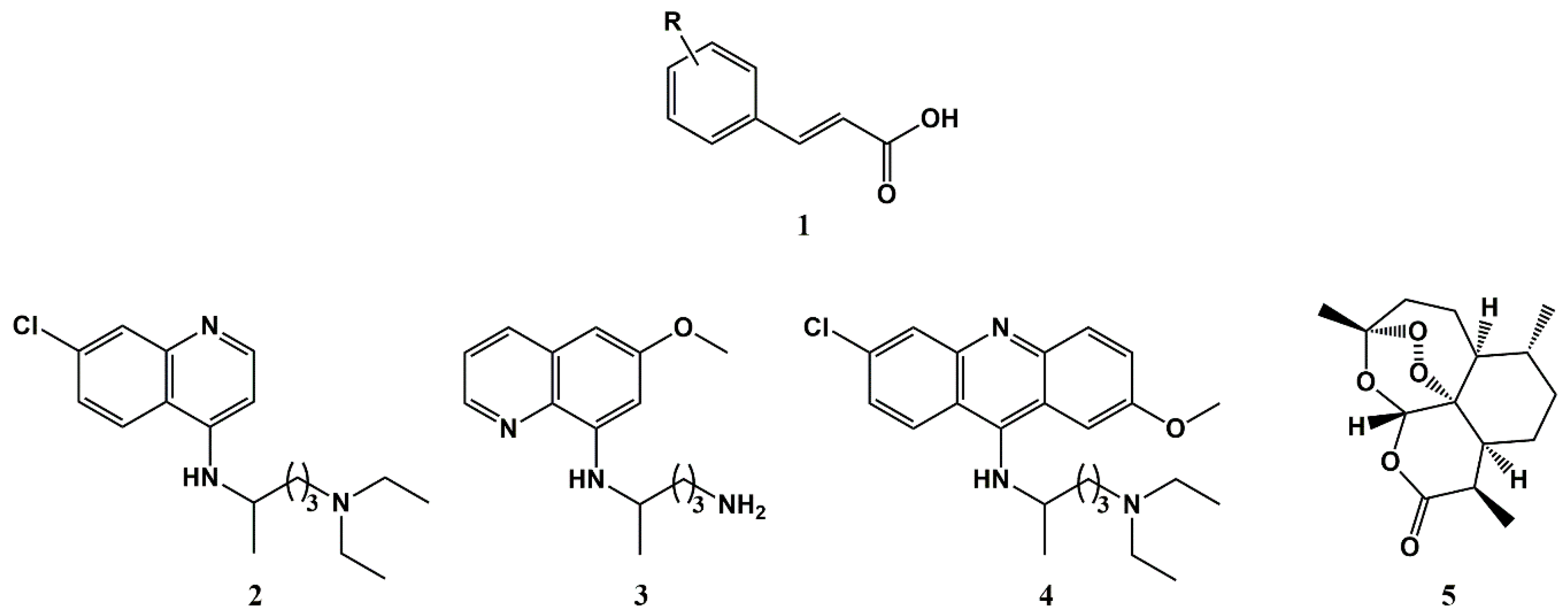
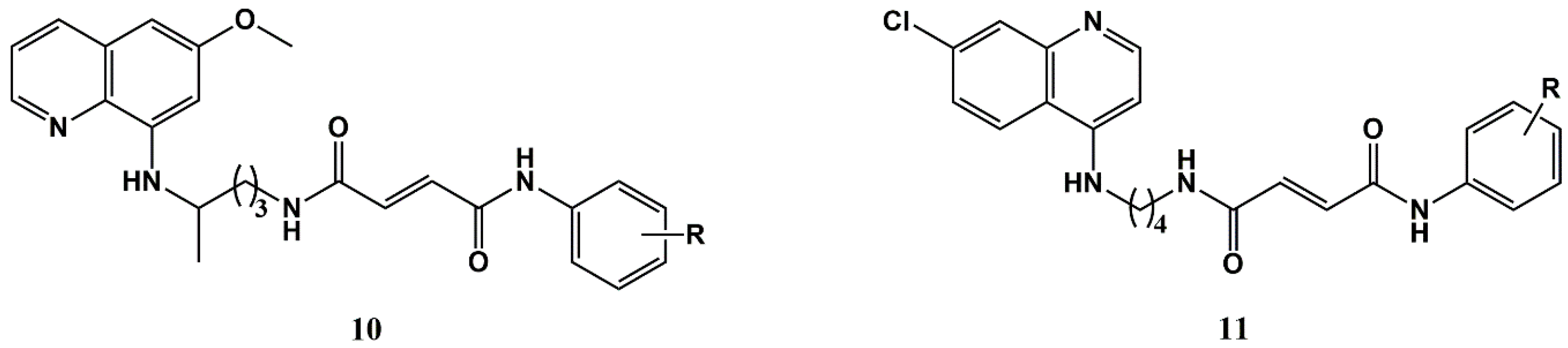
2. Cinnamic Acid Conjugation in the Rescuing of Classical Antimalarial Drugs
3. Cinnamic Acids in the Repurposing of Antimalarial Drugs
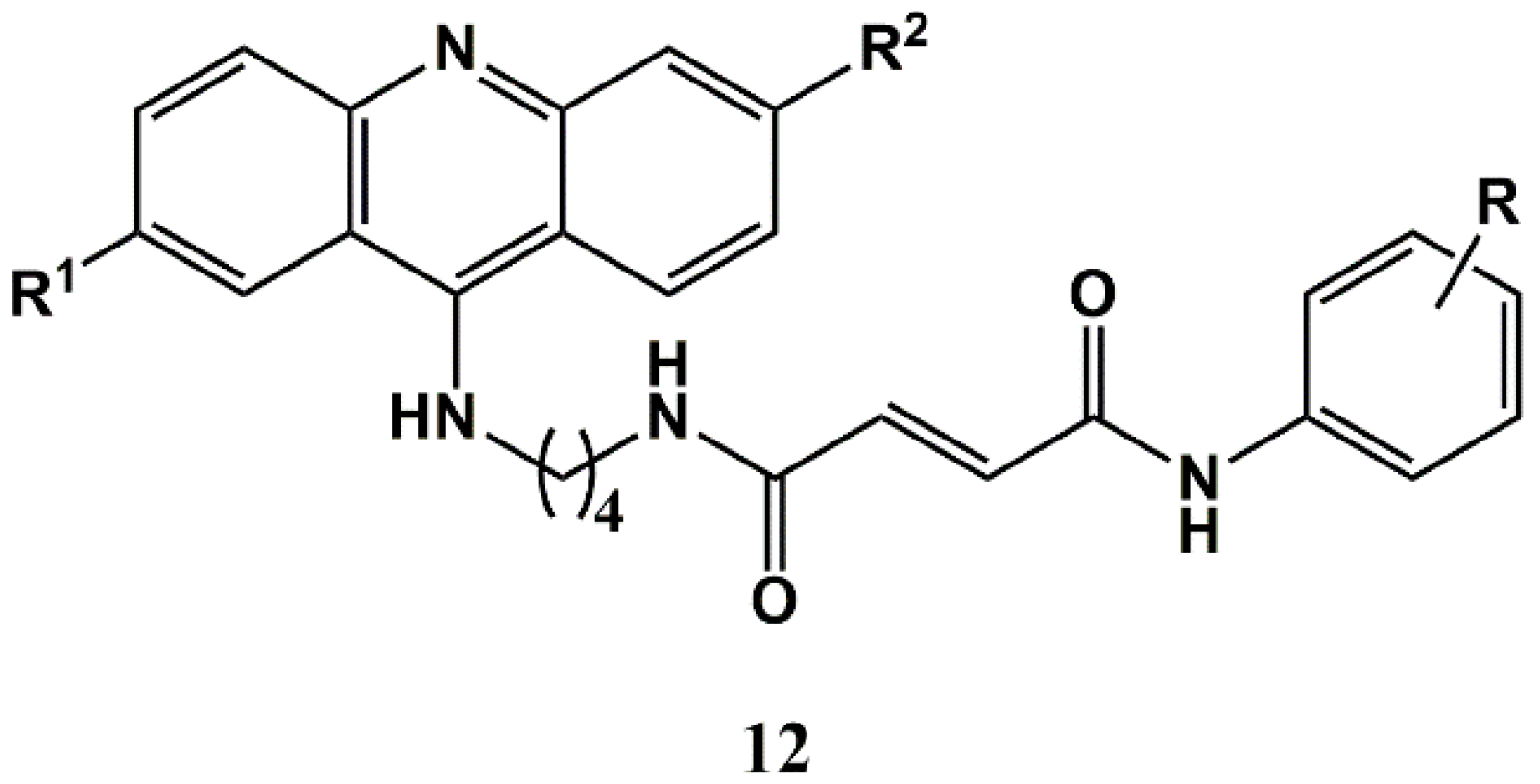
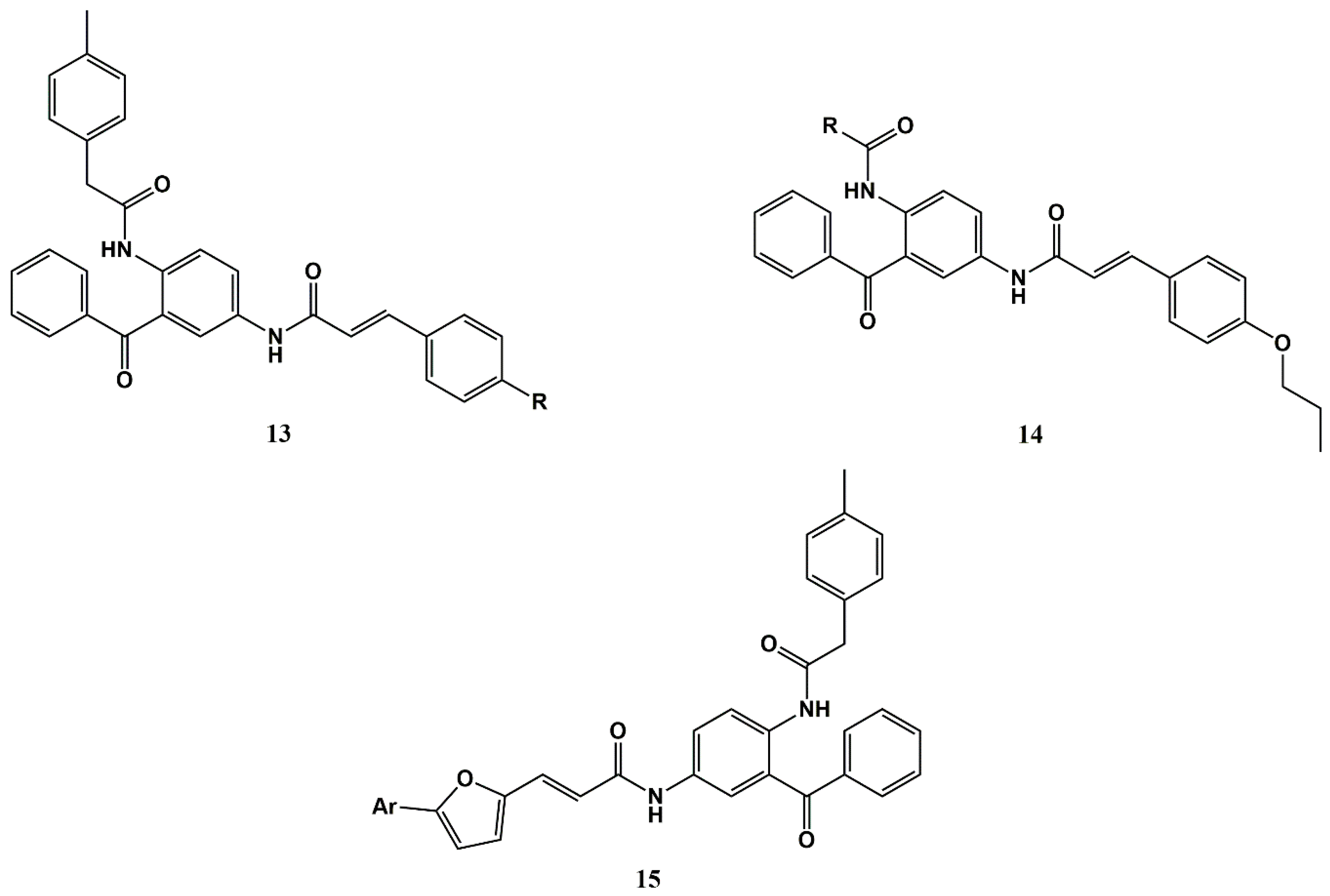
3.1. Repurposing Antimalarials for Cancer via Conjugation to Cinnamic Acids
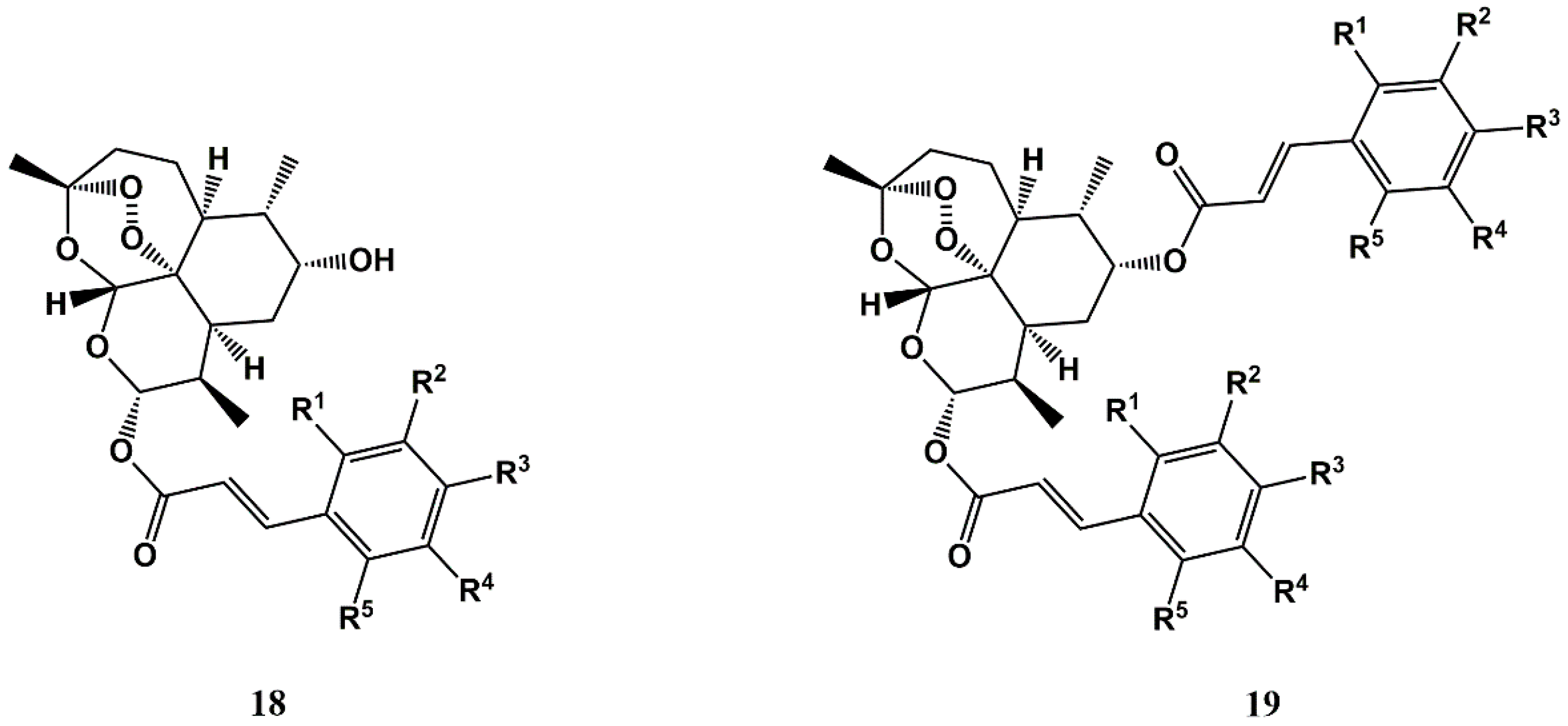
3.2. Repurposing Antimalarials for Other Infections via Conjugation to Cinnamic Acids
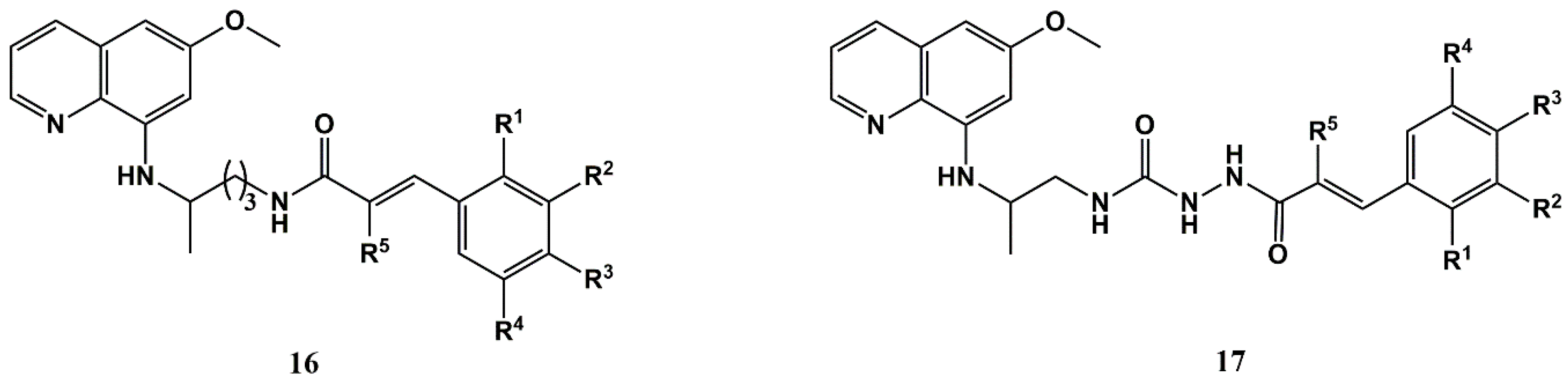
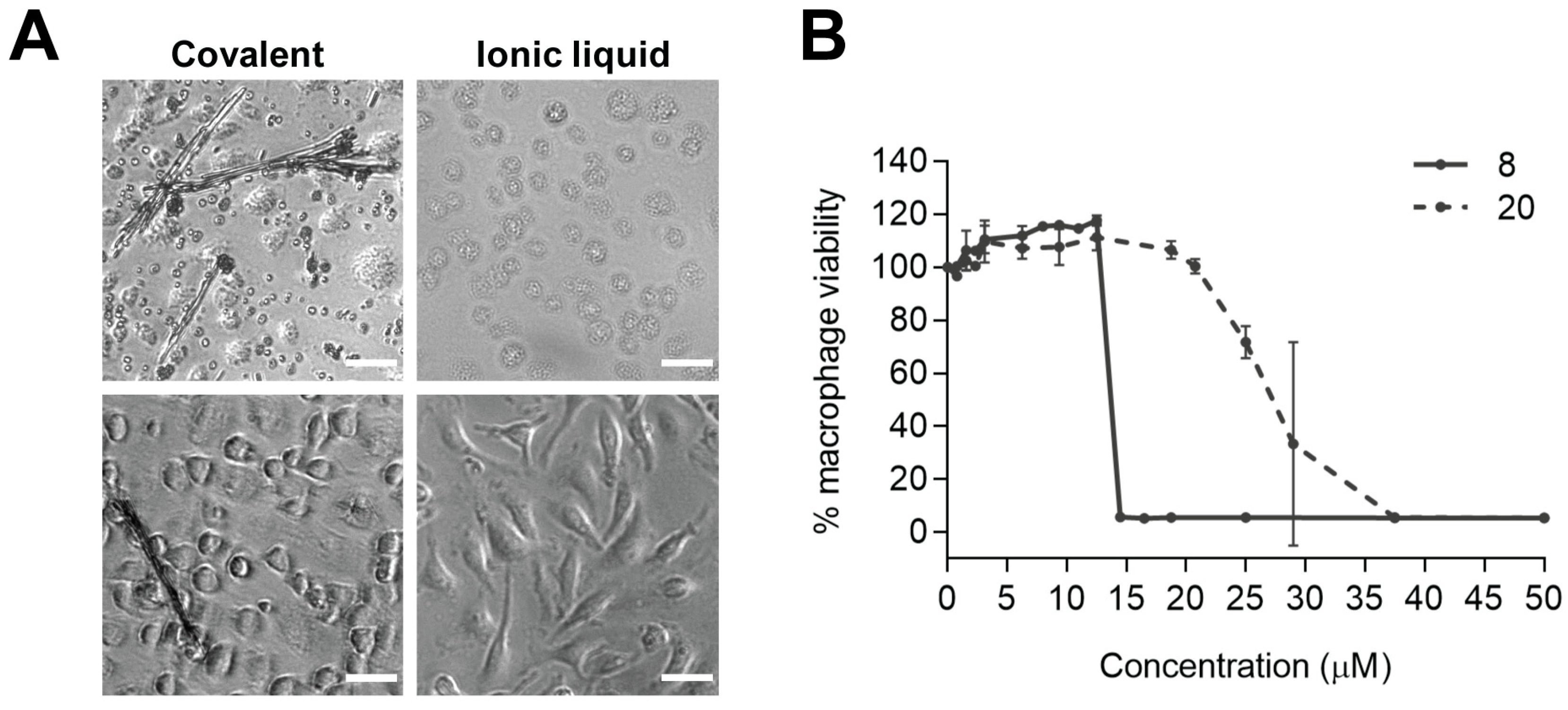
4. New Trends and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.-G.; Jo, Y.; Ameer, K.; Kwon, J.-H. Optimization of green extraction methods for cinnamic acid and cinnamaldehyde from cinnamon (Cinnamomum cassia) by response surface methodology. Food Sci. Biotechnol. 2018, 27, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Lingbeck, J.M.; O’Bryan, C.A.; Martin, E.M.; Adams, J.P.; Crandall, P.G. Sweetgum: An ancient source of beneficial compounds with modern benefits. Pharmacogn. Rev. 2015, 9, 1–11. [Google Scholar] [PubMed]
- Sharma, P. Cinnamic acid derivatives: A new chapter of various pharmacological activities. J. Chem. Pharm. Res. 2011, 3, 403–423. [Google Scholar]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, W.; Lu, Y.; Wang, Y.; Chen, X.; Bai, S.; Zhao, Y.; He, T.; Lao, F.; Shang, Y.; et al. Naturally occurring cinnamic acid sugar ester derivatives. Molecules 2016, 21, 1402. [Google Scholar] [CrossRef] [PubMed]
- Salvador, V.H.; Lima, R.B.; dos Santos, W.D.; Soares, A.R.; Bohm, P.A.; Marchiosi, R.; Ferrarese Mde, L.; Ferrarese-Filho, O. Cinnamic acid increases lignin production and inhibits soybean root growth. PLoS ONE 2013, 8, e69105. [Google Scholar] [CrossRef]
- Białecka-Florjańczyk, E.; Fabiszewska, A.; Zieniuk, B. Phenolic acids derivatives—Biotechnological methods of synthesis and bioactivity. Curr. Pharm. Biotechnol. 2018, 19, 1098–1113. [Google Scholar] [CrossRef]
- Celentano, A.; Tran, A.; Testa, C.; Thayanantha, K.; Tan-Orders, W.; Tan, S.; Syamal, M.; McCullough, M.J.; Yap, T. The protective effects of kava (Piper methysticum) constituents in cancers: A systematic review. J. Oral Pathol. Med. 2019, 48, 510–529. [Google Scholar] [CrossRef]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents-a review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef]
