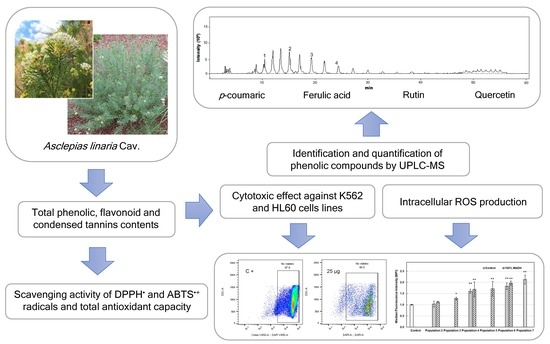
Phenolic Profile, Antioxidant and Anti-Proliferative Activities of Methanolic Extracts from Asclepias linaria Cav. Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic, Flavonoid, and Tannin Contents
2.2. Phenolic Compound Analysis by Ultra-Performance Liquid Chromatography Coupled to Mass Spectrometry (UPLC-MS)
2.3. Antioxidant Activity
2.4. Cytotoxic Activity
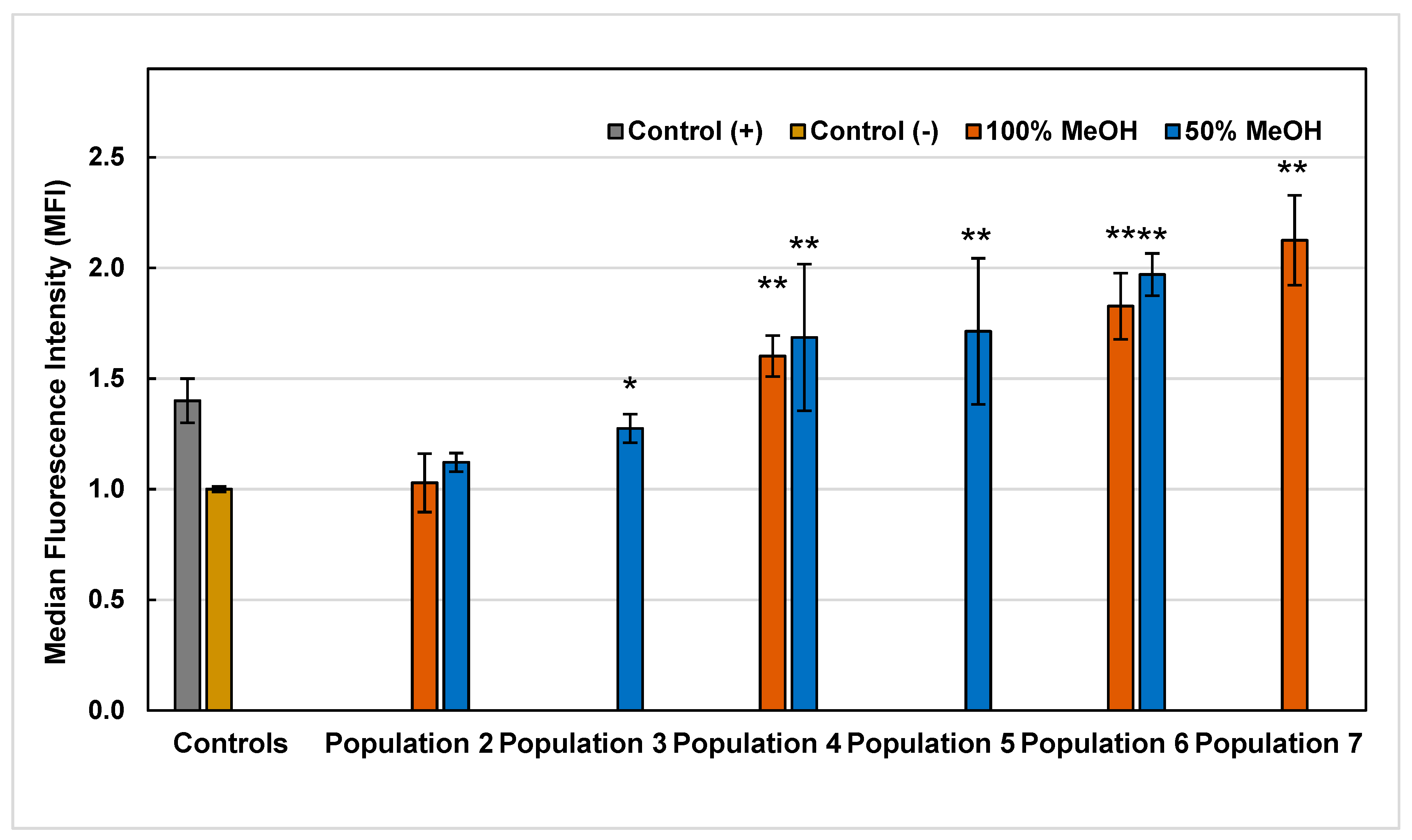
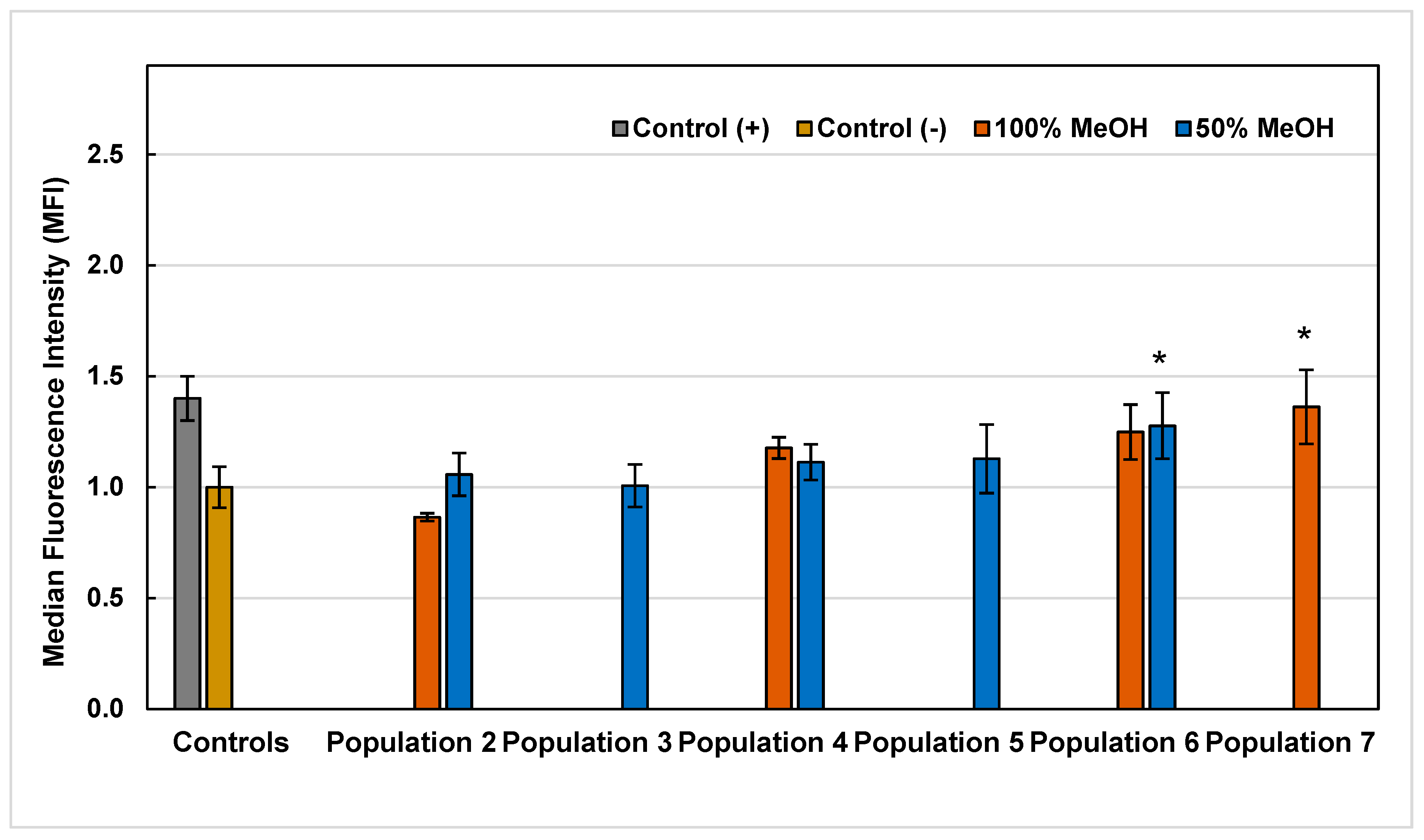
2.5. Reactive Oxygen Species (ROS)
3. Materials and Methods
3.1. Reagents and Standards
3.2. Plant Material and Sample Preparation
3.3. Determination of Total Phenolic, Flavonoid, and Tannin Contents
3.3.1. Determination of Total Phenolic Content
3.3.2. Determination of Total Flavonoid Content
3.3.3. Determination of Condensed Tannins
3.4. Phenolic Compounds Analysis by Ultra-Performance Liquid Chromatography Coupled to Mass Spectrometry (UPLC-MS)
3.4.1. Sample Preparation for Phenolic Compounds Determination by UPLC-MS
3.4.2. Identification and Quantification of Phenolic Compounds by UPLC-MS
3.5. Antioxidant Activity
3.5.1. DPPH• Free Radical Scavenging Assay
3.5.2. ABTS•+ Radical Scavenging Assay
3.5.3. Total Antioxidant Capacity
3.6. Cell Culture, Cytotoxic Effect, and Reactive Oxygen Species (ROS) Assay
3.6.1. Cell Lines
3.6.2. Cell Viability and Proliferation Assay
3.6.3. Selectivity Index
3.6.4. Reactive Oxygen Species (ROS) Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Endress, M.E.; Bruyns, P.V. A revised classification of the Apocynaceae s.l. Bot. Rev. 2000, 66, 1–56. [Google Scholar] [CrossRef] [Green Version]
- Endress, M. Apocynaceae: Brown and now. Telopea 2004, 10, 525–541. [Google Scholar]
- Kamel, E.A.R.; Sharawy, S.M.; Karakish, E.A.K. Cytotaxonomical investigations of the tribes Asclepiadeae and Ceropegieae of the subfamily Asclepiadoideae-Apocynaceae. Pakistan J. Bot. 2014, 46, 1351–1361. [Google Scholar]
- Juárez-Jaimes, V.; Alvarado-Cárdenas, L.O.; Villaseñor, J.L. La familia Apocynaceae sensu lato en México: Diversidad y distribución. Rev. Mex. Biodivers. 2007, 78, 459–482. [Google Scholar] [CrossRef] [Green Version]
