Enrichment of Polyglucosylated Isoflavones from Soybean Isoflavone Aglycones Using Optimized Amylosucrase Transglycosylation
Abstract
1. Introduction
2. Results and Discussion
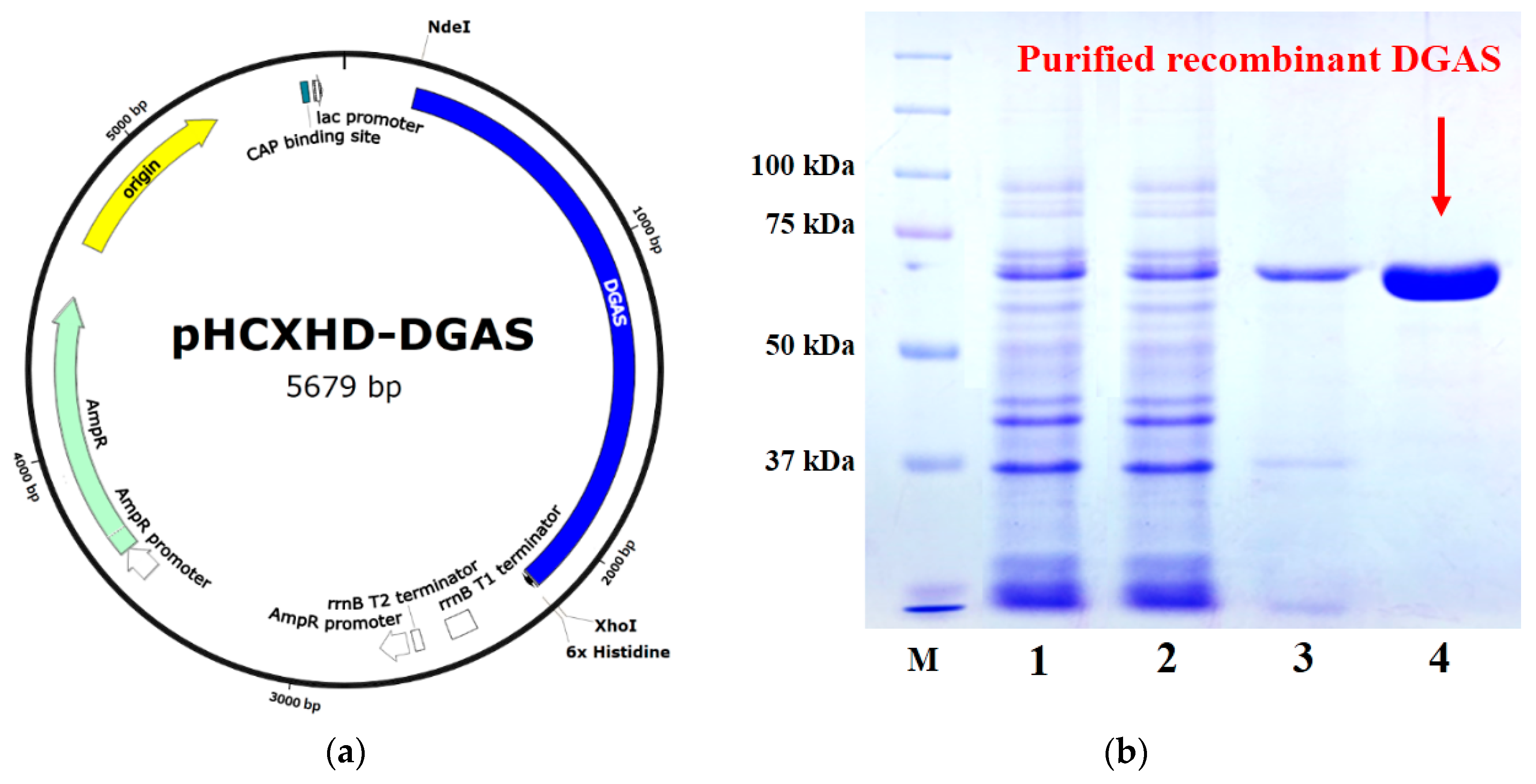
2.1. DGAS Expression and Activities
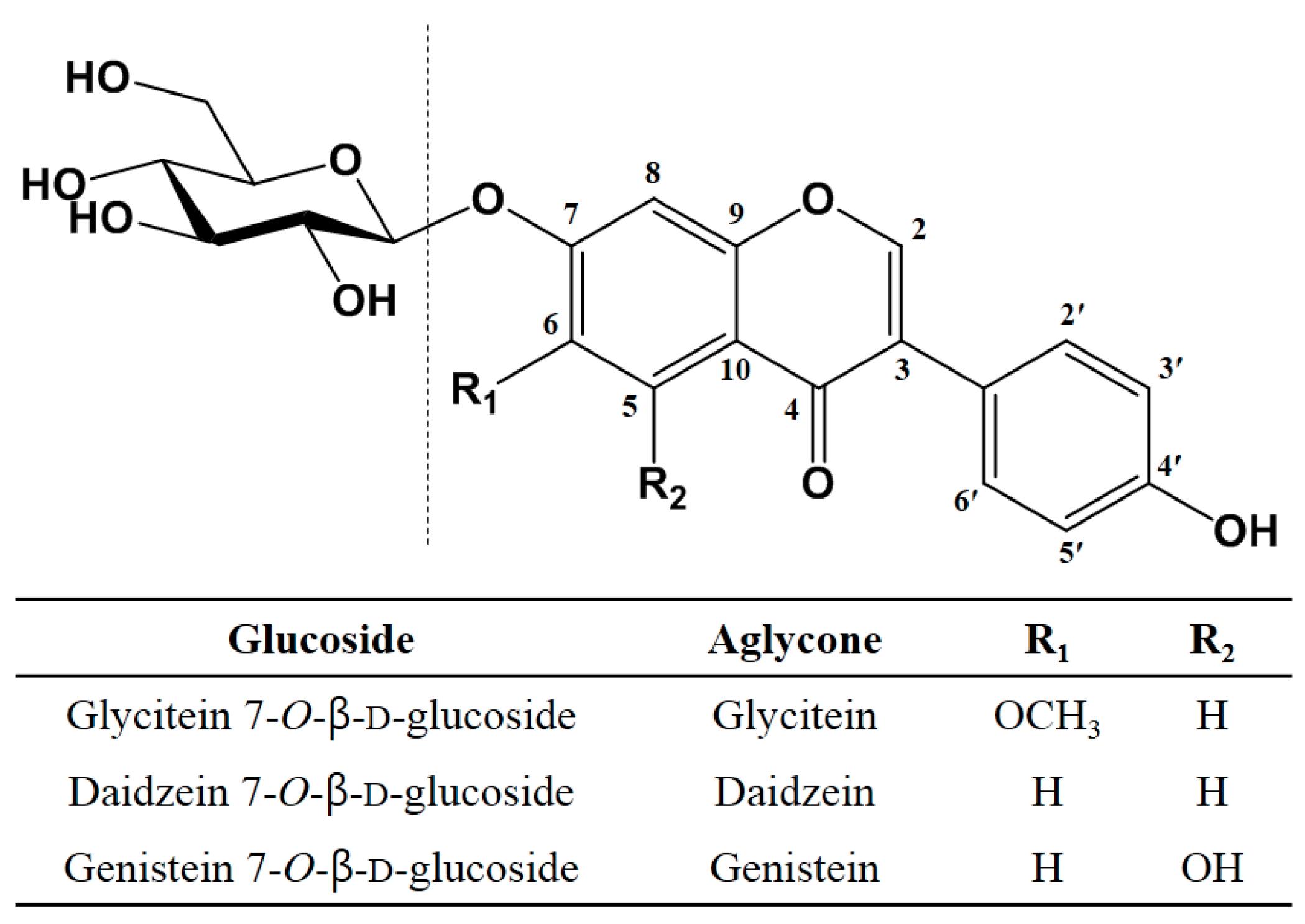
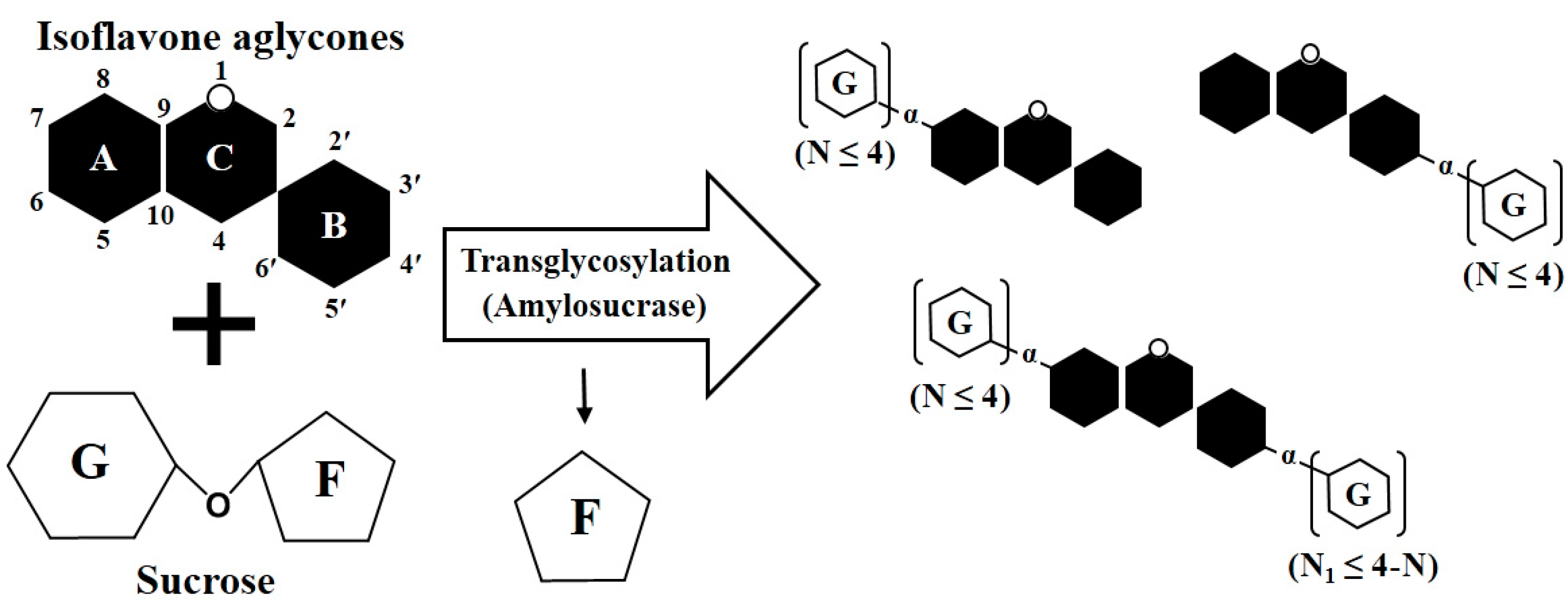
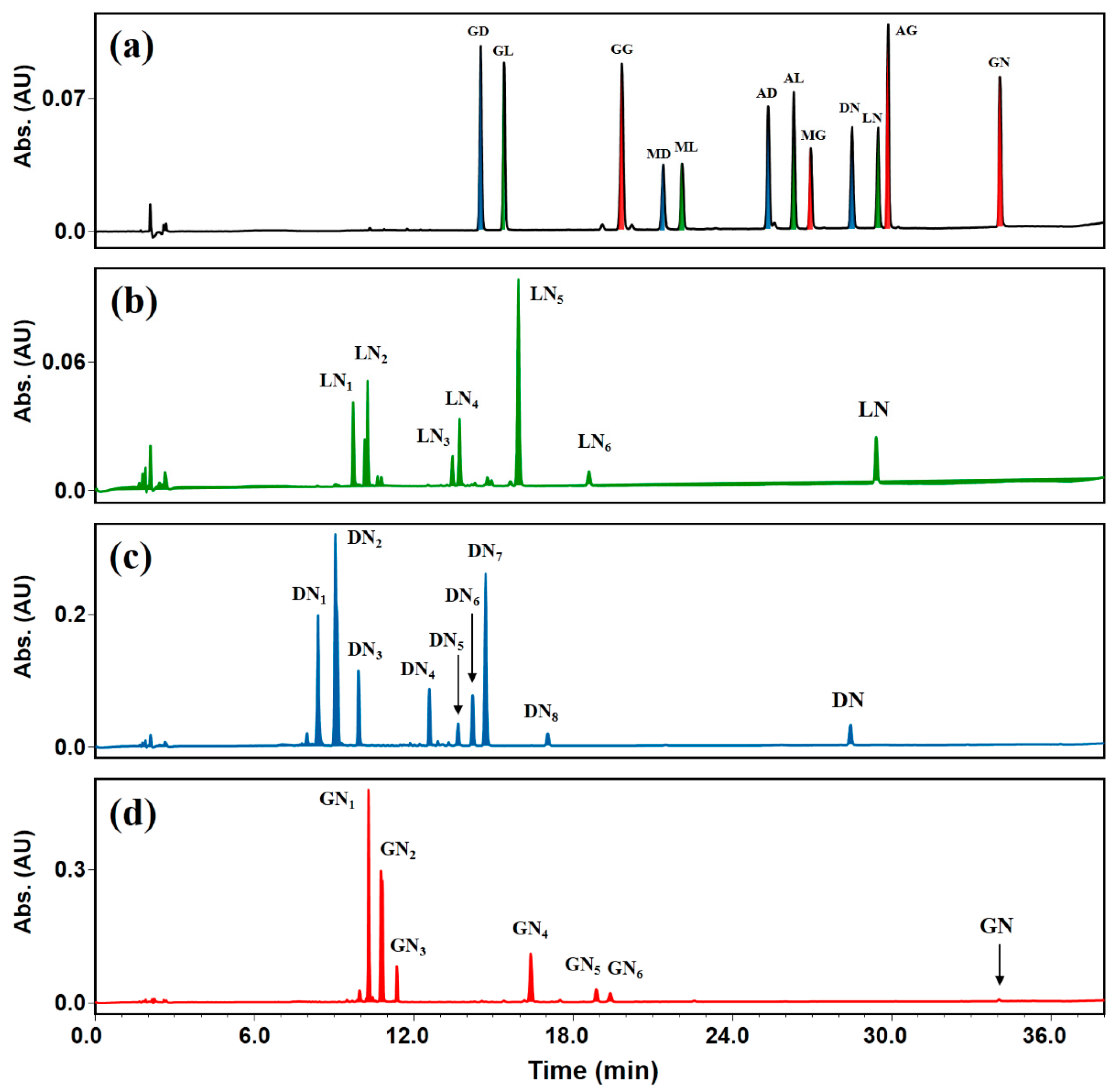
2.2. Transglycosylation of IFAs with DGAS
2.3. Effects of Reaction Conditions on DGAS Transglycosylation
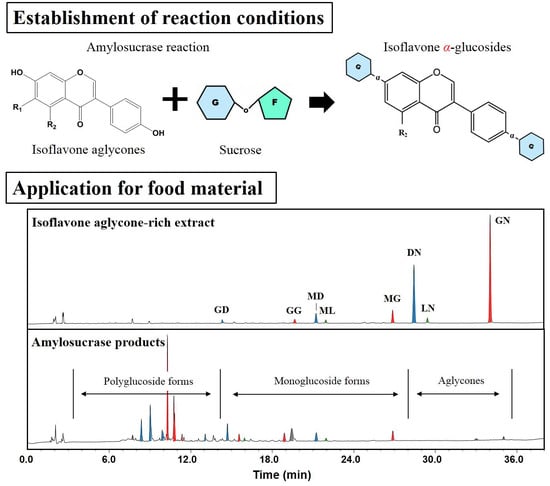
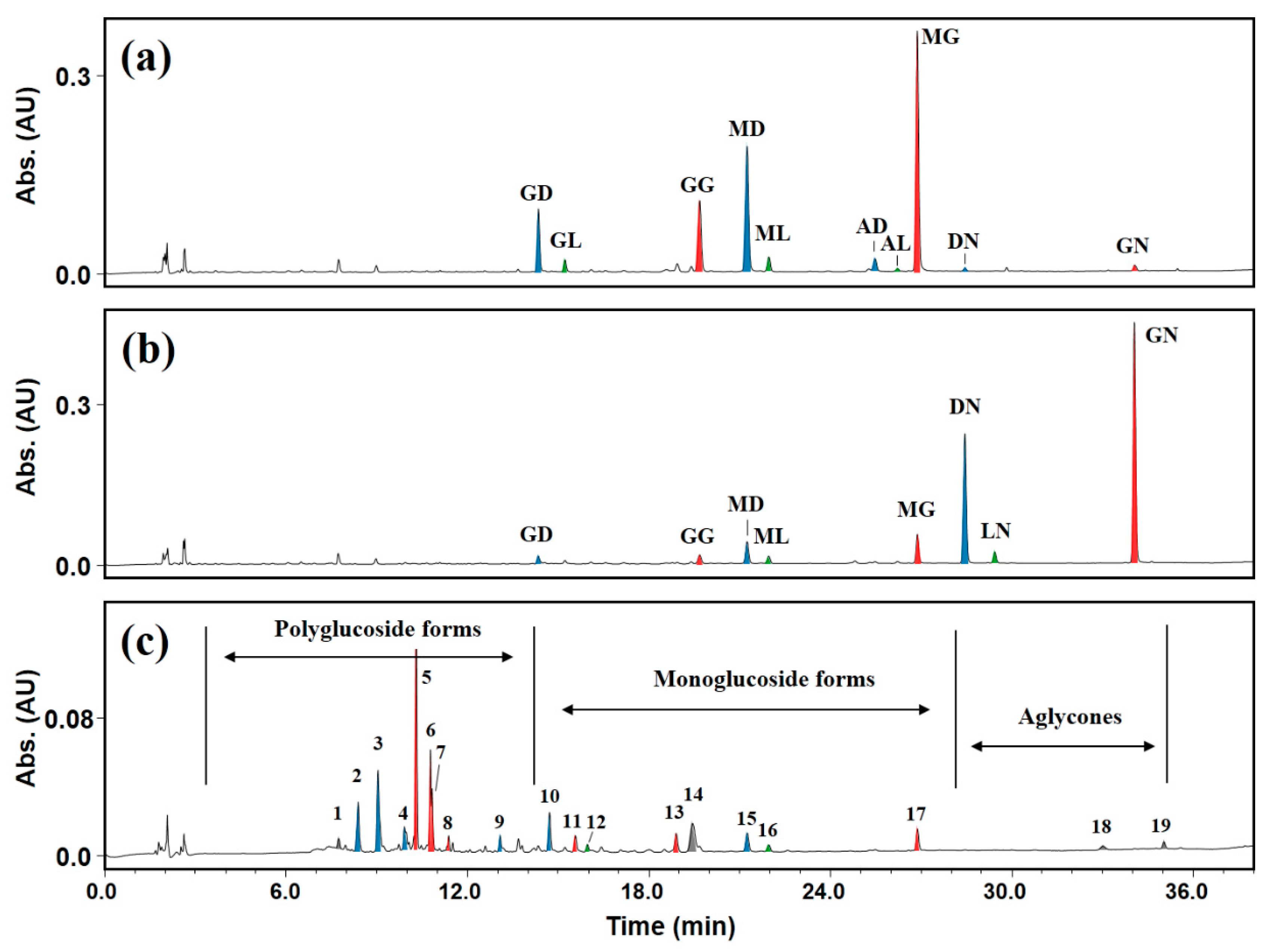
2.4. Application of DGAS to IFA-Rich Extract from Soybeans
3. Materials and Methods
3.1. Chemicals
3.2. Expression and Activity of DGAS
3.3. Transglycosylation of IFAs
3.4. Preparation and Transglycosylation of SBE
3.5. Analysis of Transglycosylated Isoflavones by HPLC and MS
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AS | amylosucrase |
| CE | cellulase |
| CE-SBE | cellulase-treated soybean extract |
| CE-SBE-DGAS | CE-SBE transglycosylated using DGAS |
| CGTase | cyclodextrin glycosyltransferase |
| DGAS | Deinococcus geothermalis amylosucrase |
| DMSO | dimethyl sulfoxide |
| HPLC | high-performance liquid chromatography |
| IFA | isoflavone aglycone |
| MS | mass spectrometry |
| SBE | soybean extract |
| QDa | quadrupole Dalton-based |
| UDP-glucose | uridine diphosphate glucose |
References
- Becker-Reshef, I.; Barkera, B.; Humber, M.; Puricelli, E.; Sanchez, A.; Sahajpal, R.; McGaughey, K.; Justice, C.; Baruth, B.; Wu, B.; et al. The GEOGLAM crop monitor for AMIS: Assessing crop conditions in the context of global markets. Glob. Food. Secur. 2019, 23, 173–181. [Google Scholar] [CrossRef]
- Veitch, N.C. Isoflavonoids of the leguminosae. Nat. Prod. Rep. 2013, 30, 988–1027. [Google Scholar] [CrossRef]
- Ko, K.-P. Isoflavones: Chemistry, analysis, functions and effects on health and cancer. Asian Pac. J. Cancer Prev. 2014, 15, 7001–7010. [Google Scholar] [CrossRef]