- Liu, L.; Hudgins, W.R.; Shack, S.; Yin, M.Q.; Samid, D. Cinnamic acid: A natural product with potential use in cancer intervention. Int. J. Cancer 1995, 62, 345–350. [Google Scholar] [CrossRef]
- Lu, M.; Li, T.; Wan, J.; Li, X.; Yuan, L.; Sun, S. Antifungal effects of phytocompounds on candida species alone and in combination with fluconazole. Int. J. Antimicrob. Agents 2017, 49, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D.; Mazumder, A.; Mazumder, R. Synthesis of cinnamanilide derivatives and their antioxidant and antimicrobial activity. J. Chem. 2015, 2015, 208910. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Abdulwanis Mohamed, Z.; Mohamed Eliaser, E.; Mazzon, E.; Rollin, P.; Cheng Lian Ee, G.; Abdull Razis, F.A. Neuroprotective potential of secondary metabolites from melicope lunu-ankenda (rutaceae). Molecules 2019, 24, 3109. [Google Scholar] [CrossRef]
- Gunia-Krzyzak, A.; Panczyk, K.; Waszkielewicz, A.M.; Marona, H. Cinnamamide derivatives for central and peripheral nervous system disorders—A review of structure-activity relationships. ChemMedChem 2015, 10, 1302–1325. [Google Scholar] [CrossRef]
- Priebe, A.; Hunke, M.; Tonello, R.; Sonawane, Y.; Berta, T.; Natarajan, A.; Bhuvanesh, N.; Pattabiraman, M.; Chandra, S. Ferulic acid dimer as a non-opioid therapeutic for acute pain. J. Pain Res. 2018, 11, 1075–1085. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Multi-target cinnamic acids for oxidative stress and inflammation: Design, synthesis, biological evaluation and modeling studies. Molecules 2018, 24, 12. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D.; et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Amano, R.; Yamashita, A.; Kasai, H.; Hori, T.; Miyasato, S.; Saito, S.; Yokoe, H.; Takahashi, K.; Tanaka, T.; Otoguro, T.; et al. Cinnamic acid derivatives inhibit hepatitis c virus replication via the induction of oxidative stress. Antivir. Res. 2017, 145, 123–130. [Google Scholar] [CrossRef] [PubMed]
- De, P.; De, K.; Veau, D.; Bedos-Belval, F.; Chassaing, S.; Baltas, M. Recent advances in the development of cinnamic-like derivatives as antituberculosis agents. Expert Opin. Ther. Pat. 2012, 22, 155–168. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Koumba Yoya, G.; Constant, P.; Bedos-Belval, F.; Duran, H.; Saffon, N.; Daffe, M.; Baltas, M. Design, synthesis, and biological evaluation of new cinnamic derivatives as antituberculosis agents. J. Med. Chem. 2011, 54, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Jain, R. New structural classes of antituberculosis agents. Med. Res. Rev. 2018, 38, 684–740. [Google Scholar] [CrossRef] [PubMed]