- Fishbein, M.; Chuba, D.; Ellison, C.; Mason-Gamer, R.J.; Lynch, S.P. Phylogenetic Relationships of Asclepias (Apocynaceae) Inferred from Non-coding Chloroplast DNA Sequences. Syst. Bot. 2011, 36, 1008–1023. [Google Scholar] [CrossRef] [Green Version]
- Fishbein, M.; Straub, S.C.K.; Boutte, J.; Hansen, K.; Cronn, R.C.; Liston, A. Evolution at the tips: Asclepias phylogenomics and new perspectives on leaf surfaces. Am. J. Bot. 2018, 105, 514–524. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Laticifers, Latex, and Their Role in Plant Defense. Trends Plant. Sci. 2019, 24, 553–567. [Google Scholar] [CrossRef]
- Roy, M.C.; Chang, F.R.; Huang, H.C.; Chiang, M.Y.N.; Wu, Y.C. Cytotoxic principles from the Formosan milkweed, Asclepias curassavica. J. Nat. Prod. 2005, 68, 1494–1499. [Google Scholar] [CrossRef]
- Zhang, R.R.; Tian, H.Y.; Tan, Y.F.; Chung, T.Y.; Sun, X.H.; Xia, X.; Ye, W.C.; Middleton, D.A.; Fedosova, N.; Esmann, M.; et al. Structures, chemotaxonomic significance, cytotoxic and Na+,K+-ATPase inhibitory activities of new cardenolides from Asclepias curassavica. Org. Biomol. Chem. 2014, 12, 8919–8929. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Zhao, X.; Kariya, Y.; Fukata, H.; Teshigawara, K.; Uchida, A. Induction of Apoptosis by Quercetin: Involvement of Heat Shock Protein. Cancer Res. 1994, 54, 4952–4957. [Google Scholar]
- Warashina, T.; Noro, T. 8,12;8,20-diepoxy-8,14-secopregnane glycosides from the aerial parts of Asclepias tuberosa. Chem. Pharm. Bull. 2010, 58, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haribal, M.; Renwick, J.A.A. Oviposition stimulants for the monarch butterfly: Flavonol glycosides from Asclepias curassavica. Phytochemistry 1996, 41, 139–144. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. 5,11-epoxymegastigmanes from the leaves of Asclepias fruticosa. Chem. Pharm. Bull. 2000, 48, 1908–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araya, J.J.; Kindscher, K.; Timmermann, B.N. Cytotoxic cardiac glycosides and other compounds from Asclepias syriaca. J. Nat. Prod. 2012, 75, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Yamauchi, T.; Honda, K.; Hayashi, N. Conduritol