- Jackson, C.-J.C.; Dini, J.P.; Lavandier, C.; Rupasinghe, H.P.V.; Faulkner, H.; Poysa, V.; Buzzell, D.; DeGrandis, S. Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochem. 2002, 37, 1117–1123. [Google Scholar] [CrossRef]
- Zaheer, K.; Akhtar, M.H. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Stancanelli, R.; Mazzaglia, A.; Tommasini, S.; Calabrò, M.L.; Villari, V.; Guardo, M.; Ficarra, P.; Ficarra, R. The enhancement of isoflavones water solubility by complexation with modified cyclodextrins: A spectroscopic investigation with implications in the pharmaceutical analysis. J. Pharm. Biomed. Anal. 2007, 44, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, A.; Saito, A.; Homma, Y.; Nakao, M.; Sasaki, N.; Nishino, T.; Takahashi, S.; Nakayama, T. A UDP-glucose: Isoflavone 7-O-glucosyltransferase from the roots of soybean (Glycine max) seedlings: Purification, gene cloning, phylogenetics, and an implication for an alternative strategy of enzyme catalysis. J. Biol. Chem. 2007, 282, 23581–23590. [Google Scholar] [CrossRef] [PubMed]
- Szeja, W.; Grynkiewicz, G.; Rusin, A. Isoflavones, their glycosides and glycoconjugates. Synthesis and biological activity. Curr. Org. Chem. 2017, 21, 218–235. [Google Scholar] [CrossRef]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef]
- Nidetzky, B.; Gutmann, A.; Zhong, C. Leloir glycosyltransferases as biocatalysts for chemical production. Acs Catal. 2018, 8, 6283–6300. [Google Scholar] [CrossRef]
- Yang, M.; Davies, G.J.; Davis, B.G. A glycosynthase catalyst for the synthesis of flavonoid glycosides. Angew. Chem. Int. Ed. 2007, 46, 3885–3888. [Google Scholar] [CrossRef] [PubMed]
- De Winter, K.; Verlinden, K.; Křen, V.; Weignerová, L.; Soetaert, W.; Desmet, T. Ionic liquids as cosolvents for glycosylation by sucrose phosphorylase: Balancing acceptor solubility and enzyme stability. Green Chem. 2013, 15, 1949–1955. [Google Scholar] [CrossRef]