- Prithwiraj, D.; Florence, B.-B.; Corinne, V.-B.; Michel, B. Cinnamic acid derivatives in tuberculosis, malaria and cardiovascular diseases—A review. Curr. Org. Chem. 2012, 16, 747–768. [Google Scholar] [CrossRef]
- Kanaani, J.; Ginsburg, H. Effects of cinnamic acid derivatives on in vitro growth of Plasmodium falciparum and on the permeability of the membrane of malaria-infected erythrocytes. Antimicrob. Agents Chemother. 1992, 36, 1102–1108. [Google Scholar] [CrossRef]
- Seck, R.; Mansaly, M.; Gassama, A.; Cavé, C.; Cojean, S. Synthesis and antimalarial activity of cinnamic acid derivatives. Eur. J. Biomed. Pharm. Sci. 2019, 6, 450–454. [Google Scholar]
- Wiesner, J.; Mitsch, A.; Wißner, P.; Jomaa, H.; Schlitzer, M. Structure–activity relationships of novel anti-malarial agents. Part 2: Cinnamic acid derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 423–424. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lanteri, P.; Clement, Y.; Leonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and qsar (quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Cai, R. Antibacterial activity and mechanism of cinnamic acid and chlorogenic acid against Alicyclobacillus acidoterrestris vegetative cells in apple juice. Int. J. Food Sci. Tech. 2019, 54, 1697–1705. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Reddy, D.B.; Padukudru, M.A.; Chitturi, C.M.K.; Vimalambike, M.G.; Madhunapantula, S.V. Induction of colon and cervical cancer cell death by cinnamic acid derivatives is mediated through the inhibition of histone deacetylases (hdac). PLoS ONE 2017, 12, e0186208. [Google Scholar] [CrossRef] [PubMed]
- Hunke, M.; Martinez, W.; Kashyap, A.; Bokoskie, T.; Pattabiraman, M.; Chandra, S. Antineoplastic actions of cinnamic acids and their dimers in breast cancer cells: A comparative study. Anticancer Res. 2018, 38, 4469–4474. [Google Scholar] [CrossRef]
- Ka, H.; Park, H.J.; Jung, H.J.; Choi, J.W.; Cho, K.S.; Ha, J.; Lee, K.T. Cinnamaldehyde induces apoptosis by ros-mediated mitochondrial permeability transition in human promyelocytic leukemia hl-60 cells. Cancer Lett. 2003, 196, 143–152. [Google Scholar] [CrossRef]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.Y. ROS-mediated cancer cell killing through dietary phytochemicals. Oxid. Med. Cell Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef]