F glucosides and terpenoid glucosides from Cynanchum liukiuense and distribution of conduritol F glucosides in several Asclepiadaceous plants. Chem. Pharm. Bull. 2000, 48, 1090–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Brewer, A.; Juárez-Jaimes, V.; Cortés-Zárraga, L. Usos de las especies del género Asclepias L. (Apocynaceae, Asclepiadoideae), información del Herbario Nacional de México, MEXU. Polibotánica 2008, 155–171. [Google Scholar]
- Vázquez-Sánchez, M.; Medina-Medrano, J.R.; Cortez-Madrigal, H.; Angoa-Pérez, M.V.; Muñoz-Ruíz, C.V.; Villar-Luna, E. Nematicidal activity of wild plant extracts against second-stage juveniles of Nacobbus aberrans. Nematropica 2018, 48, 136–144. [Google Scholar]
- Ignat, I.; Radu, D.G.; Volf, I.; Pag, A.I.; Popa, V.I. Antioxidant and antibacterial activities of some natural polyphenols. Cellul. Chem. Technol. 2013, 47, 387–399. [Google Scholar]
- Sikorska, M.; Matławska, I.; Głowniak, K.; Zgórka, G. Qualitative and quantitative analysis of phenolic acids in Asclepias syriaca L. Acta. Pol. Pharm.—Drug Res. 2000, 57, 69–72. [Google Scholar]
- Zavaleta, J.; Muñoz, A.M.; Blanco, T.; Alvarado-Ortiz, C.; Loja, B. Capacidad antioxidante y principales ácidos fenólicos y flavonoides de algunos alimentos TT - Antioxidant capacity and main phenolic acids and flavonoids of some foods. Horiz. méd. 2005, 5, 29–38. [Google Scholar]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT - Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Vidal, N.A.; Motidome, M.; Mancini, F.J.; Tanae, M.M.; Fallarero, L.A.; Brandão, T.L.; Lapa, A.J. Actividad antioxidante y ácidos fenólicos del alga marina Bryothamnion. Brazilian J. Pharm. Sci. 2001, 37, 373–382. [Google Scholar]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.P.; Yang, J.S.; Lin, J.J.; Lai, K.C.; Lu, H.F.; Ma, C.Y.; Sai-Chuen Wu, R.; Wu, K.C.; Chueh, F.S.; Gibson Wood, W.; et al. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Environ. Toxicol. 2012, 27, 480–484. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I. The Role of Phenolic Compounds in the Fight against Cancer—A Review. Anticancer. Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef]