- De Roode, B.M.; Franssen, M.C.R.; van der Padt, A.; Boom, R.M. Perspectives for the industrial enzymatic production of glycosides. Biotechnol. Progr. 2003, 19, 1391–1402. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Bai, Y.; Zhang, T.; Jiang, B.; Mu, W. Identification of an α-(1,4)-glucan-synthesizing amylosucrase from Cellulomonas carboniz T26. J. Agric. Food Chem. 2017, 65, 2110–2119. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Xu, Z.; Li, M.; Chen, K.; Zhang, Y.; Hu, Z.; Zhang, M.; Zhang, Z.; Qiao, X.; et al. Highly promiscuous flavonoid 3-O-glycosyltransferase from Scutellaria baicalensis. Org. Lett. 2019, 21, 2241–2545. [Google Scholar] [CrossRef]
- Li, D.; Roh, S.-A.; Shim, J.-H.; Mikami, B.; Baik, M.-Y.; Park, C.-S.; Park, K.-H. Glycosylation of genistin into soluble inclusion complex form of cyclic glucans by enzymatic modification. J. Agric. Food Chem. 2005, 53, 6516–6524. [Google Scholar] [CrossRef]
- Kometani, T.; Nishimura, T.; Nakae, T.; Takii, H.; Okada, S. Synthesis of neohesperidin glycosides and naringin glycosides by cyclodextrin glucanotransferase from an alkalophilic Bacillus species. Biosci. Biotechnol. Biochem. 1996, 60, 645–649. [Google Scholar] [CrossRef]
- Sato, T.; Nakagawa, H.; Kurosu, J.; Yoshida, K.; Tsugane, T.; Shimura, S.; Kirimura, K.; Kino, K.; Usami, S. α-Anomer-selective glucosylation of (+)-catechin by the crude enzyme, showing glucosyl transfer activity, of Xanthomonas campestris WU-9701. J. Biosci. Bioeng. 2000, 90, 625–630. [Google Scholar] [CrossRef]
- Křen, V.; Martínková, L. Glycosides in medicine: “The role of glycosidic residue in biological activity”. Curr. Med. Chem. 2001, 8, 1303–1328. [Google Scholar] [CrossRef]
- Cho, H.-K.; Kim, H.-H.; Seo, D.-H.; Jung, J.-H.; Park, J.-H.; Baek, N.-I.; Kim, M.-J.; Yoo, S.-H.; Cha, J.; Kim, Y.-R.; et al. Biosynthesis of (+)-catechin glycosides using recombinant amylosucrase from Deinococcus geothermalis DSM 11300. Enzym. Microb. Technol. 