- Niero, E.L.; Machado-Santelli, G.M. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J. Exp. Clin. Cancer Res. 2013, 32, 31. [Google Scholar] [CrossRef]
- Qi, G.; Chen, J.; Shi, C.; Wang, Y.; Mi, S.; Shao, W.; Yu, X.; Ma, Y.; Ling, J.; Huang, J. Cinnamic acid (cinn) induces apoptosis and proliferation in human nasopharyngeal carcinoma cells. Cell Physiol. Biochem. 2016, 40, 589–596. [Google Scholar] [CrossRef]
- Zhu, B.; Shang, B.; Li, Y.; Zhen, Y. Inhibition of histone deacetylases by trans-cinnamic acid and its antitumor effect against colon cancer xenografts in athymic mice. Mol. Med. Rep. 2016, 13, 4159–4166. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Synergistic interaction of phenylpropanoids with antibiotics against bacteria. J. Med. Microbiol. 2010, 59, 1469–1476. [Google Scholar] [CrossRef]
- Peperidou, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Voulgari, E.; Avgoustakis, K. Multifunctional cinnamic acid derivatives. Molecules 2017, 22, 1247. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Peperidou, A.; Fotopoulos, I.; Hadjipavlou-Litina, D. Cinnamate hybrids: A unique family of compounds with multiple biological activities. Curr. Pharm. Biotechnol. 2018, 19, 1019–1048. [Google Scholar] [CrossRef] [PubMed]
- Zofou, D.; Tene, M.; Tane, P.; Titanji, V.P. Antimalarial drug interactions of compounds isolated from Kigelia africana (bignoniaceae) and their synergism with artemether, against the multidrug-resistant w2mef plasmodium falciparum strain. Parasitol. Res. 2012, 110, 539–544. [Google Scholar] [CrossRef]
- Marchetti, R.V.; Lehane, A.M.; Shafik, S.H.; Winterberg, M.; Martin, R.E.; Kirk, K. A lactate and formate transporter in the intraerythrocytic malaria parasite, Plasmodium falciparum. Nat. Commun. 2015, 6, 6721. [Google Scholar] [CrossRef] [PubMed]
- Zolg, J.W.; Alexander, J.M.; Scaife, J.G.; Beaudoin, R.L. The accumulation of lactic acid and its influence on the growth of Plasmodium falciparum in synchronized cultures. In Vitro 1984, 20, 205–215. [Google Scholar] [CrossRef]
- Ginsburg, H.; Krugliak, M.; Eidelman, O.; Ioav Cabantchik, Z. New permeability pathways induced in membranes of Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 1983, 8, 177–190. [Google Scholar] [CrossRef]
- Kirk, K.; Lehane, A.M. Membrane transport in the malaria parasite and its host erythrocyte. Biochem. J. 2014, 457, 1–18. [Google Scholar] [CrossRef]