- Conesa, C.M.; Ortega, V.V.; Yáñez-Gascón, M.J.; Baños, M.A.; Jordana, M.C.; Benavente-García, O.; Castillo, J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J. Agric. Food Chem. 2005, 53, 6791–6797. [Google Scholar] [CrossRef]
- Araújo, K.C.F.; Eula, E.M.; Pazini, F.; Valadares, M.C.; De Oliveira, V. Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products. Food Chem. Toxicol. 2013, 51, 93–96. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A. Los polifenoles de los alimentos y la salud. ANS. Aliment. Nutr. y salud 2003, 10, 41–53. [Google Scholar]
- Laughton, M.J.; Halliwell, B.; Evans, P.J.; Robin, J.; Hoult, S. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin. Effects on lipid peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochem. Pharmacol. 1989, 38, 2859–2865. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Miura, Y.H.; Tomita, I.; Watanabe, T.; Hirayama, T.; Fukui, S. Active oxygens generation by flavonoids. Biol. Pharm. Bull. 1998, 21, 93–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Flórez, S.; González-Gallego, J.; Culebras, J.M.; Tuñón, M.J. Los flavonoides: Propiedades y acciones antioxidantes. Nutr. Hosp. 2002, 17, 271–278. [Google Scholar] [PubMed]
- Escamilla, C.I.; Cuevas, E.Y.; Fonseca, J.G. Flavonoides y sus acciones antioxidantes. Rev. la Fac. Med. 2009, 52, 73–75. [Google Scholar]
- Nurmi, K.; Ossipov, V.; Haukioja, E.; Pihlaja, K. Variation of total phenolic content and individual low-molecular-weight phenolics in foliage of mountain birch trees (Betula pubescens ssp. tortuosa). J. Chem. Ecol. 1996, 22, 2023–2040. [Google Scholar] [CrossRef] [PubMed]
- Lamaison, J.L.; Carnart, A. Teneurs en principaux flavonoides des fleurs et des feuilles de Crataegus monogyna Jacq. et de Crataegus laevigata (poiret) dc. en fonction de la periode de vegetation. Plantes Med. Phyther. 1991, 25, 12–16. [Google Scholar]
- Seabra, I.J.; Dias, A.M.A.; Braga, M.E.M.; De Sousa, H.C. High pressure solvent extraction of maritime pine bark: Study of fractionation, solvent flow rate and solvent composition. J. Supercrit. Fluids 2012, 62, 135–148. [Google Scholar] [CrossRef]