2011, 49, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Gantt, R.W.; Peltier-Pain, P.; Cournoyer, W.J.; Thorson, J.S. Using simple donors to drive the equilibria of glycosyltransferase-catalyzed reactions. Nat. Chem. Biol. 2011, 7, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Woo, J.-B.; Ryu, S.-I.; Moon, S.-K.; Han, N.S.; Lee, S.-B. Glucosylation of flavonol and flavanones by Bacillus cyclodextrin glucosyltransferase to enhance their solubility and stability. Food Chem. 2017, 229, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, J.; Park, J.-H.; Baek, N.-I.; Park, C.-S.; Lee, H.-S.; Cha, J. Bioconversion of piceid to piceid glucoside using amylosucrase from Alteromonas macleodii deep ecotype. J. Microbiol. Biotech. 2012, 22, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Rha, C.-S.; Jung, Y.S.; Seo, D.-H.; Kim, D.-O.; Park, C.-S. Site-specific α-glycosylation of hydroxyflavones and hydroxyflavanones by amylosucrase from Deinococcus geothermalis. Enzym. Microb. Technol. 2019, 129. [Google Scholar] [CrossRef]
- Skov, L.K.; Mirza, O.; Henriksen, A.; De Montalk, G.P.; Remaud-Simeon, M.; Sarçabal, P.; Willemot, R.-M.; Monsan, P.; Gajhede, M. Amylosucrase, a glucan-synthesizing enzyme from the α-amylase Family. J. Biol. Chem. 2001, 276, 25273–25278. [Google Scholar] [CrossRef]
- Park, C.-S.; Park, I. The structural characteristics of amylosucrase-treated waxy corn starch and relationship between its in vitro digestibility. Food Sci. Biotechnol. 2017, 26, 381–387. [Google Scholar] [CrossRef]
- Seo, D.-H.; Jung, J.-H.; Jung, D.-H.; Park, S.; Yoo, S.-H.; Kim, Y.-R.; Park, C.-S. An unusual chimeric amylosucrase generated by domain-swapping mutagenesis. Enzym. Microb. Technol. 2016, 86, 7–16. [Google Scholar] [CrossRef]
- Kim, M.-D.; Jung, D.-H.; Seo, D.-H.; Jung, J.-H.; Seo, E.-J.; Baek, N.-I.; Yoo, S.-H.; Park, C.-S. Acceptor specificity of amylosucrase from Deinococcus radiopugnans and its application for synthesis of rutin derivatives. J. Microbiol. Biotech. 2016, 26, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.-S.; Choi, J.-M.; Jung, Y.S.; Kim, E.-R.; Ko, M.J.; Seo, D.-H.; Kim, D.