- Sherman, I.W. Reflections on a century of malaria biochemistry. Adv. Parasitol. 2009, 67, 1–402. [Google Scholar] [PubMed]
- Staines, H.M.; Ellory, J.C.; Chibale, K. The new permeability pathways: Targets and selective routes for the development of new antimalarial agents. Comb. Chem. High Throughput Screen. 2005, 8, 81–88. [Google Scholar] [CrossRef]
- Masic, A.; Valencia Hernandez, A.M.; Hazra, S.; Glaser, J.; Holzgrabe, U.; Hazra, B.; Schurigt, U. Cinnamic acid bornyl ester derivatives from Valeriana wallichii exhibit antileishmanial in vivo activity in Leishmania major-infected BALB/c mice. PLoS ONE 2015, 10, e0142386. [Google Scholar] [CrossRef]
- Santos, A.; Fialho, S.N.; Medeiros, D.S.S.; Garay, A.F.G.; Diaz, J.A.R.; Gomez, M.C.V.; Teles, C.B.G.; Calderon, L.A. Antiprotozoal action of synthetic cinnamic acid analogs. Rev. Soc. Bras. Med. Trop. 2018, 51, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Silveira, G.R.; Campelo, K.A.; Lima, G.R.S.; Carvalho, L.P.; Samarao, S.S.; Vieira-da-Motta, O.; Mathias, L.; Matos, C.R.R.; Vieira, I.J.C.; Melo, E.J.T.; et al. In vitro anti-Toxoplasma gondii and antimicrobial activity of amides derived from cinnamic acid. Molecules 2018, 23, 774. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.; Teixeira, C.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.; Gomes, P. Cinnamic acid/chloroquinoline conjugates as potent agents against chloroquine-resistant Plasmodium falciparum. ChemMedChem 2012, 7, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.C.; Teixeira, C.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.B.; Prudêncio, M.; Gomes, P. N-cinnamoylated chloroquine analogues as dual-stage antimalarial leads. J. Med. Chem. 2013, 56, 556–567. [Google Scholar] [CrossRef]
- Pérez, B.C.; Teixeira, C.; Figueiras, M.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.B.; Gomes, P. Novel cinnamic acid/4-aminoquinoline conjugates bearing non-proteinogenic amino acids: Towards the development of potential dual action antimalarials. Eur. J. Med. Chem. 2012, 54, 887–899. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Benoit-Vical, F.; Robert, A.; Meunier, B. Preparation and antimalarial activities of “trioxaquines”, new modular molecules with a trioxane skeleton linked to a 4-aminoquinoline. ChemBioChem 2000, 1, 281–283. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- Nqoro, X.; Tobeka, N.; Aderibigbe, B.A. Quinoline-based hybrid compounds with antimalarial activity. Molecules 2017, 22, 2268. [Google Scholar] [CrossRef]