- Barriada-Bernal, L.G.; Almaraz-Abarca, N.; Delgado-Alvarado, E.A.; Gallardo-Velázquez, T.; Ávila-Reyes, J.A.; Torres-Morán, M.I.; González-Elizondo, M.D.S.; Herrera-Arrieta, Y. Flavonoid composition and antioxidant capacity of the edible flowers of Agave durangensis (Agavaceae). CYTA - J. Food 2014, 12, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evidence-based Complement. Altern. Med. 2015, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Alvarado-Moreno, J.A.; Hernandez-Lopez, R.; Chavez-Gonzalez, A.; Yoder, M.C.; Rangel-Corona, R.; Isordia-Salas, I.; Hernandez-Juarez, J.; Cerbulo-Vazquez, A.; Gonzalez-Jimenez, M.A.; Majluf-Cruz, A. Endothelial colony-forming cells: Biological and functional abnormalities in patients with recurrent, unprovoked venous thromboembolic disease. Thromb. Res. 2016, 137, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Lorenzana, D.; Avilés-Vazquez, S.; Sandoval-Esquivel, M.A.; Alvarado-Moreno, A.; Ortiz-Navarrete, V.; Torres-Martínez, H.; Ayala-Sánchez, M.; Mayani, H.; Chavez-Gonzalez, A. CDKIs p18 INK4c and p57 Kip2 are involved in quiescence of CML leukemic stem cells after treatment with TKI. Cell Cycle 2016, 15, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
Sample Availability:Asclepias linaria Cav. and phenolic standards are available from the authors. |
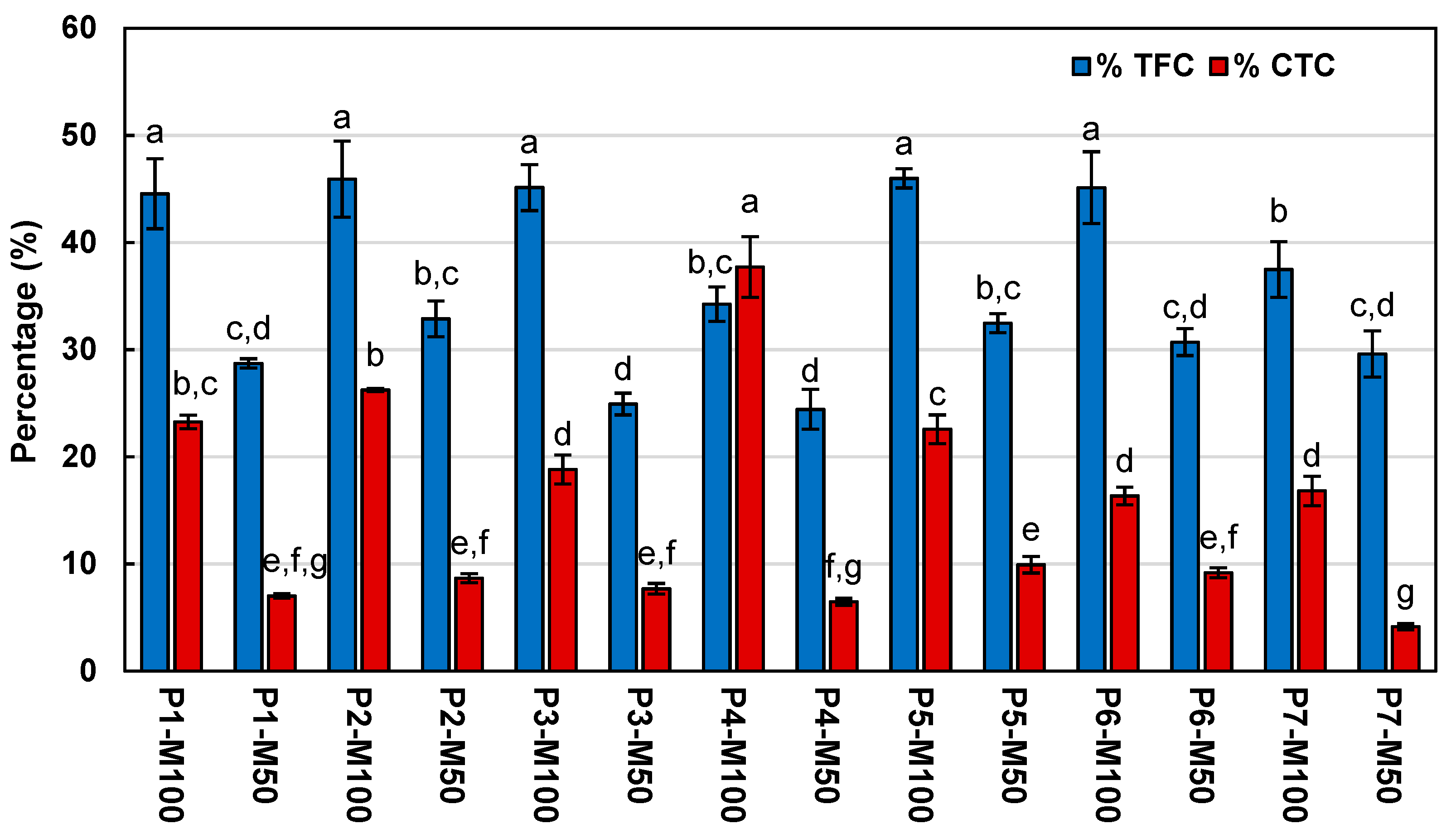
| Extract | TPC (mg GAE/g DT) | TFC (mg CE/g DT) | CTC (mg CE/g DT) | ABTS•+ | DPPH• | TAC (mg AAE/g DT) |
|---|---|---|---|---|---|---|
| IC50 (mg/mL) | ||||||
| P1-M100 | 7.17 ± 0.46 g | 3.19 ± 0.05 d,e,f | 1.67 ± 0.06 d | 0.82 ± 0.016 h | 0.16 ± 0.001 d | 63.78 ± 2.22 c |
| P1-M50 | 9.32 ± 0.27 b,c,d,e | 2.68 ± 0.11 e,f | 0.65 ± 0.02 h | 0.53 ± 0.009 a | 0.12 ± 0.001 a | 17.96 ± 1.47 g |
| P2-M100 | 8.77 ± 0.09 d,e | 4.02 ± 0.31 a,b | 2.30 ± 0.03 b | 0.74 ± 0.008 g | 0.16 ± 0.001 c,d | 74.47 ± 6.08 b |
| P2-M50 | 9.76 ± 0.37 a,b,c | 3.21 ± 0.28 d,e,f | 0.85 ± 0.03 f,g,h | 0.67 ± 0.002 f | 0.15 ± 0.001 b,c | 60.56 ± 0.78 c,d |
| P3-M100 | 8.68 ± 0.20 d,e | 3.92 ± 0.26 a,b,c | 1.63 ± 0.14 d | 0.85 ± 0.012 i | 0.16 ± 0.001 c,d | 75.89 ± 3.85 b |