-O.; Park, C.-S. High-efficiency enzymatic production of α-isoquercitrin glucosides by amylosucrase from Deinococcus geothermalis. Enzym. Microb. Technol. 2019, 120, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-W.; Cho, C.H.; Jung, Y.-S.; Rha, C.; Nam, T.-G.; Kim, D.-O.; Lee, Y.-G.; Baek, N.-I.; Park, C.-S.; Lee, B.-H.; et al. Enzymatic synthesis of α-flavone glucoside via regioselective transglucosylation by amylosucrase from Deinococcus geothermalis. PLoS ONE 2018, 13, e0207466. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef]
- Demchenko, A.V. 1,2-cis O-Glycosylation: Methods, strategies, principles. Curr. Org. Chem. 2003, 7, 35–79. [Google Scholar] [CrossRef]
- Kim, E.-R.; Rha, C.-S.; Jung, Y.S.; Choi, J.-M.; Kim, G.-T.; Jung, D.-H.; Kim, T.-J.; Seo, D.-H.; Kim, D.-O.; Park, C.-S. Enzymatic modification of daidzin using heterologously expressed amylosucrase in Bacillus subtilis. Food Sci. Biotechnol. 2019, 28, 165–174. [Google Scholar] [CrossRef]
- Nyska, A.; Hayashi, S.-m.; Koyanagi, M.; Davis, J.P.; Jokinen, M.P.; Ramot, Y.; Maronpot, R.R. Ninety-day toxicity and single-dose toxicokinetics study of alpha-glycosyl isoquercitrin in Sprague-Dawley rats. Food Chem. Toxicol. 2016, 97, 354–366. [Google Scholar] [CrossRef]
- Yatsu, F.K.J.; Koester, L.S.; Lula, I.; Passos, J.J.; Sinisterra, R.; Bassani, V.L. Multiple complexation of cyclodextrin with soy isoflavones present in an enriched fraction. Carbohydr. Polym. 2013, 98, 726–735. [Google Scholar] [CrossRef]
- Overwin, H.; Wray, V.; Hofer, B. Biotransformation of phloretin by amylosucrase yields three novel dihydrochalcone glucosides. J. Biotechnol. 2015, 211, 103–106. [Google Scholar] [CrossRef]
- Te Poele, E.M.; Valk, V.; Devlamynck, T.; van Leeuwen, S.S.; Dijkhuizen, L. Catechol glucosides act as donor/acceptor substrates of glucansucrase enzymes of Lactobacillus reuteri. Appl. Microbiol. Biotechnol. 2017, 101, 4495–4505. [Google Scholar] [CrossRef]