- Moles, E.; Galiano, S.; Gomes, A.; Quiliano, M.; Teixeira, C.; Aldana, I.; Gomes, P.; Fernandez-Busquets, X. Immunopegliposomes for the targeted delivery of novel lipophilic drugs to red blood cells in a falciparum malaria murine model. Biomaterials 2017, 145, 178–191. [Google Scholar] [CrossRef]
- Vale, N.; Moreira, R.; Gomes, P. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 2009, 44, 937–953. [Google Scholar] [CrossRef]
- Pérez, B.; Teixeira, C.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Prudêncio, M.; Gomes, P. Primacins, N-cinnamoyl-primaquine conjugates, with improved liver-stage antimalarial activity. MedChemComm 2012, 3, 1170–1172. [Google Scholar] [CrossRef]
- Beus, M.; Fontinha, D.; Held, J.; Rajic, Z.; Uzelac, L.; Kralj, M.; Prudêncio, M.; Zorc, B. Primaquine and chloroquine fumardiamides as promising antiplasmodial agents. Molecules 2019, 24, 2812. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Vale, N.; Pérez, B.; Gomes, A.; Gomes, J.R.; Gomes, P. “Recycling” classical drugs for malaria. Chem. Rev. 2014, 114, 11164–11220. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Pérez, B.; Albuquerque, I.; Machado, M.; Prudêncio, M.; Nogueira, F.; Teixeira, C.; Gomes, P. N-cinnamoylation of antimalarial classics: Quinacrine analogues with decreased toxicity and dual-stage activity. ChemMedChem 2014, 9, 305–310. [Google Scholar] [CrossRef]
- Pérez, B.; Teixeira, C.; Gomes, A.S.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Prudêncio, M.; Gomes, P. In vitro efficiency of 9-(N-cinnamoylbutyl)aminoacridines against blood- and liver-stage malaria parasites. Bioorg. Med. Chem. Lett. 2013, 23, 610–613. [Google Scholar] [CrossRef]
- Gomes, A.; Machado, M.; Lobo, L.; Nogueira, F.; Prudêncio, M.; Teixeira, C.; Gomes, P. N-cinnamoylation of antimalarial classics: Effects of using acyl groups other than cinnamoyl toward dual-stage antimalarials. ChemMedChem 2015, 10, 1344–1349. [Google Scholar] [CrossRef]
- Wiesner, J.; Kettler, K.; Jomaa, H.; Schlitzer, M. Structure–activity relationships of novel anti-malarial agents. Part 3: N-(4-acylamino-3-benzoylphenyl)-4-propoxycinnamic acid amides. Bioorg. Med. Chem. Lett. 2002, 12, 543–545. [Google Scholar] [CrossRef]