| P3-M50 | 10.49 ± 0.47 a | 2.62 ± 0.21 e,f | 0.81 ± 0.02 f,g,h | 0.51 ± 0.003 a | 0.18 ± 0.002 e,f | 49.29 ± 1.55 e |
| P4-M100 | 8.38 ± 0.26 e,f | 2.87 ± 0.22 d,e,f | 3.16 ± 0.16 a | 0.68 ± 0.009 f | 0.19 ± 0.001 f | 78.74 ± 0.62 b |
| P4-M50 | 10.50 ± 0.27 a | 2.56 ± 0.26 f | 0.68 ± 0.03 g,h | 0.59 ± 0.003 b,c | 0.15 ± 0.001 b,c | 60.82 ± 0.82 c,d |
| P5-M100 | 9.07 ± 0.51 c,d,e | 4.17 ± 0.31 a | 2.05 ± 0.06 c | 0.60 ± 0.008 c,d | 0.15 ± 0.001 b,c | 81.24 ± 3.86 b |
| P5-M50 | 10.10 ± 0.15 a,b | 3.28 ± 0.04 c,d,e | 1.00 ± 0.08 f | 0.58 ± 0.007 b,c | 0.16 ± 0.001 c,d | 36.08 ± 0.39 f |
| P6-M100 | 7.48 ± 0.33 f,g | 3.38 ± 0.31 b,c,d | 1.22 ± 0.06 e | 0.75 ± 0.001 g | 0.18 ± 0.002 e | 99.77 ± 4.32 a |
| P6-M50 | 9.58 ± 0.22 a,b,c,d | 2.94 ± 0.10 d,e,f | 0.88 ± 0.05 f,g | 0.64 ± 0.008 e | 0.14 ± 0.001 b | 29.61 ± 1.25 f |
| P7-M100 | 8.66 ± 0.53 d,e | 3.24 ± 0.17 c,d,e | 1.46 ± 0.06 d | 0.62 ± 0.004 d,e | 0.15 ± 0.001 b,c | 95.49 ± 4.32 a |
| P7-M50 | 10.44 ± 0.09 a | 3.09 ± 0.25 d,e,f | 0.43 ± 0.03 i | 0.57 ± 0.002 b | 0.15 ± 0.001 b,c | 54.99 ± 0.60 d,e |
| Extract | p-coumaric acid | Ferulic acid | Rutin | Quercetin | Total | |
|---|---|---|---|---|---|---|
| P1-M100 | tR (min) | ND | 6.29 ± 0.01 | 7.15 ± 0.01 | 8.90 ± 0.00 | |
| µg/mL | ND | 0.49 ± 0.02 g | 37.53 ± 1.10 e | 11.33 ± 0.23 c,d | 49.35 ± 0.62 f,g | |
| P1-M50 | tR (min) | ND | 6.27 ± 0.01 | 7.15 ± 0.00 | 8.87 ± 0.06 | |
| µg/mL | ND | 0.40 ± 0.00 g,h | 30.00 ± 0.40 f | 13.47 ± 0.61 a,b,c | 43.87 ± 0.21 g | |
| P2-M100 | tR (min) | ND | 6.29 ± 0.01 | 7.15 ± 0.01 | 8.91 ± 0.01 | |
| µg/mL | ND | 0.82 ± 0.06 e | 49.33 ± 0.57 d | 6.00 ± 0.00 h | 56.15 ± 0.13 d,e | |
| P2-M50 | tR (min) | ND | 6.29 ± 0.01 | 7.15 ± 0.00 | 8.91 ± 0.01 | |
| µg/mL | ND | 0.72 ± 0.06 e,f | 41.33 ± 1.52 e | 10.00 ± 1.00 d,e,f | 52.05 ± 0.42 e,f | |
| P3-M100 | tR (min) | ND | 6.28 ± 0.01 | 7.15 ± 0.00 | 8.90 ± 0.00 | |
| µg/mL | ND | 0.96 ± 0.00 d | 28.00 ± 0.00 f | 5.17 ± 0.58 h | 34.13 ± 1.34 h | |
| P3-M50 | tR (min) | ND | 6.28 ± 0.01 | 7.15 ± 0.01 | 8.91 ± 0.00 | |
| µg/mL | ND | 0.34 ± 0.02 h | 25.67 ± 0.70 f | 8.40 ± 0.69 f,g | 34.41 ± 0.50 h | |