- Bertrand, A.; Morel, S.; Lefoulon, F.; Rolland, Y.; Monsan, P.; Remaud-Simeon, M. Leuconostoc mesenteroides glucansucrase synthesis of flavonoid glucosides by acceptor reactions in aqueous-organic solvents. Carbohydr. Res. 2006, 341, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Galviz-Quezada, A.; Ochoa-Aristizábal, A.M.; Zabala, M.E.A.; Ochoa, S.; Osorio-Tobón, J.F. Valorization of iraca (Carludovica palmata, Ruiz & Pav.) infructescence by ultrasound-assisted extraction: An economic evaluation. Food. Bioprod. Process. 2019, 118, 91–102. [Google Scholar]
- Fic, E.; Kędracka-Krok, S.; Jankowska, U.; Pirog, A.; Dziedzicka-Wasylewska, M. Comparison of protein precipitation methods for various rat brain structures prior to proteomic analysis. Electrophoresis 2010, 31, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Giusti, M.M. Effect of solvent polarity and acidity on the extraction efficiency of isoflavones from soybeans (Glycine max). J. Agric. Food Chem. 2005, 53, 3795–3800. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.L.; Chitchumroonchokchai, C.; Vodovotz, Y.; Failla, M.L. Isoflavone retention during processing, bioaccessibility, and transport by Caco-2 cells: Effects of source and amount of fat in a soy soft pretzel. J. Agric. Food Chem. 2012, 60, 12196–12203. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-M.; Sun, B.-L.; Han, F.-X.; Yan, S.-R.; Yang, H.; Akio, K. Rapid HPLC method for determination of 12 isoflavone components in soybean seeds. Agric. Sci. China 2011, 10, 70–77. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| DGAS (Units) | Sucrose (M) | Glycitein (mM) | Daidzein (mM) | Genistein (mM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 | 1.0 | 4.0 | 0.2 | 5.0 | 20 | 0.2 | 5.0 | 20 | ||
| 0.5 | 0.1 | 0.0 ± 0.0 c 1 | 1.9 ± 1.7 c | 3.9 ± 0.2 b | 0.0 ± 0.0 f | 4.3 ± 0.6 de | 2.4 ± 0.4 ef | 10.9 ± 2.3 c | 3.1 ± 1.3 de | 2.1 ± 0.1 e |
| 1.0 | 0.0 ± 0.0 c | 6.8 ± 0.8 a | 4.2 ± 0.2 b | 20.0 ± 2.5 b | 8.3 ± 1.0 cd | 2.7 ± 0.1 ef | 59.8 ± 0.4 b | 5.3 ± 0.3 d | 0.8 ± 0.0 e | |