- Calderwood, D.J.; Johnston, D.N.; Munschauer, R.; Rafferty, P. Pyrrolo [2,3-d]pyrimidines containing diverse n-7 substituents as potent inhibitors of lck. Bioorg. Med. Chem. Lett. 2002, 12, 1683–1686. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Maes, H.; Schaaf, M.B.; Sukhatme, V.P.; Pantziarka, P.; Sukhatme, V.; Agostinis, P.; Bouche, G. Repurposing drugs in oncology (redo)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancer 2017, 11, 781. [Google Scholar] [CrossRef]
- Oien, D.B.; Pathoulas, C.L.; Ray, U.; Thirusangu, P.; Kalogera, E.; Shridhar, V. Repurposing quinacrine for treatment-refractory cancer. Semin. Cancer Biol. 2019, in press. [Google Scholar] [CrossRef]
- Yan, H.; Bian, A.; Gao, X.; Li, H.; Chen, Z.; Liu, X. Novel applications for an established antimalarial drug: Tumoricidal activity of quinacrine. Future Oncol. 2018, 14, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.C.; Fernandes, I.; Mateus, N.; Teixeira, C.; Gomes, P. Recycling antimalarial leads for cancer: Antiproliferative properties of n-cinnamoyl chloroquine analogues. Bioorg. Med. Chem. Lett. 2013, 23, 6769–6772. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, I.; Teixeira, C.; Mateus, N.; Sottomayor, M.J.; Gomes, P. A quinacrine analogue selective against gastric cancer cells: Insight from biochemical and biophysical studies. ChemMedChem 2016, 11, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Pavic, K.; Perkovic, I.; Gilja, P.; Kozlina, F.; Ester, K.; Kralj, M.; Schols, D.; Hadjipavlou-Litina, D.; Pontiki, E.; Zorc, B. Design, synthesis and biological evaluation of novel primaquine-cinnamic acid conjugates of the amide and acylsemicarbazide type. Molecules 2016, 21, 1629. [Google Scholar] [CrossRef]
- Mabeta, P.; Pavic, K.; Zorc, B. Insights into the mechanism of antiproliferative effects of primaquine-cinnamic acid conjugates on mcf-7 cells. Acta Pharm. 2018, 68, 337–348. [Google Scholar] [CrossRef]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012, 2012, 247597. [Google Scholar] [CrossRef]
- Slezáková, S.R.J. Anticancer activity of artemisinin and its derivatives. Anticancer Res. 2017, 37, 5995–6003. [Google Scholar]
- Xu, C.-C.; Deng, T.; Fan, M.-L.; Lv, W.-B.; Liu, J.-H.; Yu, B.-Y. Synthesis and in vitro antitumor evaluation of dihydroartemisinin-cinnamic acid ester derivatives. Eur. J. Med. Chem. 2016, 107, 192–203. [Google Scholar] [CrossRef]
- Uniprotkb—c3veh9. Available online: https://www.Uniprot.Org/uniprot/c3veh9 (accessed on 13 November 2019).