| P4-M100 | tR (min) | 5.9 ± 0.01 | 6.27 ± 0.01 | 7.14 ± 0.00 | 8.90 ± 0.01 | |
| µg/mL | 1.0 ± 0.00 a | 0.75 ± 0.00 e,f | 51.50 ± 0.00 d | 6.72 ± 0.96 g,h | 59.97 ± 2.83 d | |
| P4-M50 | tR (min) | ND | 6.28 ± 0.02 | 7.15 ± 0.01 | 8.91 ± 0.01 | |
| µg/mL | ND | 0.49 ± 0.00 g | 37.67 ± 0.76 e | 13.00 ± 0.50 b,c | 51.16 ± 0.32 e,f | |
| P5-M100 | tR (min) | 5.89 ± 0.00 | 6.29 ± 0.03 | 7.15 ± 0.00 | 8.91 ± 0.01 | |
| µg/mL | 0.25 ± 0.01 b | 0.68 ± 0.10 f | 61.33 ± 4.50 c | 9.00 ± 1.00 e,f | 71.26 ± 1.34 c | |
| P5-M50 | tR (min) | ND | ND | 7.15 ± 0.00 | 8.90 ± 0.00 | |
| µg/mL | ND | ND | 16.53 ± 1.00 g | 15.00 ± 0.60 a,b | 31.53 ± 0.76 h | |
| P6-M100 | tR (min) | ND | 6.28 ± 0.01 | 7.15 ± 0.01 | 8.90 ± 0.01 | |
| µg/mL | ND | 1.13 ± 0.01 c | 72.33 ± 1.52 b | 6.33 ± 0.58 | 79.79 ± 0.82 b | |
| P6-M50 | tR (min) | ND | ND | 7.16 ± 0.01 | ND | |
| µg/mL | ND | ND | 0.73 ± 0.05 h | ND | 0.73 ± 0.45 i | |
| P7-M100 | tR (min) | ND | 6.31 ± 0.02 | 7.16 ± 0.01 | 8.99 ± 0.00 | |
| µg/mL | ND | 1.90 ± 0.12 a | 79.67 ± 2.51 a | 10.67 ± 0.58 d,e | 92.24 ± 1.37 a | |
| P7-M50 | tR (min) | ND | 6.28 ± 0.01 | 7.16 ± 0.01 | 8.91 ± 0.00 | |
| µg/mL | ND | 1.58 ± 0.04 b | 76.67 ± 3.51 a,b | 15.67 ± 1.53 a | 93.92 ± 0.30 a |
| Extract | K562 IC50 (µg/mL) | HL60 IC50 (µg/mL) | CMN IC50 (µg/mL) | SI K562 | SI HL60 |
|---|---|---|---|---|---|
| P1-M100 | 72.13 ± 7.59 b | 17.33 ± 0.43 d | 116.7 ± 13.57 c,d,e,f | 1.61 | 6.73 |
| P1-M50 | 28.22 ± 4.09 a | 16.79 ± 0.86 c,d | 18.7 ± 0.48 a,b | 0.66 | 1.11 |
| P2-M100 | 79.57 ± 10.98 b | 14.25 ± 0.85 b,c | 155 ± 1.94 d,e,f | 1.94 | 10.88 |
| P2-M50 | 12.05 ± 1.60 a | 11.31 ± 0.15 a | 33.6 ± 5.07 a,b,c | 2.77 | 2.97 |
| P3-M100 | 29.40 ± 2.03 a | 14.28 ± 0.94 b,c | 66.8 ± 7.14 a,b,c,d | 2.27 | 4.68 |
| P3-M50 | 153.47 ± 28.77 c | 17.53 ± 1.41d | 187.3 ± 43.56 f | 1.22 | 10.68 |
| P4-M100 | 18.78 ± 2.95 a | 15.54 ± 0.42 b,c,d | 100.4 ± 8.95 b,c,d,e,f | 5.34 | 6.46 |
| P4-M50 | 167.42 ± 24.03 c | 16.00 ± 0.89 b,c,d | 130.3 ± 10.74 d,e,f | 0.77 | 8.14 |
| P5-M100 | >400 d | 15.50 ± 0.83 b,c,d | ND | ND | ND |
| P5-M50 | 15.45 ± 0.56 a | 20.89 ± 1.59 e | 108.3 ± 15.49 c,d,e,f | 7.0 | 5.18 |