| 2.0 | 0.0 ± 0.0 c | 6.5 ± 0.8 a | 4.9 ± 0.4 ab | 28.8 ± 3.0 a | 9.2 ± 1.0 c | 1.8 ± 0.2 ef | 68.6 ± 1.0 a | 3.5 ± 0.1 de | 1.2 ± 0.0 e | |
| 1.0 | 0.1 | 0.0 ± 0.0 d | 7.0 ± 0.2 c | 6.0 ± 0.3 c | 22.5 ± 3.2 d | 12.0 ± 0.9 e | 4.4 ± 1.1 f | 19.3 ± 1.9 d | 7.1 ± 0.6 e | 1.3 ± 0.0 e |
| 1.0 | 13.3 ± 2.5 b | 19.0 ± 1.7 a | 8.8 ± 0.4 bc | 50.0 ± 2.4 b | 32.5 ± 0.7 c | 6.9 ± 0.1 f | 53.1 ± 4.2 b | 28.5 ± 0.2 c | 4.8 ± 0.0 e | |
| 2.0 | 8.6 ± 3.6 bc | 20.8 ± 0.4 a | 23.2 ± 3.7 a | 55.5 ± 2.2 a | 32.4 ± 0.8 c | 4.8 ± 0.2 f | 62.5 ± 4.7 a | 24.7 ± 0.2 cd | 2.9 ± 0.2 e | |
| 5.0 | 0.1 | 20.8 ± 6.4 e | 35.0 ± 1.6 d | 28.2 ± 0.8 de | 72.0 ± 1.5 b | 65.0 ± 0.8 c | 23.3 ± 0.3 f | 77.3 ± 2.7 b | 74.8 ± 0.5 bc | 23.4 ± 0.0 e |
| 1.0 | 16.3 ± 1.5 e | 69.5 ± 9.2 b | 56.8 ± 2.2 c | 97.2 ± 2.8 a | 95.0 ± 0.1 a | 50.5 ± 0.0 d | 92.2 ± 5.1 a | 96.6 ± 1.0 a | 66.0 ± 0.2 d | |
| 2.0 | 18.0 ± 2.8 e | 88.8 ± 3.8 a | 52.1 ± 1.3 c | 97.5 ± 0.7 a | 96.9 ± 0.2 a | 36.9 ± 0.1 f | 92.1 ± 3.2 a | 98.2 ± 0.1 a | 70.1 ± 0.1 cd | |
| Isoflavone | SBE | CE-SBE | CE-SBE-DGAS |
|---|---|---|---|
| Glycitein | 0.0 ± 0.0 b 1 | 3.98 ± 0.2 a | 0.0 ± 0.0 b |
| Daidzein | 0.79 ± 0.13 b | 41.98 ± 1.26 a | 0.0 ± 0.0 c |
| Genistein | 1.0 ± 0.03 b | 53.63 ± 4.35 a | 0.0 ± 0.0 c |
| Peak No. | Time (min) | λmax (nm) | [M]+ (m/z) | Aglycone | Identified Compound |
|---|---|---|---|---|---|
| 1 | 7.74 | 219.6, 276.6 | n.d 1 | Unknown | Unknown |
| 2 | 8.38 | 248.1, 296.5 | 903.1 | Daidzein | Daidzein tetraglucoside |
| 3 | 9.04 | 248.1, 298.1 | 741.1 | Daidzein | Daidzein triglucoside |
| 4 | 9.91 | 248.1, 298.1 | 741.1 | Daidzein | Daidzein triglucoside |
| 5 | 10.30 | 257.1, 322.0 | 757.2 | Genistein | Genistein triglucoside |
| 6 | 10.77 | 257.1, 322.0 | 757.2 | Genistein | Genistein triglucoside |
| 7 | 10.83 | 257.6, 322.0 | 757.2 | Genistein | Genistein triglucoside |
| 8 | 11.38 | 257.6, 329.2 | 595.2 | Genistein | Genistein diglucoside |
| 9 | 13.08 | 248.1, 298.1 | 417.1 | Daidzein | Daidzein diglucoside |