- Pérez, B.; Antunes, S.; Goncalves, L.M.; Domingos, A.; Gomes, J.R.; Gomes, P.; Teixeira, C. Toward the discovery of inhibitors of babesipain-1, a Babesia bigemina cysteine protease: In vitro evaluation, homology modeling and molecular docking studies. J. Comput. Aided Mol. Des. 2013, 27, 823–835. [Google Scholar] [CrossRef]
- Vale-Costa, S.; Costa-Gouveia, J.; Pérez, B.; Silva, T.; Teixeira, C.; Gomes, P.; Gomes, M.S. N-cinnamoylated aminoquinolines as promising antileishmanial agents. Antimicrob. Agents Chemother. 2013, 57, 5112–5115. [Google Scholar] [CrossRef]
- Loiseau, P.M.; Cojean, S.; Schrevel, J. Sitamaquine as a putative antileishmanial drug candidate: From the mechanism of action to the risk of drug resistance. Parasite 2011, 18, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Yardley, V.; Gamarro, F.; Croft, S.L. Antileishmanial and antitrypanosomal activities of the 8-aminoquinoline tafenoquine. Antimicrob. Agents Chemother. 2010, 54, 5356–5358. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.C.; Pérez, B.; Teixeira, C.; Gomes, P.; Figueiredo, L.M. In vitro activity of cinnamic acid conjugates of classic antimalarials against Trypanossoma brucei brucei. bioRxiv 2020. Manuscript in preparation. [Google Scholar]
- Gomes, A.; Ferraz, R.; Ficker, L.; Collins, M.S.; Prudêncio, C.; Cushion, M.T.; Teixeira, C.; Gomes, P. Chloroquine analogues as leads against pneumocystis lung pathogens. Antimicrob. Agents Chemother. 2018, 62, e00983-18. [Google Scholar] [CrossRef] [PubMed]
- Benfield, T.; Atzori, C.; Miller, R.F.; Helweg-Larsen, J. Second-line salvage treatment of aids-associated Pneumocystis jirovecii pneumonia: A case series and systematic review. J. Acquir. Immune Defic. Syndr. 2008, 48, 63–67. [Google Scholar] [CrossRef]
- Porollo, A.; Meller, J.; Joshi, Y.; Jaiswal, V.; Smulian, A.G.; Cushion, M.T. Analysis of current antifungal agents and their targets within the Pneumocystis carinii genome. Curr. Drug Targets 2012, 13, 1575–1585. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Ferraz, R.; Teixeira, C.; Gomes, P.; Prudêncio, C. Chapter 16—Bioactivity of ionic liquids. In Ionic Liquid Devices; The Royal Society of Chemistry: London, UK, 2018; pp. 404–422. [Google Scholar]
- Shamshina, J.L.; Berton, P.; Wang, H.; Zhou, X.; Gurau, G.; Rogers, R.D. Chapter 20–Ionic liquids in pharmaceutical industry. In Green Techniques for Organic Synthesis and Medicinal Chemistry, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 539–577. [Google Scholar]
- Shamshina, J.L.; Rogers, R.D. Overcoming the problems of solid state drug formulations with ionic liquids. Ther. Delivery 2014, 5, 489–491. [Google Scholar] [CrossRef]
- Ferraz, R.; Noronha, J.; Murtinheira, F.; Nogueira, F.; Machado, M.; Prudêncio, M.; Parapini, S.; D’Alessandro, S.; Teixeira, C.; Gomes, A.; et al. Primaquine-based ionic liquids as a novel class of antimalarial hits. RSC Adv. 2016, 6, 56134–56138. [Google Scholar] [CrossRef]
- Ferraz, R.; Pinheiro, M.; Gomes, A.; Teixeira, C.; Prudêncio, C.; Reis, S.; Gomes, P. Effects of novel triple-stage antimalarial ionic liquids on lipid membrane models. Bioorg. Med. Chem. Lett. 2017, 27, 4190–4193. [Google Scholar] [CrossRef]
- Silva, A.T.; Fontinha, D.; Lobo, L.; Oliveira, I.; Nogueira, F.; Prudêncio, M.; Marques, E.F.M.; Teixeira, C.; Ferraz, R.; Gomes, P. Combination of chloroquine with natural carboxylic acids: From poorly active cinnamates to potent antimalarial surface-active ionic liquids derived from fatty acids. J. Mol. Liq. 2020. Manuscript in preparation. [Google Scholar]
- Santos, M.M.; Raposo, L.R.; Carrera, G.; Costa, A.; Dionisio, M.; Baptista, P.V.; Fernandes, A.R.; Branco, L.C. Ionic liquids and salts from ibuprofen as promising innovative formulations of an old drug. ChemMedChem 2019, 14, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Deng, Z.; Zhou, B.; Qi, M.; Hong, M.; Ren, G. Improved transdermal permeability of ibuprofen by ionic liquid technology: Correlation between counterion structure and the physicochemical and biological properties. J. Mol. Liq. 2019, 283, 399–409. [Google Scholar] [CrossRef]
- Bento, C. Evaluation of the Effects of Selected Ionic Liquids Against Mycobacterium avium. Master’s Thesis, Universidade do Porto, Porto, Portugal, September 2019. [Google Scholar]
- Park, H.J.; Prausnitz, M.R. Lidocaine-ibuprofen ionic liquid for dermal anesthesia. AIChE J. 2015, 61, 2732–2738. [Google Scholar] [CrossRef]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic liquids as potential and synergistic permeation enhancers for transdermal drug delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef]
- No Time to Wait: Securing the Future from Drug-Resistant Infections. Available online: https://www.Who.Int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.Pdf?Sfvrsn=5b424d7_6 (accessed on 12 November 2019).

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.T.; Bento, C.M.; Pena, A.C.; Figueiredo, L.M.; Prudêncio, C.; Aguiar, L.; Silva, T.; Ferraz, R.; Gomes, M.S.; Teixeira, C.; et al. Cinnamic Acid Conjugates in the Rescuing and Repurposing of Classical Antimalarial Drugs. Molecules 2020, 25, 66. https://doi.org/10.3390/molecules25010066
Silva AT, Bento CM, Pena AC, Figueiredo LM, Prudêncio C, Aguiar L, Silva T, Ferraz R, Gomes MS, Teixeira C, et al. Cinnamic Acid Conjugates in the Rescuing and Repurposing of Classical Antimalarial Drugs. Molecules. 2020; 25(1):66. https://doi.org/10.3390/molecules25010066
Chicago/Turabian StyleSilva, Ana Teresa, Clara M. Bento, Ana C. Pena, Luísa M. Figueiredo, Cristina Prudêncio, Luísa Aguiar, Tânia Silva, Ricardo Ferraz, Maria Salomé Gomes, Cátia Teixeira, and et al. 2020. "Cinnamic Acid Conjugates in the Rescuing and Repurposing of Classical Antimalarial Drugs" Molecules 25, no. 1: 66. https://doi.org/10.3390/molecules25010066
APA StyleSilva, A. T., Bento, C. M., Pena, A. C., Figueiredo, L. M., Prudêncio, C., Aguiar, L., Silva, T., Ferraz, R., Gomes, M. S., Teixeira, C., & Gomes, P. (2020). Cinnamic Acid Conjugates in the Rescuing and Repurposing of Classical Antimalarial Drugs. Molecules, 25(1), 66. https://doi.org/10.3390/molecules25010066