| P6-M100 | 10.07 ± 0.08 a | 17.45 ± 1.34 d | 96.8 ± 4.88 b,c,d,e | 9.58 | 5.55 |
| P6-M50 | 8.96 ± 0.29 a | 13.52 ± 0.87 a,b | 184.2 ± 13.19 e,f | 20.46 | 13.62 |
| P7-M100 | 9.52 ± 1.28 a | 11.27 ± 0.27 a | 155 ± 13.91 d,e,f | 16.31 | 13.75 |
| P7-M50 | 133.55 ± 14.36 c | 11.10 ± 0.48 a | 244.9 ± 96.16 f | 1.80 | 22.06 |
| Compound | tR (min) | Ion Mode | MRM (m/z) | Cone (V) | Collision (eV) |
|---|---|---|---|---|---|
| Vanillin | 5.46 | + | 153.01 ˃ 125.03 | 40 | 0 |
| (+)-Catechin | 3.97 | + | 291.15 ˃ 123.10 291.15 ˃ 165.10 | 13 13 | 13 10 |
| p-coumaric acid | 5.84 | - | 163.10 ˃ 119.00 | 20 | 10 |
| Gallic acid | 1.45 | - | 169.10 ˃ 125.00 | 15 | 12 |
| Caffeic acid | 4.61 | - | 179.10 ˃ 135.00 | 20 | 10 |
| Ferulic acid | 6.53 | - | 193.07 ˃ 133.90 193.07 ˃ 178.04 | 10 10 | 13 17 |
| Quercetin | 8.86 | - | 301.02 ˃ 150.99 301.02 ˃ 178.98 | 20 20 | 10 10 |
| Naringin | 9.11 | + | 273.20 ˃ 147.10 273.20 ˃ 153.05 | 35 35 | 20 20 |
| Apigenin | 9.97 | - | 269.15 ˃ 117.00 269.15 ˃ 151.00 | 30 30 | 37 23 |
| Rutin | 7.15 | - | 609.10 > 300.0 | 80 | 28 |
| Chlorogenic acid | 4.26 | - | 353.05 > 191.03 | 20 | 15 |
| Cinnamic acid | 9.01 | - | 146.80 > 103.00 | 10 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gutiérrez, J.A.; Moreno-Lorenzana, D.; Álvarez-Bernal, D.; Rodríguez-Campos, J.; Medina-Medrano, J.R. Phenolic Profile, Antioxidant and Anti-Proliferative Activities of Methanolic Extracts from Asclepias linaria Cav. Leaves. Molecules 2020, 25, 54. https://doi.org/10.3390/molecules25010054
Sánchez-Gutiérrez JA, Moreno-Lorenzana D, Álvarez-Bernal D, Rodríguez-Campos J, Medina-Medrano JR. Phenolic Profile, Antioxidant and Anti-Proliferative Activities of Methanolic Extracts from Asclepias linaria Cav. Leaves. Molecules. 2020; 25(1):54. https://doi.org/10.3390/molecules25010054
Chicago/Turabian StyleSánchez-Gutiérrez, José Alejandro, Dafné Moreno-Lorenzana, Dioselina Álvarez-Bernal, Jacobo Rodríguez-Campos, and José Roberto Medina-Medrano. 2020. "Phenolic Profile, Antioxidant and Anti-Proliferative Activities of Methanolic Extracts from Asclepias linaria Cav. Leaves" Molecules 25, no. 1: 54. https://doi.org/10.3390/molecules25010054