| 10 | 14.77 | 245.7, 300.5 | 417.1 | Daidzein | Daidzein monoglucoside |
| 11 | 15.57 | 257.6, 322.0 | 595.2 | Genistein | Genistein diglucoside |
| 12 | 15.97 | 252.8, 319.6 | 447.1 | Glycitein | Glycitein monoglucoside |
| 13 | 18.90 | 257.6, 322.0 | 433.1 | Genistein | Genistein monoglucoside |
| 14 | 19.45 | 229.1, 271.8 | n.d | Unknown | Unknown |
| 15 | 21.25 | 248.1, 298.1 | n.d | Daidzein | Malonyldaidzin |
| 16 | 21.97 | 257.6, 317.2 | n.d | Glycitein | Malonylglycitin |
| 17 | 26.88 | 257.6, 324.4 | n.d | Genistein | Malonylgenistin |
| 18 | 33.02 | 267.1 | n.d | Unknown | Unknown |
| 19 | 35.04 | 233.8, 276.6 | n.d | Unknown | Unknown |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.S.; Kim, Y.-J.; Kim, A.T.; Jang, D.; Kim, M.-S.; Seo, D.-H.; Nam, T.G.; Rha, C.-S.; Park, C.-S.; Kim, D.-O. Enrichment of Polyglucosylated Isoflavones from Soybean Isoflavone Aglycones Using Optimized Amylosucrase Transglycosylation. Molecules 2020, 25, 181. https://doi.org/10.3390/molecules25010181
Jung YS, Kim Y-J, Kim AT, Jang D, Kim M-S, Seo D-H, Nam TG, Rha C-S, Park C-S, Kim D-O. Enrichment of Polyglucosylated Isoflavones from Soybean Isoflavone Aglycones Using Optimized Amylosucrase Transglycosylation. Molecules. 2020; 25(1):181. https://doi.org/10.3390/molecules25010181
Chicago/Turabian StyleJung, Young Sung, Ye-Jin Kim, Aaron Taehwan Kim, Davin Jang, Mi-Seon Kim, Dong-Ho Seo, Tae Gyu Nam, Chan-Su Rha, Cheon-Seok Park, and Dae-Ok Kim. 2020. "Enrichment of Polyglucosylated Isoflavones from Soybean Isoflavone Aglycones Using Optimized Amylosucrase Transglycosylation" Molecules 25, no. 1: 181. https://doi.org/10.3390/molecules25010181
APA StyleJung, Y. S., Kim, Y.-J., Kim, A. T., Jang, D., Kim, M.-S., Seo, D.-H., Nam, T. G., Rha, C.-S., Park, C.-S., & Kim, D.-O. (2020). Enrichment of Polyglucosylated Isoflavones from Soybean Isoflavone Aglycones Using Optimized Amylosucrase Transglycosylation. Molecules, 25(1), 181. https://doi.org/10.3390/molecules25010181